User login
Cutaneous lupus erythematosus (CLE) is a heterogeneous autoimmune disease that involves the skin. Cutaneous lupus erythematosus can be classified into various subtypes.1 These include, but are not limited to, acute CLE, subacute CLE, chronic CLE, intermittent CLE, lupus tumidus, and lupus profundus.1,2 The CLE subtypes have variable associations with systemic lupus erythematosus. For instance, some subtypes, such as acute CLE, are more strongly associated with systemic lupus erythematosus.
Treatment of CLE is similar to other autoimmune disorders. Although the US Food and Drug Administration (FDA) has not approved any treatments for CLE,3,4 the most common therapeutic options are disease-modifying antirheumatic drugs. Unfortunately, many of these treatments carry teratogenic effects. Because CLE predominantly affects women, particularly those of childbearing age, it is imperative to understand the available treatment options for those who are pregnant or considering pregnancy for an informed discussion with patients.5
For years, the gold standard when considering a medication during pregnancy was the FDA’s classification system. According to this system, medications were classified into 5 letter categories based on their potential teratogenicity, including A (no fetal risk), B (potential animal risk but inconclusive human studies), C (risk cannot be ruled out), D (evidence of fetal risk), and X (contraindicated in pregnancy). In 2014, the FDA decided to no longer use this classification system for medications approved after 2000.6 However, because many proposed treatment options for CLE were approved prior to 2001, we have summarized the commonly prescribed medications for CLE according to their prior FDA letter categories.
Treatment Options for CLE During Pregnancy
Prior to initiating systemic medications for the treatment of CLE, topical medications should be considered. Recommended treatment options include corticosteroids and calcineurin inhibitors.7 Compared with systemic medications, topical treatments carry minimal side effects, such as skin atrophy, that typically remain localized to areas of application.8 Moreover, even with extensive application, no correlation has been found between topical corticosteroid use and fetal growth,9 which suggests that topical steroids are safe in pregnancy and should be considered as a first-line treatment option for CLE. Calcineurin inhibitors also are considered safe based on their low level of absorption through the skin and are considered second-line topical treatment options in pregnancy.10
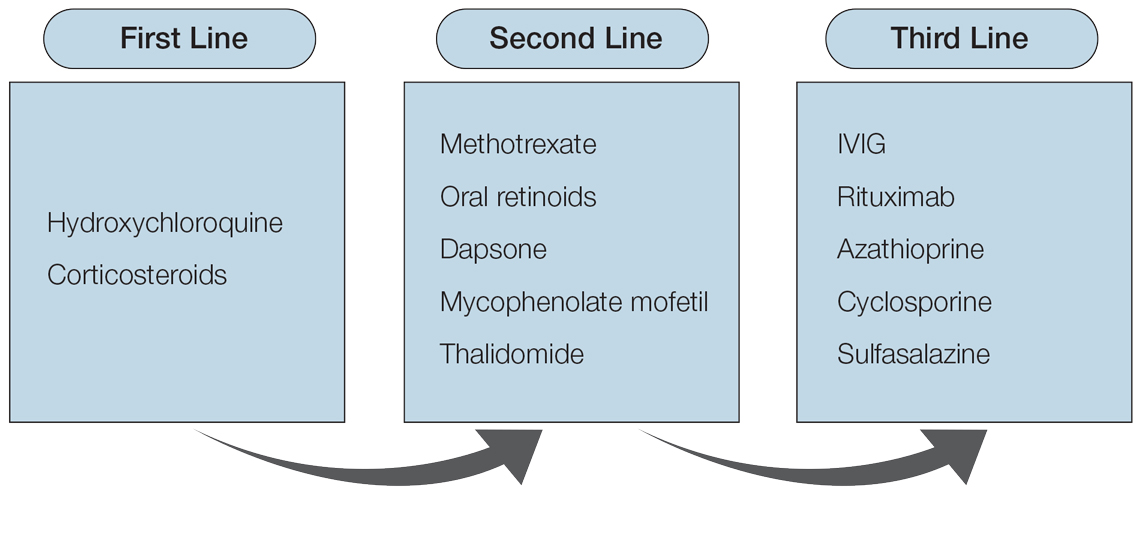
Although topical medications are effective for the treatment of CLE, many patients require the administration of systemic therapeutics for severe or refractory disease. Based on previously published reports, Figure 1 describes the current recommended systemic treatment options for CLE.11 Unfortunately, many of these medications carry teratogenic risks during pregnancy. The risks and side effects of the medications are described in detail in the following sections and summarized in the eTable.

Category B
Systemic Steroids—Systemic steroids are one of the most prescribed medications during pregnancy.12 Oral steroids have been associated with fast symptom relief, making this class of medications particularly effective during CLE flares; however, long-term management is not recommended because of the side effects, which include osteoporosis and impaired glucose metabolism.13
With low transmission across the placenta, there are 3 glucocorticoids that carry the safest profile in pregnancy: prednisone, cortisone, and hydrocortisone.14 Dexamethasone and betamethasone should be avoided, as both readily cross the placenta and increase fetal exposure.15 Although teratogenic effects have been associated with steroid use, most studies involving pregnant patients have inconclusive results. For instance, one study described an association between cleft lip/palate with in utero glucocorticoid exposure.16 However, multiple follow-up studies found no association between the two.17,18 Studies investigating the relationship between steroids and miscarriages or steroids and low birth weight also are inconclusive. Of note, if used throughout pregnancy, administration of a loading dose of glucocorticoids prior to delivery is recommended because of the increased stress brought on during labor.19
Sulfasalazine—Sulfasalazine is an immunomodulator commonly used for the treatment of inflammatory bowel disease and rheumatoid arthritis. However, studies also have shown that sulfasalazine is an effective treatment of CLE if standard treatments have failed.20,21
During pregnancy, patients exposed to sulfasalazine experienced minimal side effects despite transportation across the placenta.22 In comparison with control, pregnant women taking sulfasalazine experienced no increased risk for low fetal weight,23 congenital abnormalities,24 or spontaneous abortions.25 Of note, sulfasalazine can affect sperm, so male patients also should be counselled.
Category C
Hydroxychloroquine—Hydroxychloroquine is considered a first-line medication for those with CLE based on a symptomatic relief rate of 50% to 70%.26 For those taking hydroxychloroquine during pregnancy, the majority of studies have shown no association between the medication and adverse fetal events, including congenital abnormalities, prematurity, or spontaneous abortions.27-29 Therefore, hydroxychloroquine is considered safe in pregnancy, and those on the medication should continue standard monitoring, including retinopathy screening.30
Of note, hydroxychloroquine can be stored in tissue for weeks to months after discontinuation.5 Therefore, if patients wish to avoid hydroxychloroquine in pregnancy, one should stop taking the medication several months prior to conception.
Dapsone—Dapsone, a medication with both antimicrobial and immunomodulatory properties, is an effective second-line therapy for CLE.31 Although large-scale human trials have not been performed, multiple case reports and observational studies have supported the safe use of dapsone in pregnancy.32-34 However, there are notable side effects, including dose-dependent hemolysis, methemoglobinemia, and hypersensitivity reactions.13 Therefore, once treatment is initiated or continued, folic acid supplementation (5 mg daily) and regular serum analysis, including complete blood cell counts, are recommended in pregnant patients.19
Rituximab—Recent studies have demonstrated that rituximab can be an effective treatment of subacute and chronic CLE.35,36 Through inhibition of CD20, rituximab causes a decrease in circulating B cells and a reduced immune response. Therefore, experts recommend discontinuation of rituximab for 12 months prior to conception to reduce potential side effects to the fetus, which may include a transient reduction of circulating fetal B cells.37
If continued during pregnancy, most studies suggest discontinuation of rituximab during the third trimester, as it has been associated with neonatal infections and congenital abnormalities.19,37 However, these results are based on limited case reports, and thus robust research is needed to better understand the effect of rituximab in utero.
Intravenous Immunoglobulin Infusion—Intravenous immunoglobulin (IVIG) infusion is a well-tolerated treatment for many autoimmune disorders.38 Although not first line, limited case studies have demonstrated remission of refractory CLE following IVIG.39,40 Although no studies have directly investigated the effect of IVIG on fetal development, it has been frequently administered and well tolerated during pregnancy, especially in those with multiple sclerosis or antiphospholipid syndrome.41 Commonly reported side effects include headache and fatigue, and a rare associated side effect to be aware of is embolic events.42,43
Cyclosporine—Cyclosporine rarely is used in the treatment of localized CLE due to its extensive side-effect profile, most notably nephrotoxicity.44 However, studies have shown that cyclosporine may be efficacious if symptoms extend beyond the skin, involve multiple organs, and/or other treatments have failed.39 For those who are pregnant and wish to continue cyclosporine use, studies have associated low birth weight and premature delivery with its exposure in utero.44
Category D
Mycophenolate Mofetil—In conjunction with standard therapy, mycophenolate mofetil (MMF) is an adequate treatment of refractory CLE.45 Unfortunately, case reports have demonstrated an increased risk for fetal congenital abnormalities and first-trimester spontaneous abortion with use of MMF during pregnancy.46,47 Therefore, it is recommended that patients on MMF discontinue the medication at least 6 weeks prior to conception.46
Azathioprine—Although azathioprine has been shown to provide relief of discoid lupus erythematosus symptoms,48 it currently is only utilized for refractory disease, largely due to notable side effects that particularly affect the gastrointestinal tract and liver.4 Moreover, azathioprine use during pregnancy has been associated with prematurity, congenital anomalies, fetal cytopenia, and low birth weight.49 With that said, and although not recommended, if patients decide to continue treatment, experts recommend limiting the dose to 2 mg/kg daily to reduce potential adverse events.
Category X
Oral Retinoids—According to the American Academy of Dermatology, retinoids such as isotretinoin and acitretin are considered second-line therapy for CLE.50 With that being said, there are well-documented effects on fetal development associated with oral retinoid use, including central nervous system, cardiovascular system, and craniofacial abnormalities.51 Therefore, its use is contraindicated during pregnancy. To prevent pregnancy while taking isotretinoin, patients must enroll in an online monitoring program called iPLEDGE. This program requires monthly updates by both the physician and the patient, including a negative pregnancy test every month for female patients actively taking the medication.52
The half-lives of the oral retinoids isotretinoin and acitretin are 10 to 20 hours and 50 to 60 hours, respectively.53,54 However, alcohol consumption converts acitretin into the metabolite etretinate, which can remain in tissue for up to 120 days.54,55 Therefore, women are advised to avoid alcohol while taking acitretin and avoid conception for 2 to 3 years after cessation of the medication.55 For those wishing to restart retinoids after pregnancy, studies show the medication can be safely reinstated 35 days after delivery for those interested in continued treatment.56
Thalidomide—Although low-dose thalidomide can treat refractory CLE, its use is restricted because of its known teratogenicity, most notably limb deformities.57 If prescribed thalidomide, women will need to enroll in the System for Thalidomide Education and Prescribing Safety program, similar to the iPLEDGE program, and use 2 forms of contraception when sexually active.58 Contraception should be continued for 4 weeks following the last dose of thalidomide. After this point, conception is considered safe.59
Methotrexate—For nonpregnant patients, low-dose methotrexate (MTX) with folate supplementation is a treatment option for CLE.60 However, for those who are pregnant, low-dose MTX is an abortive agent and has been associated with aminopterin syndrome, which includes skull deficits, craniofacial abnormalities, and limb deformities in live births.19,61 Therefore, MTX is not recommended in pregnancy. Of note, MTX can affect sperm; male patients also should be counselled.
Final Thoughts
Overall, it is recommended to limit medication use as much as possible in pregnancy. To reduce these exposures, it is imperative to reduce triggers that may lead to symptomatic flares of CLE. Because CLE can be triggered by sun exposure, we advise topical sunscreen to prevent CLE flares that may require additional oral medication.62,63
Various medications are considered safe for the treatment of CLE in pregnant patients (Figure 2). Based on studies in animal and clinical trials, hydroxychloroquine is considered a safe and effective medication for CLE in pregnancy and is a first-line therapy in nonpregnant patients.26,27 If flares occur, IVIG or a short course of oral steroids should be considered to manage symptoms.13,39 For those with severe flares, treatment is difficult, and personalized approaches may be necessary.
Part of the question for the childbearing population is when a patient would like to conceive. For severe cases when hydroxychloroquine is not effective as monotherapy, using a treatment that can encourage remission prior to conception attempts can be a beneficial strategy. Rituximab is an excellent example of such a therapy, as the therapeutic effect outlasts the immunosuppressive effect and therefore is unlikely to affect a future fetus.64 Thalidomide also is a potential option prior to conception, based on its short washout period and its ability to achieve notable remission rates in patients with CLE.57,59 Regardless, patients with CLE should still consult their dermatologist and rheumatologist (if applicable) prior to conception.
Patients of childbearing potential represent a population in which discussion about life goals greatly affects medication options. Having these discussions early and often allows for an open, more successful approach so that treatment regimens are not derailed at the time of conception.
- Renner R, Sticherling M. The different faces of cutaneous lupus erythematosus. G Ital Dermatol Venereol. 2009;144:135-147.
- Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48:14-19.
- Shi H, Gudjonsson J, Kahlenberg J. Treatment of cutaneous lupus erythematosus: current approaches and future strategies. Curr Opin Rheumatol. 2020;32:208-214.
- Winkelmann RR, Kim GK, Del Rosso JQ. Treatment of cutaneous lupus erythematosus: review and assessment of treatment benefits based on Oxford Centre for Evidence-based Medicine criteria. J Clin Aesthet Dermatol. 2013;6:27-38.
- Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223-243.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;41:713-715.
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736-745.
- Kuhn A, Aberer E, Bata‐Csörgö Z, et al. S2k guideline for treatment of cutaneous lupus erythematosus—guided by the European Dermatology Forum (EDF) in cooperation with the European Academyof Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2017;31:389-404.
- Andersson NW, Skov L, Andersen JT. Evaluation of topical corticosteroid use in pregnancy and risk of newborns being small for gestational age and having low birth weight. JAMA Dermatol. 2021;157:788-795.
- Undre NA, Moloney FJ, Ahmadi S, et al. Skin and systemic pharmacokinetics of tacrolimus following topical application of tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2009;160:665-669.
- Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2014;10:97-107.
- Kuriya B, Hernández‐Díaz S, Liu J, et al. Patterns of medication use during pregnancy in rheumatoid arthritis. Arthritis Care Res. 2011;63:721-728.
- Chang A, Werth V. Treatment of cutaneous lupus. Curr Rheumatol Rep. 2011;13:300-307.
- Beitins IZ, Bayard F, Ances IG, et al. The transplacental passage of prednisone and prednisolone in pregnancy near term. J Pediatr. 1972;81:936-945.
- Ogueh O, Johnson MR. The metabolic effect of antenatal corticosteroid therapy. Hum Reprod Update. 2000;6:169-176.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Bay Bjørn A, Ehrenstein V, Hundborg HH, et al. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. Am J Ther. 2014;21:73-80.
- Hviid A, Mølgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ. 2011;183:796-804.
- Krause ML, Amin S, Makol A. Use of DMARDs and biologics during pregnancy and lactation in rheumatoid arthritis: what the rheumatologist needs to know. Ther Adv Musculoskelet Dis. 2014;6:169-184.
- Artuz F, Lenk N, Deniz N, et al. Efficacy of sulfasalazine in discoid lupus erythematosus. Int J Dermatol. 1996;35:746-748.
- Delaporte E, Catteau B, Sabbagh N, et al. Treatment of discoid lupus erythematosus with sulfasalazine: 11 cases [in French]. Ann Dermatol Venereol. 1997;124:151-156.
- Järnerot G, Into-Malmberg MB, Esbjörner E. Placental transfer of sulphasalazine and sulphapyridine and some of its metabolites. Scand J Gastroenterol. 1981;16:693-697.
- Norgard B, Pedersen L, Christensen LA, et al. Therapeutic drug use in women with Crohn’s disease and birth outcomes: a Danish nationwide cohort study. Am J Gastroenterol. 2007;102:1406-1413.
- Nørgård B, Czeizel AE, Rockenbauer M, et al. Population-based case control study of the safety of sulfasalazine use during pregnancy. Aliment Pharmacol Ther. 2001;15:483-486.
- Rahimi R, Nikfar S, Rezaie A, et al. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod Toxicol. 2008;25:271-275.
- Callen JP. Chronic cutaneous lupus erythematosus: clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416.
- Buchanan NM, Toubi E, Khamashta MA, et al. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann Rheum Dis. 1996;55:486-488.
- Costedoat‐Chalumeau N, Amoura Z, Duhaut P, et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty‐three cases compared with a control group. Arthritis Rheum. 2003;48:3207-3211.
- Sperber K, Hom C, Chao CP, et al. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009;7:9.
- Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377-1382.
- Klebes M, Wutte N, Aberer E. Dapsone as second-line treatment for cutaneous lupus erythematosus? a retrospective analysis of 34 patients and a review of the literature. Dermatology. 2016;232:91-96.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Varghese L, Viswabandya A, Mathew AJ. Dapsone, danazol, and intrapartum splenectomy in refractory ITP complicating pregnancy. Indian J Med Sci. 2008;62:452-455.
- Kahn G. Dapsone is safe during pregnancy. J Am Acad Dermatol. 1985;13:838-839.
- Quelhas da Costa R, Aguirre-Alastuey ME, Isenberg DA, et al. Assessment of response to B-cell depletion using rituximab in cutaneous lupus erythematosus. JAMA Dermatol. 2018;154:1432-1440.
- Alsanafi S, Kovarik C, Mermelstein A, et al. Rituximab in thetreatment of bullous systemic lupus erythematosus. J Clin Rheumatol. 2011;17:142-144.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506.
- Fernandez AP, Kerdel FA. The use of i.v. IG therapy in dermatology. Dermatol Ther. 2007;20:288-305.
- Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part II. J Am Acad Dermatol. 2011;65:E195-E213.
- Singh H, Naidu G, Sharma A. Intravenous immunoglobulin for the rescue in refractory cutaneous lupus. Indian Dermatol Online J. 2020;11:1003-1004.
- Clark AL. Clinical uses of intravenous immunoglobulin in pregnancy. Clin Obstet Gynecol. 1999;42:368-380.
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747-755.
- Woodruff RK, Grigg AP, Firkin FC, et al. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet. 1986;2:217-218.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Gammon B, Hansen C, Costner MI. Efficacy of mycophenolate mofetil in antimalarial-resistant cutaneous lupus erythematosus. J Am Acad Dermatol. 2010;65:717-721.e2.
- Abdulaziz HM, Shemies RS, Taman M, et al. Fetal proximal and distal limb anomalies following exposure to mycophenolate mofetil during pregnancy: a case report and review of the literature. Lupus. 2021;30:1522-1525.
- Pisoni CN, D’Cruz DP. The safety of mycophenolate mofetil in pregnancy. Exp Opin Drug Saf. 2008;7:219-222.
- Ashinoff R, Werth VP, Franks AG. Resistant discoid lupus erythematosus of palms and soles: successful treatment with azathioprine. J Am Acad Dermatol. 1988;19:961-965. doi:10.1016/S0190-9622(88)70259-5
- Goldstein LH, Dolinsky G, Greenberg R, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79:696-701.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for cutaneous lupus erythematosus. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:830-836.
- Sladden MJ, Harman KE. What is the chance of a normal pregnancy in a woman whose fetus has been exposed to isotretinoin? Arch Dermatol. 2007;143:1187-1188.
- Shin J, Cheetham TC, Wong L, et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- Brazzell RK, Colburn WA. Pharmacokinetics of the retinoids isotretinoin and etretinate. J Am Acad Dermatol. 1982;6:643-651.
- Pilkington T, Brogden RN. Acitretin: a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Gronhoj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
- Jajoria H, Mysore V. Washout period for pregnancy post isotretinoin therapy. Indian Dermatol Online J. 2020;11:239-242.
- Cortés-Hernández J, Torres-Salido M, Castro-Marrero J, et al. Thalidomide in the treatment of refractory cutaneous lupus erythematosus: prognostic factors of clinical outcome. Br J Dermatol. 2012;166:616-623.
- Zeldis JB, Williams BA, Thomas SD, et al. S.T.E.P.S.™: a comprehensive program for controlling and monitoring access to thalidomide. Clin Ther. 1999;21:319-330.
- C.S. Mott Children’s Hospital. University of Michigan Health. Thalidomide. Updated March 26, 2020. Accessed January 14, 2022. https://www.mottchildren.org/health-library/d04331a1
- Boehm IB, Boehm GA, Bauer R. Management of cutaneous lupus erythematosus with low-dose methotrexate: indication for modulation of inflammatory mechanisms. Rheumatol Int. 1998;18:59-62.
- Buckley LM, Bullaboy CA, Leichtman L, et al. Multiple congenital anomalies associated with weekly low‐dose methotrexate treatment of the mother. Arthritis Rheum. 1997;40:971-973.
- Foering K, Okawa J, Rose M, et al. Characterization of photosensitivity and poor quality of life in lupus. J Invest Dermatol. 2010;130(suppl):S10.
- Kuhn A, Herrmann M, Kleber S, et al. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 2006;54:939-950.
- Lake EP, Huang Y, Aronson IK. Rituximab treatment of pemphigus in women of childbearing age: experience with two patients. J Dermatol Treat. 2017;28:751-752.
Cutaneous lupus erythematosus (CLE) is a heterogeneous autoimmune disease that involves the skin. Cutaneous lupus erythematosus can be classified into various subtypes.1 These include, but are not limited to, acute CLE, subacute CLE, chronic CLE, intermittent CLE, lupus tumidus, and lupus profundus.1,2 The CLE subtypes have variable associations with systemic lupus erythematosus. For instance, some subtypes, such as acute CLE, are more strongly associated with systemic lupus erythematosus.
Treatment of CLE is similar to other autoimmune disorders. Although the US Food and Drug Administration (FDA) has not approved any treatments for CLE,3,4 the most common therapeutic options are disease-modifying antirheumatic drugs. Unfortunately, many of these treatments carry teratogenic effects. Because CLE predominantly affects women, particularly those of childbearing age, it is imperative to understand the available treatment options for those who are pregnant or considering pregnancy for an informed discussion with patients.5
For years, the gold standard when considering a medication during pregnancy was the FDA’s classification system. According to this system, medications were classified into 5 letter categories based on their potential teratogenicity, including A (no fetal risk), B (potential animal risk but inconclusive human studies), C (risk cannot be ruled out), D (evidence of fetal risk), and X (contraindicated in pregnancy). In 2014, the FDA decided to no longer use this classification system for medications approved after 2000.6 However, because many proposed treatment options for CLE were approved prior to 2001, we have summarized the commonly prescribed medications for CLE according to their prior FDA letter categories.
Treatment Options for CLE During Pregnancy
Prior to initiating systemic medications for the treatment of CLE, topical medications should be considered. Recommended treatment options include corticosteroids and calcineurin inhibitors.7 Compared with systemic medications, topical treatments carry minimal side effects, such as skin atrophy, that typically remain localized to areas of application.8 Moreover, even with extensive application, no correlation has been found between topical corticosteroid use and fetal growth,9 which suggests that topical steroids are safe in pregnancy and should be considered as a first-line treatment option for CLE. Calcineurin inhibitors also are considered safe based on their low level of absorption through the skin and are considered second-line topical treatment options in pregnancy.10

Although topical medications are effective for the treatment of CLE, many patients require the administration of systemic therapeutics for severe or refractory disease. Based on previously published reports, Figure 1 describes the current recommended systemic treatment options for CLE.11 Unfortunately, many of these medications carry teratogenic risks during pregnancy. The risks and side effects of the medications are described in detail in the following sections and summarized in the eTable.

Category B
Systemic Steroids—Systemic steroids are one of the most prescribed medications during pregnancy.12 Oral steroids have been associated with fast symptom relief, making this class of medications particularly effective during CLE flares; however, long-term management is not recommended because of the side effects, which include osteoporosis and impaired glucose metabolism.13
With low transmission across the placenta, there are 3 glucocorticoids that carry the safest profile in pregnancy: prednisone, cortisone, and hydrocortisone.14 Dexamethasone and betamethasone should be avoided, as both readily cross the placenta and increase fetal exposure.15 Although teratogenic effects have been associated with steroid use, most studies involving pregnant patients have inconclusive results. For instance, one study described an association between cleft lip/palate with in utero glucocorticoid exposure.16 However, multiple follow-up studies found no association between the two.17,18 Studies investigating the relationship between steroids and miscarriages or steroids and low birth weight also are inconclusive. Of note, if used throughout pregnancy, administration of a loading dose of glucocorticoids prior to delivery is recommended because of the increased stress brought on during labor.19
Sulfasalazine—Sulfasalazine is an immunomodulator commonly used for the treatment of inflammatory bowel disease and rheumatoid arthritis. However, studies also have shown that sulfasalazine is an effective treatment of CLE if standard treatments have failed.20,21
During pregnancy, patients exposed to sulfasalazine experienced minimal side effects despite transportation across the placenta.22 In comparison with control, pregnant women taking sulfasalazine experienced no increased risk for low fetal weight,23 congenital abnormalities,24 or spontaneous abortions.25 Of note, sulfasalazine can affect sperm, so male patients also should be counselled.
Category C
Hydroxychloroquine—Hydroxychloroquine is considered a first-line medication for those with CLE based on a symptomatic relief rate of 50% to 70%.26 For those taking hydroxychloroquine during pregnancy, the majority of studies have shown no association between the medication and adverse fetal events, including congenital abnormalities, prematurity, or spontaneous abortions.27-29 Therefore, hydroxychloroquine is considered safe in pregnancy, and those on the medication should continue standard monitoring, including retinopathy screening.30
Of note, hydroxychloroquine can be stored in tissue for weeks to months after discontinuation.5 Therefore, if patients wish to avoid hydroxychloroquine in pregnancy, one should stop taking the medication several months prior to conception.
Dapsone—Dapsone, a medication with both antimicrobial and immunomodulatory properties, is an effective second-line therapy for CLE.31 Although large-scale human trials have not been performed, multiple case reports and observational studies have supported the safe use of dapsone in pregnancy.32-34 However, there are notable side effects, including dose-dependent hemolysis, methemoglobinemia, and hypersensitivity reactions.13 Therefore, once treatment is initiated or continued, folic acid supplementation (5 mg daily) and regular serum analysis, including complete blood cell counts, are recommended in pregnant patients.19
Rituximab—Recent studies have demonstrated that rituximab can be an effective treatment of subacute and chronic CLE.35,36 Through inhibition of CD20, rituximab causes a decrease in circulating B cells and a reduced immune response. Therefore, experts recommend discontinuation of rituximab for 12 months prior to conception to reduce potential side effects to the fetus, which may include a transient reduction of circulating fetal B cells.37
If continued during pregnancy, most studies suggest discontinuation of rituximab during the third trimester, as it has been associated with neonatal infections and congenital abnormalities.19,37 However, these results are based on limited case reports, and thus robust research is needed to better understand the effect of rituximab in utero.
Intravenous Immunoglobulin Infusion—Intravenous immunoglobulin (IVIG) infusion is a well-tolerated treatment for many autoimmune disorders.38 Although not first line, limited case studies have demonstrated remission of refractory CLE following IVIG.39,40 Although no studies have directly investigated the effect of IVIG on fetal development, it has been frequently administered and well tolerated during pregnancy, especially in those with multiple sclerosis or antiphospholipid syndrome.41 Commonly reported side effects include headache and fatigue, and a rare associated side effect to be aware of is embolic events.42,43
Cyclosporine—Cyclosporine rarely is used in the treatment of localized CLE due to its extensive side-effect profile, most notably nephrotoxicity.44 However, studies have shown that cyclosporine may be efficacious if symptoms extend beyond the skin, involve multiple organs, and/or other treatments have failed.39 For those who are pregnant and wish to continue cyclosporine use, studies have associated low birth weight and premature delivery with its exposure in utero.44
Category D
Mycophenolate Mofetil—In conjunction with standard therapy, mycophenolate mofetil (MMF) is an adequate treatment of refractory CLE.45 Unfortunately, case reports have demonstrated an increased risk for fetal congenital abnormalities and first-trimester spontaneous abortion with use of MMF during pregnancy.46,47 Therefore, it is recommended that patients on MMF discontinue the medication at least 6 weeks prior to conception.46
Azathioprine—Although azathioprine has been shown to provide relief of discoid lupus erythematosus symptoms,48 it currently is only utilized for refractory disease, largely due to notable side effects that particularly affect the gastrointestinal tract and liver.4 Moreover, azathioprine use during pregnancy has been associated with prematurity, congenital anomalies, fetal cytopenia, and low birth weight.49 With that said, and although not recommended, if patients decide to continue treatment, experts recommend limiting the dose to 2 mg/kg daily to reduce potential adverse events.
Category X
Oral Retinoids—According to the American Academy of Dermatology, retinoids such as isotretinoin and acitretin are considered second-line therapy for CLE.50 With that being said, there are well-documented effects on fetal development associated with oral retinoid use, including central nervous system, cardiovascular system, and craniofacial abnormalities.51 Therefore, its use is contraindicated during pregnancy. To prevent pregnancy while taking isotretinoin, patients must enroll in an online monitoring program called iPLEDGE. This program requires monthly updates by both the physician and the patient, including a negative pregnancy test every month for female patients actively taking the medication.52
The half-lives of the oral retinoids isotretinoin and acitretin are 10 to 20 hours and 50 to 60 hours, respectively.53,54 However, alcohol consumption converts acitretin into the metabolite etretinate, which can remain in tissue for up to 120 days.54,55 Therefore, women are advised to avoid alcohol while taking acitretin and avoid conception for 2 to 3 years after cessation of the medication.55 For those wishing to restart retinoids after pregnancy, studies show the medication can be safely reinstated 35 days after delivery for those interested in continued treatment.56
Thalidomide—Although low-dose thalidomide can treat refractory CLE, its use is restricted because of its known teratogenicity, most notably limb deformities.57 If prescribed thalidomide, women will need to enroll in the System for Thalidomide Education and Prescribing Safety program, similar to the iPLEDGE program, and use 2 forms of contraception when sexually active.58 Contraception should be continued for 4 weeks following the last dose of thalidomide. After this point, conception is considered safe.59
Methotrexate—For nonpregnant patients, low-dose methotrexate (MTX) with folate supplementation is a treatment option for CLE.60 However, for those who are pregnant, low-dose MTX is an abortive agent and has been associated with aminopterin syndrome, which includes skull deficits, craniofacial abnormalities, and limb deformities in live births.19,61 Therefore, MTX is not recommended in pregnancy. Of note, MTX can affect sperm; male patients also should be counselled.
Final Thoughts
Overall, it is recommended to limit medication use as much as possible in pregnancy. To reduce these exposures, it is imperative to reduce triggers that may lead to symptomatic flares of CLE. Because CLE can be triggered by sun exposure, we advise topical sunscreen to prevent CLE flares that may require additional oral medication.62,63
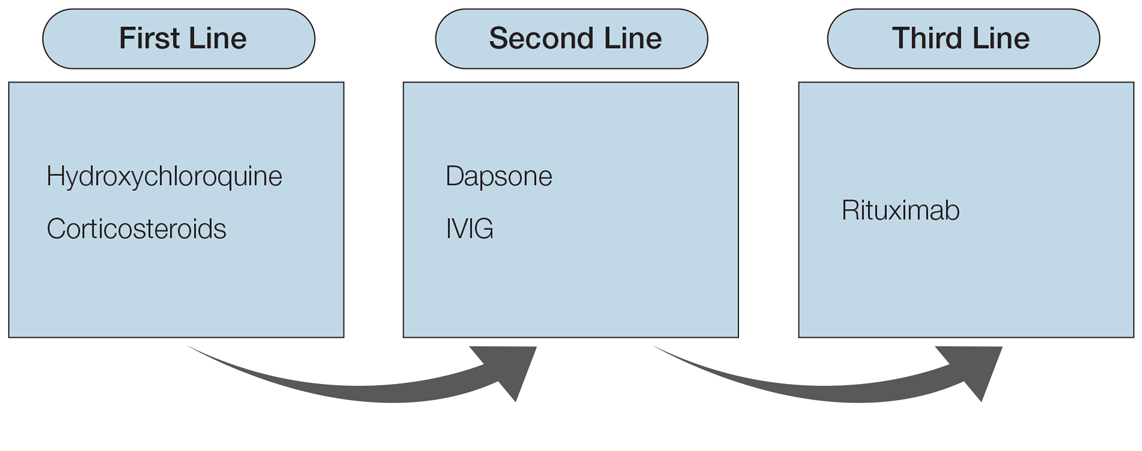
Various medications are considered safe for the treatment of CLE in pregnant patients (Figure 2). Based on studies in animal and clinical trials, hydroxychloroquine is considered a safe and effective medication for CLE in pregnancy and is a first-line therapy in nonpregnant patients.26,27 If flares occur, IVIG or a short course of oral steroids should be considered to manage symptoms.13,39 For those with severe flares, treatment is difficult, and personalized approaches may be necessary.

Part of the question for the childbearing population is when a patient would like to conceive. For severe cases when hydroxychloroquine is not effective as monotherapy, using a treatment that can encourage remission prior to conception attempts can be a beneficial strategy. Rituximab is an excellent example of such a therapy, as the therapeutic effect outlasts the immunosuppressive effect and therefore is unlikely to affect a future fetus.64 Thalidomide also is a potential option prior to conception, based on its short washout period and its ability to achieve notable remission rates in patients with CLE.57,59 Regardless, patients with CLE should still consult their dermatologist and rheumatologist (if applicable) prior to conception.
Patients of childbearing potential represent a population in which discussion about life goals greatly affects medication options. Having these discussions early and often allows for an open, more successful approach so that treatment regimens are not derailed at the time of conception.
Cutaneous lupus erythematosus (CLE) is a heterogeneous autoimmune disease that involves the skin. Cutaneous lupus erythematosus can be classified into various subtypes.1 These include, but are not limited to, acute CLE, subacute CLE, chronic CLE, intermittent CLE, lupus tumidus, and lupus profundus.1,2 The CLE subtypes have variable associations with systemic lupus erythematosus. For instance, some subtypes, such as acute CLE, are more strongly associated with systemic lupus erythematosus.
Treatment of CLE is similar to other autoimmune disorders. Although the US Food and Drug Administration (FDA) has not approved any treatments for CLE,3,4 the most common therapeutic options are disease-modifying antirheumatic drugs. Unfortunately, many of these treatments carry teratogenic effects. Because CLE predominantly affects women, particularly those of childbearing age, it is imperative to understand the available treatment options for those who are pregnant or considering pregnancy for an informed discussion with patients.5
For years, the gold standard when considering a medication during pregnancy was the FDA’s classification system. According to this system, medications were classified into 5 letter categories based on their potential teratogenicity, including A (no fetal risk), B (potential animal risk but inconclusive human studies), C (risk cannot be ruled out), D (evidence of fetal risk), and X (contraindicated in pregnancy). In 2014, the FDA decided to no longer use this classification system for medications approved after 2000.6 However, because many proposed treatment options for CLE were approved prior to 2001, we have summarized the commonly prescribed medications for CLE according to their prior FDA letter categories.
Treatment Options for CLE During Pregnancy
Prior to initiating systemic medications for the treatment of CLE, topical medications should be considered. Recommended treatment options include corticosteroids and calcineurin inhibitors.7 Compared with systemic medications, topical treatments carry minimal side effects, such as skin atrophy, that typically remain localized to areas of application.8 Moreover, even with extensive application, no correlation has been found between topical corticosteroid use and fetal growth,9 which suggests that topical steroids are safe in pregnancy and should be considered as a first-line treatment option for CLE. Calcineurin inhibitors also are considered safe based on their low level of absorption through the skin and are considered second-line topical treatment options in pregnancy.10

Although topical medications are effective for the treatment of CLE, many patients require the administration of systemic therapeutics for severe or refractory disease. Based on previously published reports, Figure 1 describes the current recommended systemic treatment options for CLE.11 Unfortunately, many of these medications carry teratogenic risks during pregnancy. The risks and side effects of the medications are described in detail in the following sections and summarized in the eTable.

Category B
Systemic Steroids—Systemic steroids are one of the most prescribed medications during pregnancy.12 Oral steroids have been associated with fast symptom relief, making this class of medications particularly effective during CLE flares; however, long-term management is not recommended because of the side effects, which include osteoporosis and impaired glucose metabolism.13
With low transmission across the placenta, there are 3 glucocorticoids that carry the safest profile in pregnancy: prednisone, cortisone, and hydrocortisone.14 Dexamethasone and betamethasone should be avoided, as both readily cross the placenta and increase fetal exposure.15 Although teratogenic effects have been associated with steroid use, most studies involving pregnant patients have inconclusive results. For instance, one study described an association between cleft lip/palate with in utero glucocorticoid exposure.16 However, multiple follow-up studies found no association between the two.17,18 Studies investigating the relationship between steroids and miscarriages or steroids and low birth weight also are inconclusive. Of note, if used throughout pregnancy, administration of a loading dose of glucocorticoids prior to delivery is recommended because of the increased stress brought on during labor.19
Sulfasalazine—Sulfasalazine is an immunomodulator commonly used for the treatment of inflammatory bowel disease and rheumatoid arthritis. However, studies also have shown that sulfasalazine is an effective treatment of CLE if standard treatments have failed.20,21
During pregnancy, patients exposed to sulfasalazine experienced minimal side effects despite transportation across the placenta.22 In comparison with control, pregnant women taking sulfasalazine experienced no increased risk for low fetal weight,23 congenital abnormalities,24 or spontaneous abortions.25 Of note, sulfasalazine can affect sperm, so male patients also should be counselled.
Category C
Hydroxychloroquine—Hydroxychloroquine is considered a first-line medication for those with CLE based on a symptomatic relief rate of 50% to 70%.26 For those taking hydroxychloroquine during pregnancy, the majority of studies have shown no association between the medication and adverse fetal events, including congenital abnormalities, prematurity, or spontaneous abortions.27-29 Therefore, hydroxychloroquine is considered safe in pregnancy, and those on the medication should continue standard monitoring, including retinopathy screening.30
Of note, hydroxychloroquine can be stored in tissue for weeks to months after discontinuation.5 Therefore, if patients wish to avoid hydroxychloroquine in pregnancy, one should stop taking the medication several months prior to conception.
Dapsone—Dapsone, a medication with both antimicrobial and immunomodulatory properties, is an effective second-line therapy for CLE.31 Although large-scale human trials have not been performed, multiple case reports and observational studies have supported the safe use of dapsone in pregnancy.32-34 However, there are notable side effects, including dose-dependent hemolysis, methemoglobinemia, and hypersensitivity reactions.13 Therefore, once treatment is initiated or continued, folic acid supplementation (5 mg daily) and regular serum analysis, including complete blood cell counts, are recommended in pregnant patients.19
Rituximab—Recent studies have demonstrated that rituximab can be an effective treatment of subacute and chronic CLE.35,36 Through inhibition of CD20, rituximab causes a decrease in circulating B cells and a reduced immune response. Therefore, experts recommend discontinuation of rituximab for 12 months prior to conception to reduce potential side effects to the fetus, which may include a transient reduction of circulating fetal B cells.37
If continued during pregnancy, most studies suggest discontinuation of rituximab during the third trimester, as it has been associated with neonatal infections and congenital abnormalities.19,37 However, these results are based on limited case reports, and thus robust research is needed to better understand the effect of rituximab in utero.
Intravenous Immunoglobulin Infusion—Intravenous immunoglobulin (IVIG) infusion is a well-tolerated treatment for many autoimmune disorders.38 Although not first line, limited case studies have demonstrated remission of refractory CLE following IVIG.39,40 Although no studies have directly investigated the effect of IVIG on fetal development, it has been frequently administered and well tolerated during pregnancy, especially in those with multiple sclerosis or antiphospholipid syndrome.41 Commonly reported side effects include headache and fatigue, and a rare associated side effect to be aware of is embolic events.42,43
Cyclosporine—Cyclosporine rarely is used in the treatment of localized CLE due to its extensive side-effect profile, most notably nephrotoxicity.44 However, studies have shown that cyclosporine may be efficacious if symptoms extend beyond the skin, involve multiple organs, and/or other treatments have failed.39 For those who are pregnant and wish to continue cyclosporine use, studies have associated low birth weight and premature delivery with its exposure in utero.44
Category D
Mycophenolate Mofetil—In conjunction with standard therapy, mycophenolate mofetil (MMF) is an adequate treatment of refractory CLE.45 Unfortunately, case reports have demonstrated an increased risk for fetal congenital abnormalities and first-trimester spontaneous abortion with use of MMF during pregnancy.46,47 Therefore, it is recommended that patients on MMF discontinue the medication at least 6 weeks prior to conception.46
Azathioprine—Although azathioprine has been shown to provide relief of discoid lupus erythematosus symptoms,48 it currently is only utilized for refractory disease, largely due to notable side effects that particularly affect the gastrointestinal tract and liver.4 Moreover, azathioprine use during pregnancy has been associated with prematurity, congenital anomalies, fetal cytopenia, and low birth weight.49 With that said, and although not recommended, if patients decide to continue treatment, experts recommend limiting the dose to 2 mg/kg daily to reduce potential adverse events.
Category X
Oral Retinoids—According to the American Academy of Dermatology, retinoids such as isotretinoin and acitretin are considered second-line therapy for CLE.50 With that being said, there are well-documented effects on fetal development associated with oral retinoid use, including central nervous system, cardiovascular system, and craniofacial abnormalities.51 Therefore, its use is contraindicated during pregnancy. To prevent pregnancy while taking isotretinoin, patients must enroll in an online monitoring program called iPLEDGE. This program requires monthly updates by both the physician and the patient, including a negative pregnancy test every month for female patients actively taking the medication.52
The half-lives of the oral retinoids isotretinoin and acitretin are 10 to 20 hours and 50 to 60 hours, respectively.53,54 However, alcohol consumption converts acitretin into the metabolite etretinate, which can remain in tissue for up to 120 days.54,55 Therefore, women are advised to avoid alcohol while taking acitretin and avoid conception for 2 to 3 years after cessation of the medication.55 For those wishing to restart retinoids after pregnancy, studies show the medication can be safely reinstated 35 days after delivery for those interested in continued treatment.56
Thalidomide—Although low-dose thalidomide can treat refractory CLE, its use is restricted because of its known teratogenicity, most notably limb deformities.57 If prescribed thalidomide, women will need to enroll in the System for Thalidomide Education and Prescribing Safety program, similar to the iPLEDGE program, and use 2 forms of contraception when sexually active.58 Contraception should be continued for 4 weeks following the last dose of thalidomide. After this point, conception is considered safe.59
Methotrexate—For nonpregnant patients, low-dose methotrexate (MTX) with folate supplementation is a treatment option for CLE.60 However, for those who are pregnant, low-dose MTX is an abortive agent and has been associated with aminopterin syndrome, which includes skull deficits, craniofacial abnormalities, and limb deformities in live births.19,61 Therefore, MTX is not recommended in pregnancy. Of note, MTX can affect sperm; male patients also should be counselled.
Final Thoughts
Overall, it is recommended to limit medication use as much as possible in pregnancy. To reduce these exposures, it is imperative to reduce triggers that may lead to symptomatic flares of CLE. Because CLE can be triggered by sun exposure, we advise topical sunscreen to prevent CLE flares that may require additional oral medication.62,63
Various medications are considered safe for the treatment of CLE in pregnant patients (Figure 2). Based on studies in animal and clinical trials, hydroxychloroquine is considered a safe and effective medication for CLE in pregnancy and is a first-line therapy in nonpregnant patients.26,27 If flares occur, IVIG or a short course of oral steroids should be considered to manage symptoms.13,39 For those with severe flares, treatment is difficult, and personalized approaches may be necessary.

Part of the question for the childbearing population is when a patient would like to conceive. For severe cases when hydroxychloroquine is not effective as monotherapy, using a treatment that can encourage remission prior to conception attempts can be a beneficial strategy. Rituximab is an excellent example of such a therapy, as the therapeutic effect outlasts the immunosuppressive effect and therefore is unlikely to affect a future fetus.64 Thalidomide also is a potential option prior to conception, based on its short washout period and its ability to achieve notable remission rates in patients with CLE.57,59 Regardless, patients with CLE should still consult their dermatologist and rheumatologist (if applicable) prior to conception.
Patients of childbearing potential represent a population in which discussion about life goals greatly affects medication options. Having these discussions early and often allows for an open, more successful approach so that treatment regimens are not derailed at the time of conception.
- Renner R, Sticherling M. The different faces of cutaneous lupus erythematosus. G Ital Dermatol Venereol. 2009;144:135-147.
- Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48:14-19.
- Shi H, Gudjonsson J, Kahlenberg J. Treatment of cutaneous lupus erythematosus: current approaches and future strategies. Curr Opin Rheumatol. 2020;32:208-214.
- Winkelmann RR, Kim GK, Del Rosso JQ. Treatment of cutaneous lupus erythematosus: review and assessment of treatment benefits based on Oxford Centre for Evidence-based Medicine criteria. J Clin Aesthet Dermatol. 2013;6:27-38.
- Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223-243.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;41:713-715.
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736-745.
- Kuhn A, Aberer E, Bata‐Csörgö Z, et al. S2k guideline for treatment of cutaneous lupus erythematosus—guided by the European Dermatology Forum (EDF) in cooperation with the European Academyof Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2017;31:389-404.
- Andersson NW, Skov L, Andersen JT. Evaluation of topical corticosteroid use in pregnancy and risk of newborns being small for gestational age and having low birth weight. JAMA Dermatol. 2021;157:788-795.
- Undre NA, Moloney FJ, Ahmadi S, et al. Skin and systemic pharmacokinetics of tacrolimus following topical application of tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2009;160:665-669.
- Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2014;10:97-107.
- Kuriya B, Hernández‐Díaz S, Liu J, et al. Patterns of medication use during pregnancy in rheumatoid arthritis. Arthritis Care Res. 2011;63:721-728.
- Chang A, Werth V. Treatment of cutaneous lupus. Curr Rheumatol Rep. 2011;13:300-307.
- Beitins IZ, Bayard F, Ances IG, et al. The transplacental passage of prednisone and prednisolone in pregnancy near term. J Pediatr. 1972;81:936-945.
- Ogueh O, Johnson MR. The metabolic effect of antenatal corticosteroid therapy. Hum Reprod Update. 2000;6:169-176.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Bay Bjørn A, Ehrenstein V, Hundborg HH, et al. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. Am J Ther. 2014;21:73-80.
- Hviid A, Mølgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ. 2011;183:796-804.
- Krause ML, Amin S, Makol A. Use of DMARDs and biologics during pregnancy and lactation in rheumatoid arthritis: what the rheumatologist needs to know. Ther Adv Musculoskelet Dis. 2014;6:169-184.
- Artuz F, Lenk N, Deniz N, et al. Efficacy of sulfasalazine in discoid lupus erythematosus. Int J Dermatol. 1996;35:746-748.
- Delaporte E, Catteau B, Sabbagh N, et al. Treatment of discoid lupus erythematosus with sulfasalazine: 11 cases [in French]. Ann Dermatol Venereol. 1997;124:151-156.
- Järnerot G, Into-Malmberg MB, Esbjörner E. Placental transfer of sulphasalazine and sulphapyridine and some of its metabolites. Scand J Gastroenterol. 1981;16:693-697.
- Norgard B, Pedersen L, Christensen LA, et al. Therapeutic drug use in women with Crohn’s disease and birth outcomes: a Danish nationwide cohort study. Am J Gastroenterol. 2007;102:1406-1413.
- Nørgård B, Czeizel AE, Rockenbauer M, et al. Population-based case control study of the safety of sulfasalazine use during pregnancy. Aliment Pharmacol Ther. 2001;15:483-486.
- Rahimi R, Nikfar S, Rezaie A, et al. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod Toxicol. 2008;25:271-275.
- Callen JP. Chronic cutaneous lupus erythematosus: clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416.
- Buchanan NM, Toubi E, Khamashta MA, et al. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann Rheum Dis. 1996;55:486-488.
- Costedoat‐Chalumeau N, Amoura Z, Duhaut P, et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty‐three cases compared with a control group. Arthritis Rheum. 2003;48:3207-3211.
- Sperber K, Hom C, Chao CP, et al. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009;7:9.
- Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377-1382.
- Klebes M, Wutte N, Aberer E. Dapsone as second-line treatment for cutaneous lupus erythematosus? a retrospective analysis of 34 patients and a review of the literature. Dermatology. 2016;232:91-96.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Varghese L, Viswabandya A, Mathew AJ. Dapsone, danazol, and intrapartum splenectomy in refractory ITP complicating pregnancy. Indian J Med Sci. 2008;62:452-455.
- Kahn G. Dapsone is safe during pregnancy. J Am Acad Dermatol. 1985;13:838-839.
- Quelhas da Costa R, Aguirre-Alastuey ME, Isenberg DA, et al. Assessment of response to B-cell depletion using rituximab in cutaneous lupus erythematosus. JAMA Dermatol. 2018;154:1432-1440.
- Alsanafi S, Kovarik C, Mermelstein A, et al. Rituximab in thetreatment of bullous systemic lupus erythematosus. J Clin Rheumatol. 2011;17:142-144.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506.
- Fernandez AP, Kerdel FA. The use of i.v. IG therapy in dermatology. Dermatol Ther. 2007;20:288-305.
- Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part II. J Am Acad Dermatol. 2011;65:E195-E213.
- Singh H, Naidu G, Sharma A. Intravenous immunoglobulin for the rescue in refractory cutaneous lupus. Indian Dermatol Online J. 2020;11:1003-1004.
- Clark AL. Clinical uses of intravenous immunoglobulin in pregnancy. Clin Obstet Gynecol. 1999;42:368-380.
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747-755.
- Woodruff RK, Grigg AP, Firkin FC, et al. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet. 1986;2:217-218.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Gammon B, Hansen C, Costner MI. Efficacy of mycophenolate mofetil in antimalarial-resistant cutaneous lupus erythematosus. J Am Acad Dermatol. 2010;65:717-721.e2.
- Abdulaziz HM, Shemies RS, Taman M, et al. Fetal proximal and distal limb anomalies following exposure to mycophenolate mofetil during pregnancy: a case report and review of the literature. Lupus. 2021;30:1522-1525.
- Pisoni CN, D’Cruz DP. The safety of mycophenolate mofetil in pregnancy. Exp Opin Drug Saf. 2008;7:219-222.
- Ashinoff R, Werth VP, Franks AG. Resistant discoid lupus erythematosus of palms and soles: successful treatment with azathioprine. J Am Acad Dermatol. 1988;19:961-965. doi:10.1016/S0190-9622(88)70259-5
- Goldstein LH, Dolinsky G, Greenberg R, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79:696-701.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for cutaneous lupus erythematosus. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:830-836.
- Sladden MJ, Harman KE. What is the chance of a normal pregnancy in a woman whose fetus has been exposed to isotretinoin? Arch Dermatol. 2007;143:1187-1188.
- Shin J, Cheetham TC, Wong L, et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- Brazzell RK, Colburn WA. Pharmacokinetics of the retinoids isotretinoin and etretinate. J Am Acad Dermatol. 1982;6:643-651.
- Pilkington T, Brogden RN. Acitretin: a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Gronhoj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
- Jajoria H, Mysore V. Washout period for pregnancy post isotretinoin therapy. Indian Dermatol Online J. 2020;11:239-242.
- Cortés-Hernández J, Torres-Salido M, Castro-Marrero J, et al. Thalidomide in the treatment of refractory cutaneous lupus erythematosus: prognostic factors of clinical outcome. Br J Dermatol. 2012;166:616-623.
- Zeldis JB, Williams BA, Thomas SD, et al. S.T.E.P.S.™: a comprehensive program for controlling and monitoring access to thalidomide. Clin Ther. 1999;21:319-330.
- C.S. Mott Children’s Hospital. University of Michigan Health. Thalidomide. Updated March 26, 2020. Accessed January 14, 2022. https://www.mottchildren.org/health-library/d04331a1
- Boehm IB, Boehm GA, Bauer R. Management of cutaneous lupus erythematosus with low-dose methotrexate: indication for modulation of inflammatory mechanisms. Rheumatol Int. 1998;18:59-62.
- Buckley LM, Bullaboy CA, Leichtman L, et al. Multiple congenital anomalies associated with weekly low‐dose methotrexate treatment of the mother. Arthritis Rheum. 1997;40:971-973.
- Foering K, Okawa J, Rose M, et al. Characterization of photosensitivity and poor quality of life in lupus. J Invest Dermatol. 2010;130(suppl):S10.
- Kuhn A, Herrmann M, Kleber S, et al. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 2006;54:939-950.
- Lake EP, Huang Y, Aronson IK. Rituximab treatment of pemphigus in women of childbearing age: experience with two patients. J Dermatol Treat. 2017;28:751-752.
- Renner R, Sticherling M. The different faces of cutaneous lupus erythematosus. G Ital Dermatol Venereol. 2009;144:135-147.
- Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48:14-19.
- Shi H, Gudjonsson J, Kahlenberg J. Treatment of cutaneous lupus erythematosus: current approaches and future strategies. Curr Opin Rheumatol. 2020;32:208-214.
- Winkelmann RR, Kim GK, Del Rosso JQ. Treatment of cutaneous lupus erythematosus: review and assessment of treatment benefits based on Oxford Centre for Evidence-based Medicine criteria. J Clin Aesthet Dermatol. 2013;6:27-38.
- Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223-243.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;41:713-715.
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736-745.
- Kuhn A, Aberer E, Bata‐Csörgö Z, et al. S2k guideline for treatment of cutaneous lupus erythematosus—guided by the European Dermatology Forum (EDF) in cooperation with the European Academyof Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2017;31:389-404.
- Andersson NW, Skov L, Andersen JT. Evaluation of topical corticosteroid use in pregnancy and risk of newborns being small for gestational age and having low birth weight. JAMA Dermatol. 2021;157:788-795.
- Undre NA, Moloney FJ, Ahmadi S, et al. Skin and systemic pharmacokinetics of tacrolimus following topical application of tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2009;160:665-669.
- Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2014;10:97-107.
- Kuriya B, Hernández‐Díaz S, Liu J, et al. Patterns of medication use during pregnancy in rheumatoid arthritis. Arthritis Care Res. 2011;63:721-728.
- Chang A, Werth V. Treatment of cutaneous lupus. Curr Rheumatol Rep. 2011;13:300-307.
- Beitins IZ, Bayard F, Ances IG, et al. The transplacental passage of prednisone and prednisolone in pregnancy near term. J Pediatr. 1972;81:936-945.
- Ogueh O, Johnson MR. The metabolic effect of antenatal corticosteroid therapy. Hum Reprod Update. 2000;6:169-176.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Bay Bjørn A, Ehrenstein V, Hundborg HH, et al. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. Am J Ther. 2014;21:73-80.
- Hviid A, Mølgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ. 2011;183:796-804.
- Krause ML, Amin S, Makol A. Use of DMARDs and biologics during pregnancy and lactation in rheumatoid arthritis: what the rheumatologist needs to know. Ther Adv Musculoskelet Dis. 2014;6:169-184.
- Artuz F, Lenk N, Deniz N, et al. Efficacy of sulfasalazine in discoid lupus erythematosus. Int J Dermatol. 1996;35:746-748.
- Delaporte E, Catteau B, Sabbagh N, et al. Treatment of discoid lupus erythematosus with sulfasalazine: 11 cases [in French]. Ann Dermatol Venereol. 1997;124:151-156.
- Järnerot G, Into-Malmberg MB, Esbjörner E. Placental transfer of sulphasalazine and sulphapyridine and some of its metabolites. Scand J Gastroenterol. 1981;16:693-697.
- Norgard B, Pedersen L, Christensen LA, et al. Therapeutic drug use in women with Crohn’s disease and birth outcomes: a Danish nationwide cohort study. Am J Gastroenterol. 2007;102:1406-1413.
- Nørgård B, Czeizel AE, Rockenbauer M, et al. Population-based case control study of the safety of sulfasalazine use during pregnancy. Aliment Pharmacol Ther. 2001;15:483-486.
- Rahimi R, Nikfar S, Rezaie A, et al. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod Toxicol. 2008;25:271-275.
- Callen JP. Chronic cutaneous lupus erythematosus: clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416.
- Buchanan NM, Toubi E, Khamashta MA, et al. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann Rheum Dis. 1996;55:486-488.
- Costedoat‐Chalumeau N, Amoura Z, Duhaut P, et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty‐three cases compared with a control group. Arthritis Rheum. 2003;48:3207-3211.
- Sperber K, Hom C, Chao CP, et al. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009;7:9.
- Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377-1382.
- Klebes M, Wutte N, Aberer E. Dapsone as second-line treatment for cutaneous lupus erythematosus? a retrospective analysis of 34 patients and a review of the literature. Dermatology. 2016;232:91-96.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Varghese L, Viswabandya A, Mathew AJ. Dapsone, danazol, and intrapartum splenectomy in refractory ITP complicating pregnancy. Indian J Med Sci. 2008;62:452-455.
- Kahn G. Dapsone is safe during pregnancy. J Am Acad Dermatol. 1985;13:838-839.
- Quelhas da Costa R, Aguirre-Alastuey ME, Isenberg DA, et al. Assessment of response to B-cell depletion using rituximab in cutaneous lupus erythematosus. JAMA Dermatol. 2018;154:1432-1440.
- Alsanafi S, Kovarik C, Mermelstein A, et al. Rituximab in thetreatment of bullous systemic lupus erythematosus. J Clin Rheumatol. 2011;17:142-144.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506.
- Fernandez AP, Kerdel FA. The use of i.v. IG therapy in dermatology. Dermatol Ther. 2007;20:288-305.
- Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part II. J Am Acad Dermatol. 2011;65:E195-E213.
- Singh H, Naidu G, Sharma A. Intravenous immunoglobulin for the rescue in refractory cutaneous lupus. Indian Dermatol Online J. 2020;11:1003-1004.
- Clark AL. Clinical uses of intravenous immunoglobulin in pregnancy. Clin Obstet Gynecol. 1999;42:368-380.
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747-755.
- Woodruff RK, Grigg AP, Firkin FC, et al. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet. 1986;2:217-218.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Gammon B, Hansen C, Costner MI. Efficacy of mycophenolate mofetil in antimalarial-resistant cutaneous lupus erythematosus. J Am Acad Dermatol. 2010;65:717-721.e2.
- Abdulaziz HM, Shemies RS, Taman M, et al. Fetal proximal and distal limb anomalies following exposure to mycophenolate mofetil during pregnancy: a case report and review of the literature. Lupus. 2021;30:1522-1525.
- Pisoni CN, D’Cruz DP. The safety of mycophenolate mofetil in pregnancy. Exp Opin Drug Saf. 2008;7:219-222.
- Ashinoff R, Werth VP, Franks AG. Resistant discoid lupus erythematosus of palms and soles: successful treatment with azathioprine. J Am Acad Dermatol. 1988;19:961-965. doi:10.1016/S0190-9622(88)70259-5
- Goldstein LH, Dolinsky G, Greenberg R, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79:696-701.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for cutaneous lupus erythematosus. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:830-836.
- Sladden MJ, Harman KE. What is the chance of a normal pregnancy in a woman whose fetus has been exposed to isotretinoin? Arch Dermatol. 2007;143:1187-1188.
- Shin J, Cheetham TC, Wong L, et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- Brazzell RK, Colburn WA. Pharmacokinetics of the retinoids isotretinoin and etretinate. J Am Acad Dermatol. 1982;6:643-651.
- Pilkington T, Brogden RN. Acitretin: a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Gronhoj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
- Jajoria H, Mysore V. Washout period for pregnancy post isotretinoin therapy. Indian Dermatol Online J. 2020;11:239-242.
- Cortés-Hernández J, Torres-Salido M, Castro-Marrero J, et al. Thalidomide in the treatment of refractory cutaneous lupus erythematosus: prognostic factors of clinical outcome. Br J Dermatol. 2012;166:616-623.
- Zeldis JB, Williams BA, Thomas SD, et al. S.T.E.P.S.™: a comprehensive program for controlling and monitoring access to thalidomide. Clin Ther. 1999;21:319-330.
- C.S. Mott Children’s Hospital. University of Michigan Health. Thalidomide. Updated March 26, 2020. Accessed January 14, 2022. https://www.mottchildren.org/health-library/d04331a1
- Boehm IB, Boehm GA, Bauer R. Management of cutaneous lupus erythematosus with low-dose methotrexate: indication for modulation of inflammatory mechanisms. Rheumatol Int. 1998;18:59-62.
- Buckley LM, Bullaboy CA, Leichtman L, et al. Multiple congenital anomalies associated with weekly low‐dose methotrexate treatment of the mother. Arthritis Rheum. 1997;40:971-973.
- Foering K, Okawa J, Rose M, et al. Characterization of photosensitivity and poor quality of life in lupus. J Invest Dermatol. 2010;130(suppl):S10.
- Kuhn A, Herrmann M, Kleber S, et al. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 2006;54:939-950.
- Lake EP, Huang Y, Aronson IK. Rituximab treatment of pemphigus in women of childbearing age: experience with two patients. J Dermatol Treat. 2017;28:751-752.
Practice Points
- Patients should consult their primary dermatologist when discussing medication options for cutaneous lupus erythematosus (CLE) prior to pregnancy.
- Hydroxychloroquine is a first-line medication for maintenance treatment of CLE, while oral steroids are effective for CLE flares in pregnancy. Second-line medications include dapsone and intravenous immunoglobulin. These classes of medications are considered safe in pregnancy.
- Cutaneous lupus erythematosus medications contraindicated in pregnancy include oral retinoids, mycophenolate mofetil, thalidomide, and methotrexate.