User login
Dermatology Immediate Care: A Game Changer for the Health Care System?
Dermatology Immediate Care: A Game Changer for the Health Care System?
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
Emergency departments (EDs) and immediate care (IC) facilities often do not have prompt dermatologic care available for triage and treatment. Many EDs do not have staff dermatologists on call, instead relying on input from other specialists or quick outpatient dermatology appointments. It can be challenging to obtain a prompt appointment with a board-certified dermatologist, which is preferred for complex cases such as severe drug reactions or infection. In the United States, there are few well-established IC centers equipped to address dermatologic needs. The orthopedic specialty has modeled a concept that has led to the establishment of orthopedic urgent care/IC in many larger institutions,1 and many private practice clinics serve their communities as well. We present a rationale for why a similar IC concept for dermatology would be beneficial, particularly within a large institution or health system.
Dermatology Consultation Changes Disease Management
There is diagnostic and therapeutic utility in dermatology evaluation. In a prospective study of 591 patients who were either hospitalized or evaluated in an ED/urgent care setting, treatment was changed in more than 60% of cases when dermatology consultation was utilized.2 In another prospective review of 691 cases on an inpatient service, dermatology consultation resulted in treatment changes more than 80% of the time.3
Cellulitis has been a particularly well-studied diagnosis. Dermatologists often change the diagnosis of cellulitis in the hospital setting and reduce antibiotic exposure. In a prospective cohort study of 116 patients, 33.6% had their diagnosis of cellulitis changed to pseudocellulitis following evaluation by the dermatologist; of 34 patients who had started antibiotic therapy, 82.4% were recommended to discontinue the treatment, and all 39 patients with pseudocellulitis had a proven stable clinical course at 1-month follow-up.4 In another trial, 175 patients with presumed cellulitis were given standard management (provided by the medicine inpatient team) either alone or with the addition of dermatology consultation. Duration of antibiotic treatment (including intravenous therapy) was reduced when dermatology was consulted. Two weeks after discharge, patients who had dermatology consultations demonstrated greater clinical improvement.5
Improving ED and IC Access to Dermatology
Emergency department and IC teams across the United States work tirelessly to meet the demands of patients presenting with medically urgent conditions. In a study examining 861 ED cases, dermatology made up only 9.5% of specialist consultations, and in the opinion of the on-call dermatology resident, 51.0% (439/861) of cases warranted ED-level care.6
Data from the 2021 National Hospital Ambulatory Medical Care Survey showed that the mean wait time to see a physician, nurse, or physician assistant in an ED was 37.5 minutes, but wait times could range from less than 15 minutes to more than 6 hours.7 According to a study of 35,849 ED visits at nonfederal hospitals in the United States, only 47.7% of EDs admitted more than 90% of their patients within 6 hours.8 Moreover, perceived wait times in the ED have been shown to greatly impact patient satisfaction. Two predictors of perceived wait time include appropriate assessment of emergency level and the feeling of being forgotten.9 In a study of 2377 ED visits with primary dermatologic diagnoses, only 5.5% led to admission.10 This suggests many patients who come to the ED for dermatologic needs do not require inpatient hospital care. In these cases, patients with primary dermatologic concerns may experience longer ED wait times, as higher acuity or emergency cases take precedence. Studies also have shown that more vulnerable populations are utilizing ED visits most for primary dermatologic concerns.10,11 This includes individuals of lower income and/or those with Medicaid/Medicare or those without insurance.11 Predictors of high ED use for dermatologic concerns include prior frequent use of the ED (for nondermatologic concerns) instead of outpatient care, income below the poverty level, and lack of insurance; older individuals (>65 years) also were found to use the ED more frequently for dermatologic concerns when compared to younger individuals.10
Importantly, there is a great need for urgent dermatology consultation for pediatric patients. A single-institution study showed that over a 36-month period, there were 347 pediatric dermatology consultations from the pediatric ED mostly for children aged 0 days to 5 years; nearly half of these consultations required outpatient clinic follow-up.12 However, dermatology outpatient follow-up can be difficult to obtain, especially for vulnerable groups. In a study of 611 dermatology clinics, patients with Medicaid were shown to have longer wait times and less success in obtaining dermatology appointments compared to those with Medicare or private insurance.13 Only about 30% of private dermatology practices accept Medicaid patients, likely pushing these patients toward utilization of emergency services for dermatologic concerns.13,14
There is a clear role for a dermatology IC in our health care system, and the concept already has been identified and trialed in several institutions. At Oregon Health and Science University (Portland, Oregon), a retrospective chart review of patients with diagnoses of Morgellons disease and neurotic excoriations seen in dermatology urgent care between 2018 and 2020 showed an 88% decrease in annual rates of health care visits and a 77% decrease in ED visits after dermatology services were engaged compared to before the opening of the dermatology urgent care.15 Another study showed that uninsured or self-pay patients were more than 14 times more likely to access dermatology urgent care than to schedule a routine clinic appointment, suggesting that there is a barrier to making outpatient dermatologic appointments for uninsured patients. An urgent access model may facilitate the ability of underinsured patients to access care.16
Improving Dermatology Access for Other Specialties
Needs for dermatologic care are encountered in many other specialties. Having direct access to immediate dermatologic treatment is best for patients and may avoid inpatient care and trips to the ED for consultation access. Ideally, a dermatology IC would allow direct care to be provided alongside the oncology outpatient team. New immunologic therapies (cytotoxic T-lymphocyte–associated protein 4 and programmed cell death protein 1/programmed death-ligand 1 treatments) can cause dermatologic reactions in more than 40% of patients.17 Paraneoplastic syndromes can manifest with cutaneous symptoms, as can acute graft-vs-host-disease.18 In a study at Memorial Sloan Kettering (New York, New York) analyzing 426 same-day outpatient dermatology consultations, 17% of patients experienced interruptions in their cancer therapy, but 83% responded quickly to dermatologic treatment and resumed oncologic therapy—19% of them at a reduced dose.19 This is an important demonstration of prompt dermatologic consultation in an outpatient setting reducing interruptions to anticancer therapy. The heterogeneity of the cutaneous reactions seen from oncologic and immunomodulatory medications is profound, with more than 140 different types of skin-specific reactions.20
Solid-organ transplant recipients also could benefit from urgent access to dermatology services. These patients are at a much higher risk for skin cancers, and a study showed that those who receive referrals to dermatology are seen sooner after transplantation (5.6 years) than those who self-refer (7.2 years). Importantly, annual skin cancer screenings are recommended to begin 1 year after transplantation.21
Direct access to dermatology care could benefit patients with complicated rheumatologic conditions who present with skin findings; for example, patients with lupus erythematosus or dermatomyositis can have a spectrum of disease ranging from skin-predominant to systemic manifestations. Identification and treatment of such diseases require collaboration between dermatologists and rheumatologists.22 Likewise, a study of a joint rheumatology-dermatology clinic for psoriatic arthritis showed that a multidisciplinary approach to management leads to decreased time for patients to obtain proper rheumatologic and dermatologic examination and a faster time to diagnosis; however, such multidisciplinary clinic models and approaches to care often are found only at large university-based hospitals.23 In a patient population for whom time to diagnosis is crucial to avoid permanent changes such as joint destruction, a dermatology IC could fill this role in community hospitals and clinics. A dermatology IC also can serve patients with specific diagnoses who would benefit from more direct access to care; for example, in 2017 there were 131,430 ED visits for hidradenitis suppurativa (HS) in the United States. While HS is not uncommon, it usually is underdiagnosed because it can be challenging to differentiate from an uncomplicated abscess. Emergency department visits often are utilized for first-time presentations as well as flares of HS. In these situations, ED doctors can provide palliative treatment, but prompt referrals to dermatologists should be made for disease management to decrease recurrence.24
Final Thoughts
A huge caveat to the dermatology urgent care system is determining what is deemed “urgent.” We propose starting with a referral-based system only from other physicians (including IC and urgent care) rather than having patients walk in directly. Ideally, as support and staff increases, the availability can increase as well. In our institution, we suggested half-day clinics staffed by varying physicians, with compensation models similar to an ED or IC physician rather than by productivity. Each group considering this kind of addition to patient care will need to assess these points in building an IC for dermatology. The University of Pennsylvania’s (Philadelphia, Pennsylvania) system of rapid-access clinics to facilitate access to care for patients requiring urgent appointments may function as a model for future similar clinics.25 Creating a specialized IC/urgent care is not a novel concept. Orthopedic urgent care centers have increased greatly in the past decade, reducing ED burden for musculoskeletal complaints. In a study evaluating the utility of orthopedic urgent care settings, time to see an orthopedic specialist and cost were both greatly reduced with this system.1 The same has been shown in same-day access ophthalmology clinics, which are organized similarly to an urgent care.26
In 2021, there were 107.4 million treat-and-release visits to the ED in the United States for a total cost of $80.3 billion.27 This emphasizes the need to consider care models that not only provide excellent clinical care and treat the most acute diagnoses promptly and accurately but also reduce overall costs. While this may be convoluted for other specialties given the difficulty of having patients self-triage, dermatologic concerns are similar to orthopedic concerns for the patient to decipher the etiology of the concern. As in orthopedics, a dermatology IC could function similarly, increasing access, decreasing ED and IC wait times, saving overall health care spending, and allowing underserved and publicly insured individuals to have improved, prompt care.
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
- Anderson TJ, Althausen PL. The role of dedicated musculoskeletal urgent care centers in reducing cost and improving access to orthopaedic care. J Orthop Trauma. 2016;30:S3-S6.
- Falanga V, Schachner LA, Rae V, et al. Dermatologic consultations in the hospital setting. Arch Dermatol. 1994;130:1022-1025.
- Galimberti F, Guren L, Fernandez AP, et al. Dermatology consultations significantly contribute quality to care of hospitalized patients: a prospective study of dermatology inpatient consults at a tertiary care center. Int J Dermatol. 2016;55:E547-E551.
- Li DG, Xia FD, Khosravi H, et al. Outcomes of early dermatology consultation for inpatients diagnosed with cellulitis. JAMA Dermatol. 2018;154:537-543.
- Ko LN, Garza-Mayers AC, St John J, et al. Effect of dermatology consultation on outcomes for patients with presumed cellulitis: a randomized clinical trial. JAMA Dermatol. 2018;154:529-536.
- Grillo E, Vañó-Galván S, Jiménez-Gómez N, et al. Dermatologic emergencies: descriptive analysis of 861 patients in a tertiary care teaching hospital. Actas Dermosifiliogr. 2013;104:316-324.
- National Center for Health Statistics. National Hospital Ambulatory Medical Care Survey, 2021. Accessed September 23, 2025. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2021-nhamcs-ed-web-tables-508.pdf
- Horwitz LI, Green J, Bradley EH. US emergency department performance on wait time and length of visit. Ann Emerg Med. 2010;55:133-141.
- Spechbach H, Rochat J, Gaspoz JM, et al. Patients’ time perception in the waiting room of an ambulatory emergency unit: a cross-sectional study. BMC Emerg Med. 2019;19:41.
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483.
- Chen CL, Fitzpatrick L, Kamel H. Who uses the emergency department for dermatologic care? a statewide analysis. J Am Acad Dermatol. 2014;71:308-313.
- Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173-1177.
- Creadore A, Desai S, Li SJ, et al. Insurance acceptance, appointment wait time, and dermatologist access across practice types in the US. JAMA Dermatol. 2021;157:181-188.
- Mazmudar RS, Gupta N, Desai BJ, et al. Dermatologist appointment access and waiting times: a comparative study of insurance types. J Am Acad Dermatol. 2020;83:1468-1470.
- Johnson J, Cutler B, Latour E, et al. Dermatology urgent care model reduces costs and healthcare utilization for psychodermatology patients-a retrospective chart review. Dermatol Online J. 2022;28:5.
- Wintringham JA, Strock DM, Perkins-Holtsclaw K, et al. Dermatology in the urgent care setting: a retrospective review of patients seen in an urgent access dermatology clinic. J Am Acad Dermatol. 2023;89:1271-1273.
- Yoo MJ, Long B, Brady WJ, et al. Immune checkpoint inhibitors: an emergency medicine focused review. Am J Emerg Med. 2021;50:335-344.
- Merlo G, Cozzani E, Canale F, et al. Cutaneous manifestations of hematologic malignancies the experience of an Italian dermatology department. Hematol Oncol. 2019;37:285-290.
- Barrios D, Phillips G, Freites-Martinez A, et al. Outpatient dermatology consultations for oncology patients with acute dermatologic adverse events impact anticancer therapy interruption: a retrospective study.J Eur Acad Dermatol Venereol. 2020;34:1340-1347.
- Salah S, Kerob D, Pages Laurent C, et al. Evaluation of anticancer therapy-related dermatologic adverse events: insights from Food and Drug Administration’s Adverse Event Reporting System dataset. J Am Acad Dermatol. 2024;91:863-871. doi:10.1016/j.jaad.2024.07.1456
- Shope C, Andrews L, Girvin A, et al. Referrals to dermatology following solid organ transplant. J Am Acad Dermatol. 2023;88:1159-1160. doi:10.1016/j.jaad.2022.11.052
- Werth VP, Askanase AD, Lundberg IE. Importance of collaboration of dermatology and rheumatology to advance the field for lupus and dermatomyositis. Int J Womens Dermatol. 2021;7:583-587.
- Ziob J, Behning C, Brossart P, et al. Specialized dermatological-rheumatological patient management improves diagnostic outcome and patient journey in psoriasis and psoriatic arthritis: a four-year analysis. BMC Rheumatol. 2021;5:1-8. doi:10.1186/s41927-021-00217-z
- Okun MM, Flamm A, Werley EB, et al. Hidradenitis suppurativa: diagnosis and management in the emergency department. J Emerg Med. 2022;63:636-644.
- Jayakumar KL, Samimi SS, Vittorio CC, et al. Expediting patient appointments with dermatology rapid access clinics. Dermatol Online J. 2018;24:13030/qt2zv07510.
- Singman EL, Smith K, Mehta R, et al. Cost and visit duration of same-day access at an academic ophthalmology department vs emergency department. JAMA Ophthalmol. 2019;137:729-735. doi:10.1001/jamaophthalmol.2019.0864
- Roemer M. Costs of treat-and-release emergency department visits in the United States, 2021. Agency for Healthcare Research and Quality. Published September 2024. Accessed September 16, 2025. https://hcup-us.ahrq.gov/reports/statbriefs/sb311-ED-visit-costs-2021.pdf
Dermatology Immediate Care: A Game Changer for the Health Care System?
Dermatology Immediate Care: A Game Changer for the Health Care System?
Practice Points
- Emergency departments and most immediate care (IC) centers often lack prompt access to board-certified dermatologists.
- A dermatology urgent care/IC model may shorten wait times, improve access for vulnerable patients and pediatric populations, and reduce unnecessary hospital admissions and costs.
- Increased access to dermatology benefits other specialties by enabling multidisciplinary care leading to faster diagnosis and treatment.
- A staged referral-first dermatology IC pilot with defined staffing and triage rules is a practical path to demonstrate value and scale the service.
Treatments for Hidradenitis Suppurativa Comorbidities Help With Pain Management
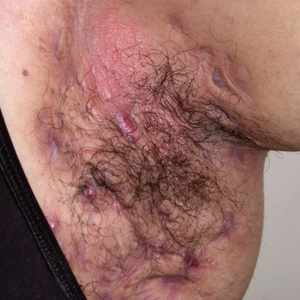
Hidradenitis suppurativa (HS) has an unpredictable disease course and poses substantial therapeutic challenges. It carries an increased risk for adverse cardiovascular outcomes and all-cause mortality. It also is associated with comorbidities including mood disorders, tobacco smoking, obesity, diabetes mellitus, sleep disorders, sexual dysfunction, and autoimmune diseases, which can complicate its management and considerably affect patients’ quality of life (QOL).1 Hidradenitis suppurativa also disproportionately affects minority groups and has far-reaching inequities; for example, the condition has a notable economic impact on patients, including higher unemployment and disability rates, lower-paying jobs, less paid time off, and other indirect costs.2,3 Race can impact how pain itself is treated. In one study (N = 217), Black patients with extremity fractures presenting to anemergency department were significantly less likely to receive analgesia compared to White patients despite reporting similar pain (57% vs 74%, respectively; P = .01).4 In another study, Hispanic patients were 7-times less likely to be treated with opioids compared to non-Hispanic patients with long-bone fractures.5 Herein, we highlight pain management disparities in HS patients.
Treating HS Comorbidities Helps Improve Pain
Pain is reported by almost all HS patients and is the symptom most associated with QOL impairment.6,7 Pain in HS is multifactorial, with other symptoms and comorbidities affecting its severity. Treatment of acute flares often is painful and procedural, including intralesional steroid injections or incision and drainage.8 Algorithms for addressing pain through the treatment of comorbidities also have been developed.6 Although there are few studies on the medications that treat related comorbidities in HS, there is evidence of their benefits in similar diseases; for example, treating depression in patients with irritable bowel disease (IBD) improved pain perception, cognitive function, and sexual dysfunction.9
Depression exacerbates pain, and higher levels of depression have been observed in severe HS.10,11 Additionally, more than 80% of individuals with HS report tobacco smoking.1 Nicotine not only increases pain sensitivity and decreases pain tolerance but also worsens neuropathic, nociceptive, and psychosocial pain, as well as mood disorders and sleep disturbances.12 Given the higher prevalence of depression and smoking in HS patients and the impact on pain, addressing these comorbidities is crucial. Additionally, poor sleep amplifies pain sensitivity and affects neurologic pain modulation.13 Chronic pain also is associated with obesity and sleep dysfunction.14
Treatments Targeting Pain and Comorbidities
Treatments that target comorbidities and other symptoms of HS also may improve pain. Bupropion is a well-studied antidepressant and first-line option to aid in smoking cessation. It provides acute and chronic pain relief associated with IBD and may perform similarly in patients with HS.15-18 Bupropion also demonstrated dose-dependent weight reduction in obese and overweight individuals.19,20 Additionally, varenicline is a first-line option to aid in smoking cessation and can be combined with bupropion to increase long-term efficacy.21,22
Other antidepressants may alleviate HS pain. The selective norepinephrine reuptake inhibitors duloxetine and venlafaxine are recommended for chronic pain in HS.6 Selective serotonin reuptake inhibitors such as citalopram, escitalopram, and paroxetine are inexpensive and widely available antidepressants. Citalopram is as efficacious as duloxetine for chronic pain with fewer side effects.23 Paroxetine has been shown to improve pain and pruritus, QOL, and depression in patients with IBD.24 Benefits such as improved weight and sexual dysfunction also have been reported.25
Metformin is well studied in Black patients, and greater glycemic response supports its efficacy for diabetes as well as HS, which disproportionately affects individuals with skin of color.26 Metformin also targets other comorbidities of HS, such as improving insulin resistance, polycystic ovary syndrome, acne vulgaris, weight loss, hyperlipidemia, cardiovascular risk, and neuropsychologic conditions.27 Growing evidence supports the use of metformin as a new agent in chronic pain management, specifically for patients with HS.28,29
Final Thoughts
Hidradenitis suppurativa is a complex medical condition seen disproportionately in minority groups. Understanding common comorbidities as well as the biases associated with pain management will allow providers to treat HS patients more effectively. Dermatologists who see many HS patients should become more familiar with treating these associated comorbidities to provide patient care that is more holistic and effective.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Yang H, Mu F, et al. Impact of hidradenitis suppurativa on work loss, indirect costs and income. Br J Dermatol. 2019;181:147-154. doi:10.1111/bjd.17101
- Udechukwu NS, Fleischer AB. Higher risk of care for hidradenitis suppurativa in African American and non-Hispanic patients in the United States. J Natl Med Assoc. 2017;109:44-48. doi:10.1016/j.jnma.2016.09.002
- Todd KH, Deaton C, D’Adamo AP, et al. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35:11-16. doi:10.1016/s0196-0644(00)70099-0
- Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537-1539.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Matusiak Ł, Szcze˛ch J, Kaaz K, et al. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol. 2018;98:191-194. doi:10.2340/00015555-2815
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen Hosp Psychiatry. 1996;18:220-229. doi:10.1016/0163-8343(96)00036-9
- Phan K, Huo YR, Smith SD. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: systematic review and meta-analysis. Ann Transl Med. 2020;8:821. doi:10.21037/atm-20-1028
- Woo AK. Depression and anxiety in pain. Rev Pain. 2010;4:8-12. doi:10.1177/204946371000400103
- Iida H, Yamaguchi S, Goyagi T, et al. Consensus statement on smoking cessation in patients with pain. J Anesth. 2022;36:671-687. doi:10.1007/s00540-022-03097-w
- Krause AJ, Prather AA, Wager TD, et al. The pain of sleep loss: a brain characterization in humans. J Neurosci. 2019;39:2291-2300. doi:10.1523/JNEUROSCI.2408-18.2018
- Mundal I, Gråwe RW, Bjørngaard JH, et al. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: the HUNT study. BMC Musculoskelet Disord. 2014;15:213. doi:10.1186/1471-2474-15-213
- Aubin H-J. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;(62 suppl 2):45-52. doi:10.2165/00003495-200262002-00005
- Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care. 2010;27:333-336. doi:10.1177/1049909110361229
- Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219:25-50. doi:10.1016/j.psychres.2014.05.013
- Walker PW, Cole JO, Gardner EA, et al. Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry. 1993;54:459-465.
- Sherman MM, Ungureanu S, Rey JA. Naltrexone/bupropion ER (contrave): newly approved treatment option for chronic weight management in obese adults. P T. 2016;41:164-172.
- Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633-641. doi:10.1038/oby.2002.86
- Kalkhoran S, Benowitz NL, Rigotti NA. Prevention and treatment of tobacco use: JACC health promotion series. J Am Coll Cardiol. 2018;72:1030-1045. doi:10.1016/j.jacc.2018.06.036
- Singh D, Saadabadi A. Varenicline. StatPearls Publishing; 2023.
- Mazza M, Mazza O, Pazzaglia C, et al. Escitalopram 20 mg versus duloxetine 60 mg for the treatment of chronic low back pain. Expert Opin Pharmacother. 2010;11:1049-1052. doi:10.1517/14656561003730413
- Docherty MJ, Jones RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:592-601.
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. StatPearls Publishing; 2022.
- Williams LK, Padhukasahasram B, Ahmedani BK, et al. Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab. 2014;99:3160-3168. doi:10.1210/jc.2014-1539
- Sharma S, Mathur DK, Paliwal V, et al. Efficacy of metformin in the treatment of acne in women with polycystic ovarian syndrome: a newer approach to acne therapy. J Clin Aesthet Dermatol. 2019;12:34-38.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1. doi:10.5070/D35VW402NF
- Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, et al. Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol. 2020;11:558474. doi:10.3389/fphar.2020.558474
Hidradenitis suppurativa (HS) has an unpredictable disease course and poses substantial therapeutic challenges. It carries an increased risk for adverse cardiovascular outcomes and all-cause mortality. It also is associated with comorbidities including mood disorders, tobacco smoking, obesity, diabetes mellitus, sleep disorders, sexual dysfunction, and autoimmune diseases, which can complicate its management and considerably affect patients’ quality of life (QOL).1 Hidradenitis suppurativa also disproportionately affects minority groups and has far-reaching inequities; for example, the condition has a notable economic impact on patients, including higher unemployment and disability rates, lower-paying jobs, less paid time off, and other indirect costs.2,3 Race can impact how pain itself is treated. In one study (N = 217), Black patients with extremity fractures presenting to anemergency department were significantly less likely to receive analgesia compared to White patients despite reporting similar pain (57% vs 74%, respectively; P = .01).4 In another study, Hispanic patients were 7-times less likely to be treated with opioids compared to non-Hispanic patients with long-bone fractures.5 Herein, we highlight pain management disparities in HS patients.
Treating HS Comorbidities Helps Improve Pain
Pain is reported by almost all HS patients and is the symptom most associated with QOL impairment.6,7 Pain in HS is multifactorial, with other symptoms and comorbidities affecting its severity. Treatment of acute flares often is painful and procedural, including intralesional steroid injections or incision and drainage.8 Algorithms for addressing pain through the treatment of comorbidities also have been developed.6 Although there are few studies on the medications that treat related comorbidities in HS, there is evidence of their benefits in similar diseases; for example, treating depression in patients with irritable bowel disease (IBD) improved pain perception, cognitive function, and sexual dysfunction.9
Depression exacerbates pain, and higher levels of depression have been observed in severe HS.10,11 Additionally, more than 80% of individuals with HS report tobacco smoking.1 Nicotine not only increases pain sensitivity and decreases pain tolerance but also worsens neuropathic, nociceptive, and psychosocial pain, as well as mood disorders and sleep disturbances.12 Given the higher prevalence of depression and smoking in HS patients and the impact on pain, addressing these comorbidities is crucial. Additionally, poor sleep amplifies pain sensitivity and affects neurologic pain modulation.13 Chronic pain also is associated with obesity and sleep dysfunction.14
Treatments Targeting Pain and Comorbidities
Treatments that target comorbidities and other symptoms of HS also may improve pain. Bupropion is a well-studied antidepressant and first-line option to aid in smoking cessation. It provides acute and chronic pain relief associated with IBD and may perform similarly in patients with HS.15-18 Bupropion also demonstrated dose-dependent weight reduction in obese and overweight individuals.19,20 Additionally, varenicline is a first-line option to aid in smoking cessation and can be combined with bupropion to increase long-term efficacy.21,22
Other antidepressants may alleviate HS pain. The selective norepinephrine reuptake inhibitors duloxetine and venlafaxine are recommended for chronic pain in HS.6 Selective serotonin reuptake inhibitors such as citalopram, escitalopram, and paroxetine are inexpensive and widely available antidepressants. Citalopram is as efficacious as duloxetine for chronic pain with fewer side effects.23 Paroxetine has been shown to improve pain and pruritus, QOL, and depression in patients with IBD.24 Benefits such as improved weight and sexual dysfunction also have been reported.25
Metformin is well studied in Black patients, and greater glycemic response supports its efficacy for diabetes as well as HS, which disproportionately affects individuals with skin of color.26 Metformin also targets other comorbidities of HS, such as improving insulin resistance, polycystic ovary syndrome, acne vulgaris, weight loss, hyperlipidemia, cardiovascular risk, and neuropsychologic conditions.27 Growing evidence supports the use of metformin as a new agent in chronic pain management, specifically for patients with HS.28,29
Final Thoughts
Hidradenitis suppurativa is a complex medical condition seen disproportionately in minority groups. Understanding common comorbidities as well as the biases associated with pain management will allow providers to treat HS patients more effectively. Dermatologists who see many HS patients should become more familiar with treating these associated comorbidities to provide patient care that is more holistic and effective.
Hidradenitis suppurativa (HS) has an unpredictable disease course and poses substantial therapeutic challenges. It carries an increased risk for adverse cardiovascular outcomes and all-cause mortality. It also is associated with comorbidities including mood disorders, tobacco smoking, obesity, diabetes mellitus, sleep disorders, sexual dysfunction, and autoimmune diseases, which can complicate its management and considerably affect patients’ quality of life (QOL).1 Hidradenitis suppurativa also disproportionately affects minority groups and has far-reaching inequities; for example, the condition has a notable economic impact on patients, including higher unemployment and disability rates, lower-paying jobs, less paid time off, and other indirect costs.2,3 Race can impact how pain itself is treated. In one study (N = 217), Black patients with extremity fractures presenting to anemergency department were significantly less likely to receive analgesia compared to White patients despite reporting similar pain (57% vs 74%, respectively; P = .01).4 In another study, Hispanic patients were 7-times less likely to be treated with opioids compared to non-Hispanic patients with long-bone fractures.5 Herein, we highlight pain management disparities in HS patients.
Treating HS Comorbidities Helps Improve Pain
Pain is reported by almost all HS patients and is the symptom most associated with QOL impairment.6,7 Pain in HS is multifactorial, with other symptoms and comorbidities affecting its severity. Treatment of acute flares often is painful and procedural, including intralesional steroid injections or incision and drainage.8 Algorithms for addressing pain through the treatment of comorbidities also have been developed.6 Although there are few studies on the medications that treat related comorbidities in HS, there is evidence of their benefits in similar diseases; for example, treating depression in patients with irritable bowel disease (IBD) improved pain perception, cognitive function, and sexual dysfunction.9
Depression exacerbates pain, and higher levels of depression have been observed in severe HS.10,11 Additionally, more than 80% of individuals with HS report tobacco smoking.1 Nicotine not only increases pain sensitivity and decreases pain tolerance but also worsens neuropathic, nociceptive, and psychosocial pain, as well as mood disorders and sleep disturbances.12 Given the higher prevalence of depression and smoking in HS patients and the impact on pain, addressing these comorbidities is crucial. Additionally, poor sleep amplifies pain sensitivity and affects neurologic pain modulation.13 Chronic pain also is associated with obesity and sleep dysfunction.14
Treatments Targeting Pain and Comorbidities
Treatments that target comorbidities and other symptoms of HS also may improve pain. Bupropion is a well-studied antidepressant and first-line option to aid in smoking cessation. It provides acute and chronic pain relief associated with IBD and may perform similarly in patients with HS.15-18 Bupropion also demonstrated dose-dependent weight reduction in obese and overweight individuals.19,20 Additionally, varenicline is a first-line option to aid in smoking cessation and can be combined with bupropion to increase long-term efficacy.21,22
Other antidepressants may alleviate HS pain. The selective norepinephrine reuptake inhibitors duloxetine and venlafaxine are recommended for chronic pain in HS.6 Selective serotonin reuptake inhibitors such as citalopram, escitalopram, and paroxetine are inexpensive and widely available antidepressants. Citalopram is as efficacious as duloxetine for chronic pain with fewer side effects.23 Paroxetine has been shown to improve pain and pruritus, QOL, and depression in patients with IBD.24 Benefits such as improved weight and sexual dysfunction also have been reported.25
Metformin is well studied in Black patients, and greater glycemic response supports its efficacy for diabetes as well as HS, which disproportionately affects individuals with skin of color.26 Metformin also targets other comorbidities of HS, such as improving insulin resistance, polycystic ovary syndrome, acne vulgaris, weight loss, hyperlipidemia, cardiovascular risk, and neuropsychologic conditions.27 Growing evidence supports the use of metformin as a new agent in chronic pain management, specifically for patients with HS.28,29
Final Thoughts
Hidradenitis suppurativa is a complex medical condition seen disproportionately in minority groups. Understanding common comorbidities as well as the biases associated with pain management will allow providers to treat HS patients more effectively. Dermatologists who see many HS patients should become more familiar with treating these associated comorbidities to provide patient care that is more holistic and effective.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Yang H, Mu F, et al. Impact of hidradenitis suppurativa on work loss, indirect costs and income. Br J Dermatol. 2019;181:147-154. doi:10.1111/bjd.17101
- Udechukwu NS, Fleischer AB. Higher risk of care for hidradenitis suppurativa in African American and non-Hispanic patients in the United States. J Natl Med Assoc. 2017;109:44-48. doi:10.1016/j.jnma.2016.09.002
- Todd KH, Deaton C, D’Adamo AP, et al. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35:11-16. doi:10.1016/s0196-0644(00)70099-0
- Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537-1539.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Matusiak Ł, Szcze˛ch J, Kaaz K, et al. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol. 2018;98:191-194. doi:10.2340/00015555-2815
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen Hosp Psychiatry. 1996;18:220-229. doi:10.1016/0163-8343(96)00036-9
- Phan K, Huo YR, Smith SD. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: systematic review and meta-analysis. Ann Transl Med. 2020;8:821. doi:10.21037/atm-20-1028
- Woo AK. Depression and anxiety in pain. Rev Pain. 2010;4:8-12. doi:10.1177/204946371000400103
- Iida H, Yamaguchi S, Goyagi T, et al. Consensus statement on smoking cessation in patients with pain. J Anesth. 2022;36:671-687. doi:10.1007/s00540-022-03097-w
- Krause AJ, Prather AA, Wager TD, et al. The pain of sleep loss: a brain characterization in humans. J Neurosci. 2019;39:2291-2300. doi:10.1523/JNEUROSCI.2408-18.2018
- Mundal I, Gråwe RW, Bjørngaard JH, et al. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: the HUNT study. BMC Musculoskelet Disord. 2014;15:213. doi:10.1186/1471-2474-15-213
- Aubin H-J. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;(62 suppl 2):45-52. doi:10.2165/00003495-200262002-00005
- Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care. 2010;27:333-336. doi:10.1177/1049909110361229
- Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219:25-50. doi:10.1016/j.psychres.2014.05.013
- Walker PW, Cole JO, Gardner EA, et al. Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry. 1993;54:459-465.
- Sherman MM, Ungureanu S, Rey JA. Naltrexone/bupropion ER (contrave): newly approved treatment option for chronic weight management in obese adults. P T. 2016;41:164-172.
- Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633-641. doi:10.1038/oby.2002.86
- Kalkhoran S, Benowitz NL, Rigotti NA. Prevention and treatment of tobacco use: JACC health promotion series. J Am Coll Cardiol. 2018;72:1030-1045. doi:10.1016/j.jacc.2018.06.036
- Singh D, Saadabadi A. Varenicline. StatPearls Publishing; 2023.
- Mazza M, Mazza O, Pazzaglia C, et al. Escitalopram 20 mg versus duloxetine 60 mg for the treatment of chronic low back pain. Expert Opin Pharmacother. 2010;11:1049-1052. doi:10.1517/14656561003730413
- Docherty MJ, Jones RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:592-601.
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. StatPearls Publishing; 2022.
- Williams LK, Padhukasahasram B, Ahmedani BK, et al. Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab. 2014;99:3160-3168. doi:10.1210/jc.2014-1539
- Sharma S, Mathur DK, Paliwal V, et al. Efficacy of metformin in the treatment of acne in women with polycystic ovarian syndrome: a newer approach to acne therapy. J Clin Aesthet Dermatol. 2019;12:34-38.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1. doi:10.5070/D35VW402NF
- Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, et al. Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol. 2020;11:558474. doi:10.3389/fphar.2020.558474
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Yang H, Mu F, et al. Impact of hidradenitis suppurativa on work loss, indirect costs and income. Br J Dermatol. 2019;181:147-154. doi:10.1111/bjd.17101
- Udechukwu NS, Fleischer AB. Higher risk of care for hidradenitis suppurativa in African American and non-Hispanic patients in the United States. J Natl Med Assoc. 2017;109:44-48. doi:10.1016/j.jnma.2016.09.002
- Todd KH, Deaton C, D’Adamo AP, et al. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35:11-16. doi:10.1016/s0196-0644(00)70099-0
- Todd KH, Samaroo N, Hoffman JR. Ethnicity as a risk factor for inadequate emergency department analgesia. JAMA. 1993;269:1537-1539.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Matusiak Ł, Szcze˛ch J, Kaaz K, et al. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol. 2018;98:191-194. doi:10.2340/00015555-2815
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen Hosp Psychiatry. 1996;18:220-229. doi:10.1016/0163-8343(96)00036-9
- Phan K, Huo YR, Smith SD. Hidradenitis suppurativa and psychiatric comorbidities, suicides and substance abuse: systematic review and meta-analysis. Ann Transl Med. 2020;8:821. doi:10.21037/atm-20-1028
- Woo AK. Depression and anxiety in pain. Rev Pain. 2010;4:8-12. doi:10.1177/204946371000400103
- Iida H, Yamaguchi S, Goyagi T, et al. Consensus statement on smoking cessation in patients with pain. J Anesth. 2022;36:671-687. doi:10.1007/s00540-022-03097-w
- Krause AJ, Prather AA, Wager TD, et al. The pain of sleep loss: a brain characterization in humans. J Neurosci. 2019;39:2291-2300. doi:10.1523/JNEUROSCI.2408-18.2018
- Mundal I, Gråwe RW, Bjørngaard JH, et al. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: the HUNT study. BMC Musculoskelet Disord. 2014;15:213. doi:10.1186/1471-2474-15-213
- Aubin H-J. Tolerability and safety of sustained-release bupropion in the management of smoking cessation. Drugs. 2002;(62 suppl 2):45-52. doi:10.2165/00003495-200262002-00005
- Shah TH, Moradimehr A. Bupropion for the treatment of neuropathic pain. Am J Hosp Palliat Care. 2010;27:333-336. doi:10.1177/1049909110361229
- Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219:25-50. doi:10.1016/j.psychres.2014.05.013
- Walker PW, Cole JO, Gardner EA, et al. Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry. 1993;54:459-465.
- Sherman MM, Ungureanu S, Rey JA. Naltrexone/bupropion ER (contrave): newly approved treatment option for chronic weight management in obese adults. P T. 2016;41:164-172.
- Anderson JW, Greenway FL, Fujioka K, et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002;10:633-641. doi:10.1038/oby.2002.86
- Kalkhoran S, Benowitz NL, Rigotti NA. Prevention and treatment of tobacco use: JACC health promotion series. J Am Coll Cardiol. 2018;72:1030-1045. doi:10.1016/j.jacc.2018.06.036
- Singh D, Saadabadi A. Varenicline. StatPearls Publishing; 2023.
- Mazza M, Mazza O, Pazzaglia C, et al. Escitalopram 20 mg versus duloxetine 60 mg for the treatment of chronic low back pain. Expert Opin Pharmacother. 2010;11:1049-1052. doi:10.1517/14656561003730413
- Docherty MJ, Jones RCW, Wallace MS. Managing pain in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:592-601.
- Shrestha P, Fariba KA, Abdijadid S. Paroxetine. StatPearls Publishing; 2022.
- Williams LK, Padhukasahasram B, Ahmedani BK, et al. Differing effects of metformin on glycemic control by race-ethnicity. J Clin Endocrinol Metab. 2014;99:3160-3168. doi:10.1210/jc.2014-1539
- Sharma S, Mathur DK, Paliwal V, et al. Efficacy of metformin in the treatment of acne in women with polycystic ovarian syndrome: a newer approach to acne therapy. J Clin Aesthet Dermatol. 2019;12:34-38.
- Scheinfeld N. Hidradenitis suppurativa: a practical review of possible medical treatments based on over 350 hidradenitis patients. Dermatol Online J. 2013;19:1. doi:10.5070/D35VW402NF
- Baeza-Flores GDC, Guzmán-Priego CG, Parra-Flores LI, et al. Metformin: a prospective alternative for the treatment of chronic pain. Front Pharmacol. 2020;11:558474. doi:10.3389/fphar.2020.558474
Juvenile Dermatomyositis–Associated Panniculitis
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.
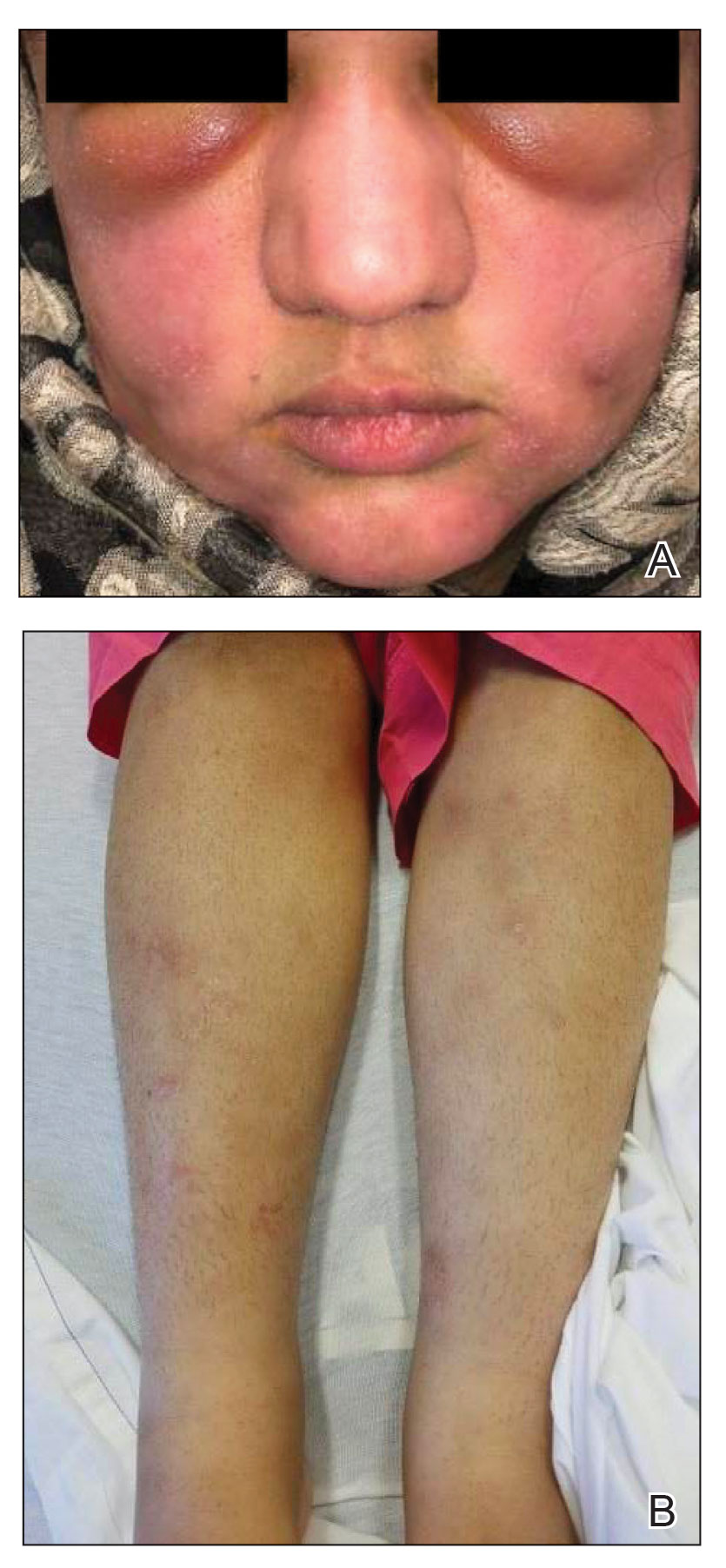
A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.
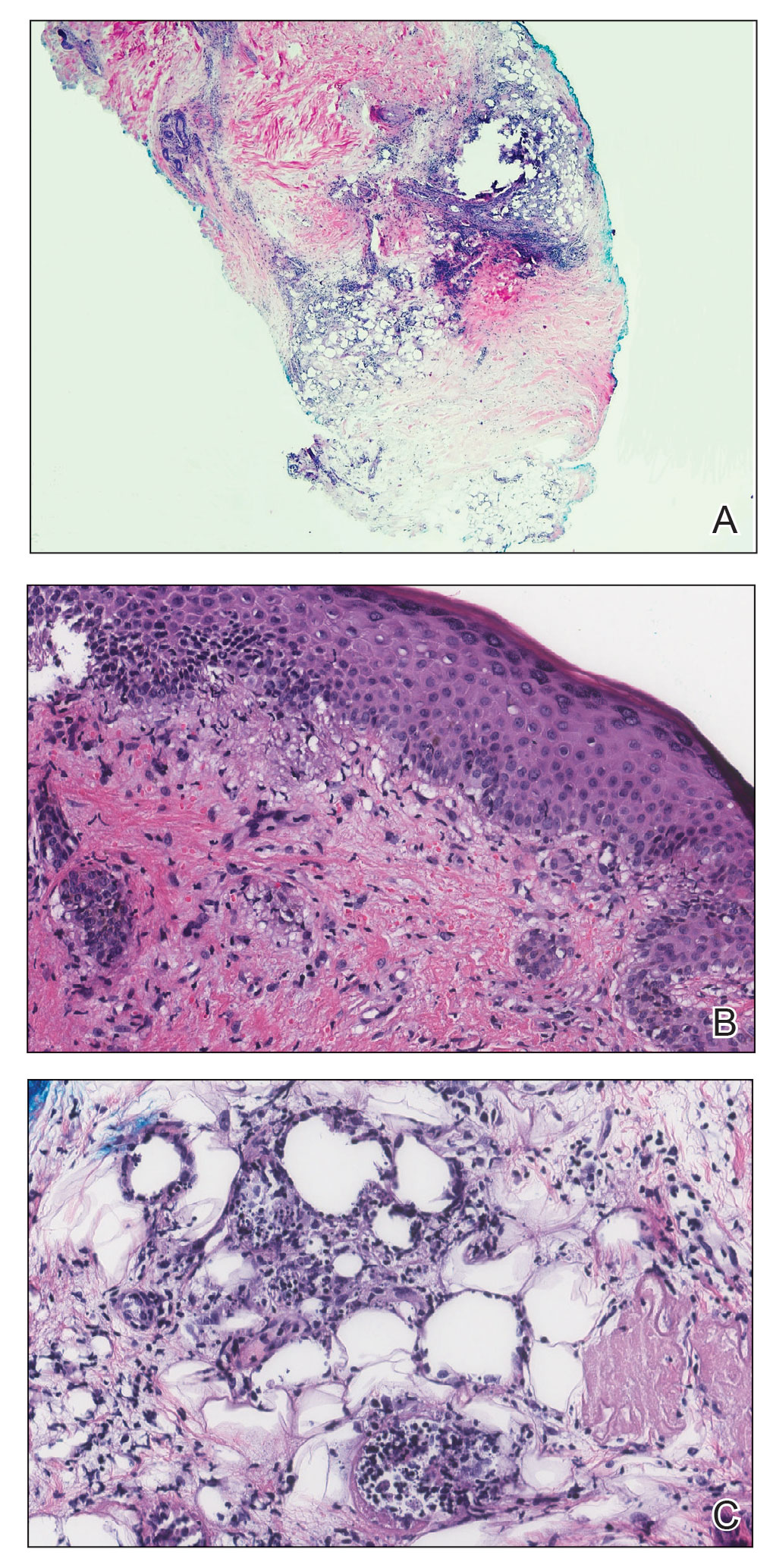
Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
Practice Points
- Juvenile dermatomyositis is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin.
- Juvenile dermatomyositis has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant, dermatomyositis (DM).
- Panniculitis is a rare but severe complication of DM, and this subset of DM may be challenging to treat, requiring aggressive therapy.
Bullous Dermatoses and Quality of Life: A Summary of Tools to Assess Psychosocial Health
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.
Results
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.
The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.
To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.

Results
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.

The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.

To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.

Results
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.

The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.

To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
Practice Points
- Autoimmune bullous dermatoses cause cutaneous lesions that are painful and disfiguring. These conditions affect a patient’s ability to perform everyday tasks, and individual lesions can take years to heal.
- Providers should take necessary steps to address patient well-being, especially at disease onset in patients with bullous dermatoses.
Current Recommendations for the Systemic Treatment of Cutaneous Lupus Erythematosus During Pregnancy
Cutaneous lupus erythematosus (CLE) is a heterogeneous autoimmune disease that involves the skin. Cutaneous lupus erythematosus can be classified into various subtypes.1 These include, but are not limited to, acute CLE, subacute CLE, chronic CLE, intermittent CLE, lupus tumidus, and lupus profundus.1,2 The CLE subtypes have variable associations with systemic lupus erythematosus. For instance, some subtypes, such as acute CLE, are more strongly associated with systemic lupus erythematosus.
Treatment of CLE is similar to other autoimmune disorders. Although the US Food and Drug Administration (FDA) has not approved any treatments for CLE,3,4 the most common therapeutic options are disease-modifying antirheumatic drugs. Unfortunately, many of these treatments carry teratogenic effects. Because CLE predominantly affects women, particularly those of childbearing age, it is imperative to understand the available treatment options for those who are pregnant or considering pregnancy for an informed discussion with patients.5
For years, the gold standard when considering a medication during pregnancy was the FDA’s classification system. According to this system, medications were classified into 5 letter categories based on their potential teratogenicity, including A (no fetal risk), B (potential animal risk but inconclusive human studies), C (risk cannot be ruled out), D (evidence of fetal risk), and X (contraindicated in pregnancy). In 2014, the FDA decided to no longer use this classification system for medications approved after 2000.6 However, because many proposed treatment options for CLE were approved prior to 2001, we have summarized the commonly prescribed medications for CLE according to their prior FDA letter categories.
Treatment Options for CLE During Pregnancy
Prior to initiating systemic medications for the treatment of CLE, topical medications should be considered. Recommended treatment options include corticosteroids and calcineurin inhibitors.7 Compared with systemic medications, topical treatments carry minimal side effects, such as skin atrophy, that typically remain localized to areas of application.8 Moreover, even with extensive application, no correlation has been found between topical corticosteroid use and fetal growth,9 which suggests that topical steroids are safe in pregnancy and should be considered as a first-line treatment option for CLE. Calcineurin inhibitors also are considered safe based on their low level of absorption through the skin and are considered second-line topical treatment options in pregnancy.10
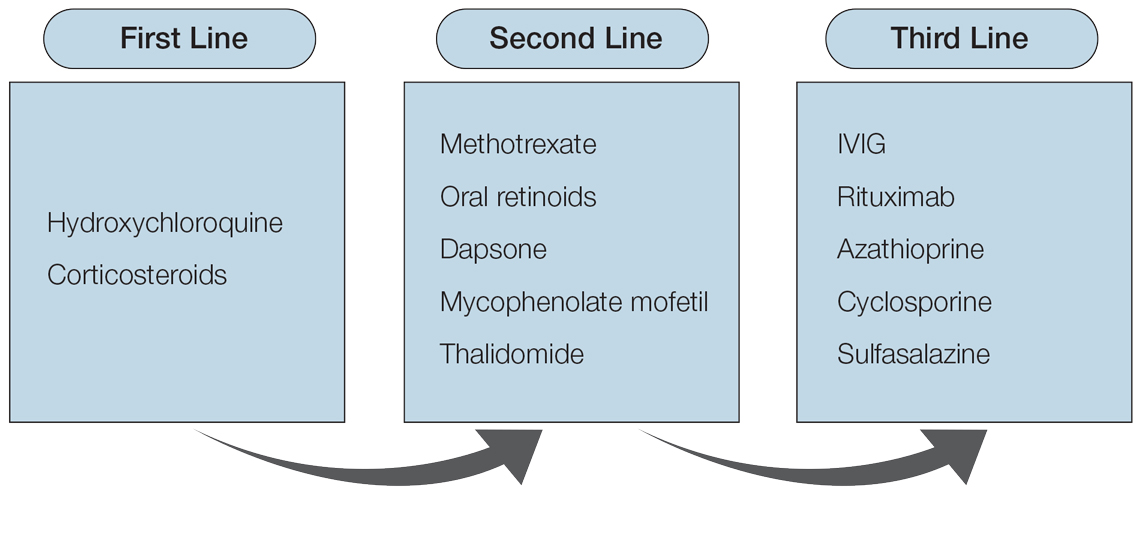
Although topical medications are effective for the treatment of CLE, many patients require the administration of systemic therapeutics for severe or refractory disease. Based on previously published reports, Figure 1 describes the current recommended systemic treatment options for CLE.11 Unfortunately, many of these medications carry teratogenic risks during pregnancy. The risks and side effects of the medications are described in detail in the following sections and summarized in the eTable.

Category B
Systemic Steroids—Systemic steroids are one of the most prescribed medications during pregnancy.12 Oral steroids have been associated with fast symptom relief, making this class of medications particularly effective during CLE flares; however, long-term management is not recommended because of the side effects, which include osteoporosis and impaired glucose metabolism.13
With low transmission across the placenta, there are 3 glucocorticoids that carry the safest profile in pregnancy: prednisone, cortisone, and hydrocortisone.14 Dexamethasone and betamethasone should be avoided, as both readily cross the placenta and increase fetal exposure.15 Although teratogenic effects have been associated with steroid use, most studies involving pregnant patients have inconclusive results. For instance, one study described an association between cleft lip/palate with in utero glucocorticoid exposure.16 However, multiple follow-up studies found no association between the two.17,18 Studies investigating the relationship between steroids and miscarriages or steroids and low birth weight also are inconclusive. Of note, if used throughout pregnancy, administration of a loading dose of glucocorticoids prior to delivery is recommended because of the increased stress brought on during labor.19
Sulfasalazine—Sulfasalazine is an immunomodulator commonly used for the treatment of inflammatory bowel disease and rheumatoid arthritis. However, studies also have shown that sulfasalazine is an effective treatment of CLE if standard treatments have failed.20,21
During pregnancy, patients exposed to sulfasalazine experienced minimal side effects despite transportation across the placenta.22 In comparison with control, pregnant women taking sulfasalazine experienced no increased risk for low fetal weight,23 congenital abnormalities,24 or spontaneous abortions.25 Of note, sulfasalazine can affect sperm, so male patients also should be counselled.
Category C
Hydroxychloroquine—Hydroxychloroquine is considered a first-line medication for those with CLE based on a symptomatic relief rate of 50% to 70%.26 For those taking hydroxychloroquine during pregnancy, the majority of studies have shown no association between the medication and adverse fetal events, including congenital abnormalities, prematurity, or spontaneous abortions.27-29 Therefore, hydroxychloroquine is considered safe in pregnancy, and those on the medication should continue standard monitoring, including retinopathy screening.30
Of note, hydroxychloroquine can be stored in tissue for weeks to months after discontinuation.5 Therefore, if patients wish to avoid hydroxychloroquine in pregnancy, one should stop taking the medication several months prior to conception.
Dapsone—Dapsone, a medication with both antimicrobial and immunomodulatory properties, is an effective second-line therapy for CLE.31 Although large-scale human trials have not been performed, multiple case reports and observational studies have supported the safe use of dapsone in pregnancy.32-34 However, there are notable side effects, including dose-dependent hemolysis, methemoglobinemia, and hypersensitivity reactions.13 Therefore, once treatment is initiated or continued, folic acid supplementation (5 mg daily) and regular serum analysis, including complete blood cell counts, are recommended in pregnant patients.19
Rituximab—Recent studies have demonstrated that rituximab can be an effective treatment of subacute and chronic CLE.35,36 Through inhibition of CD20, rituximab causes a decrease in circulating B cells and a reduced immune response. Therefore, experts recommend discontinuation of rituximab for 12 months prior to conception to reduce potential side effects to the fetus, which may include a transient reduction of circulating fetal B cells.37
If continued during pregnancy, most studies suggest discontinuation of rituximab during the third trimester, as it has been associated with neonatal infections and congenital abnormalities.19,37 However, these results are based on limited case reports, and thus robust research is needed to better understand the effect of rituximab in utero.
Intravenous Immunoglobulin Infusion—Intravenous immunoglobulin (IVIG) infusion is a well-tolerated treatment for many autoimmune disorders.38 Although not first line, limited case studies have demonstrated remission of refractory CLE following IVIG.39,40 Although no studies have directly investigated the effect of IVIG on fetal development, it has been frequently administered and well tolerated during pregnancy, especially in those with multiple sclerosis or antiphospholipid syndrome.41 Commonly reported side effects include headache and fatigue, and a rare associated side effect to be aware of is embolic events.42,43
Cyclosporine—Cyclosporine rarely is used in the treatment of localized CLE due to its extensive side-effect profile, most notably nephrotoxicity.44 However, studies have shown that cyclosporine may be efficacious if symptoms extend beyond the skin, involve multiple organs, and/or other treatments have failed.39 For those who are pregnant and wish to continue cyclosporine use, studies have associated low birth weight and premature delivery with its exposure in utero.44
Category D
Mycophenolate Mofetil—In conjunction with standard therapy, mycophenolate mofetil (MMF) is an adequate treatment of refractory CLE.45 Unfortunately, case reports have demonstrated an increased risk for fetal congenital abnormalities and first-trimester spontaneous abortion with use of MMF during pregnancy.46,47 Therefore, it is recommended that patients on MMF discontinue the medication at least 6 weeks prior to conception.46
Azathioprine—Although azathioprine has been shown to provide relief of discoid lupus erythematosus symptoms,48 it currently is only utilized for refractory disease, largely due to notable side effects that particularly affect the gastrointestinal tract and liver.4 Moreover, azathioprine use during pregnancy has been associated with prematurity, congenital anomalies, fetal cytopenia, and low birth weight.49 With that said, and although not recommended, if patients decide to continue treatment, experts recommend limiting the dose to 2 mg/kg daily to reduce potential adverse events.
Category X
Oral Retinoids—According to the American Academy of Dermatology, retinoids such as isotretinoin and acitretin are considered second-line therapy for CLE.50 With that being said, there are well-documented effects on fetal development associated with oral retinoid use, including central nervous system, cardiovascular system, and craniofacial abnormalities.51 Therefore, its use is contraindicated during pregnancy. To prevent pregnancy while taking isotretinoin, patients must enroll in an online monitoring program called iPLEDGE. This program requires monthly updates by both the physician and the patient, including a negative pregnancy test every month for female patients actively taking the medication.52
The half-lives of the oral retinoids isotretinoin and acitretin are 10 to 20 hours and 50 to 60 hours, respectively.53,54 However, alcohol consumption converts acitretin into the metabolite etretinate, which can remain in tissue for up to 120 days.54,55 Therefore, women are advised to avoid alcohol while taking acitretin and avoid conception for 2 to 3 years after cessation of the medication.55 For those wishing to restart retinoids after pregnancy, studies show the medication can be safely reinstated 35 days after delivery for those interested in continued treatment.56
Thalidomide—Although low-dose thalidomide can treat refractory CLE, its use is restricted because of its known teratogenicity, most notably limb deformities.57 If prescribed thalidomide, women will need to enroll in the System for Thalidomide Education and Prescribing Safety program, similar to the iPLEDGE program, and use 2 forms of contraception when sexually active.58 Contraception should be continued for 4 weeks following the last dose of thalidomide. After this point, conception is considered safe.59
Methotrexate—For nonpregnant patients, low-dose methotrexate (MTX) with folate supplementation is a treatment option for CLE.60 However, for those who are pregnant, low-dose MTX is an abortive agent and has been associated with aminopterin syndrome, which includes skull deficits, craniofacial abnormalities, and limb deformities in live births.19,61 Therefore, MTX is not recommended in pregnancy. Of note, MTX can affect sperm; male patients also should be counselled.
Final Thoughts
Overall, it is recommended to limit medication use as much as possible in pregnancy. To reduce these exposures, it is imperative to reduce triggers that may lead to symptomatic flares of CLE. Because CLE can be triggered by sun exposure, we advise topical sunscreen to prevent CLE flares that may require additional oral medication.62,63
Various medications are considered safe for the treatment of CLE in pregnant patients (Figure 2). Based on studies in animal and clinical trials, hydroxychloroquine is considered a safe and effective medication for CLE in pregnancy and is a first-line therapy in nonpregnant patients.26,27 If flares occur, IVIG or a short course of oral steroids should be considered to manage symptoms.13,39 For those with severe flares, treatment is difficult, and personalized approaches may be necessary.
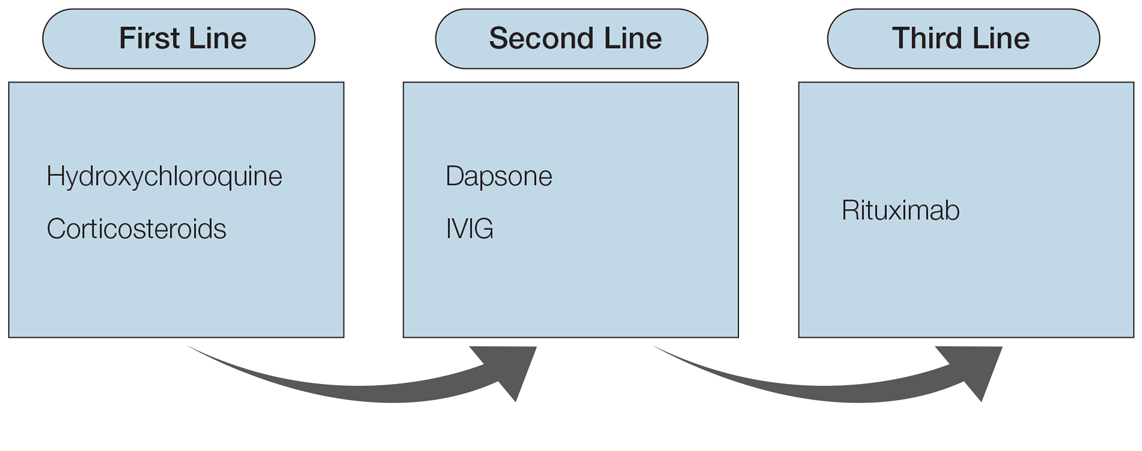
Part of the question for the childbearing population is when a patient would like to conceive. For severe cases when hydroxychloroquine is not effective as monotherapy, using a treatment that can encourage remission prior to conception attempts can be a beneficial strategy. Rituximab is an excellent example of such a therapy, as the therapeutic effect outlasts the immunosuppressive effect and therefore is unlikely to affect a future fetus.64 Thalidomide also is a potential option prior to conception, based on its short washout period and its ability to achieve notable remission rates in patients with CLE.57,59 Regardless, patients with CLE should still consult their dermatologist and rheumatologist (if applicable) prior to conception.
Patients of childbearing potential represent a population in which discussion about life goals greatly affects medication options. Having these discussions early and often allows for an open, more successful approach so that treatment regimens are not derailed at the time of conception.
- Renner R, Sticherling M. The different faces of cutaneous lupus erythematosus. G Ital Dermatol Venereol. 2009;144:135-147.
- Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48:14-19.
- Shi H, Gudjonsson J, Kahlenberg J. Treatment of cutaneous lupus erythematosus: current approaches and future strategies. Curr Opin Rheumatol. 2020;32:208-214.
- Winkelmann RR, Kim GK, Del Rosso JQ. Treatment of cutaneous lupus erythematosus: review and assessment of treatment benefits based on Oxford Centre for Evidence-based Medicine criteria. J Clin Aesthet Dermatol. 2013;6:27-38.
- Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223-243.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;41:713-715.
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736-745.
- Kuhn A, Aberer E, Bata‐Csörgö Z, et al. S2k guideline for treatment of cutaneous lupus erythematosus—guided by the European Dermatology Forum (EDF) in cooperation with the European Academyof Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2017;31:389-404.
- Andersson NW, Skov L, Andersen JT. Evaluation of topical corticosteroid use in pregnancy and risk of newborns being small for gestational age and having low birth weight. JAMA Dermatol. 2021;157:788-795.
- Undre NA, Moloney FJ, Ahmadi S, et al. Skin and systemic pharmacokinetics of tacrolimus following topical application of tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2009;160:665-669.
- Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2014;10:97-107.
- Kuriya B, Hernández‐Díaz S, Liu J, et al. Patterns of medication use during pregnancy in rheumatoid arthritis. Arthritis Care Res. 2011;63:721-728.
- Chang A, Werth V. Treatment of cutaneous lupus. Curr Rheumatol Rep. 2011;13:300-307.
- Beitins IZ, Bayard F, Ances IG, et al. The transplacental passage of prednisone and prednisolone in pregnancy near term. J Pediatr. 1972;81:936-945.
- Ogueh O, Johnson MR. The metabolic effect of antenatal corticosteroid therapy. Hum Reprod Update. 2000;6:169-176.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Bay Bjørn A, Ehrenstein V, Hundborg HH, et al. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. Am J Ther. 2014;21:73-80.
- Hviid A, Mølgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ. 2011;183:796-804.
- Krause ML, Amin S, Makol A. Use of DMARDs and biologics during pregnancy and lactation in rheumatoid arthritis: what the rheumatologist needs to know. Ther Adv Musculoskelet Dis. 2014;6:169-184.
- Artuz F, Lenk N, Deniz N, et al. Efficacy of sulfasalazine in discoid lupus erythematosus. Int J Dermatol. 1996;35:746-748.
- Delaporte E, Catteau B, Sabbagh N, et al. Treatment of discoid lupus erythematosus with sulfasalazine: 11 cases [in French]. Ann Dermatol Venereol. 1997;124:151-156.
- Järnerot G, Into-Malmberg MB, Esbjörner E. Placental transfer of sulphasalazine and sulphapyridine and some of its metabolites. Scand J Gastroenterol. 1981;16:693-697.
- Norgard B, Pedersen L, Christensen LA, et al. Therapeutic drug use in women with Crohn’s disease and birth outcomes: a Danish nationwide cohort study. Am J Gastroenterol. 2007;102:1406-1413.
- Nørgård B, Czeizel AE, Rockenbauer M, et al. Population-based case control study of the safety of sulfasalazine use during pregnancy. Aliment Pharmacol Ther. 2001;15:483-486.
- Rahimi R, Nikfar S, Rezaie A, et al. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod Toxicol. 2008;25:271-275.
- Callen JP. Chronic cutaneous lupus erythematosus: clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416.
- Buchanan NM, Toubi E, Khamashta MA, et al. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann Rheum Dis. 1996;55:486-488.
- Costedoat‐Chalumeau N, Amoura Z, Duhaut P, et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty‐three cases compared with a control group. Arthritis Rheum. 2003;48:3207-3211.
- Sperber K, Hom C, Chao CP, et al. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009;7:9.
- Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377-1382.
- Klebes M, Wutte N, Aberer E. Dapsone as second-line treatment for cutaneous lupus erythematosus? a retrospective analysis of 34 patients and a review of the literature. Dermatology. 2016;232:91-96.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Varghese L, Viswabandya A, Mathew AJ. Dapsone, danazol, and intrapartum splenectomy in refractory ITP complicating pregnancy. Indian J Med Sci. 2008;62:452-455.
- Kahn G. Dapsone is safe during pregnancy. J Am Acad Dermatol. 1985;13:838-839.
- Quelhas da Costa R, Aguirre-Alastuey ME, Isenberg DA, et al. Assessment of response to B-cell depletion using rituximab in cutaneous lupus erythematosus. JAMA Dermatol. 2018;154:1432-1440.
- Alsanafi S, Kovarik C, Mermelstein A, et al. Rituximab in thetreatment of bullous systemic lupus erythematosus. J Clin Rheumatol. 2011;17:142-144.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506.
- Fernandez AP, Kerdel FA. The use of i.v. IG therapy in dermatology. Dermatol Ther. 2007;20:288-305.
- Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part II. J Am Acad Dermatol. 2011;65:E195-E213.
- Singh H, Naidu G, Sharma A. Intravenous immunoglobulin for the rescue in refractory cutaneous lupus. Indian Dermatol Online J. 2020;11:1003-1004.
- Clark AL. Clinical uses of intravenous immunoglobulin in pregnancy. Clin Obstet Gynecol. 1999;42:368-380.
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747-755.
- Woodruff RK, Grigg AP, Firkin FC, et al. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet. 1986;2:217-218.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Gammon B, Hansen C, Costner MI. Efficacy of mycophenolate mofetil in antimalarial-resistant cutaneous lupus erythematosus. J Am Acad Dermatol. 2010;65:717-721.e2.
- Abdulaziz HM, Shemies RS, Taman M, et al. Fetal proximal and distal limb anomalies following exposure to mycophenolate mofetil during pregnancy: a case report and review of the literature. Lupus. 2021;30:1522-1525.
- Pisoni CN, D’Cruz DP. The safety of mycophenolate mofetil in pregnancy. Exp Opin Drug Saf. 2008;7:219-222.
- Ashinoff R, Werth VP, Franks AG. Resistant discoid lupus erythematosus of palms and soles: successful treatment with azathioprine. J Am Acad Dermatol. 1988;19:961-965. doi:10.1016/S0190-9622(88)70259-5
- Goldstein LH, Dolinsky G, Greenberg R, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79:696-701.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for cutaneous lupus erythematosus. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:830-836.
- Sladden MJ, Harman KE. What is the chance of a normal pregnancy in a woman whose fetus has been exposed to isotretinoin? Arch Dermatol. 2007;143:1187-1188.
- Shin J, Cheetham TC, Wong L, et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- Brazzell RK, Colburn WA. Pharmacokinetics of the retinoids isotretinoin and etretinate. J Am Acad Dermatol. 1982;6:643-651.
- Pilkington T, Brogden RN. Acitretin: a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Gronhoj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
- Jajoria H, Mysore V. Washout period for pregnancy post isotretinoin therapy. Indian Dermatol Online J. 2020;11:239-242.
- Cortés-Hernández J, Torres-Salido M, Castro-Marrero J, et al. Thalidomide in the treatment of refractory cutaneous lupus erythematosus: prognostic factors of clinical outcome. Br J Dermatol. 2012;166:616-623.
- Zeldis JB, Williams BA, Thomas SD, et al. S.T.E.P.S.™: a comprehensive program for controlling and monitoring access to thalidomide. Clin Ther. 1999;21:319-330.
- C.S. Mott Children’s Hospital. University of Michigan Health. Thalidomide. Updated March 26, 2020. Accessed January 14, 2022. https://www.mottchildren.org/health-library/d04331a1
- Boehm IB, Boehm GA, Bauer R. Management of cutaneous lupus erythematosus with low-dose methotrexate: indication for modulation of inflammatory mechanisms. Rheumatol Int. 1998;18:59-62.
- Buckley LM, Bullaboy CA, Leichtman L, et al. Multiple congenital anomalies associated with weekly low‐dose methotrexate treatment of the mother. Arthritis Rheum. 1997;40:971-973.
- Foering K, Okawa J, Rose M, et al. Characterization of photosensitivity and poor quality of life in lupus. J Invest Dermatol. 2010;130(suppl):S10.
- Kuhn A, Herrmann M, Kleber S, et al. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 2006;54:939-950.
- Lake EP, Huang Y, Aronson IK. Rituximab treatment of pemphigus in women of childbearing age: experience with two patients. J Dermatol Treat. 2017;28:751-752.
Cutaneous lupus erythematosus (CLE) is a heterogeneous autoimmune disease that involves the skin. Cutaneous lupus erythematosus can be classified into various subtypes.1 These include, but are not limited to, acute CLE, subacute CLE, chronic CLE, intermittent CLE, lupus tumidus, and lupus profundus.1,2 The CLE subtypes have variable associations with systemic lupus erythematosus. For instance, some subtypes, such as acute CLE, are more strongly associated with systemic lupus erythematosus.
Treatment of CLE is similar to other autoimmune disorders. Although the US Food and Drug Administration (FDA) has not approved any treatments for CLE,3,4 the most common therapeutic options are disease-modifying antirheumatic drugs. Unfortunately, many of these treatments carry teratogenic effects. Because CLE predominantly affects women, particularly those of childbearing age, it is imperative to understand the available treatment options for those who are pregnant or considering pregnancy for an informed discussion with patients.5
For years, the gold standard when considering a medication during pregnancy was the FDA’s classification system. According to this system, medications were classified into 5 letter categories based on their potential teratogenicity, including A (no fetal risk), B (potential animal risk but inconclusive human studies), C (risk cannot be ruled out), D (evidence of fetal risk), and X (contraindicated in pregnancy). In 2014, the FDA decided to no longer use this classification system for medications approved after 2000.6 However, because many proposed treatment options for CLE were approved prior to 2001, we have summarized the commonly prescribed medications for CLE according to their prior FDA letter categories.
Treatment Options for CLE During Pregnancy
Prior to initiating systemic medications for the treatment of CLE, topical medications should be considered. Recommended treatment options include corticosteroids and calcineurin inhibitors.7 Compared with systemic medications, topical treatments carry minimal side effects, such as skin atrophy, that typically remain localized to areas of application.8 Moreover, even with extensive application, no correlation has been found between topical corticosteroid use and fetal growth,9 which suggests that topical steroids are safe in pregnancy and should be considered as a first-line treatment option for CLE. Calcineurin inhibitors also are considered safe based on their low level of absorption through the skin and are considered second-line topical treatment options in pregnancy.10

Although topical medications are effective for the treatment of CLE, many patients require the administration of systemic therapeutics for severe or refractory disease. Based on previously published reports, Figure 1 describes the current recommended systemic treatment options for CLE.11 Unfortunately, many of these medications carry teratogenic risks during pregnancy. The risks and side effects of the medications are described in detail in the following sections and summarized in the eTable.

Category B
Systemic Steroids—Systemic steroids are one of the most prescribed medications during pregnancy.12 Oral steroids have been associated with fast symptom relief, making this class of medications particularly effective during CLE flares; however, long-term management is not recommended because of the side effects, which include osteoporosis and impaired glucose metabolism.13
With low transmission across the placenta, there are 3 glucocorticoids that carry the safest profile in pregnancy: prednisone, cortisone, and hydrocortisone.14 Dexamethasone and betamethasone should be avoided, as both readily cross the placenta and increase fetal exposure.15 Although teratogenic effects have been associated with steroid use, most studies involving pregnant patients have inconclusive results. For instance, one study described an association between cleft lip/palate with in utero glucocorticoid exposure.16 However, multiple follow-up studies found no association between the two.17,18 Studies investigating the relationship between steroids and miscarriages or steroids and low birth weight also are inconclusive. Of note, if used throughout pregnancy, administration of a loading dose of glucocorticoids prior to delivery is recommended because of the increased stress brought on during labor.19
Sulfasalazine—Sulfasalazine is an immunomodulator commonly used for the treatment of inflammatory bowel disease and rheumatoid arthritis. However, studies also have shown that sulfasalazine is an effective treatment of CLE if standard treatments have failed.20,21
During pregnancy, patients exposed to sulfasalazine experienced minimal side effects despite transportation across the placenta.22 In comparison with control, pregnant women taking sulfasalazine experienced no increased risk for low fetal weight,23 congenital abnormalities,24 or spontaneous abortions.25 Of note, sulfasalazine can affect sperm, so male patients also should be counselled.
Category C
Hydroxychloroquine—Hydroxychloroquine is considered a first-line medication for those with CLE based on a symptomatic relief rate of 50% to 70%.26 For those taking hydroxychloroquine during pregnancy, the majority of studies have shown no association between the medication and adverse fetal events, including congenital abnormalities, prematurity, or spontaneous abortions.27-29 Therefore, hydroxychloroquine is considered safe in pregnancy, and those on the medication should continue standard monitoring, including retinopathy screening.30
Of note, hydroxychloroquine can be stored in tissue for weeks to months after discontinuation.5 Therefore, if patients wish to avoid hydroxychloroquine in pregnancy, one should stop taking the medication several months prior to conception.
Dapsone—Dapsone, a medication with both antimicrobial and immunomodulatory properties, is an effective second-line therapy for CLE.31 Although large-scale human trials have not been performed, multiple case reports and observational studies have supported the safe use of dapsone in pregnancy.32-34 However, there are notable side effects, including dose-dependent hemolysis, methemoglobinemia, and hypersensitivity reactions.13 Therefore, once treatment is initiated or continued, folic acid supplementation (5 mg daily) and regular serum analysis, including complete blood cell counts, are recommended in pregnant patients.19
Rituximab—Recent studies have demonstrated that rituximab can be an effective treatment of subacute and chronic CLE.35,36 Through inhibition of CD20, rituximab causes a decrease in circulating B cells and a reduced immune response. Therefore, experts recommend discontinuation of rituximab for 12 months prior to conception to reduce potential side effects to the fetus, which may include a transient reduction of circulating fetal B cells.37
If continued during pregnancy, most studies suggest discontinuation of rituximab during the third trimester, as it has been associated with neonatal infections and congenital abnormalities.19,37 However, these results are based on limited case reports, and thus robust research is needed to better understand the effect of rituximab in utero.
Intravenous Immunoglobulin Infusion—Intravenous immunoglobulin (IVIG) infusion is a well-tolerated treatment for many autoimmune disorders.38 Although not first line, limited case studies have demonstrated remission of refractory CLE following IVIG.39,40 Although no studies have directly investigated the effect of IVIG on fetal development, it has been frequently administered and well tolerated during pregnancy, especially in those with multiple sclerosis or antiphospholipid syndrome.41 Commonly reported side effects include headache and fatigue, and a rare associated side effect to be aware of is embolic events.42,43
Cyclosporine—Cyclosporine rarely is used in the treatment of localized CLE due to its extensive side-effect profile, most notably nephrotoxicity.44 However, studies have shown that cyclosporine may be efficacious if symptoms extend beyond the skin, involve multiple organs, and/or other treatments have failed.39 For those who are pregnant and wish to continue cyclosporine use, studies have associated low birth weight and premature delivery with its exposure in utero.44
Category D
Mycophenolate Mofetil—In conjunction with standard therapy, mycophenolate mofetil (MMF) is an adequate treatment of refractory CLE.45 Unfortunately, case reports have demonstrated an increased risk for fetal congenital abnormalities and first-trimester spontaneous abortion with use of MMF during pregnancy.46,47 Therefore, it is recommended that patients on MMF discontinue the medication at least 6 weeks prior to conception.46
Azathioprine—Although azathioprine has been shown to provide relief of discoid lupus erythematosus symptoms,48 it currently is only utilized for refractory disease, largely due to notable side effects that particularly affect the gastrointestinal tract and liver.4 Moreover, azathioprine use during pregnancy has been associated with prematurity, congenital anomalies, fetal cytopenia, and low birth weight.49 With that said, and although not recommended, if patients decide to continue treatment, experts recommend limiting the dose to 2 mg/kg daily to reduce potential adverse events.
Category X
Oral Retinoids—According to the American Academy of Dermatology, retinoids such as isotretinoin and acitretin are considered second-line therapy for CLE.50 With that being said, there are well-documented effects on fetal development associated with oral retinoid use, including central nervous system, cardiovascular system, and craniofacial abnormalities.51 Therefore, its use is contraindicated during pregnancy. To prevent pregnancy while taking isotretinoin, patients must enroll in an online monitoring program called iPLEDGE. This program requires monthly updates by both the physician and the patient, including a negative pregnancy test every month for female patients actively taking the medication.52
The half-lives of the oral retinoids isotretinoin and acitretin are 10 to 20 hours and 50 to 60 hours, respectively.53,54 However, alcohol consumption converts acitretin into the metabolite etretinate, which can remain in tissue for up to 120 days.54,55 Therefore, women are advised to avoid alcohol while taking acitretin and avoid conception for 2 to 3 years after cessation of the medication.55 For those wishing to restart retinoids after pregnancy, studies show the medication can be safely reinstated 35 days after delivery for those interested in continued treatment.56
Thalidomide—Although low-dose thalidomide can treat refractory CLE, its use is restricted because of its known teratogenicity, most notably limb deformities.57 If prescribed thalidomide, women will need to enroll in the System for Thalidomide Education and Prescribing Safety program, similar to the iPLEDGE program, and use 2 forms of contraception when sexually active.58 Contraception should be continued for 4 weeks following the last dose of thalidomide. After this point, conception is considered safe.59
Methotrexate—For nonpregnant patients, low-dose methotrexate (MTX) with folate supplementation is a treatment option for CLE.60 However, for those who are pregnant, low-dose MTX is an abortive agent and has been associated with aminopterin syndrome, which includes skull deficits, craniofacial abnormalities, and limb deformities in live births.19,61 Therefore, MTX is not recommended in pregnancy. Of note, MTX can affect sperm; male patients also should be counselled.
Final Thoughts
Overall, it is recommended to limit medication use as much as possible in pregnancy. To reduce these exposures, it is imperative to reduce triggers that may lead to symptomatic flares of CLE. Because CLE can be triggered by sun exposure, we advise topical sunscreen to prevent CLE flares that may require additional oral medication.62,63
Various medications are considered safe for the treatment of CLE in pregnant patients (Figure 2). Based on studies in animal and clinical trials, hydroxychloroquine is considered a safe and effective medication for CLE in pregnancy and is a first-line therapy in nonpregnant patients.26,27 If flares occur, IVIG or a short course of oral steroids should be considered to manage symptoms.13,39 For those with severe flares, treatment is difficult, and personalized approaches may be necessary.

Part of the question for the childbearing population is when a patient would like to conceive. For severe cases when hydroxychloroquine is not effective as monotherapy, using a treatment that can encourage remission prior to conception attempts can be a beneficial strategy. Rituximab is an excellent example of such a therapy, as the therapeutic effect outlasts the immunosuppressive effect and therefore is unlikely to affect a future fetus.64 Thalidomide also is a potential option prior to conception, based on its short washout period and its ability to achieve notable remission rates in patients with CLE.57,59 Regardless, patients with CLE should still consult their dermatologist and rheumatologist (if applicable) prior to conception.
Patients of childbearing potential represent a population in which discussion about life goals greatly affects medication options. Having these discussions early and often allows for an open, more successful approach so that treatment regimens are not derailed at the time of conception.
Cutaneous lupus erythematosus (CLE) is a heterogeneous autoimmune disease that involves the skin. Cutaneous lupus erythematosus can be classified into various subtypes.1 These include, but are not limited to, acute CLE, subacute CLE, chronic CLE, intermittent CLE, lupus tumidus, and lupus profundus.1,2 The CLE subtypes have variable associations with systemic lupus erythematosus. For instance, some subtypes, such as acute CLE, are more strongly associated with systemic lupus erythematosus.
Treatment of CLE is similar to other autoimmune disorders. Although the US Food and Drug Administration (FDA) has not approved any treatments for CLE,3,4 the most common therapeutic options are disease-modifying antirheumatic drugs. Unfortunately, many of these treatments carry teratogenic effects. Because CLE predominantly affects women, particularly those of childbearing age, it is imperative to understand the available treatment options for those who are pregnant or considering pregnancy for an informed discussion with patients.5
For years, the gold standard when considering a medication during pregnancy was the FDA’s classification system. According to this system, medications were classified into 5 letter categories based on their potential teratogenicity, including A (no fetal risk), B (potential animal risk but inconclusive human studies), C (risk cannot be ruled out), D (evidence of fetal risk), and X (contraindicated in pregnancy). In 2014, the FDA decided to no longer use this classification system for medications approved after 2000.6 However, because many proposed treatment options for CLE were approved prior to 2001, we have summarized the commonly prescribed medications for CLE according to their prior FDA letter categories.
Treatment Options for CLE During Pregnancy
Prior to initiating systemic medications for the treatment of CLE, topical medications should be considered. Recommended treatment options include corticosteroids and calcineurin inhibitors.7 Compared with systemic medications, topical treatments carry minimal side effects, such as skin atrophy, that typically remain localized to areas of application.8 Moreover, even with extensive application, no correlation has been found between topical corticosteroid use and fetal growth,9 which suggests that topical steroids are safe in pregnancy and should be considered as a first-line treatment option for CLE. Calcineurin inhibitors also are considered safe based on their low level of absorption through the skin and are considered second-line topical treatment options in pregnancy.10

Although topical medications are effective for the treatment of CLE, many patients require the administration of systemic therapeutics for severe or refractory disease. Based on previously published reports, Figure 1 describes the current recommended systemic treatment options for CLE.11 Unfortunately, many of these medications carry teratogenic risks during pregnancy. The risks and side effects of the medications are described in detail in the following sections and summarized in the eTable.

Category B
Systemic Steroids—Systemic steroids are one of the most prescribed medications during pregnancy.12 Oral steroids have been associated with fast symptom relief, making this class of medications particularly effective during CLE flares; however, long-term management is not recommended because of the side effects, which include osteoporosis and impaired glucose metabolism.13
With low transmission across the placenta, there are 3 glucocorticoids that carry the safest profile in pregnancy: prednisone, cortisone, and hydrocortisone.14 Dexamethasone and betamethasone should be avoided, as both readily cross the placenta and increase fetal exposure.15 Although teratogenic effects have been associated with steroid use, most studies involving pregnant patients have inconclusive results. For instance, one study described an association between cleft lip/palate with in utero glucocorticoid exposure.16 However, multiple follow-up studies found no association between the two.17,18 Studies investigating the relationship between steroids and miscarriages or steroids and low birth weight also are inconclusive. Of note, if used throughout pregnancy, administration of a loading dose of glucocorticoids prior to delivery is recommended because of the increased stress brought on during labor.19
Sulfasalazine—Sulfasalazine is an immunomodulator commonly used for the treatment of inflammatory bowel disease and rheumatoid arthritis. However, studies also have shown that sulfasalazine is an effective treatment of CLE if standard treatments have failed.20,21
During pregnancy, patients exposed to sulfasalazine experienced minimal side effects despite transportation across the placenta.22 In comparison with control, pregnant women taking sulfasalazine experienced no increased risk for low fetal weight,23 congenital abnormalities,24 or spontaneous abortions.25 Of note, sulfasalazine can affect sperm, so male patients also should be counselled.
Category C
Hydroxychloroquine—Hydroxychloroquine is considered a first-line medication for those with CLE based on a symptomatic relief rate of 50% to 70%.26 For those taking hydroxychloroquine during pregnancy, the majority of studies have shown no association between the medication and adverse fetal events, including congenital abnormalities, prematurity, or spontaneous abortions.27-29 Therefore, hydroxychloroquine is considered safe in pregnancy, and those on the medication should continue standard monitoring, including retinopathy screening.30
Of note, hydroxychloroquine can be stored in tissue for weeks to months after discontinuation.5 Therefore, if patients wish to avoid hydroxychloroquine in pregnancy, one should stop taking the medication several months prior to conception.
Dapsone—Dapsone, a medication with both antimicrobial and immunomodulatory properties, is an effective second-line therapy for CLE.31 Although large-scale human trials have not been performed, multiple case reports and observational studies have supported the safe use of dapsone in pregnancy.32-34 However, there are notable side effects, including dose-dependent hemolysis, methemoglobinemia, and hypersensitivity reactions.13 Therefore, once treatment is initiated or continued, folic acid supplementation (5 mg daily) and regular serum analysis, including complete blood cell counts, are recommended in pregnant patients.19
Rituximab—Recent studies have demonstrated that rituximab can be an effective treatment of subacute and chronic CLE.35,36 Through inhibition of CD20, rituximab causes a decrease in circulating B cells and a reduced immune response. Therefore, experts recommend discontinuation of rituximab for 12 months prior to conception to reduce potential side effects to the fetus, which may include a transient reduction of circulating fetal B cells.37
If continued during pregnancy, most studies suggest discontinuation of rituximab during the third trimester, as it has been associated with neonatal infections and congenital abnormalities.19,37 However, these results are based on limited case reports, and thus robust research is needed to better understand the effect of rituximab in utero.
Intravenous Immunoglobulin Infusion—Intravenous immunoglobulin (IVIG) infusion is a well-tolerated treatment for many autoimmune disorders.38 Although not first line, limited case studies have demonstrated remission of refractory CLE following IVIG.39,40 Although no studies have directly investigated the effect of IVIG on fetal development, it has been frequently administered and well tolerated during pregnancy, especially in those with multiple sclerosis or antiphospholipid syndrome.41 Commonly reported side effects include headache and fatigue, and a rare associated side effect to be aware of is embolic events.42,43
Cyclosporine—Cyclosporine rarely is used in the treatment of localized CLE due to its extensive side-effect profile, most notably nephrotoxicity.44 However, studies have shown that cyclosporine may be efficacious if symptoms extend beyond the skin, involve multiple organs, and/or other treatments have failed.39 For those who are pregnant and wish to continue cyclosporine use, studies have associated low birth weight and premature delivery with its exposure in utero.44
Category D
Mycophenolate Mofetil—In conjunction with standard therapy, mycophenolate mofetil (MMF) is an adequate treatment of refractory CLE.45 Unfortunately, case reports have demonstrated an increased risk for fetal congenital abnormalities and first-trimester spontaneous abortion with use of MMF during pregnancy.46,47 Therefore, it is recommended that patients on MMF discontinue the medication at least 6 weeks prior to conception.46
Azathioprine—Although azathioprine has been shown to provide relief of discoid lupus erythematosus symptoms,48 it currently is only utilized for refractory disease, largely due to notable side effects that particularly affect the gastrointestinal tract and liver.4 Moreover, azathioprine use during pregnancy has been associated with prematurity, congenital anomalies, fetal cytopenia, and low birth weight.49 With that said, and although not recommended, if patients decide to continue treatment, experts recommend limiting the dose to 2 mg/kg daily to reduce potential adverse events.
Category X
Oral Retinoids—According to the American Academy of Dermatology, retinoids such as isotretinoin and acitretin are considered second-line therapy for CLE.50 With that being said, there are well-documented effects on fetal development associated with oral retinoid use, including central nervous system, cardiovascular system, and craniofacial abnormalities.51 Therefore, its use is contraindicated during pregnancy. To prevent pregnancy while taking isotretinoin, patients must enroll in an online monitoring program called iPLEDGE. This program requires monthly updates by both the physician and the patient, including a negative pregnancy test every month for female patients actively taking the medication.52
The half-lives of the oral retinoids isotretinoin and acitretin are 10 to 20 hours and 50 to 60 hours, respectively.53,54 However, alcohol consumption converts acitretin into the metabolite etretinate, which can remain in tissue for up to 120 days.54,55 Therefore, women are advised to avoid alcohol while taking acitretin and avoid conception for 2 to 3 years after cessation of the medication.55 For those wishing to restart retinoids after pregnancy, studies show the medication can be safely reinstated 35 days after delivery for those interested in continued treatment.56
Thalidomide—Although low-dose thalidomide can treat refractory CLE, its use is restricted because of its known teratogenicity, most notably limb deformities.57 If prescribed thalidomide, women will need to enroll in the System for Thalidomide Education and Prescribing Safety program, similar to the iPLEDGE program, and use 2 forms of contraception when sexually active.58 Contraception should be continued for 4 weeks following the last dose of thalidomide. After this point, conception is considered safe.59
Methotrexate—For nonpregnant patients, low-dose methotrexate (MTX) with folate supplementation is a treatment option for CLE.60 However, for those who are pregnant, low-dose MTX is an abortive agent and has been associated with aminopterin syndrome, which includes skull deficits, craniofacial abnormalities, and limb deformities in live births.19,61 Therefore, MTX is not recommended in pregnancy. Of note, MTX can affect sperm; male patients also should be counselled.
Final Thoughts
Overall, it is recommended to limit medication use as much as possible in pregnancy. To reduce these exposures, it is imperative to reduce triggers that may lead to symptomatic flares of CLE. Because CLE can be triggered by sun exposure, we advise topical sunscreen to prevent CLE flares that may require additional oral medication.62,63
Various medications are considered safe for the treatment of CLE in pregnant patients (Figure 2). Based on studies in animal and clinical trials, hydroxychloroquine is considered a safe and effective medication for CLE in pregnancy and is a first-line therapy in nonpregnant patients.26,27 If flares occur, IVIG or a short course of oral steroids should be considered to manage symptoms.13,39 For those with severe flares, treatment is difficult, and personalized approaches may be necessary.

Part of the question for the childbearing population is when a patient would like to conceive. For severe cases when hydroxychloroquine is not effective as monotherapy, using a treatment that can encourage remission prior to conception attempts can be a beneficial strategy. Rituximab is an excellent example of such a therapy, as the therapeutic effect outlasts the immunosuppressive effect and therefore is unlikely to affect a future fetus.64 Thalidomide also is a potential option prior to conception, based on its short washout period and its ability to achieve notable remission rates in patients with CLE.57,59 Regardless, patients with CLE should still consult their dermatologist and rheumatologist (if applicable) prior to conception.
Patients of childbearing potential represent a population in which discussion about life goals greatly affects medication options. Having these discussions early and often allows for an open, more successful approach so that treatment regimens are not derailed at the time of conception.
- Renner R, Sticherling M. The different faces of cutaneous lupus erythematosus. G Ital Dermatol Venereol. 2009;144:135-147.
- Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48:14-19.
- Shi H, Gudjonsson J, Kahlenberg J. Treatment of cutaneous lupus erythematosus: current approaches and future strategies. Curr Opin Rheumatol. 2020;32:208-214.
- Winkelmann RR, Kim GK, Del Rosso JQ. Treatment of cutaneous lupus erythematosus: review and assessment of treatment benefits based on Oxford Centre for Evidence-based Medicine criteria. J Clin Aesthet Dermatol. 2013;6:27-38.
- Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223-243.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;41:713-715.
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736-745.
- Kuhn A, Aberer E, Bata‐Csörgö Z, et al. S2k guideline for treatment of cutaneous lupus erythematosus—guided by the European Dermatology Forum (EDF) in cooperation with the European Academyof Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2017;31:389-404.
- Andersson NW, Skov L, Andersen JT. Evaluation of topical corticosteroid use in pregnancy and risk of newborns being small for gestational age and having low birth weight. JAMA Dermatol. 2021;157:788-795.
- Undre NA, Moloney FJ, Ahmadi S, et al. Skin and systemic pharmacokinetics of tacrolimus following topical application of tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2009;160:665-669.
- Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2014;10:97-107.
- Kuriya B, Hernández‐Díaz S, Liu J, et al. Patterns of medication use during pregnancy in rheumatoid arthritis. Arthritis Care Res. 2011;63:721-728.
- Chang A, Werth V. Treatment of cutaneous lupus. Curr Rheumatol Rep. 2011;13:300-307.
- Beitins IZ, Bayard F, Ances IG, et al. The transplacental passage of prednisone and prednisolone in pregnancy near term. J Pediatr. 1972;81:936-945.
- Ogueh O, Johnson MR. The metabolic effect of antenatal corticosteroid therapy. Hum Reprod Update. 2000;6:169-176.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Bay Bjørn A, Ehrenstein V, Hundborg HH, et al. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. Am J Ther. 2014;21:73-80.
- Hviid A, Mølgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ. 2011;183:796-804.
- Krause ML, Amin S, Makol A. Use of DMARDs and biologics during pregnancy and lactation in rheumatoid arthritis: what the rheumatologist needs to know. Ther Adv Musculoskelet Dis. 2014;6:169-184.
- Artuz F, Lenk N, Deniz N, et al. Efficacy of sulfasalazine in discoid lupus erythematosus. Int J Dermatol. 1996;35:746-748.
- Delaporte E, Catteau B, Sabbagh N, et al. Treatment of discoid lupus erythematosus with sulfasalazine: 11 cases [in French]. Ann Dermatol Venereol. 1997;124:151-156.
- Järnerot G, Into-Malmberg MB, Esbjörner E. Placental transfer of sulphasalazine and sulphapyridine and some of its metabolites. Scand J Gastroenterol. 1981;16:693-697.
- Norgard B, Pedersen L, Christensen LA, et al. Therapeutic drug use in women with Crohn’s disease and birth outcomes: a Danish nationwide cohort study. Am J Gastroenterol. 2007;102:1406-1413.
- Nørgård B, Czeizel AE, Rockenbauer M, et al. Population-based case control study of the safety of sulfasalazine use during pregnancy. Aliment Pharmacol Ther. 2001;15:483-486.
- Rahimi R, Nikfar S, Rezaie A, et al. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod Toxicol. 2008;25:271-275.
- Callen JP. Chronic cutaneous lupus erythematosus: clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416.
- Buchanan NM, Toubi E, Khamashta MA, et al. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann Rheum Dis. 1996;55:486-488.
- Costedoat‐Chalumeau N, Amoura Z, Duhaut P, et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty‐three cases compared with a control group. Arthritis Rheum. 2003;48:3207-3211.
- Sperber K, Hom C, Chao CP, et al. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009;7:9.
- Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377-1382.
- Klebes M, Wutte N, Aberer E. Dapsone as second-line treatment for cutaneous lupus erythematosus? a retrospective analysis of 34 patients and a review of the literature. Dermatology. 2016;232:91-96.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Varghese L, Viswabandya A, Mathew AJ. Dapsone, danazol, and intrapartum splenectomy in refractory ITP complicating pregnancy. Indian J Med Sci. 2008;62:452-455.
- Kahn G. Dapsone is safe during pregnancy. J Am Acad Dermatol. 1985;13:838-839.
- Quelhas da Costa R, Aguirre-Alastuey ME, Isenberg DA, et al. Assessment of response to B-cell depletion using rituximab in cutaneous lupus erythematosus. JAMA Dermatol. 2018;154:1432-1440.
- Alsanafi S, Kovarik C, Mermelstein A, et al. Rituximab in thetreatment of bullous systemic lupus erythematosus. J Clin Rheumatol. 2011;17:142-144.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506.
- Fernandez AP, Kerdel FA. The use of i.v. IG therapy in dermatology. Dermatol Ther. 2007;20:288-305.
- Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part II. J Am Acad Dermatol. 2011;65:E195-E213.
- Singh H, Naidu G, Sharma A. Intravenous immunoglobulin for the rescue in refractory cutaneous lupus. Indian Dermatol Online J. 2020;11:1003-1004.
- Clark AL. Clinical uses of intravenous immunoglobulin in pregnancy. Clin Obstet Gynecol. 1999;42:368-380.
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747-755.
- Woodruff RK, Grigg AP, Firkin FC, et al. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet. 1986;2:217-218.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Gammon B, Hansen C, Costner MI. Efficacy of mycophenolate mofetil in antimalarial-resistant cutaneous lupus erythematosus. J Am Acad Dermatol. 2010;65:717-721.e2.
- Abdulaziz HM, Shemies RS, Taman M, et al. Fetal proximal and distal limb anomalies following exposure to mycophenolate mofetil during pregnancy: a case report and review of the literature. Lupus. 2021;30:1522-1525.
- Pisoni CN, D’Cruz DP. The safety of mycophenolate mofetil in pregnancy. Exp Opin Drug Saf. 2008;7:219-222.
- Ashinoff R, Werth VP, Franks AG. Resistant discoid lupus erythematosus of palms and soles: successful treatment with azathioprine. J Am Acad Dermatol. 1988;19:961-965. doi:10.1016/S0190-9622(88)70259-5
- Goldstein LH, Dolinsky G, Greenberg R, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79:696-701.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for cutaneous lupus erythematosus. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:830-836.
- Sladden MJ, Harman KE. What is the chance of a normal pregnancy in a woman whose fetus has been exposed to isotretinoin? Arch Dermatol. 2007;143:1187-1188.
- Shin J, Cheetham TC, Wong L, et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- Brazzell RK, Colburn WA. Pharmacokinetics of the retinoids isotretinoin and etretinate. J Am Acad Dermatol. 1982;6:643-651.
- Pilkington T, Brogden RN. Acitretin: a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Gronhoj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
- Jajoria H, Mysore V. Washout period for pregnancy post isotretinoin therapy. Indian Dermatol Online J. 2020;11:239-242.
- Cortés-Hernández J, Torres-Salido M, Castro-Marrero J, et al. Thalidomide in the treatment of refractory cutaneous lupus erythematosus: prognostic factors of clinical outcome. Br J Dermatol. 2012;166:616-623.
- Zeldis JB, Williams BA, Thomas SD, et al. S.T.E.P.S.™: a comprehensive program for controlling and monitoring access to thalidomide. Clin Ther. 1999;21:319-330.
- C.S. Mott Children’s Hospital. University of Michigan Health. Thalidomide. Updated March 26, 2020. Accessed January 14, 2022. https://www.mottchildren.org/health-library/d04331a1
- Boehm IB, Boehm GA, Bauer R. Management of cutaneous lupus erythematosus with low-dose methotrexate: indication for modulation of inflammatory mechanisms. Rheumatol Int. 1998;18:59-62.
- Buckley LM, Bullaboy CA, Leichtman L, et al. Multiple congenital anomalies associated with weekly low‐dose methotrexate treatment of the mother. Arthritis Rheum. 1997;40:971-973.
- Foering K, Okawa J, Rose M, et al. Characterization of photosensitivity and poor quality of life in lupus. J Invest Dermatol. 2010;130(suppl):S10.
- Kuhn A, Herrmann M, Kleber S, et al. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 2006;54:939-950.
- Lake EP, Huang Y, Aronson IK. Rituximab treatment of pemphigus in women of childbearing age: experience with two patients. J Dermatol Treat. 2017;28:751-752.
- Renner R, Sticherling M. The different faces of cutaneous lupus erythematosus. G Ital Dermatol Venereol. 2009;144:135-147.
- Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48:14-19.
- Shi H, Gudjonsson J, Kahlenberg J. Treatment of cutaneous lupus erythematosus: current approaches and future strategies. Curr Opin Rheumatol. 2020;32:208-214.
- Winkelmann RR, Kim GK, Del Rosso JQ. Treatment of cutaneous lupus erythematosus: review and assessment of treatment benefits based on Oxford Centre for Evidence-based Medicine criteria. J Clin Aesthet Dermatol. 2013;6:27-38.
- Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223-243.
- Pernia S, DeMaagd G. The new pregnancy and lactation labeling rule. P T. 2016;41:713-715.
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78:736-745.
- Kuhn A, Aberer E, Bata‐Csörgö Z, et al. S2k guideline for treatment of cutaneous lupus erythematosus—guided by the European Dermatology Forum (EDF) in cooperation with the European Academyof Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. 2017;31:389-404.
- Andersson NW, Skov L, Andersen JT. Evaluation of topical corticosteroid use in pregnancy and risk of newborns being small for gestational age and having low birth weight. JAMA Dermatol. 2021;157:788-795.
- Undre NA, Moloney FJ, Ahmadi S, et al. Skin and systemic pharmacokinetics of tacrolimus following topical application of tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol. 2009;160:665-669.
- Xiong W, Lahita RG. Pragmatic approaches to therapy for systemic lupus erythematosus. Nat Rev Rheumatol. 2014;10:97-107.
- Kuriya B, Hernández‐Díaz S, Liu J, et al. Patterns of medication use during pregnancy in rheumatoid arthritis. Arthritis Care Res. 2011;63:721-728.
- Chang A, Werth V. Treatment of cutaneous lupus. Curr Rheumatol Rep. 2011;13:300-307.
- Beitins IZ, Bayard F, Ances IG, et al. The transplacental passage of prednisone and prednisolone in pregnancy near term. J Pediatr. 1972;81:936-945.
- Ogueh O, Johnson MR. The metabolic effect of antenatal corticosteroid therapy. Hum Reprod Update. 2000;6:169-176.
- Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385-392.
- Bay Bjørn A, Ehrenstein V, Hundborg HH, et al. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. Am J Ther. 2014;21:73-80.
- Hviid A, Mølgaard-Nielsen D. Corticosteroid use during pregnancy and risk of orofacial clefts. CMAJ. 2011;183:796-804.
- Krause ML, Amin S, Makol A. Use of DMARDs and biologics during pregnancy and lactation in rheumatoid arthritis: what the rheumatologist needs to know. Ther Adv Musculoskelet Dis. 2014;6:169-184.
- Artuz F, Lenk N, Deniz N, et al. Efficacy of sulfasalazine in discoid lupus erythematosus. Int J Dermatol. 1996;35:746-748.
- Delaporte E, Catteau B, Sabbagh N, et al. Treatment of discoid lupus erythematosus with sulfasalazine: 11 cases [in French]. Ann Dermatol Venereol. 1997;124:151-156.
- Järnerot G, Into-Malmberg MB, Esbjörner E. Placental transfer of sulphasalazine and sulphapyridine and some of its metabolites. Scand J Gastroenterol. 1981;16:693-697.
- Norgard B, Pedersen L, Christensen LA, et al. Therapeutic drug use in women with Crohn’s disease and birth outcomes: a Danish nationwide cohort study. Am J Gastroenterol. 2007;102:1406-1413.
- Nørgård B, Czeizel AE, Rockenbauer M, et al. Population-based case control study of the safety of sulfasalazine use during pregnancy. Aliment Pharmacol Ther. 2001;15:483-486.
- Rahimi R, Nikfar S, Rezaie A, et al. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod Toxicol. 2008;25:271-275.
- Callen JP. Chronic cutaneous lupus erythematosus: clinical, laboratory, therapeutic, and prognostic examination of 62 patients. Arch Dermatol. 1982;118:412-416.
- Buchanan NM, Toubi E, Khamashta MA, et al. Hydroxychloroquine and lupus pregnancy: review of a series of 36 cases. Ann Rheum Dis. 1996;55:486-488.
- Costedoat‐Chalumeau N, Amoura Z, Duhaut P, et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases: a study of one hundred thirty‐three cases compared with a control group. Arthritis Rheum. 2003;48:3207-3211.
- Sperber K, Hom C, Chao CP, et al. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr Rheumatol Online J. 2009;7:9.
- Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377-1382.
- Klebes M, Wutte N, Aberer E. Dapsone as second-line treatment for cutaneous lupus erythematosus? a retrospective analysis of 34 patients and a review of the literature. Dermatology. 2016;232:91-96.
- Tuffanelli DL. Successful pregnancy in a patient with dermatitis herpetiformis treated with low-dose dapsone. Arch Dermatol. 1982;118:876.
- Varghese L, Viswabandya A, Mathew AJ. Dapsone, danazol, and intrapartum splenectomy in refractory ITP complicating pregnancy. Indian J Med Sci. 2008;62:452-455.
- Kahn G. Dapsone is safe during pregnancy. J Am Acad Dermatol. 1985;13:838-839.
- Quelhas da Costa R, Aguirre-Alastuey ME, Isenberg DA, et al. Assessment of response to B-cell depletion using rituximab in cutaneous lupus erythematosus. JAMA Dermatol. 2018;154:1432-1440.
- Alsanafi S, Kovarik C, Mermelstein A, et al. Rituximab in thetreatment of bullous systemic lupus erythematosus. J Clin Rheumatol. 2011;17:142-144.
- Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506.
- Fernandez AP, Kerdel FA. The use of i.v. IG therapy in dermatology. Dermatol Ther. 2007;20:288-305.
- Kuhn A, Ruland V, Bonsmann G. Cutaneous lupus erythematosus: update of therapeutic options part II. J Am Acad Dermatol. 2011;65:E195-E213.
- Singh H, Naidu G, Sharma A. Intravenous immunoglobulin for the rescue in refractory cutaneous lupus. Indian Dermatol Online J. 2020;11:1003-1004.
- Clark AL. Clinical uses of intravenous immunoglobulin in pregnancy. Clin Obstet Gynecol. 1999;42:368-380.
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001;345:747-755.
- Woodruff RK, Grigg AP, Firkin FC, et al. Fatal thrombotic events during treatment of autoimmune thrombocytopenia with intravenous immunoglobulin in elderly patients. Lancet. 1986;2:217-218.
- Paziana K, Del Monaco M, Cardonick E, et al. Ciclosporin use during pregnancy. Drug Saf. 2013;36:279-294.
- Gammon B, Hansen C, Costner MI. Efficacy of mycophenolate mofetil in antimalarial-resistant cutaneous lupus erythematosus. J Am Acad Dermatol. 2010;65:717-721.e2.
- Abdulaziz HM, Shemies RS, Taman M, et al. Fetal proximal and distal limb anomalies following exposure to mycophenolate mofetil during pregnancy: a case report and review of the literature. Lupus. 2021;30:1522-1525.
- Pisoni CN, D’Cruz DP. The safety of mycophenolate mofetil in pregnancy. Exp Opin Drug Saf. 2008;7:219-222.
- Ashinoff R, Werth VP, Franks AG. Resistant discoid lupus erythematosus of palms and soles: successful treatment with azathioprine. J Am Acad Dermatol. 1988;19:961-965. doi:10.1016/S0190-9622(88)70259-5
- Goldstein LH, Dolinsky G, Greenberg R, et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res A Clin Mol Teratol. 2007;79:696-701.
- Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for cutaneous lupus erythematosus. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:830-836.
- Sladden MJ, Harman KE. What is the chance of a normal pregnancy in a woman whose fetus has been exposed to isotretinoin? Arch Dermatol. 2007;143:1187-1188.
- Shin J, Cheetham TC, Wong L, et al. The impact of the iPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- Brazzell RK, Colburn WA. Pharmacokinetics of the retinoids isotretinoin and etretinate. J Am Acad Dermatol. 1982;6:643-651.
- Pilkington T, Brogden RN. Acitretin: a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Gronhoj Larsen F, Steinkjer B, Jakobsen P, et al. Acitretin is converted to etretinate only during concomitant alcohol intake. Br J Dermatol. 2000;143:1164-1169.
- Jajoria H, Mysore V. Washout period for pregnancy post isotretinoin therapy. Indian Dermatol Online J. 2020;11:239-242.
- Cortés-Hernández J, Torres-Salido M, Castro-Marrero J, et al. Thalidomide in the treatment of refractory cutaneous lupus erythematosus: prognostic factors of clinical outcome. Br J Dermatol. 2012;166:616-623.
- Zeldis JB, Williams BA, Thomas SD, et al. S.T.E.P.S.™: a comprehensive program for controlling and monitoring access to thalidomide. Clin Ther. 1999;21:319-330.
- C.S. Mott Children’s Hospital. University of Michigan Health. Thalidomide. Updated March 26, 2020. Accessed January 14, 2022. https://www.mottchildren.org/health-library/d04331a1
- Boehm IB, Boehm GA, Bauer R. Management of cutaneous lupus erythematosus with low-dose methotrexate: indication for modulation of inflammatory mechanisms. Rheumatol Int. 1998;18:59-62.
- Buckley LM, Bullaboy CA, Leichtman L, et al. Multiple congenital anomalies associated with weekly low‐dose methotrexate treatment of the mother. Arthritis Rheum. 1997;40:971-973.
- Foering K, Okawa J, Rose M, et al. Characterization of photosensitivity and poor quality of life in lupus. J Invest Dermatol. 2010;130(suppl):S10.
- Kuhn A, Herrmann M, Kleber S, et al. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 2006;54:939-950.
- Lake EP, Huang Y, Aronson IK. Rituximab treatment of pemphigus in women of childbearing age: experience with two patients. J Dermatol Treat. 2017;28:751-752.
Practice Points
- Patients should consult their primary dermatologist when discussing medication options for cutaneous lupus erythematosus (CLE) prior to pregnancy.
- Hydroxychloroquine is a first-line medication for maintenance treatment of CLE, while oral steroids are effective for CLE flares in pregnancy. Second-line medications include dapsone and intravenous immunoglobulin. These classes of medications are considered safe in pregnancy.
- Cutaneous lupus erythematosus medications contraindicated in pregnancy include oral retinoids, mycophenolate mofetil, thalidomide, and methotrexate.
Pediatric Dermatology Emergencies
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.
Differential Diagnosis
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1
Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9
Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13
Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.
Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24

The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34
IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.

Differential Diagnosis
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1

Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9

Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13

Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.

Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24

The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34

IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
Many pediatric skin conditions can be safely monitored with minimal intervention, but certain skin conditions are emergent and require immediate attention and proper assessment of the neonate, infant, or child. The skin may provide the first presentation of a potentially fatal disease with serious sequelae. Cutaneous findings may indicate the need for further evaluation. Therefore, it is important to differentiate skin conditions with benign etiologies from those that require immediate diagnosis and treatment, as early intervention of some of these conditions can be lifesaving. Herein, we discuss pertinent pediatric dermatology emergencies that dermatologists should keep in mind so that these diagnoses are never missed.
Staphylococcal Scalded Skin Syndrome
Presentation
Staphylococcal scalded skin syndrome (SSSS), or Ritter disease, is a potentially fatal pediatric emergency, especially in newborns.1 The mortality rate for SSSS in the United States is 3.6% to 11% in children.2 It typically presents with a prodrome of tenderness, fever, and confluent erythematous patches on the folds of the skin such as the groin, axillae, nose, and ears, with eventual spread to the legs and trunk.1,2 Within 24 to 48 hours of symptom onset, blistering and fluid accumulation will appear diffusely. Bullae are flaccid, and tangential and gentle pressure on involved unblistered skin may lead to shearing of the epithelium, which is a positive Nikolsky sign.1,2
Causes
Staphylococcal scalded skin syndrome is caused by exfoliative toxins A and B, toxigenic strains of Staphylococcus aureus. Exfoliative toxins A and B are serine proteases that target and cleave desmoglein 1, which binds keratinocytes in the stratum granulosum.1,3 Exfoliative toxins disrupt the adhesion of keratinocytes, resulting in bullae formation and subsequently diffuse sheetlike desquamation.1,4,5 Although up to 30% of the human population are asymptomatically and permanently colonized with nasal S aureus,6 the exfoliative toxins are produced by only 5% of species.1
In neonates, the immune and renal systems are underdeveloped; therefore, patients are susceptible to SSSS due to lack of neutralizing antibodies and decreased renal toxin excretion.4 Potential complications of SSSS are deeper soft-tissue infection, septicemia (blood-borne infection), and fluid and electrolyte imbalance.1,4
Diagnosis and Treatment
The condition is diagnosed clinically based on the findings of tender erythroderma, bullae, and desquamation with a scalded appearance, especially in friction zones; periorificial crusting; positive Nikolsky sign; and lack of mucosal involvement (Figure 1).1 Histopathology can aid in complicated clinical scenarios as well as culture from affected areas, including the upper respiratory tract, diaper region, and umbilicus.1,4 Hospitalization is required for SSSS for intravenous antibiotics, fluids, and electrolyte repletion.

Differential Diagnosis
There are multiple diagnoses to consider in the setting of flaccid bullae in the pediatric population. Stevens-Johnson syndrome or toxic epidermal necrolysis also can present with fever and superficial desquamation or bullae; however, exposure to medications and mucosal involvement often are absent in SSSS (Figure 2).2 Pemphigus, particularly paraneoplastic pemphigus, also often includes mucosal involvement and scalding thermal burns that are often geometric or focal. Epidermolysis bullosa and toxic shock syndrome also should be considered.1

Impetigo
Presentation
Impetigo is the most common bacterial skin infection in children caused by S aureus or Streptococcus pyogenes.7-9 It begins as erythematous papules transitioning to thin-walled vesicles that rapidly rupture and result in honey-crusted papules.7,9,10 Individuals of any age can be affected by nonbullous impetigo, but it is the most common skin infection in children aged 2 to 5 years.7
Bullous impetigo primarily is seen in children, especially infants, and rarely can occur in teenagers or adults.7 It most commonly is caused by the exfoliative toxins of S aureus. Bullous impetigo presents as small vesicles that may converge into larger flaccid bullae or pustules.7-10 Once the bullae rupture, an erythematous base with a collarette of scale remains without the formation of a honey-colored crust.8 Bullous impetigo usually affects moist intertriginous areas such as the axillae, neck, and diaper area8,10 (Figure 3). Complications may result in cellulitis, septicemia, osteomyelitis, poststreptococcal glomerulonephritis associated with S pyogenes, and S aureus–induced SSSS.7-9

Diagnosis
Nonbullous and bullous impetigo are largely clinical diagnoses that can be confirmed by culture of a vesicle or pustular fluid.10 Treatment of impetigo includes topical or systemic antibiotics.7,10 Patients should be advised to keep lesions covered and avoid contact with others until all lesions resolve, as lesions are contagious.9
Eczema Herpeticum
Presentation
Eczema herpeticum (EH), also known as Kaposi varicelliform eruption, is a disseminated herpes simplex virus infection of impaired skin, most commonly in patients with atopic dermatitis (AD).11 Eczema herpeticum presents as a widespread eruption of erythematous monomorphic vesicles that progress to punched-out erosions with hemorrhagic crusting (Figure 4). Patients may have associated fever or lymphadenopathy.12,13

Causes
The number of children hospitalized annually for EH in the United States is approximately 4 to 7 cases per million children. Less than 3% of pediatric AD patients are affected, with a particularly increased risk in patients with severe and earlier-onset AD.12-15 Patients with AD have skin barrier defects, and decreased IFN-γ expression and cathelicidins predispose patients with AD to developing EH.12,16,17
Diagnosis
Viral polymerase chain reaction for herpes simplex virus types 1 and 2 is the standard for confirmatory diagnosis. Herpes simplex virus cultures from cutaneous scrapings, direct fluorescent antibody testing, or Tzanck test revealing multinucleated giant cells also may help establish the diagnosis.11,12,17
Management
Individuals with severe AD and other dermatologic conditions with cutaneous barrier compromise are at risk for developing EH, which is a medical emergency requiring hospitalization and prompt treatment with antiviral therapy such as acyclovir, often intravenously, as death can result if left untreated.11,17 Topical or systemic antibiotic therapy should be initiated if there is suspicion for secondary bacterial superinfection. Patients should be evaluated for multiorgan involvement such as keratoconjunctivitis, meningitis, encephalitis, and systemic viremia due to increased mortality, especially in infants.12,15,16
Langerhans Cell Histiocytosis
Presentation
Langerhans cell histiocytosis (LCH) has a variable clinical presentation and can involve a single or multiple organ systems, including the bones and skin. Cutaneous LCH can present as violaceous papules, nodules, or ulcerations and crusted erosions (Figure 5). The lymph nodes, liver, spleen, oral mucosa, and respiratory and central nervous systems also may be involved.

Langerhans cell histiocytosis affects individuals of any age group but more often is seen in pediatric patients. The incidence of LCH is approximately 4.6 cases per million children.18 The pathogenesis is secondary to pathologic Langerhans cells, characterized as a clonal myeloid malignancy and dysregulation of the immune system.18,19
Diagnosis
A thorough physical examination is essential in patients with suspected LCH. Additionally, diagnosis of LCH is heavily based on histopathology of tissue from the involved organ system(s) with features of positive S-100 protein, CD1a, and CD207, and identification of Birbeck granules.20 Imaging and laboratory studies also are indicated and can include a skeletal survey (to assess osteolytic and organ involvement), a complete hematologic panel, coagulation studies, and liver function tests.18,21
Management
Management of LCH varies based on the organ system(s) involved along with the extent of the disease. Dermatology referral may be indicated in patients presenting with nonresolving cutaneous lesions as well as in severe cases. Single-organ and multisystem disease may require one treatment modality or a combination of chemotherapy, surgery, radiation, and/or immunotherapy.21
Infantile Hemangioma
Presentation
Infantile hemangioma (IH) is the most common benign tumor of infancy and usually is apparent a few weeks after birth. Lesions appear as bright red papules, nodules, or plaques. Deep or subcutaneous lesions present as raised, flesh-colored nodules with a blue hue and bruiselike appearance with or without a central patch of telangiectasia22-24 (Figure 6). Although all IHs eventually resolve, residual skin changes such as scarring, atrophy, and fibrosis can persist.24

The incidence of IH has been reported to occur in up to 4% to 5% of infants in the United States.23,25 Infantile hemangiomas also have been found to be more common among white, preterm, and multiple-gestation infants.25 The proposed pathogenesis of IHs includes angiogenic and vasogenic factors that cause rapid proliferation of blood vessels, likely driven by tissue hypoxia.23,26,27
Diagnosis
Infantile hemangioma is diagnosed clinically; however, immunohistochemical staining showing positivity for glucose transporter 1 also is helpful.26,27 Imaging modalities such as ultrasonography and magnetic resonance imaging also can be utilized to visualize the extent of lesions if necessary.25
Management
Around 15% to 25% of IHs are considered complicated and require intervention.25,27 Infantile hemangiomas can interfere with function depending on location or have potentially fatal complications. Based on the location and extent of involvement, these findings can include ulceration; hemorrhage; impairment of feeding, hearing, and/or vision; facial deformities; airway obstruction; hypothyroidism; and congestive heart failure.25,28 Early treatment with topical or oral beta-blockers is imperative for potentially life-threatening IHs, which can be seen due to large size or dangerous location.28,29 Because the rapid proliferative phase of IHs is thought to begin around 6 weeks of life, treatment should be initiated as early as possible. Initiation of beta-blocker therapy in the first few months of life can prevent functional impairment, ulceration, and permanent cosmetic changes. Additionally, surgery or pulsed dye laser treatment have been found to be effective for skin changes found after involution of IH.25,29
Differential Diagnosis
The differential diagnosis for IH includes vascular malformations, which are present at birth and do not undergo rapid proliferation; sarcoma; and kaposiform hemangioendothelioma, which causes the Kasabach-Merritt phenomenon secondary to platelet trapping. Careful attention to the history of the skin lesion provides good support for diagnosis of IH in most cases.
IgA Vasculitis
Presentation
IgA vasculitis, or Henoch-Schönlein purpura, classically presents as a tetrad of palpable purpura, acute-onset arthritis or arthralgia, abdominal pain, and renal disease with proteinuria or hematuria.30 Skin involvement is seen in almost all cases and is essential for diagnosis of IgA vasculitis. The initial dermatosis may be pruritic and present as an erythematous macular or urticarial wheal that evolves into petechiae, along with palpable purpura that is most frequently located on the legs or buttocks (Figure 7).30-34

IgA vasculitis is an immune-mediated small vessel vasculitis with deposition of IgA in the small vessels. The underlying cause remains unknown, though infection, dietary allergens, drugs, vaccinations, and chemical triggers have been recognized in literature.32,35,36 IgA vasculitis is largely a pediatric diagnosis, with 90% of affected individuals younger than 10 years worldwide.37 In the pediatric population, the incidence has been reported to be 3 to 26.7 cases per 100,000 children.32
Diagnosis
Diagnosis is based on the clinical presentation and histopathology.30 On direct immunofluorescence, IgA deposition is seen in the vessel walls.35 Laboratory testing is not diagnostic, but urinalysis is mandatory to identify involvement of renal vasculature. Imaging studies may be used in patients with abdominal symptoms, as an ultrasound can be used to visualize bowel structure and abnormalities such as intussusception.33
Management
The majority of cases of IgA vasculitis recover spontaneously, with patients requiring hospital admission based on severity of symptoms.30 The primary approach to management involves providing supportive care including hydration, adequate rest, and symptomatic pain relief of the joints and abdomen with oral analgesics. Systemic corticosteroids or steroid-sparing agents such as dapsone or colchicine can be used to treat cutaneous manifestations in addition to severe pain symptoms.30,31 Patients with IgA vasculitis must be monitored for proteinuria or hematuria to assess the extent of renal involvement. Although much more common in adults, long-term renal impairment can result from childhood cases of IgA vasculitis.34
Final Thoughts
Pediatric dermatology emergencies can be difficult to detect and accurately diagnose. Many of these diseases are potential emergencies that that may result in delayed treatment and considerable morbidity and mortality if missed. Clinicians should be aware that timely recognition and diagnosis, along with possible referral to pediatric dermatology, are essential to avoid complications.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
- Leung AKC, Barankin B, Leong KF. Staphylococcal-scalded skin syndrome: evaluation, diagnosis, and management. World J Pediatr. 2018;14:116-120.
- Handler MZ, Schwartz RA. Staphylococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418-1423.
- Davidson J, Polly S, Hayes P, et al. Recurrent staphylococcal scalded skin syndrome in an extremely low-birth-weight neonate. AJP Rep. 2017;7:E134-E137.
- Mishra AK, Yadav P, Mishra A. A systemic review on staphylococcal scalded skin syndrome (SSSS): a rare and critical disease of neonates. Open Microbiol J. 2016;10:150-159.
- Berk D. Staphylococcal scalded skin syndrome. Cancer Therapy Advisor website. https://www.cancertherapyadvisor.com/home/decision-support-in-medicine/pediatrics/staphylococcal-scalded-skin-syndrome/. Published 2017. Accessed February 19, 2020.
- Sakr A, Brégeon F, Mège JL, et al. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections [published online October 8, 2018]. Front Microbiol. 2018;9:2419.
- Pereira LB. Impetigo review. An Bras Dermatol. 2014;89:293-299.
- Nardi NM, Schaefer TJ. Impetigo. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2019. https://www.ncbi.nlm.nih.gov/books/NBK430974/. Accessed February 21, 2020.
- Koning S, van der Sande R, Verhagen AP, et al. Interventions for impetigo. Cochrane Database Syst Rev. 2012;1:CD003261.
- Sommer LL, Reboli AC, Heymann WR. Bacterial diseases. In: Bolognia, JL Schaffer, JV Cerroni L, eds. Dermatology. 4th ed. Philadelphia, PA: Elsevier; 2018:1259-1295.
- Micali G, Lacarrubba F. Eczema herpeticum. N Engl J Med. 2017;377:e9.
- Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98:153-157.
- Seegräber M, Worm M, Werfel T, et al. Recurrent eczema herpeticum—a retrospective European multicenter study evaluating the clinical characteristics of eczema herpeticum cases in atopic dermatitis patients [published online November 16, 2019]. J Eur Acad Dermatology Venereol. doi:10.1111/jdv.16090.
- Sun D, Ong PY. Infectious complications in atopic dermatitis. Immunol Allergy Clin North Am. 2017;37:75-93.
- Hsu DY, Shinkai K, Silverberg JI. Epidemiology of eczema herpeticum in hospitalized U.S. children: analysis of a nationwide cohort [published online September 17, 2018]. J Invest Dermatol. 2018;138:265-272.
- Leung DY, Gao PS, Grigoryev DN, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-γ response. J Allergy Clin Immunol. 2011;127:965-73.e1-5.
- Darji K, Frisch S, Adjei Boakye E, et al. Characterization of children with recurrent eczema herpeticum and response to treatment with interferon-gamma. Pediatr Dermatol. 2017;34:686-689.
- Allen CE, Merad M, McClain KL. Langerhans-cell histiocytosis. N Engl J Med. 2018;379:856-868.
- Abla O, Weitzman S. Treatment of Langerhans cell histiocytosis: role of BRAF/MAPK inhibition. Hematology Am Soc Hematol Educ Program. 2015;2015:565-570.
- Allen CE, Li L, Peters TL, et al. Cell-specific gene expression in Langerhans cell histiocytosis lesions reveals a distinct profile compared with epidermal Langerhans cells. J Immunol. 2010;184:4557-4567.
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184.
- Holland KE, Drolet BA. Infantile hemangioma [published online August 21, 2010]. Pediatr Clin North Am. 2010;57:1069-1083.
- Chen TS, Eichenfield LF, Friedlander SF. Infantile hemangiomas: an update on pathogenesis and therapy. Pediatrics. 2013;131:99-108.
- George A, Mani V, Noufal A. Update on the classification of hemangioma. J Oral Maxillofac Pathol. 2014;18(suppl 1):S117-S120.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:786-791.
- Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907-913.
- de Jong S, Itinteang T, Withers AH, et al. Does hypoxia play a role in infantile hemangioma? Arch Dermatol Res. 2016;308:219-227.
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128:E259-E266.
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas [published online January 2019]. Pediatrics. doi:10.1542/peds.2018-3475.
- Sohagia AB, Gunturu SG, Tong TR, et al. Henoch-Schönlein purpura—a case report and review of the literature [published online May 23, 2010]. Gastroenterol Res Pract. doi:10.1155/2010/597648.
- Rigante D, Castellazzi L, Bosco A, et al. Is there a crossroad between infections, genetics, and Henoch-Schönlein purpura? Autoimmun Rev. 2013;12:1016-1021.
- Piram M, Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch–Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25:171-178.
- Carlson JA. The histological assessment of cutaneous vasculitis. Histopathology. 2010;56:3-23.
- Eleftheriou D, Batu ED, Ozen S, et al. Vasculitis in children. Nephrol Dial Transplant. 2014;30:I94-I103.
- van Timmeren MM, Heeringa P, Kallenberg CG. Infectious triggers for vasculitis. Curr Opin Rheumatol. 2014;26:416-423.
- Scott DGI, Watts RA. Epidemiology and clinical features of systemic vasculitis [published online July 11, 2013]. Clin Exp Nephrol. 2013;17:607-610.
- He X, Yu C, Zhao P, et al. The genetics of Henoch-Schönlein purpura: a systematic review and meta-analysis. Rheumatol Int. 2013;33:1387-1395.
Practice Points
- Staphylococcal scalded skin syndrome, impetigo, eczema herpeticum, Langerhans cell histiocytosis, infantile hemangiomas, and IgA vasculitis all present potential emergencies in pediatric patients in dermatologic settings.
- Early and accurate identification and management of these entities is critical to avoid short-term and long-term negative sequalae.
Outpatient Management and Follow-up Recommendations for Adverse Drug Reactions: Guidelines for Posthospitalization Care
It has been estimated that 2 million serious adverse drug reactions (ADRs) occur annually in the United States, resulting in 100,000 deaths.1 Although the acute morbidity and mortality of these ADRs are readily apparent, postdischarge sequalae are critical aspects of a patient’s care. Herein, we present an approach to outpatient dermatologic follow-up of 3 ADRs: acute generalized exanthematous pustulosis (AGEP), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, and Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). For these ADRs, the first step is prompt diagnosis and discontinuation of any potentially causative medications.
ACUTE GENERALIZED EXANTHEMATOUS PUSTULOSIS
Ninety percent of the time, AGEP is caused by medications, most commonly antibiotics, and less often it is caused by viruses.2-4 It presents as a cutaneous eruption with nonfollicular sterile pustules, fever, and leukocytosis, usually within 5 days after starting a causative medication.5 After stopping the medication, cutaneous findings generally improve within 1 week, and leukocytosis often resolves within 1 week.3
Notable Sequelae
Although AGEP typically is considered benign,2 there have been reports of severe sequelae including death from a systemic inflammatory response and complications such as bacterial superinfection and sepsis.6,7 Visceral involvement can be seen in up to 20% of AGEP patients, with systemic symptoms similar to those seen in DRESS syndrome. Mortality has been reported in up to 5% of cases, mainly in patients with comorbidities and notable mucosal involvement.8 More severe disease can be seen in patients with known dermatologic disease, as AGEP can provoke an isomorphic phenomenon.9 Laboratory alterations typically seen in AGEP include neutrophilia, eosinophilia, and elevated liver enzymes.2
Follow-up Recommendations
Patients should be informed of the expected timeline for resolution and should be counseled on the possibility of rare systemic symptoms. Laboratory abnormalities should be monitored every 2 to 4 weeks until normalized.
DRESS SYNDROME
DRESS syndrome is characterized by a morbilliform eruption that can be accompanied by fever; eosinophilia; purpura; facial edema; lymphadenopathy; and liver, renal, or other organ dysfunction. DRESS syndrome most often presents within 8 weeks of exposure to a causative drug.10,11 The most common causative agents are anticonvulsants, antimicrobials, and allopurinol.12 Treatment includes topical corticosteroids and systemic corticosteroids for internal organ involvement.10
Short-term Sequelae
Several potential sequelae may occur within 6 months of resolution of DRESS syndrome, resulting from both the ADR itself and/or systemic corticosteroids that often are required for treatment.13 Complications secondary to herpesviruses have been reported.14 Cases of cytomegalovirus-induced gastric ulcers can lead to gastrointestinal tract bleeds.15
Infections including Cryptococcus species and herpes zoster also have been reported.16 Patients, particularly those treated with systemic corticosteroids, should be monitored with close follow-up for infectious complications and treatment-related adverse effects.13
Long-term Sequelae
Endocrine
Thyroid gland abnormalities secondary to DRESS syndrome include Graves disease and Hashimoto disease as well as variations in biomarkers including elevated free thyroxine and low and elevated thyroid-stimulating hormone levels.16,17 Type 1 diabetes mellitus also has been seen after DRESS syndrome, developing within the first 10 months after onset with unknown pathogenesis.18
Autoimmune
Other reported sequelae of DRESS syndrome include elevated antinuclear antibodies with possible development into systemic lupus erythematosus, autoimmune hemolytic anemia, vitiligo, and rheumatoid arthritis.11,16 Symptoms may be exacerbated in patients with preexisting autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, and patients with preexisting renal disease are at an increased risk for requiring lifelong hemodialysis after DRESS syndrome.16
Other
Studies have demonstrated that pneumonia, thrombosis, and alopecia can be complications of DRESS syndrome.11,16 Psychiatric disturbances including fear of taking new medications, anxiety, and depression also have been reported.19 Children with DRESS syndrome may develop vitiligo, alopecia, sclerodermatous lesions, photophobia, uveitis, and Vogt-Koyanagi-Harada disease.17
Follow-up Recommendations
It is important to inform patients of both the potential short-term and long-term sequelae of DRESS syndrome, including those associated with treatment. A thorough review of systems should be performed at each visit, along with laboratory evaluation including a complete blood cell count with differential and liver function testing every 1 to 2 weeks after discharge until normalized, with monthly monitoring of glucose, thyroid-stimulating hormone, and free thyroxine levels for 3 months after discharge.
STEVENS-JOHNSON SYNDROME/TOXIC EPIDERMAL NECROLYSIS
Stevens-Johnson syndrome/toxic epidermal necrolysis are severe ADRs that present with dusky violaceous macules. Inciting medications include nonsteroidal anti-inflammatory drugs, allopurinol, antibiotics, and anticonvulsants, and symptoms begin 1 to 3 weeks after medication exposure.12 Initially, the lesions often begin on the trunk and can progress to full-body erythema and exfoliation with a necrotic epidermis and mucosal involvement.12,20
Notable Sequelae
Cutaneous
Chronic eczema can present at any time and can vary in severity in SJS/TEN patients.21 Xerosis and pruritus can be treated with emollients.11 Dyschromia is common. Hypertrophic and keloidal scarring can result from surgical debridement and are best prevented with the use of nonadherent dressings.22 Nail changes such as anonychia, dystrophy, longitudinal ridges, and pterygium also are seen, and topical steroids can be helpful. Other reported dermatologic sequelae include dyschromia and eruption of ectopic sebaceous glands.21,22
Ocular
Ocular sequelae include dry eyes, photophobia, symblepharon, corneal scarring, corneal neovascularization, corneal xerosis, trichiasis, reduced visual acuity, blindness, and subconjunctival fibrosis. The most common sequelae are bilateral conjunctivitis and corneal ulcerations.22,23 Early and regular ophthalmologic follow-up is recommended, as SJS/TEN-induced blindness can result from delayed therapy, destroying corneal stem cells.21 Amniotic membrane transplantation replaces the damaged corneal membrane, which may reduce corneal inflammation.24
Chronic dry eye syndrome can recur for years after SJS/TEN resolves and progresses over time.22 Frequent use of nonpreserved artificial tears and salivary gland transplantation can be helpful.24 Unfortunately, ocular disease may develop months after discharge; therefore, it is recommended that dermatologists ask all SJS/TEN patients about ocular symptoms in follow-up visits. If ocular involvement was present initially, patients should be followed by ophthalmology for at least 1 year after discharge.23
Genitourinary
Genitourinary sequelae in SJS/TEN include adhesions, particularly in the female urethra and vaginal opening; vaginal adenosis; vulvovaginal endometriosis; and persistent genital ulcerations most commonly reported in females.22 Prompt inpatient gynecologic or urologic consultation is critical to reduce these potentially permanent outcomes. Topical corticosteroid therapy is recommended in the acute phase.22
Psychologic
Posttraumatic stress disorder may occur in patients with SJS/TEN. One study showed that 23% (7/30) of patients had posttraumatic stress disorder 6 months after hospitalization for SJS/TEN. The investigators recommended routine psychiatric assessment in the acute disease period and for at least 1 year after discharge.25
Pulmonary, Gastrointestinal, and Renal
Interstitial pneumonia and obliterative bronchitis/bronchiolitis can be caused by SJS/TEN. Interstitial pneumonia tends to occur during the acute course, while obliterative airway disease manifests after resolution of SJS/TEN.21,22 Abnormal pulmonary function testing can be seen in more than half of SJS/TEN patients 2 months after the ADR.22 Gastrointestinal sequelae include esophageal strictures, intestinal ulceration, and cholestasis.22 Renal sequelae include acute kidney injury and glomerulonephritis, which may be secondary to the volume loss seen in SJS/TEN but may be irreversible.21
Special Populations
A correlation with infertility in women has been documented in patients with SJS/TEN; thus, follow-up with obstetrics and gynecology is recommended in women of child-bearing potential. The most considerable risk in pregnant women with SJS/TEN is premature birth, and mucosal necrosis of SJS/TEN can impair vaginal delivery.26 Antiretrovirals can be a cause of SJS/TEN in the human immunodeficiency virus–positive population.27 In those cases, it is best to discontinue the medication and find an alternative.
Risk factors for children can be different and can include viral and febrile illnesses as well as mycoplasma infection.28 Children also can be at an increased risk for poor ocular outcomes, such as permanent deficiency in visual acuity and blindness.29
Follow-up Recommendations
Patients should be counseled regarding sequelae and the multisystem nature of SJS/TEN. Inpatient referrals should be given as needed. It is important to watch for ocular symptoms for 1 year after SJS/TEN resolution. When ocular involvement is present, follow-up with ophthalmology is recommended within 1 month of discharge and then at the discretion of the ophthalmologist. Pulmonary function should be monitored for 1 year after SJS/TEN, starting 1 month after discharge and then at the discretion of the pulmonologist. Patients also should be screened for psychologic sequelae for at least 1 year after discharge.
FINAL THOUGHTS
Adverse drug reactions are notable causes of inpatient hospitalization and may lead to considerable sequelae. These ADRs range in severity from more common and benign maculopapular exanthems to severe multiorgan ADRs such as DRESS syndrome and SJS/TEN.
In AGEP, it is important to monitor patients with preexisting dermatologic diseases and to screen for visceral involvement. DRESS syndrome has the potential to cause immune dysregulation and variable long-term adverse sequelae, both from the disease itself and from corticosteroid therapy. Mucocutaneous sequelae of SJS/TEN can potentially affect a patient’s cutaneous, ocular, genitourinary, mental, pulmonary, gastrointestinal, and renal health.
The baseline recommendations provided here warrant more frequent monitoring if the findings and symptoms are severe. In all of these cases, if a causative medication is identified, it should be added to the patient’s allergy list and the patient should be counseled extensively to avoid this medication and other medications in the same class. If a single agent cannot be identified, referrals for patch testing may be of some utility, particularly in AGEP and DRESS syndrome.30,31
- Preventable adverse drug reactions: a focus on drug interactions. US Food and Drug Administration website. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm110632.htm. Updated March 6, 2018. Accessed April 12, 2019.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;3:1-8.
- Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010
;49:507-512. - Ropars N, Darrieux L, Tisseau L, et al. Acute generalized exanthematous pustulosis associated with primary Epstein-Barr virus infection. JAAD Case Rep. 2014;1:9-11.
- Hattem S, Beerthuizen G, Kardaun S. Severe flucloxacillin‐induced acute generalized exanthematous pustulosis (AGEP), with toxic epidermal necrolysis (TEN)‐like features: does overlap between AGEP and TEN exist? clinical report and review of the literature. Br J Dermatol. 2014;171:1539-1545.
- Tajmir-Riahi A, Wörl P, Harrer T, et al. Life-threatening atypical case of acute generalized exanthematous pustulosis. Int Arch Allergy Immunol. 2017;174:108-111.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP). a review and update. J Am Acad Dermatol. 2015;73:843-848.
- Totonchy MB, McNiff JM, Bunick CG. Koebnerization of Hailey-Hailey disease into a cutaneous drug eruption of acute generalized exanthematous pustulosis associated with systemic symptoms. J Cutan Pathol. 2016;43:1031-1035.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9; quiz 718-720.
- Kano Y, Shiohara T. Long-term outcome of patients with severe cutaneous adverse reactions. Dermatologica Sinica. 2013;31:211-216.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier Saunders; 2012.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Ljungman P, Wang FZ, Clark DA, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. 2000;111:774-781.
- Asano Y, Kagawa H, Kano Y, et al. Cytomegalovirus disease during severe drug eruptions: report of 2 cases and retrospective study of 18 patients with drug-induced hypersensitivity syndrome. Arch Dermatol. 2009;145:1030-1036.
- Kano Y , Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug‐induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Morita C, Yanase T, Shiohara T, et al. Aggressive treatment in paediatric or young patients with drug-induced hypersensitivity syndrome (DiHS)/ drug reaction with eosinophilia and systemic symptoms (DRESS) is associated with future development of type III polyglandular autoimmune syndrome [published online October 27, 2018]. BMJ Case Rep. doi:10.1136/bcr-2018-225528.
- Chiang A, Shiu J, Elsensohn AN, et al. Classic autoimmune type 1 diabetes mellitus after a case of drug reaction with eosinophilia and systemic symptoms (DRESS). JAAD Case Rep. 2018;4:295-297.
- Lew TT, Creamer D, Mackenzie J, et al. Post-traumatic stress disorder following drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2015;172:836-837.
- Kumar R, Das A, Das S. Management of Stevens-Johnson syndrome-toxic epidermal necrolysis: looking beyond guidelines! Indian J Dermatol. 2018;63:117-124.
- Yang CW, Cho YT, Chen KL, et al. Long-term sequelae of Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2016;96:525-529.
- Lee HY, Walsh SA, Creamer D. Long‐term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow‐up. Br J Dermatol. 2017;177:924-935.
- Hsu M, Jayaram A, Verner R, et al. Indications and outcomes of amniotic membrane transplantation in the management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control study. Cornea. 2012;31:1394-1402.
- Sant’ Anna AE, Hazarbassanov RM, de Freitas D, et al. Minor salivary glands and labial mucous membrane graft in the treatment of severe symblepharon and dry eye in patients with Stevens-Johnson syndrome. Br J Ophthalmol. 2012;96:234-239.
- Hefez L, Zaghbib K, Sbidian E, et al. Post-traumatic stress disorder in Stevens-Johnson syndrome and toxic epidermal necrolysis: prevalence and risk factors. a prospective study of 31 patients [published online October 3, 2018]. Br J Dermatol. doi:10.1111/bjd.17267.
- Knight L, Todd G, Muloiwa R, et al. Stevens Johnson syndrome and toxic epidermal necrolysis: maternal and foetal outcomes intwenty-two consecutive pregnant HIV infected women [published online August 12, 2015]. PLoS One. doi:10.1371/journal.pone.0135501.
- Tchetnya X, Ngwasiri CA, Munge T, et al. Severe eye complications from toxic epidermal necrolysis following initiation of nevirapine based HAART regimen in a child with HIV infection: a case from Cameroon. BMC Pediatr. 2018;18:108.
- Antoon JW, Goldman JL, Lee B, et al. Incidence, outcomes, and resource use in children with Stevens-Johnson syndrome and toxic epidermal necrolysis. Pediatr Dermatol. 2018;35:182-187.
- Basu S, Shanbhag SS, Gokani A, et al. Chronic ocular sequelae of Stevens-Johnson syndrome in children: long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol. 2018;189:17-28.
- Pinho A, Marta A, Coutinho I, et al. Long‐term reproducibility of positive patch test reactions in patients with non‐immediate cutaneous adverse drug reactions to antibiotics. Contact Dermatitis. 2017;76:204-209.
- Barbaud A, Collet E, Milpied B, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555-562.
It has been estimated that 2 million serious adverse drug reactions (ADRs) occur annually in the United States, resulting in 100,000 deaths.1 Although the acute morbidity and mortality of these ADRs are readily apparent, postdischarge sequalae are critical aspects of a patient’s care. Herein, we present an approach to outpatient dermatologic follow-up of 3 ADRs: acute generalized exanthematous pustulosis (AGEP), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, and Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). For these ADRs, the first step is prompt diagnosis and discontinuation of any potentially causative medications.
ACUTE GENERALIZED EXANTHEMATOUS PUSTULOSIS
Ninety percent of the time, AGEP is caused by medications, most commonly antibiotics, and less often it is caused by viruses.2-4 It presents as a cutaneous eruption with nonfollicular sterile pustules, fever, and leukocytosis, usually within 5 days after starting a causative medication.5 After stopping the medication, cutaneous findings generally improve within 1 week, and leukocytosis often resolves within 1 week.3
Notable Sequelae
Although AGEP typically is considered benign,2 there have been reports of severe sequelae including death from a systemic inflammatory response and complications such as bacterial superinfection and sepsis.6,7 Visceral involvement can be seen in up to 20% of AGEP patients, with systemic symptoms similar to those seen in DRESS syndrome. Mortality has been reported in up to 5% of cases, mainly in patients with comorbidities and notable mucosal involvement.8 More severe disease can be seen in patients with known dermatologic disease, as AGEP can provoke an isomorphic phenomenon.9 Laboratory alterations typically seen in AGEP include neutrophilia, eosinophilia, and elevated liver enzymes.2
Follow-up Recommendations
Patients should be informed of the expected timeline for resolution and should be counseled on the possibility of rare systemic symptoms. Laboratory abnormalities should be monitored every 2 to 4 weeks until normalized.
DRESS SYNDROME
DRESS syndrome is characterized by a morbilliform eruption that can be accompanied by fever; eosinophilia; purpura; facial edema; lymphadenopathy; and liver, renal, or other organ dysfunction. DRESS syndrome most often presents within 8 weeks of exposure to a causative drug.10,11 The most common causative agents are anticonvulsants, antimicrobials, and allopurinol.12 Treatment includes topical corticosteroids and systemic corticosteroids for internal organ involvement.10
Short-term Sequelae
Several potential sequelae may occur within 6 months of resolution of DRESS syndrome, resulting from both the ADR itself and/or systemic corticosteroids that often are required for treatment.13 Complications secondary to herpesviruses have been reported.14 Cases of cytomegalovirus-induced gastric ulcers can lead to gastrointestinal tract bleeds.15
Infections including Cryptococcus species and herpes zoster also have been reported.16 Patients, particularly those treated with systemic corticosteroids, should be monitored with close follow-up for infectious complications and treatment-related adverse effects.13
Long-term Sequelae
Endocrine
Thyroid gland abnormalities secondary to DRESS syndrome include Graves disease and Hashimoto disease as well as variations in biomarkers including elevated free thyroxine and low and elevated thyroid-stimulating hormone levels.16,17 Type 1 diabetes mellitus also has been seen after DRESS syndrome, developing within the first 10 months after onset with unknown pathogenesis.18
Autoimmune
Other reported sequelae of DRESS syndrome include elevated antinuclear antibodies with possible development into systemic lupus erythematosus, autoimmune hemolytic anemia, vitiligo, and rheumatoid arthritis.11,16 Symptoms may be exacerbated in patients with preexisting autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, and patients with preexisting renal disease are at an increased risk for requiring lifelong hemodialysis after DRESS syndrome.16
Other
Studies have demonstrated that pneumonia, thrombosis, and alopecia can be complications of DRESS syndrome.11,16 Psychiatric disturbances including fear of taking new medications, anxiety, and depression also have been reported.19 Children with DRESS syndrome may develop vitiligo, alopecia, sclerodermatous lesions, photophobia, uveitis, and Vogt-Koyanagi-Harada disease.17
Follow-up Recommendations
It is important to inform patients of both the potential short-term and long-term sequelae of DRESS syndrome, including those associated with treatment. A thorough review of systems should be performed at each visit, along with laboratory evaluation including a complete blood cell count with differential and liver function testing every 1 to 2 weeks after discharge until normalized, with monthly monitoring of glucose, thyroid-stimulating hormone, and free thyroxine levels for 3 months after discharge.
STEVENS-JOHNSON SYNDROME/TOXIC EPIDERMAL NECROLYSIS
Stevens-Johnson syndrome/toxic epidermal necrolysis are severe ADRs that present with dusky violaceous macules. Inciting medications include nonsteroidal anti-inflammatory drugs, allopurinol, antibiotics, and anticonvulsants, and symptoms begin 1 to 3 weeks after medication exposure.12 Initially, the lesions often begin on the trunk and can progress to full-body erythema and exfoliation with a necrotic epidermis and mucosal involvement.12,20
Notable Sequelae
Cutaneous
Chronic eczema can present at any time and can vary in severity in SJS/TEN patients.21 Xerosis and pruritus can be treated with emollients.11 Dyschromia is common. Hypertrophic and keloidal scarring can result from surgical debridement and are best prevented with the use of nonadherent dressings.22 Nail changes such as anonychia, dystrophy, longitudinal ridges, and pterygium also are seen, and topical steroids can be helpful. Other reported dermatologic sequelae include dyschromia and eruption of ectopic sebaceous glands.21,22
Ocular
Ocular sequelae include dry eyes, photophobia, symblepharon, corneal scarring, corneal neovascularization, corneal xerosis, trichiasis, reduced visual acuity, blindness, and subconjunctival fibrosis. The most common sequelae are bilateral conjunctivitis and corneal ulcerations.22,23 Early and regular ophthalmologic follow-up is recommended, as SJS/TEN-induced blindness can result from delayed therapy, destroying corneal stem cells.21 Amniotic membrane transplantation replaces the damaged corneal membrane, which may reduce corneal inflammation.24
Chronic dry eye syndrome can recur for years after SJS/TEN resolves and progresses over time.22 Frequent use of nonpreserved artificial tears and salivary gland transplantation can be helpful.24 Unfortunately, ocular disease may develop months after discharge; therefore, it is recommended that dermatologists ask all SJS/TEN patients about ocular symptoms in follow-up visits. If ocular involvement was present initially, patients should be followed by ophthalmology for at least 1 year after discharge.23
Genitourinary
Genitourinary sequelae in SJS/TEN include adhesions, particularly in the female urethra and vaginal opening; vaginal adenosis; vulvovaginal endometriosis; and persistent genital ulcerations most commonly reported in females.22 Prompt inpatient gynecologic or urologic consultation is critical to reduce these potentially permanent outcomes. Topical corticosteroid therapy is recommended in the acute phase.22
Psychologic
Posttraumatic stress disorder may occur in patients with SJS/TEN. One study showed that 23% (7/30) of patients had posttraumatic stress disorder 6 months after hospitalization for SJS/TEN. The investigators recommended routine psychiatric assessment in the acute disease period and for at least 1 year after discharge.25
Pulmonary, Gastrointestinal, and Renal
Interstitial pneumonia and obliterative bronchitis/bronchiolitis can be caused by SJS/TEN. Interstitial pneumonia tends to occur during the acute course, while obliterative airway disease manifests after resolution of SJS/TEN.21,22 Abnormal pulmonary function testing can be seen in more than half of SJS/TEN patients 2 months after the ADR.22 Gastrointestinal sequelae include esophageal strictures, intestinal ulceration, and cholestasis.22 Renal sequelae include acute kidney injury and glomerulonephritis, which may be secondary to the volume loss seen in SJS/TEN but may be irreversible.21
Special Populations
A correlation with infertility in women has been documented in patients with SJS/TEN; thus, follow-up with obstetrics and gynecology is recommended in women of child-bearing potential. The most considerable risk in pregnant women with SJS/TEN is premature birth, and mucosal necrosis of SJS/TEN can impair vaginal delivery.26 Antiretrovirals can be a cause of SJS/TEN in the human immunodeficiency virus–positive population.27 In those cases, it is best to discontinue the medication and find an alternative.
Risk factors for children can be different and can include viral and febrile illnesses as well as mycoplasma infection.28 Children also can be at an increased risk for poor ocular outcomes, such as permanent deficiency in visual acuity and blindness.29
Follow-up Recommendations
Patients should be counseled regarding sequelae and the multisystem nature of SJS/TEN. Inpatient referrals should be given as needed. It is important to watch for ocular symptoms for 1 year after SJS/TEN resolution. When ocular involvement is present, follow-up with ophthalmology is recommended within 1 month of discharge and then at the discretion of the ophthalmologist. Pulmonary function should be monitored for 1 year after SJS/TEN, starting 1 month after discharge and then at the discretion of the pulmonologist. Patients also should be screened for psychologic sequelae for at least 1 year after discharge.
FINAL THOUGHTS
Adverse drug reactions are notable causes of inpatient hospitalization and may lead to considerable sequelae. These ADRs range in severity from more common and benign maculopapular exanthems to severe multiorgan ADRs such as DRESS syndrome and SJS/TEN.
In AGEP, it is important to monitor patients with preexisting dermatologic diseases and to screen for visceral involvement. DRESS syndrome has the potential to cause immune dysregulation and variable long-term adverse sequelae, both from the disease itself and from corticosteroid therapy. Mucocutaneous sequelae of SJS/TEN can potentially affect a patient’s cutaneous, ocular, genitourinary, mental, pulmonary, gastrointestinal, and renal health.
The baseline recommendations provided here warrant more frequent monitoring if the findings and symptoms are severe. In all of these cases, if a causative medication is identified, it should be added to the patient’s allergy list and the patient should be counseled extensively to avoid this medication and other medications in the same class. If a single agent cannot be identified, referrals for patch testing may be of some utility, particularly in AGEP and DRESS syndrome.30,31
It has been estimated that 2 million serious adverse drug reactions (ADRs) occur annually in the United States, resulting in 100,000 deaths.1 Although the acute morbidity and mortality of these ADRs are readily apparent, postdischarge sequalae are critical aspects of a patient’s care. Herein, we present an approach to outpatient dermatologic follow-up of 3 ADRs: acute generalized exanthematous pustulosis (AGEP), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, and Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN). For these ADRs, the first step is prompt diagnosis and discontinuation of any potentially causative medications.
ACUTE GENERALIZED EXANTHEMATOUS PUSTULOSIS
Ninety percent of the time, AGEP is caused by medications, most commonly antibiotics, and less often it is caused by viruses.2-4 It presents as a cutaneous eruption with nonfollicular sterile pustules, fever, and leukocytosis, usually within 5 days after starting a causative medication.5 After stopping the medication, cutaneous findings generally improve within 1 week, and leukocytosis often resolves within 1 week.3
Notable Sequelae
Although AGEP typically is considered benign,2 there have been reports of severe sequelae including death from a systemic inflammatory response and complications such as bacterial superinfection and sepsis.6,7 Visceral involvement can be seen in up to 20% of AGEP patients, with systemic symptoms similar to those seen in DRESS syndrome. Mortality has been reported in up to 5% of cases, mainly in patients with comorbidities and notable mucosal involvement.8 More severe disease can be seen in patients with known dermatologic disease, as AGEP can provoke an isomorphic phenomenon.9 Laboratory alterations typically seen in AGEP include neutrophilia, eosinophilia, and elevated liver enzymes.2
Follow-up Recommendations
Patients should be informed of the expected timeline for resolution and should be counseled on the possibility of rare systemic symptoms. Laboratory abnormalities should be monitored every 2 to 4 weeks until normalized.
DRESS SYNDROME
DRESS syndrome is characterized by a morbilliform eruption that can be accompanied by fever; eosinophilia; purpura; facial edema; lymphadenopathy; and liver, renal, or other organ dysfunction. DRESS syndrome most often presents within 8 weeks of exposure to a causative drug.10,11 The most common causative agents are anticonvulsants, antimicrobials, and allopurinol.12 Treatment includes topical corticosteroids and systemic corticosteroids for internal organ involvement.10
Short-term Sequelae
Several potential sequelae may occur within 6 months of resolution of DRESS syndrome, resulting from both the ADR itself and/or systemic corticosteroids that often are required for treatment.13 Complications secondary to herpesviruses have been reported.14 Cases of cytomegalovirus-induced gastric ulcers can lead to gastrointestinal tract bleeds.15
Infections including Cryptococcus species and herpes zoster also have been reported.16 Patients, particularly those treated with systemic corticosteroids, should be monitored with close follow-up for infectious complications and treatment-related adverse effects.13
Long-term Sequelae
Endocrine
Thyroid gland abnormalities secondary to DRESS syndrome include Graves disease and Hashimoto disease as well as variations in biomarkers including elevated free thyroxine and low and elevated thyroid-stimulating hormone levels.16,17 Type 1 diabetes mellitus also has been seen after DRESS syndrome, developing within the first 10 months after onset with unknown pathogenesis.18
Autoimmune
Other reported sequelae of DRESS syndrome include elevated antinuclear antibodies with possible development into systemic lupus erythematosus, autoimmune hemolytic anemia, vitiligo, and rheumatoid arthritis.11,16 Symptoms may be exacerbated in patients with preexisting autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, and patients with preexisting renal disease are at an increased risk for requiring lifelong hemodialysis after DRESS syndrome.16
Other
Studies have demonstrated that pneumonia, thrombosis, and alopecia can be complications of DRESS syndrome.11,16 Psychiatric disturbances including fear of taking new medications, anxiety, and depression also have been reported.19 Children with DRESS syndrome may develop vitiligo, alopecia, sclerodermatous lesions, photophobia, uveitis, and Vogt-Koyanagi-Harada disease.17
Follow-up Recommendations
It is important to inform patients of both the potential short-term and long-term sequelae of DRESS syndrome, including those associated with treatment. A thorough review of systems should be performed at each visit, along with laboratory evaluation including a complete blood cell count with differential and liver function testing every 1 to 2 weeks after discharge until normalized, with monthly monitoring of glucose, thyroid-stimulating hormone, and free thyroxine levels for 3 months after discharge.
STEVENS-JOHNSON SYNDROME/TOXIC EPIDERMAL NECROLYSIS
Stevens-Johnson syndrome/toxic epidermal necrolysis are severe ADRs that present with dusky violaceous macules. Inciting medications include nonsteroidal anti-inflammatory drugs, allopurinol, antibiotics, and anticonvulsants, and symptoms begin 1 to 3 weeks after medication exposure.12 Initially, the lesions often begin on the trunk and can progress to full-body erythema and exfoliation with a necrotic epidermis and mucosal involvement.12,20
Notable Sequelae
Cutaneous
Chronic eczema can present at any time and can vary in severity in SJS/TEN patients.21 Xerosis and pruritus can be treated with emollients.11 Dyschromia is common. Hypertrophic and keloidal scarring can result from surgical debridement and are best prevented with the use of nonadherent dressings.22 Nail changes such as anonychia, dystrophy, longitudinal ridges, and pterygium also are seen, and topical steroids can be helpful. Other reported dermatologic sequelae include dyschromia and eruption of ectopic sebaceous glands.21,22
Ocular
Ocular sequelae include dry eyes, photophobia, symblepharon, corneal scarring, corneal neovascularization, corneal xerosis, trichiasis, reduced visual acuity, blindness, and subconjunctival fibrosis. The most common sequelae are bilateral conjunctivitis and corneal ulcerations.22,23 Early and regular ophthalmologic follow-up is recommended, as SJS/TEN-induced blindness can result from delayed therapy, destroying corneal stem cells.21 Amniotic membrane transplantation replaces the damaged corneal membrane, which may reduce corneal inflammation.24
Chronic dry eye syndrome can recur for years after SJS/TEN resolves and progresses over time.22 Frequent use of nonpreserved artificial tears and salivary gland transplantation can be helpful.24 Unfortunately, ocular disease may develop months after discharge; therefore, it is recommended that dermatologists ask all SJS/TEN patients about ocular symptoms in follow-up visits. If ocular involvement was present initially, patients should be followed by ophthalmology for at least 1 year after discharge.23
Genitourinary
Genitourinary sequelae in SJS/TEN include adhesions, particularly in the female urethra and vaginal opening; vaginal adenosis; vulvovaginal endometriosis; and persistent genital ulcerations most commonly reported in females.22 Prompt inpatient gynecologic or urologic consultation is critical to reduce these potentially permanent outcomes. Topical corticosteroid therapy is recommended in the acute phase.22
Psychologic
Posttraumatic stress disorder may occur in patients with SJS/TEN. One study showed that 23% (7/30) of patients had posttraumatic stress disorder 6 months after hospitalization for SJS/TEN. The investigators recommended routine psychiatric assessment in the acute disease period and for at least 1 year after discharge.25
Pulmonary, Gastrointestinal, and Renal
Interstitial pneumonia and obliterative bronchitis/bronchiolitis can be caused by SJS/TEN. Interstitial pneumonia tends to occur during the acute course, while obliterative airway disease manifests after resolution of SJS/TEN.21,22 Abnormal pulmonary function testing can be seen in more than half of SJS/TEN patients 2 months after the ADR.22 Gastrointestinal sequelae include esophageal strictures, intestinal ulceration, and cholestasis.22 Renal sequelae include acute kidney injury and glomerulonephritis, which may be secondary to the volume loss seen in SJS/TEN but may be irreversible.21
Special Populations
A correlation with infertility in women has been documented in patients with SJS/TEN; thus, follow-up with obstetrics and gynecology is recommended in women of child-bearing potential. The most considerable risk in pregnant women with SJS/TEN is premature birth, and mucosal necrosis of SJS/TEN can impair vaginal delivery.26 Antiretrovirals can be a cause of SJS/TEN in the human immunodeficiency virus–positive population.27 In those cases, it is best to discontinue the medication and find an alternative.
Risk factors for children can be different and can include viral and febrile illnesses as well as mycoplasma infection.28 Children also can be at an increased risk for poor ocular outcomes, such as permanent deficiency in visual acuity and blindness.29
Follow-up Recommendations
Patients should be counseled regarding sequelae and the multisystem nature of SJS/TEN. Inpatient referrals should be given as needed. It is important to watch for ocular symptoms for 1 year after SJS/TEN resolution. When ocular involvement is present, follow-up with ophthalmology is recommended within 1 month of discharge and then at the discretion of the ophthalmologist. Pulmonary function should be monitored for 1 year after SJS/TEN, starting 1 month after discharge and then at the discretion of the pulmonologist. Patients also should be screened for psychologic sequelae for at least 1 year after discharge.
FINAL THOUGHTS
Adverse drug reactions are notable causes of inpatient hospitalization and may lead to considerable sequelae. These ADRs range in severity from more common and benign maculopapular exanthems to severe multiorgan ADRs such as DRESS syndrome and SJS/TEN.
In AGEP, it is important to monitor patients with preexisting dermatologic diseases and to screen for visceral involvement. DRESS syndrome has the potential to cause immune dysregulation and variable long-term adverse sequelae, both from the disease itself and from corticosteroid therapy. Mucocutaneous sequelae of SJS/TEN can potentially affect a patient’s cutaneous, ocular, genitourinary, mental, pulmonary, gastrointestinal, and renal health.
The baseline recommendations provided here warrant more frequent monitoring if the findings and symptoms are severe. In all of these cases, if a causative medication is identified, it should be added to the patient’s allergy list and the patient should be counseled extensively to avoid this medication and other medications in the same class. If a single agent cannot be identified, referrals for patch testing may be of some utility, particularly in AGEP and DRESS syndrome.30,31
- Preventable adverse drug reactions: a focus on drug interactions. US Food and Drug Administration website. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm110632.htm. Updated March 6, 2018. Accessed April 12, 2019.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;3:1-8.
- Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010
;49:507-512. - Ropars N, Darrieux L, Tisseau L, et al. Acute generalized exanthematous pustulosis associated with primary Epstein-Barr virus infection. JAAD Case Rep. 2014;1:9-11.
- Hattem S, Beerthuizen G, Kardaun S. Severe flucloxacillin‐induced acute generalized exanthematous pustulosis (AGEP), with toxic epidermal necrolysis (TEN)‐like features: does overlap between AGEP and TEN exist? clinical report and review of the literature. Br J Dermatol. 2014;171:1539-1545.
- Tajmir-Riahi A, Wörl P, Harrer T, et al. Life-threatening atypical case of acute generalized exanthematous pustulosis. Int Arch Allergy Immunol. 2017;174:108-111.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP). a review and update. J Am Acad Dermatol. 2015;73:843-848.
- Totonchy MB, McNiff JM, Bunick CG. Koebnerization of Hailey-Hailey disease into a cutaneous drug eruption of acute generalized exanthematous pustulosis associated with systemic symptoms. J Cutan Pathol. 2016;43:1031-1035.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9; quiz 718-720.
- Kano Y, Shiohara T. Long-term outcome of patients with severe cutaneous adverse reactions. Dermatologica Sinica. 2013;31:211-216.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier Saunders; 2012.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Ljungman P, Wang FZ, Clark DA, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. 2000;111:774-781.
- Asano Y, Kagawa H, Kano Y, et al. Cytomegalovirus disease during severe drug eruptions: report of 2 cases and retrospective study of 18 patients with drug-induced hypersensitivity syndrome. Arch Dermatol. 2009;145:1030-1036.
- Kano Y , Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug‐induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Morita C, Yanase T, Shiohara T, et al. Aggressive treatment in paediatric or young patients with drug-induced hypersensitivity syndrome (DiHS)/ drug reaction with eosinophilia and systemic symptoms (DRESS) is associated with future development of type III polyglandular autoimmune syndrome [published online October 27, 2018]. BMJ Case Rep. doi:10.1136/bcr-2018-225528.
- Chiang A, Shiu J, Elsensohn AN, et al. Classic autoimmune type 1 diabetes mellitus after a case of drug reaction with eosinophilia and systemic symptoms (DRESS). JAAD Case Rep. 2018;4:295-297.
- Lew TT, Creamer D, Mackenzie J, et al. Post-traumatic stress disorder following drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2015;172:836-837.
- Kumar R, Das A, Das S. Management of Stevens-Johnson syndrome-toxic epidermal necrolysis: looking beyond guidelines! Indian J Dermatol. 2018;63:117-124.
- Yang CW, Cho YT, Chen KL, et al. Long-term sequelae of Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2016;96:525-529.
- Lee HY, Walsh SA, Creamer D. Long‐term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow‐up. Br J Dermatol. 2017;177:924-935.
- Hsu M, Jayaram A, Verner R, et al. Indications and outcomes of amniotic membrane transplantation in the management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control study. Cornea. 2012;31:1394-1402.
- Sant’ Anna AE, Hazarbassanov RM, de Freitas D, et al. Minor salivary glands and labial mucous membrane graft in the treatment of severe symblepharon and dry eye in patients with Stevens-Johnson syndrome. Br J Ophthalmol. 2012;96:234-239.
- Hefez L, Zaghbib K, Sbidian E, et al. Post-traumatic stress disorder in Stevens-Johnson syndrome and toxic epidermal necrolysis: prevalence and risk factors. a prospective study of 31 patients [published online October 3, 2018]. Br J Dermatol. doi:10.1111/bjd.17267.
- Knight L, Todd G, Muloiwa R, et al. Stevens Johnson syndrome and toxic epidermal necrolysis: maternal and foetal outcomes intwenty-two consecutive pregnant HIV infected women [published online August 12, 2015]. PLoS One. doi:10.1371/journal.pone.0135501.
- Tchetnya X, Ngwasiri CA, Munge T, et al. Severe eye complications from toxic epidermal necrolysis following initiation of nevirapine based HAART regimen in a child with HIV infection: a case from Cameroon. BMC Pediatr. 2018;18:108.
- Antoon JW, Goldman JL, Lee B, et al. Incidence, outcomes, and resource use in children with Stevens-Johnson syndrome and toxic epidermal necrolysis. Pediatr Dermatol. 2018;35:182-187.
- Basu S, Shanbhag SS, Gokani A, et al. Chronic ocular sequelae of Stevens-Johnson syndrome in children: long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol. 2018;189:17-28.
- Pinho A, Marta A, Coutinho I, et al. Long‐term reproducibility of positive patch test reactions in patients with non‐immediate cutaneous adverse drug reactions to antibiotics. Contact Dermatitis. 2017;76:204-209.
- Barbaud A, Collet E, Milpied B, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555-562.
- Preventable adverse drug reactions: a focus on drug interactions. US Food and Drug Administration website. https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm110632.htm. Updated March 6, 2018. Accessed April 12, 2019.
- Thienvibul C, Vachiramon V, Chanprapaph K. Five-year retrospective review of acute generalized exanthematous pustulosis. Dermatol Res Pract. 2015;3:1-8.
- Lee HY, Chou D, Pang SM, et al. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010
;49:507-512. - Ropars N, Darrieux L, Tisseau L, et al. Acute generalized exanthematous pustulosis associated with primary Epstein-Barr virus infection. JAAD Case Rep. 2014;1:9-11.
- Hattem S, Beerthuizen G, Kardaun S. Severe flucloxacillin‐induced acute generalized exanthematous pustulosis (AGEP), with toxic epidermal necrolysis (TEN)‐like features: does overlap between AGEP and TEN exist? clinical report and review of the literature. Br J Dermatol. 2014;171:1539-1545.
- Tajmir-Riahi A, Wörl P, Harrer T, et al. Life-threatening atypical case of acute generalized exanthematous pustulosis. Int Arch Allergy Immunol. 2017;174:108-111.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:E1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP). a review and update. J Am Acad Dermatol. 2015;73:843-848.
- Totonchy MB, McNiff JM, Bunick CG. Koebnerization of Hailey-Hailey disease into a cutaneous drug eruption of acute generalized exanthematous pustulosis associated with systemic symptoms. J Cutan Pathol. 2016;43:1031-1035.
- Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: part II. management and therapeutics. J Am Acad Dermatol. 2013;68:709.e1-e9; quiz 718-720.
- Kano Y, Shiohara T. Long-term outcome of patients with severe cutaneous adverse reactions. Dermatologica Sinica. 2013;31:211-216.
- Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier Saunders; 2012.
- Ushigome Y, Kano Y, Ishida T, et al. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721-728.
- Ljungman P, Wang FZ, Clark DA, et al. High levels of human herpesvirus 6 DNA in peripheral blood leucocytes are correlated to platelet engraftment and disease in allogeneic stem cell transplant patients. Br J Haematol. 2000;111:774-781.
- Asano Y, Kagawa H, Kano Y, et al. Cytomegalovirus disease during severe drug eruptions: report of 2 cases and retrospective study of 18 patients with drug-induced hypersensitivity syndrome. Arch Dermatol. 2009;145:1030-1036.
- Kano Y , Tohyama M, Aihara M, et al. Sequelae in 145 patients with drug‐induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms: survey conducted by the Asian Research Committee on Severe Cutaneous Adverse Reactions (ASCAR). J Dermatol. 2015;42:276-282.
- Morita C, Yanase T, Shiohara T, et al. Aggressive treatment in paediatric or young patients with drug-induced hypersensitivity syndrome (DiHS)/ drug reaction with eosinophilia and systemic symptoms (DRESS) is associated with future development of type III polyglandular autoimmune syndrome [published online October 27, 2018]. BMJ Case Rep. doi:10.1136/bcr-2018-225528.
- Chiang A, Shiu J, Elsensohn AN, et al. Classic autoimmune type 1 diabetes mellitus after a case of drug reaction with eosinophilia and systemic symptoms (DRESS). JAAD Case Rep. 2018;4:295-297.
- Lew TT, Creamer D, Mackenzie J, et al. Post-traumatic stress disorder following drug reaction with eosinophilia and systemic symptoms. Br J Dermatol. 2015;172:836-837.
- Kumar R, Das A, Das S. Management of Stevens-Johnson syndrome-toxic epidermal necrolysis: looking beyond guidelines! Indian J Dermatol. 2018;63:117-124.
- Yang CW, Cho YT, Chen KL, et al. Long-term sequelae of Stevens-Johnson syndrome/toxic epidermal necrolysis. Acta Derm Venereol. 2016;96:525-529.
- Lee HY, Walsh SA, Creamer D. Long‐term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow‐up. Br J Dermatol. 2017;177:924-935.
- Hsu M, Jayaram A, Verner R, et al. Indications and outcomes of amniotic membrane transplantation in the management of acute Stevens-Johnson syndrome and toxic epidermal necrolysis: a case-control study. Cornea. 2012;31:1394-1402.
- Sant’ Anna AE, Hazarbassanov RM, de Freitas D, et al. Minor salivary glands and labial mucous membrane graft in the treatment of severe symblepharon and dry eye in patients with Stevens-Johnson syndrome. Br J Ophthalmol. 2012;96:234-239.
- Hefez L, Zaghbib K, Sbidian E, et al. Post-traumatic stress disorder in Stevens-Johnson syndrome and toxic epidermal necrolysis: prevalence and risk factors. a prospective study of 31 patients [published online October 3, 2018]. Br J Dermatol. doi:10.1111/bjd.17267.
- Knight L, Todd G, Muloiwa R, et al. Stevens Johnson syndrome and toxic epidermal necrolysis: maternal and foetal outcomes intwenty-two consecutive pregnant HIV infected women [published online August 12, 2015]. PLoS One. doi:10.1371/journal.pone.0135501.
- Tchetnya X, Ngwasiri CA, Munge T, et al. Severe eye complications from toxic epidermal necrolysis following initiation of nevirapine based HAART regimen in a child with HIV infection: a case from Cameroon. BMC Pediatr. 2018;18:108.
- Antoon JW, Goldman JL, Lee B, et al. Incidence, outcomes, and resource use in children with Stevens-Johnson syndrome and toxic epidermal necrolysis. Pediatr Dermatol. 2018;35:182-187.
- Basu S, Shanbhag SS, Gokani A, et al. Chronic ocular sequelae of Stevens-Johnson syndrome in children: long-term impact of appropriate therapy on natural history of disease. Am J Ophthalmol. 2018;189:17-28.
- Pinho A, Marta A, Coutinho I, et al. Long‐term reproducibility of positive patch test reactions in patients with non‐immediate cutaneous adverse drug reactions to antibiotics. Contact Dermatitis. 2017;76:204-209.
- Barbaud A, Collet E, Milpied B, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555-562.
Practice Points
- In the setting of an adverse drug reaction (ADR), discontinuing the concerning medication is the first and most important step.
- Acute generalized exanthematous pustulosis, drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, and Stevens-Johnson syndrome/toxic epidermal necrolysis all require specific outpatient follow-up after discharge.
Reticular Hyperpigmented Patches With Indurated Subcutaneous Plaques
The Diagnosis: Superficial Migratory Thrombophlebitis
On initial presentation, the differential diagnosis included livedoid vasculopathy, cutaneous polyarteritis nodosa, erythema ab igne, cholesterol embolism, and livedo reticularis. Laboratory investigation included antiphospholipid antibody syndrome (APS), antinuclear antibody, rheumatoid factor, antineutrophil cytoplasmic antibody, serum protein electrophoresis, and coagulation tests. Pertinent findings included transient low total complement activity but normal complement protein C2, C3, and C5 levels and negative cryoglobulins. Additional laboratory testing revealed elevated antiphosphatidylserine IgG, which remained elevated 12 weeks later.
New lesions continued to appear over the next several months as painful, erythematous, linear, pruritic nodules that resolved as hyperpigmented linear patches, which intersected to form a livedo reticularis-like pattern that covered the lower legs. Biopsy of an erythematous nodule on the right leg revealed fibrin occlusion of a medium-sized vein in the subcutaneous fat. Direct immunofluorescence was not specific. Venous duplex ultrasonography demonstrated chronic superficial thrombophlebitis and was crucial to the diagnosis. Ultimately, the patient's history, clinical presentation, laboratory results, venous studies, and histopathologic analysis were consistent with a diagnosis of superficial migratory thrombophlebitis (SMT) with resultant postinflammatory hyperpigmentation presenting in a reticular pattern that mimicked livedoid vasculopathy, livedo reticularis, or erythema ab igne.
Superficial migratory thrombophlebitis, also known as thrombophlebitis migrans, is defined as the recurrent formation of thrombi within superficial veins.1 The presence of a thrombus in a superficial vein evokes an inflammatory response, resulting in swelling, tenderness, erythema, and warmth in the affected area. Superficial migratory thrombophlebitis has been associated with several etiologies, including pregnancy, oral contraceptive use, APS, vasculitic disorders, and malignancies (eg, pancreas, lung, breast), as well as infections such as secondary syphilis.1
When SMT is associated with an occult malignancy, it is known as Trousseau syndrome. Common malignancies found in association with Trousseau syndrome include pancreatic, lung, and breast cancers.2 A systematic review from 2008 evaluated the utility of extensive cancer screening strategies in patients with newly diagnosed, unprovoked venous thromboembolic events.3 Using a wide screening strategy that included computed tomography of the abdomen and pelvis, the investigators detected a considerable number of formerly undiagnosed cancers, increasing detection rates from 49.4% to 69.7%. After the diagnosis of SMT was made in our patient, computed tomography of the chest, abdomen, and pelvis was performed, but the findings were unremarkable.
Because occult malignancy was excluded in our patient, the likely etiology of SMT was APS, an acquired autoimmune condition diagnosed based on the presence of a vascular thrombosis and/or pregnancy failure in women as well as elevation of at least one antiphospholipid antibody laboratory marker (eg, lupus anticoagulant, anticardiolipin antibody, and anti-β2 glycoprotein I antibody) on 2 or more occasions at least 12 weeks apart.4 Other antibodies such as those directed against negatively charged phospholipids (eg, antiphosphatidylserine [which was elevated in our patient], phosphatidylinositol, phosphatidic acid) have been reported in patients with APS, although their diagnostic use is controversial.5 For example, the presence of antiphosphatidylserine antibodies has been considered common but not specific in patients with APS.4 However, a recent observational study demonstrated that antiphosphatidylserine antibodies are highly specific (87%) and useful in diagnosing clinical APS cases in the presence of other negative markers.6
In our patient, diagnosis of SMT with resultant postinflammatory hyperpigmentation in a reticular pattern was based on the patient's medical history, clinical examination, and histopathologic findings, as well as laboratory results and venous studies. However, it is important to note that a livedo reticularis-like pattern also is a very common finding in APS and must be included in the differential diagnosis of a reticular network on the skin.7 Moreover, differentiating livedo reticularis from SMT has prognostic importance since SMT may be associated with underlying malignancies while livedo reticularis may be associated with Sneddon syndrome, a disorder in which neurologic vascular events (eg, cerebrovascular accidents) are present.8 While this distinction is important, there are no pathognomonic histologic findings seen in livedo reticularis, and consideration of the clinical picture and additional testing is critical.4,8
Livedo vasculopathy was excluded in our patient due to the lack of diagnostic histopathologic findings, such as fibrin deposition and thrombus formation involving the upper- and mid-dermal capillaries.9 Furthermore, characteristic direct immunofluorescence findings of a homogenous or granular deposition in the vessel wall consisting of immune complexes, complement, and fibrin were absent in our patient.9 Our patient also lacked common clinical findings found in livedo vasculopathy such as small ulcerations or atrophic, porcelain-white scars on the lower legs. Erythema ab igne also was excluded in our patient due to the absence of heat exposure and presence of fibrin occlusion in the superficial leg veins. Physiologic livedo reticularis, defined as a livedoid pattern due to physiologic changes in the skin in response to cold exposure,10 also was excluded, as our patient's cutaneous changes included an alteration in pigmentation with a brown reticular pattern and no blanching, erythematous or violaceous hue, warmth, or tenderness.
In conclusion, SMT is a disorder with multiple associations that may clinically mimic livedo reticularis and livedoid vasculopathy when postinflammatory hyperpigmentation has a lacelike or livedoid pattern. While nontraditional antibodies may be useful in diagnosis in patients suspected of having APS with otherwise negative markers, standardized assays and further studies are needed to determine the specificity and value of these antibodies, particularly when used in isolation. Our patient's elevated antiphosphatidylserine IgG may have been the cause of her hypercoagulable state causing the SMT. A livedoid pattern is a common finding in APS and also was seen in our patient with SMT, but the differentiation of the brown pigmentary change and more active erythema was critical to the appropriate clinical workup of our patient.
- Samlaska CP, James WD. Superficial thrombophlebitis. II. secondary hypercoagulable states. J Am Acad Dermatol. 1990;23:1-18.
- Rigdon EE. Trousseau's syndrome and acute arterial thrombosis. Cardiovasc Surg. 2000;8:214-218.
- Carrier M, Le Gal G, Wells PS, et al. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149:323-333.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Bertolaccini ML, Amengual O, Atsumi T, et al. 'Non-criteria' aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus. 2011;20:191-205.
- Khogeer H, Alfattani A, Al Kaff M, et al. Antiphosphatidylserine antibodies as diagnostic indicators of antiphospholipid syndrome. Lupus. 2015;24:186-190.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6 pt 1):970-982.
- Francès C, Papo T, Wechsler B, et al. Sneddon syndrome with or without antiphospholipid antibodies. a comparative study in 46 patients. Medicine (Baltimore). 1999;78:209-219.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478-488.
- James WD, Berger TG, Elston DM. Andrews' Diseases Of The Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: Elsevier Saunders; 2006.
The Diagnosis: Superficial Migratory Thrombophlebitis
On initial presentation, the differential diagnosis included livedoid vasculopathy, cutaneous polyarteritis nodosa, erythema ab igne, cholesterol embolism, and livedo reticularis. Laboratory investigation included antiphospholipid antibody syndrome (APS), antinuclear antibody, rheumatoid factor, antineutrophil cytoplasmic antibody, serum protein electrophoresis, and coagulation tests. Pertinent findings included transient low total complement activity but normal complement protein C2, C3, and C5 levels and negative cryoglobulins. Additional laboratory testing revealed elevated antiphosphatidylserine IgG, which remained elevated 12 weeks later.
New lesions continued to appear over the next several months as painful, erythematous, linear, pruritic nodules that resolved as hyperpigmented linear patches, which intersected to form a livedo reticularis-like pattern that covered the lower legs. Biopsy of an erythematous nodule on the right leg revealed fibrin occlusion of a medium-sized vein in the subcutaneous fat. Direct immunofluorescence was not specific. Venous duplex ultrasonography demonstrated chronic superficial thrombophlebitis and was crucial to the diagnosis. Ultimately, the patient's history, clinical presentation, laboratory results, venous studies, and histopathologic analysis were consistent with a diagnosis of superficial migratory thrombophlebitis (SMT) with resultant postinflammatory hyperpigmentation presenting in a reticular pattern that mimicked livedoid vasculopathy, livedo reticularis, or erythema ab igne.
Superficial migratory thrombophlebitis, also known as thrombophlebitis migrans, is defined as the recurrent formation of thrombi within superficial veins.1 The presence of a thrombus in a superficial vein evokes an inflammatory response, resulting in swelling, tenderness, erythema, and warmth in the affected area. Superficial migratory thrombophlebitis has been associated with several etiologies, including pregnancy, oral contraceptive use, APS, vasculitic disorders, and malignancies (eg, pancreas, lung, breast), as well as infections such as secondary syphilis.1
When SMT is associated with an occult malignancy, it is known as Trousseau syndrome. Common malignancies found in association with Trousseau syndrome include pancreatic, lung, and breast cancers.2 A systematic review from 2008 evaluated the utility of extensive cancer screening strategies in patients with newly diagnosed, unprovoked venous thromboembolic events.3 Using a wide screening strategy that included computed tomography of the abdomen and pelvis, the investigators detected a considerable number of formerly undiagnosed cancers, increasing detection rates from 49.4% to 69.7%. After the diagnosis of SMT was made in our patient, computed tomography of the chest, abdomen, and pelvis was performed, but the findings were unremarkable.
Because occult malignancy was excluded in our patient, the likely etiology of SMT was APS, an acquired autoimmune condition diagnosed based on the presence of a vascular thrombosis and/or pregnancy failure in women as well as elevation of at least one antiphospholipid antibody laboratory marker (eg, lupus anticoagulant, anticardiolipin antibody, and anti-β2 glycoprotein I antibody) on 2 or more occasions at least 12 weeks apart.4 Other antibodies such as those directed against negatively charged phospholipids (eg, antiphosphatidylserine [which was elevated in our patient], phosphatidylinositol, phosphatidic acid) have been reported in patients with APS, although their diagnostic use is controversial.5 For example, the presence of antiphosphatidylserine antibodies has been considered common but not specific in patients with APS.4 However, a recent observational study demonstrated that antiphosphatidylserine antibodies are highly specific (87%) and useful in diagnosing clinical APS cases in the presence of other negative markers.6
In our patient, diagnosis of SMT with resultant postinflammatory hyperpigmentation in a reticular pattern was based on the patient's medical history, clinical examination, and histopathologic findings, as well as laboratory results and venous studies. However, it is important to note that a livedo reticularis-like pattern also is a very common finding in APS and must be included in the differential diagnosis of a reticular network on the skin.7 Moreover, differentiating livedo reticularis from SMT has prognostic importance since SMT may be associated with underlying malignancies while livedo reticularis may be associated with Sneddon syndrome, a disorder in which neurologic vascular events (eg, cerebrovascular accidents) are present.8 While this distinction is important, there are no pathognomonic histologic findings seen in livedo reticularis, and consideration of the clinical picture and additional testing is critical.4,8
Livedo vasculopathy was excluded in our patient due to the lack of diagnostic histopathologic findings, such as fibrin deposition and thrombus formation involving the upper- and mid-dermal capillaries.9 Furthermore, characteristic direct immunofluorescence findings of a homogenous or granular deposition in the vessel wall consisting of immune complexes, complement, and fibrin were absent in our patient.9 Our patient also lacked common clinical findings found in livedo vasculopathy such as small ulcerations or atrophic, porcelain-white scars on the lower legs. Erythema ab igne also was excluded in our patient due to the absence of heat exposure and presence of fibrin occlusion in the superficial leg veins. Physiologic livedo reticularis, defined as a livedoid pattern due to physiologic changes in the skin in response to cold exposure,10 also was excluded, as our patient's cutaneous changes included an alteration in pigmentation with a brown reticular pattern and no blanching, erythematous or violaceous hue, warmth, or tenderness.
In conclusion, SMT is a disorder with multiple associations that may clinically mimic livedo reticularis and livedoid vasculopathy when postinflammatory hyperpigmentation has a lacelike or livedoid pattern. While nontraditional antibodies may be useful in diagnosis in patients suspected of having APS with otherwise negative markers, standardized assays and further studies are needed to determine the specificity and value of these antibodies, particularly when used in isolation. Our patient's elevated antiphosphatidylserine IgG may have been the cause of her hypercoagulable state causing the SMT. A livedoid pattern is a common finding in APS and also was seen in our patient with SMT, but the differentiation of the brown pigmentary change and more active erythema was critical to the appropriate clinical workup of our patient.
The Diagnosis: Superficial Migratory Thrombophlebitis
On initial presentation, the differential diagnosis included livedoid vasculopathy, cutaneous polyarteritis nodosa, erythema ab igne, cholesterol embolism, and livedo reticularis. Laboratory investigation included antiphospholipid antibody syndrome (APS), antinuclear antibody, rheumatoid factor, antineutrophil cytoplasmic antibody, serum protein electrophoresis, and coagulation tests. Pertinent findings included transient low total complement activity but normal complement protein C2, C3, and C5 levels and negative cryoglobulins. Additional laboratory testing revealed elevated antiphosphatidylserine IgG, which remained elevated 12 weeks later.
New lesions continued to appear over the next several months as painful, erythematous, linear, pruritic nodules that resolved as hyperpigmented linear patches, which intersected to form a livedo reticularis-like pattern that covered the lower legs. Biopsy of an erythematous nodule on the right leg revealed fibrin occlusion of a medium-sized vein in the subcutaneous fat. Direct immunofluorescence was not specific. Venous duplex ultrasonography demonstrated chronic superficial thrombophlebitis and was crucial to the diagnosis. Ultimately, the patient's history, clinical presentation, laboratory results, venous studies, and histopathologic analysis were consistent with a diagnosis of superficial migratory thrombophlebitis (SMT) with resultant postinflammatory hyperpigmentation presenting in a reticular pattern that mimicked livedoid vasculopathy, livedo reticularis, or erythema ab igne.
Superficial migratory thrombophlebitis, also known as thrombophlebitis migrans, is defined as the recurrent formation of thrombi within superficial veins.1 The presence of a thrombus in a superficial vein evokes an inflammatory response, resulting in swelling, tenderness, erythema, and warmth in the affected area. Superficial migratory thrombophlebitis has been associated with several etiologies, including pregnancy, oral contraceptive use, APS, vasculitic disorders, and malignancies (eg, pancreas, lung, breast), as well as infections such as secondary syphilis.1
When SMT is associated with an occult malignancy, it is known as Trousseau syndrome. Common malignancies found in association with Trousseau syndrome include pancreatic, lung, and breast cancers.2 A systematic review from 2008 evaluated the utility of extensive cancer screening strategies in patients with newly diagnosed, unprovoked venous thromboembolic events.3 Using a wide screening strategy that included computed tomography of the abdomen and pelvis, the investigators detected a considerable number of formerly undiagnosed cancers, increasing detection rates from 49.4% to 69.7%. After the diagnosis of SMT was made in our patient, computed tomography of the chest, abdomen, and pelvis was performed, but the findings were unremarkable.
Because occult malignancy was excluded in our patient, the likely etiology of SMT was APS, an acquired autoimmune condition diagnosed based on the presence of a vascular thrombosis and/or pregnancy failure in women as well as elevation of at least one antiphospholipid antibody laboratory marker (eg, lupus anticoagulant, anticardiolipin antibody, and anti-β2 glycoprotein I antibody) on 2 or more occasions at least 12 weeks apart.4 Other antibodies such as those directed against negatively charged phospholipids (eg, antiphosphatidylserine [which was elevated in our patient], phosphatidylinositol, phosphatidic acid) have been reported in patients with APS, although their diagnostic use is controversial.5 For example, the presence of antiphosphatidylserine antibodies has been considered common but not specific in patients with APS.4 However, a recent observational study demonstrated that antiphosphatidylserine antibodies are highly specific (87%) and useful in diagnosing clinical APS cases in the presence of other negative markers.6
In our patient, diagnosis of SMT with resultant postinflammatory hyperpigmentation in a reticular pattern was based on the patient's medical history, clinical examination, and histopathologic findings, as well as laboratory results and venous studies. However, it is important to note that a livedo reticularis-like pattern also is a very common finding in APS and must be included in the differential diagnosis of a reticular network on the skin.7 Moreover, differentiating livedo reticularis from SMT has prognostic importance since SMT may be associated with underlying malignancies while livedo reticularis may be associated with Sneddon syndrome, a disorder in which neurologic vascular events (eg, cerebrovascular accidents) are present.8 While this distinction is important, there are no pathognomonic histologic findings seen in livedo reticularis, and consideration of the clinical picture and additional testing is critical.4,8
Livedo vasculopathy was excluded in our patient due to the lack of diagnostic histopathologic findings, such as fibrin deposition and thrombus formation involving the upper- and mid-dermal capillaries.9 Furthermore, characteristic direct immunofluorescence findings of a homogenous or granular deposition in the vessel wall consisting of immune complexes, complement, and fibrin were absent in our patient.9 Our patient also lacked common clinical findings found in livedo vasculopathy such as small ulcerations or atrophic, porcelain-white scars on the lower legs. Erythema ab igne also was excluded in our patient due to the absence of heat exposure and presence of fibrin occlusion in the superficial leg veins. Physiologic livedo reticularis, defined as a livedoid pattern due to physiologic changes in the skin in response to cold exposure,10 also was excluded, as our patient's cutaneous changes included an alteration in pigmentation with a brown reticular pattern and no blanching, erythematous or violaceous hue, warmth, or tenderness.
In conclusion, SMT is a disorder with multiple associations that may clinically mimic livedo reticularis and livedoid vasculopathy when postinflammatory hyperpigmentation has a lacelike or livedoid pattern. While nontraditional antibodies may be useful in diagnosis in patients suspected of having APS with otherwise negative markers, standardized assays and further studies are needed to determine the specificity and value of these antibodies, particularly when used in isolation. Our patient's elevated antiphosphatidylserine IgG may have been the cause of her hypercoagulable state causing the SMT. A livedoid pattern is a common finding in APS and also was seen in our patient with SMT, but the differentiation of the brown pigmentary change and more active erythema was critical to the appropriate clinical workup of our patient.
- Samlaska CP, James WD. Superficial thrombophlebitis. II. secondary hypercoagulable states. J Am Acad Dermatol. 1990;23:1-18.
- Rigdon EE. Trousseau's syndrome and acute arterial thrombosis. Cardiovasc Surg. 2000;8:214-218.
- Carrier M, Le Gal G, Wells PS, et al. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149:323-333.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Bertolaccini ML, Amengual O, Atsumi T, et al. 'Non-criteria' aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus. 2011;20:191-205.
- Khogeer H, Alfattani A, Al Kaff M, et al. Antiphosphatidylserine antibodies as diagnostic indicators of antiphospholipid syndrome. Lupus. 2015;24:186-190.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6 pt 1):970-982.
- Francès C, Papo T, Wechsler B, et al. Sneddon syndrome with or without antiphospholipid antibodies. a comparative study in 46 patients. Medicine (Baltimore). 1999;78:209-219.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478-488.
- James WD, Berger TG, Elston DM. Andrews' Diseases Of The Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: Elsevier Saunders; 2006.
- Samlaska CP, James WD. Superficial thrombophlebitis. II. secondary hypercoagulable states. J Am Acad Dermatol. 1990;23:1-18.
- Rigdon EE. Trousseau's syndrome and acute arterial thrombosis. Cardiovasc Surg. 2000;8:214-218.
- Carrier M, Le Gal G, Wells PS, et al. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149:323-333.
- Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295-306.
- Bertolaccini ML, Amengual O, Atsumi T, et al. 'Non-criteria' aPL tests: report of a task force and preconference workshop at the 13th International Congress on Antiphospholipid Antibodies, Galveston, TX, USA, April 2010. Lupus. 2011;20:191-205.
- Khogeer H, Alfattani A, Al Kaff M, et al. Antiphosphatidylserine antibodies as diagnostic indicators of antiphospholipid syndrome. Lupus. 2015;24:186-190.
- Gibson GE, Su WP, Pittelkow MR. Antiphospholipid syndrome and the skin. J Am Acad Dermatol. 1997;36(6 pt 1):970-982.
- Francès C, Papo T, Wechsler B, et al. Sneddon syndrome with or without antiphospholipid antibodies. a comparative study in 46 patients. Medicine (Baltimore). 1999;78:209-219.
- Vasudevan B, Neema S, Verma R. Livedoid vasculopathy: a review of pathogenesis and principles of management. Indian J Dermatol Venereol Leprol. 2016;82:478-488.
- James WD, Berger TG, Elston DM. Andrews' Diseases Of The Skin: Clinical Dermatology. 10th ed. Philadelphia, PA: Elsevier Saunders; 2006.

A 32-year-old woman presented for evaluation of small, tender, erythematous nodules on the lower legs of 1 year's duration that had started to spread to the thighs over several months prior to presentation. The patient reported no history of ulceration or other cutaneous findings. On physical examination, a hyperpigmented, linear to reticular pattern was noted on the lower legs with a few 1-cm, erythematous, mildly indurated and tender subcutaneous nodules. The patient denied any recent medical procedures, history of malignancy or cardiovascular disease, use of tobacco or illicit drugs, prolonged contact with a heat source, recent unintentional weight loss, fevers, or night sweats. Her medical history was notable for asthma and migraines, which were treated with albuterol, fluticasone, and topiramate.