User login
Case Report
A 60-year-old Cambodian woman presented with recurrent fever (temperature, up to 38.8°C) 7 months after receiving a kidney transplant secondary to polycystic kidney disease. Fever was attributed to recurrent pyelonephritis of the native kidneys while on mycophenolate mofetil, tacrolimus, and prednisone. As a result, she underwent a bilateral native nephrectomy and was found to have peritoneal nodules. Pathology of both native kidneys and peritoneal tissue revealed caseating granulomas and acid-fast bacilli (AFB) diagnostic for kidney and peritoneal tuberculosis (TB). She had no history of TB, and a TB skin test (purified protein derivative [PPD]) upon entering the United States from Cambodia a decade earlier was negative. Additionally, her pretransplantation PPD was negative.
Treatment with isoniazid, ethambutol, pyrazinamide, and levofloxacin was initiated immediately upon diagnosis, and all of her immunosuppressive medications—mycophenolate mofetil, tacrolimus, and prednisone—were discontinued. Her symptoms subsided within 1 week, and she was discharged from the hospital. Over the next 2 months, her immunosuppressive medications were restarted, and her TB medications were periodically discontinued by the Tuberculosis Control Program at the Department of Health (Philadelphia, Pennsylvania) due to severe thrombocytopenia. During this time, she was closely monitored twice weekly in the clinic with blood draws performed weekly.
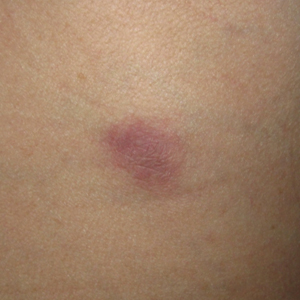
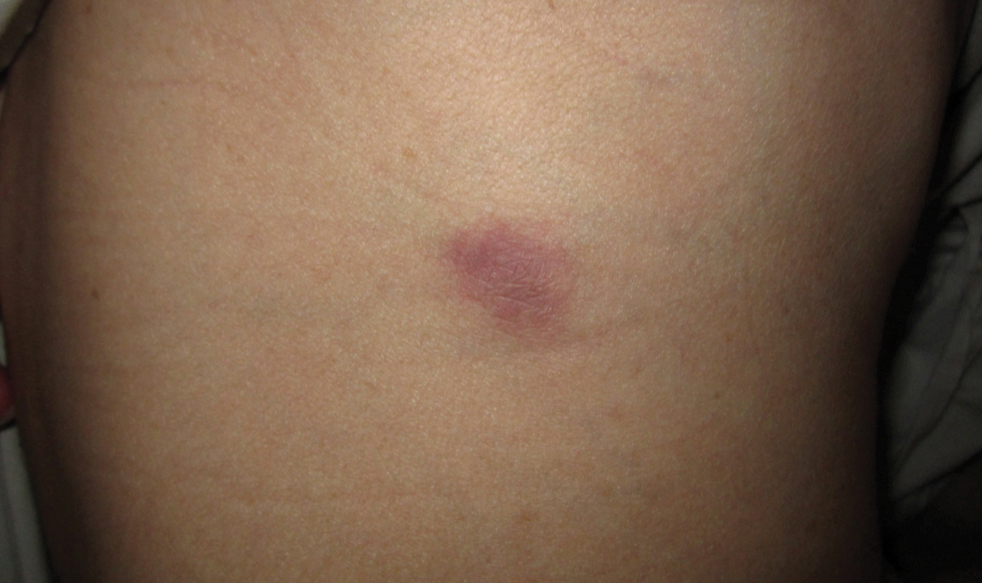
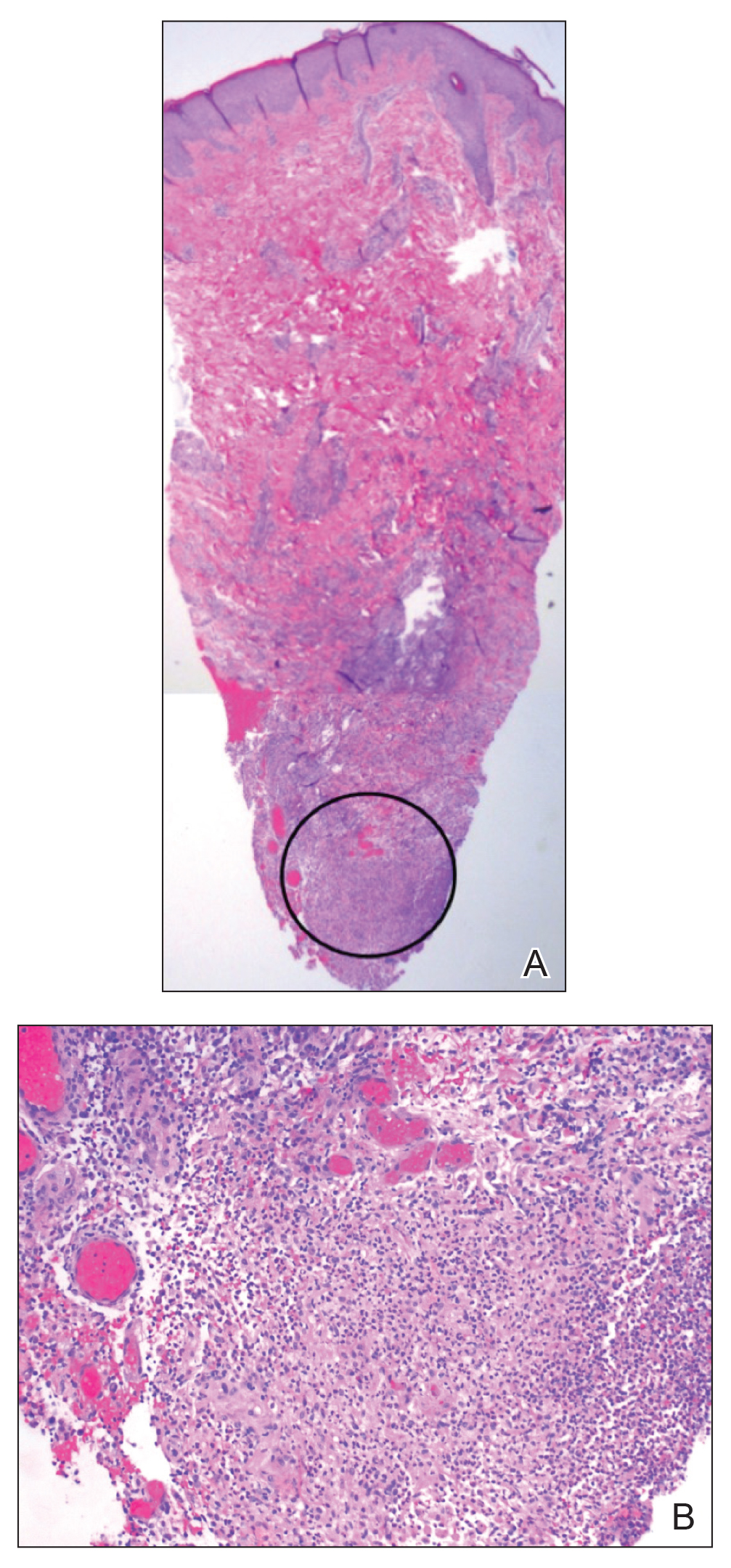
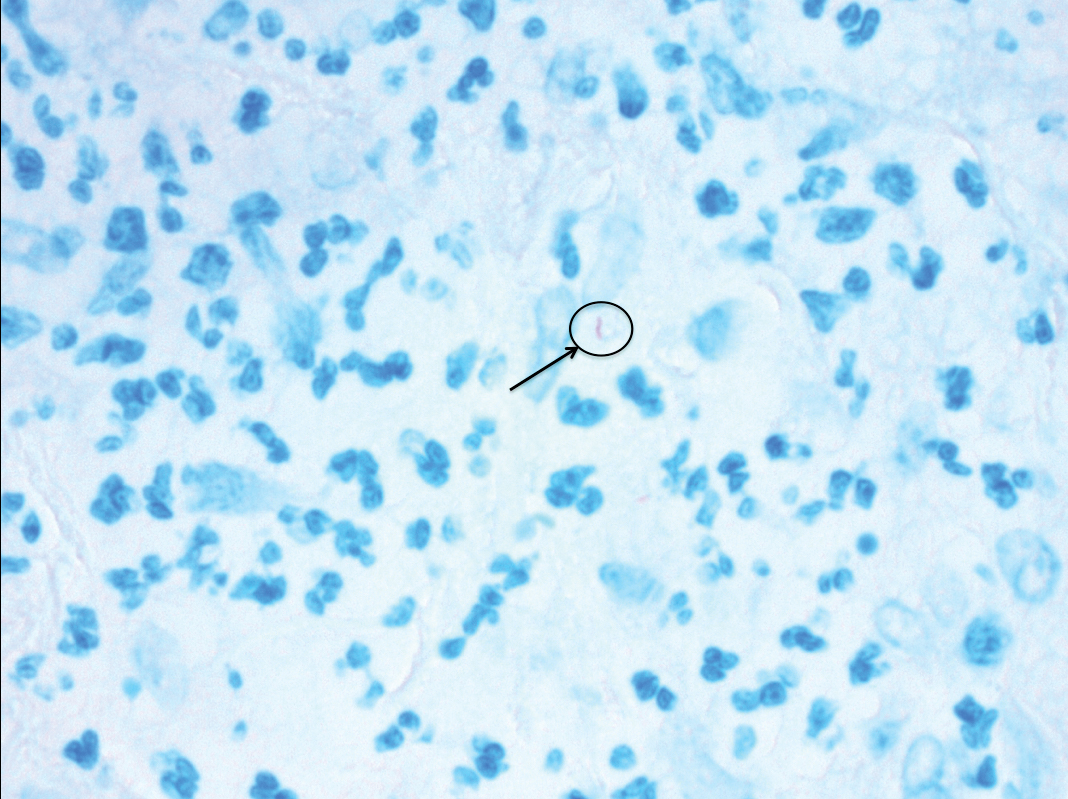
Approximately 10 weeks after initiation of treatment, she noted recurrent subjective fever (temperature, up to 38.8°C) and painful lesions on the right side of the flank, left breast, and left arm of 3 days’ duration. Physical examination revealed a warm, dull red, tender nodule on the right side of the flank (Figure 1) and subcutaneous nodules with no overlying skin changes on the left breast and left arm. A biopsy of the lesion on the right side of the flank was performed, which resulted in substantial purulent drainage. Histologic analysis showed an inflammatory infiltrate within the deep dermis composed of neutrophils, macrophages, and giant cells, indicative of suppurative granulomatous dermatitis (Figure 2). Ziehl-Neelsen stain demonstrated rare AFB within the cytoplasm of macrophages, suggestive of Mycobacterium tuberculosis infection (Figure 3). A repeat chest radiograph was normal.
Based on the patient’s history and clinical presentation, she was continued on isoniazid, ethambutol, and levofloxacin, with complete resolution of symptoms and cutaneous lesions. Over the subsequent 2 months, the therapy was modified to rifabutin, pyrazinamide, and levofloxacin, and subsequently pyrazinamide was stopped. A subsequent biopsy of the left breast and histologic analysis indicated that the specimen was benign; stains for AFB were negative. Currently, both the fever and skin lesions have completely resolved, and she remains on anti-TB therapy.
Comment
Clinical Presentation
Cutaneous TB is an uncommon manifestation of TB that can occur either exogenously or endogenously.1 It tends to occur primarily in previously infected TB patients through hematogenous, lymphatic, or contiguous spread.2 Due to their immunocompromised state, solid organ transplant recipients have an increased incidence of primary and reactivated latent TB reported to be 20 to 74 times greater than the general population.3,4 One report stated the total incidence of posttransplant TB as 0.48% in the West and 11.8% in endemic regions such as India.5 The occurrence of cutaneous TB is rare among solid organ transplant recipients.1 On average, a diagnosis of latent TB is made 9 months after transplantation because of the opportunistic nature of M tuberculosis in an immunosuppressed environment.6
TB Subtypes
Cutaneous TB can be in the form of localized disease (eg, primary tuberculous chancre, TB verrucosa cutis, lupus vulgaris, smear-negative scrofuloderma), disseminated disease (eg, disseminated TB, TB gumma, orificial TB, miliary cutaneous TB), or tuberculids (eg, papulonecrotic tuberculid, lichen scrofulosorum, erythema induratum).7 Due to the pustular epithelioid cell granulomas and AFB positivity of the involved cutaneous lesions, our patient’s TB can be classified as a metastatic TB abscess or gummatous TB.7
Metastatic TB abscess, an uncommon subtype of cutaneous TB, generally is only seen in malnourished children and notably immunocompromised individuals.2,8,9 In these individuals, systemic failure of cell-mediated immunity enables M tuberculosis to hematogenously infect various organs of the body, resulting in alternative forms of TB, such as gummatous-type TB.10 One study reported that of the 0.1% of dermatology patients presenting with cutaneous TB, only 5.4% of these individuals had the rarer gummatous form.7 These metastatic TB abscesses begin as a single or multiple nontender subcutaneous nodule(s), which breaks down and softens to form a draining sinus abscess.2,8,9 Abscesses are most commonly seen on the trunk and extremities; however, they can be found nearly anywhere on the body.8 The pathology of cutaneous TB lesions demonstrates caseating necrosis with epithelioid and giant cells forming a surrounding rim.9
Diagnosis
Diagnosis may be difficult because of the vast number of dermatologic conditions that resemble cutaneous TB, including mycoses, sarcoidosis, leishmaniasis, leprosy, syphilis, other non-TB mycobacteria, and Wegener granulomatosis.9 Thus, confirmatory diagnosis is made via clinical presentation, detailed history and physical examination, and laboratory tests.11 These tests include the Mantoux tuberculin skin test (PPD or TST) or IFN-γ release assays (QuantiFERON-TB Gold test), identification of AFB on skin biopsy, and isolation of M tuberculosis from tissue culture or polymerase chain reaction.11
At-Risk Populations
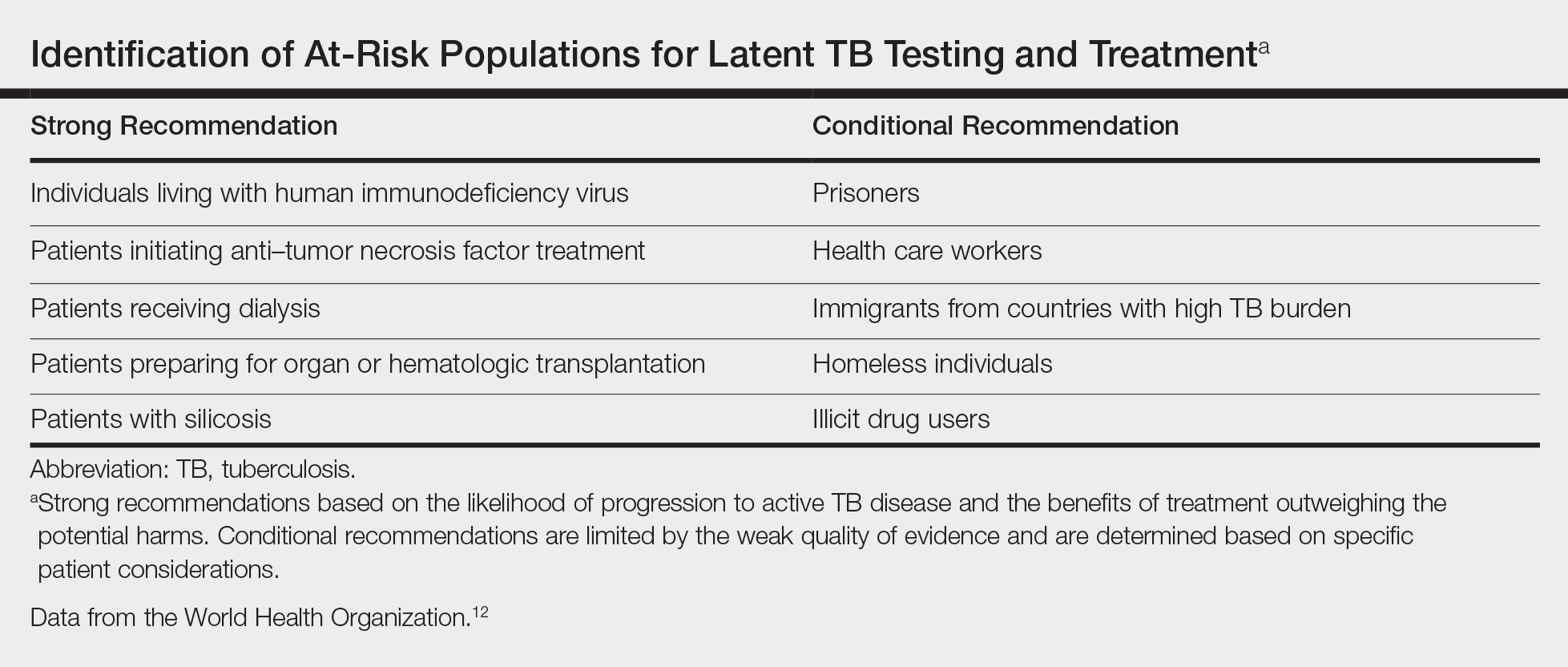
The recommendation for the identification of at-risk populations for latent TB testing and treatment have been clearly defined by the World Health Organization (Table).12 Our patient met 2 of these criteria: she had been preparing for organ transplantation and was from a country with high TB burden. Such at-risk patients should be tested for a latent TB infection with either IFN-γ release assays or PPD.12
Treatment
The recommended treatment of active TB in transplant recipients is based on randomized trials in immunocompetent hosts, and thus the same as that used by the general population.16 This anti-TB regimen includes the use of 4 drugs—typically rifampicin, isoniazid, ethambutol, and pyrazinamide—for a 6-month duration.11 Unfortunately, the management of TB in an immunocompromised patient is more challenging due to the potential side effects and drug interactions.
Finally, thrombocytopenia is an infrequent, life-threatening complication that can be acquired by immunocompromised patients on anti-TB therapy.17 Drug-induced thrombocytopenia can be caused by a variety of medications, including rifampicin, isoniazid, ethambutol, and pyrazinamide. Diagnosis of drug-induced thrombocytopenia can be confirmed only after discontinuation of the suspected drug and subsequent resolution of the thrombocytopenia.17 Our patient initially became thrombocytopenic while taking isoniazid, ethambutol, pyrazinamide, and levofloxacin. However, her platelet levels improved once the pyrazinamide was discontinued, thereby suggesting pyrazinamide-induced thrombocytopenia.
Conclusion
The risk for infectious disease reactivation in an immunocompromised patient undergoing transplant surgery is notable. Our findings emphasize the value of a comprehensive pretransplant evaluation, vigilance even when test results appear negative, and treatment of latent TB within this population.16,18,19 Furthermore, this case illustrates a noteworthy example of a rare form of cutaneous TB, which should be considered and included in the differential for cutaneous lesions in an immunosuppressed patient.
- Sakhuja V, Jha V, Varma PP, et al. The high incidence of tuberculosis among renal transplant recipients in India. Transplantation. 1996;61:211-215.
- Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
- Schultz V, Marroni CA, Amorim CS, et al. Risk factors for hepatotoxicity in solid organ transplants recipients being treated for tuberculosis. Transplant Proc. 2014;46:3606-3610.
- Tabarsi P, Farshidpour M, Marjani M, et al. Mycobacterial infection and the impact of rifabutin treatment in organ transplant recipients: a single-center study. Saudi J Kidney Dis Transpl. 2015;26:6-11.
- Rathi M, Gundlapalli S, Ramachandran R, et al. A rare case of cytomegalovirus, scedosporium apiospermum and mycobacterium tuberculosis in a renal transplant recipient. BMC Infect Dis. 2014;14:259.
- Hickey MD, Quan DJ, Chin-Hong PV, et al. Use of rifabutin for the treatment of a latent tuberculosis infection in a patient after solid organ transplantation. Liver Transpl. 2013;19:457-461.
- Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty-year prospective study. Int J Tuberc Lung Dis. 1999;3:494-500.
- Dekeyzer S, Moerman F, Callens S, et al. Cutaneous metastatic tuberculous abscess in patient with cervico-mediastinal lymphatic tuberculosis. Acta Clin Belg. 2013;68:34-36.
- Ko M, Wu C, Chiu H. Tuberculous gumma (cutaneous metastatic tuberculous abscess). Dermatol Sinica. 2005;23:27-31.
- Steger JW, Barrett TL. Cutaneous tuberculosis. In: James WD, ed. Textbook of Military Medicine: Military Dermatology. Washington, DC: Borden Institute; 1994:355-389.
- Santos JB, Figueiredo AR, Ferraz CE, et al. Cutaneous tuberculosis: diagnosis, histopathology and treatment - part II. An Bras Dermatol. 2014;89:545-555.
- Guidelines on the Management of Latent Tuberculosis Infection. Geneva, Switzerland: World Health Organization; 2015.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 pt 2):S221-S247.
- Mycobacterium tuberculosis. Am J Transplant. 2004;4(suppl 10):37-41.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284.
- Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
- Kant S, Verma SK, Gupta V, et al. Pyrazinamide induced thrombocytopenia. Indian J Pharmacol. 2010;42:108-109.
- Screening for tuberculosis and tuberculosis infection in high-risk populations. recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:19-34.
- Fischer SA, Avery RK; AST Infectious Disease Community of Practice. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant. 2009;9(suppl 4):S7-S18.
Case Report
A 60-year-old Cambodian woman presented with recurrent fever (temperature, up to 38.8°C) 7 months after receiving a kidney transplant secondary to polycystic kidney disease. Fever was attributed to recurrent pyelonephritis of the native kidneys while on mycophenolate mofetil, tacrolimus, and prednisone. As a result, she underwent a bilateral native nephrectomy and was found to have peritoneal nodules. Pathology of both native kidneys and peritoneal tissue revealed caseating granulomas and acid-fast bacilli (AFB) diagnostic for kidney and peritoneal tuberculosis (TB). She had no history of TB, and a TB skin test (purified protein derivative [PPD]) upon entering the United States from Cambodia a decade earlier was negative. Additionally, her pretransplantation PPD was negative.
Treatment with isoniazid, ethambutol, pyrazinamide, and levofloxacin was initiated immediately upon diagnosis, and all of her immunosuppressive medications—mycophenolate mofetil, tacrolimus, and prednisone—were discontinued. Her symptoms subsided within 1 week, and she was discharged from the hospital. Over the next 2 months, her immunosuppressive medications were restarted, and her TB medications were periodically discontinued by the Tuberculosis Control Program at the Department of Health (Philadelphia, Pennsylvania) due to severe thrombocytopenia. During this time, she was closely monitored twice weekly in the clinic with blood draws performed weekly.
Approximately 10 weeks after initiation of treatment, she noted recurrent subjective fever (temperature, up to 38.8°C) and painful lesions on the right side of the flank, left breast, and left arm of 3 days’ duration. Physical examination revealed a warm, dull red, tender nodule on the right side of the flank (Figure 1) and subcutaneous nodules with no overlying skin changes on the left breast and left arm. A biopsy of the lesion on the right side of the flank was performed, which resulted in substantial purulent drainage. Histologic analysis showed an inflammatory infiltrate within the deep dermis composed of neutrophils, macrophages, and giant cells, indicative of suppurative granulomatous dermatitis (Figure 2). Ziehl-Neelsen stain demonstrated rare AFB within the cytoplasm of macrophages, suggestive of Mycobacterium tuberculosis infection (Figure 3). A repeat chest radiograph was normal.



Based on the patient’s history and clinical presentation, she was continued on isoniazid, ethambutol, and levofloxacin, with complete resolution of symptoms and cutaneous lesions. Over the subsequent 2 months, the therapy was modified to rifabutin, pyrazinamide, and levofloxacin, and subsequently pyrazinamide was stopped. A subsequent biopsy of the left breast and histologic analysis indicated that the specimen was benign; stains for AFB were negative. Currently, both the fever and skin lesions have completely resolved, and she remains on anti-TB therapy.
Comment
Clinical Presentation
Cutaneous TB is an uncommon manifestation of TB that can occur either exogenously or endogenously.1 It tends to occur primarily in previously infected TB patients through hematogenous, lymphatic, or contiguous spread.2 Due to their immunocompromised state, solid organ transplant recipients have an increased incidence of primary and reactivated latent TB reported to be 20 to 74 times greater than the general population.3,4 One report stated the total incidence of posttransplant TB as 0.48% in the West and 11.8% in endemic regions such as India.5 The occurrence of cutaneous TB is rare among solid organ transplant recipients.1 On average, a diagnosis of latent TB is made 9 months after transplantation because of the opportunistic nature of M tuberculosis in an immunosuppressed environment.6
TB Subtypes
Cutaneous TB can be in the form of localized disease (eg, primary tuberculous chancre, TB verrucosa cutis, lupus vulgaris, smear-negative scrofuloderma), disseminated disease (eg, disseminated TB, TB gumma, orificial TB, miliary cutaneous TB), or tuberculids (eg, papulonecrotic tuberculid, lichen scrofulosorum, erythema induratum).7 Due to the pustular epithelioid cell granulomas and AFB positivity of the involved cutaneous lesions, our patient’s TB can be classified as a metastatic TB abscess or gummatous TB.7
Metastatic TB abscess, an uncommon subtype of cutaneous TB, generally is only seen in malnourished children and notably immunocompromised individuals.2,8,9 In these individuals, systemic failure of cell-mediated immunity enables M tuberculosis to hematogenously infect various organs of the body, resulting in alternative forms of TB, such as gummatous-type TB.10 One study reported that of the 0.1% of dermatology patients presenting with cutaneous TB, only 5.4% of these individuals had the rarer gummatous form.7 These metastatic TB abscesses begin as a single or multiple nontender subcutaneous nodule(s), which breaks down and softens to form a draining sinus abscess.2,8,9 Abscesses are most commonly seen on the trunk and extremities; however, they can be found nearly anywhere on the body.8 The pathology of cutaneous TB lesions demonstrates caseating necrosis with epithelioid and giant cells forming a surrounding rim.9
Diagnosis
Diagnosis may be difficult because of the vast number of dermatologic conditions that resemble cutaneous TB, including mycoses, sarcoidosis, leishmaniasis, leprosy, syphilis, other non-TB mycobacteria, and Wegener granulomatosis.9 Thus, confirmatory diagnosis is made via clinical presentation, detailed history and physical examination, and laboratory tests.11 These tests include the Mantoux tuberculin skin test (PPD or TST) or IFN-γ release assays (QuantiFERON-TB Gold test), identification of AFB on skin biopsy, and isolation of M tuberculosis from tissue culture or polymerase chain reaction.11
At-Risk Populations
The recommendation for the identification of at-risk populations for latent TB testing and treatment have been clearly defined by the World Health Organization (Table).12 Our patient met 2 of these criteria: she had been preparing for organ transplantation and was from a country with high TB burden. Such at-risk patients should be tested for a latent TB infection with either IFN-γ release assays or PPD.12

Treatment
The recommended treatment of active TB in transplant recipients is based on randomized trials in immunocompetent hosts, and thus the same as that used by the general population.16 This anti-TB regimen includes the use of 4 drugs—typically rifampicin, isoniazid, ethambutol, and pyrazinamide—for a 6-month duration.11 Unfortunately, the management of TB in an immunocompromised patient is more challenging due to the potential side effects and drug interactions.
Finally, thrombocytopenia is an infrequent, life-threatening complication that can be acquired by immunocompromised patients on anti-TB therapy.17 Drug-induced thrombocytopenia can be caused by a variety of medications, including rifampicin, isoniazid, ethambutol, and pyrazinamide. Diagnosis of drug-induced thrombocytopenia can be confirmed only after discontinuation of the suspected drug and subsequent resolution of the thrombocytopenia.17 Our patient initially became thrombocytopenic while taking isoniazid, ethambutol, pyrazinamide, and levofloxacin. However, her platelet levels improved once the pyrazinamide was discontinued, thereby suggesting pyrazinamide-induced thrombocytopenia.
Conclusion
The risk for infectious disease reactivation in an immunocompromised patient undergoing transplant surgery is notable. Our findings emphasize the value of a comprehensive pretransplant evaluation, vigilance even when test results appear negative, and treatment of latent TB within this population.16,18,19 Furthermore, this case illustrates a noteworthy example of a rare form of cutaneous TB, which should be considered and included in the differential for cutaneous lesions in an immunosuppressed patient.
Case Report
A 60-year-old Cambodian woman presented with recurrent fever (temperature, up to 38.8°C) 7 months after receiving a kidney transplant secondary to polycystic kidney disease. Fever was attributed to recurrent pyelonephritis of the native kidneys while on mycophenolate mofetil, tacrolimus, and prednisone. As a result, she underwent a bilateral native nephrectomy and was found to have peritoneal nodules. Pathology of both native kidneys and peritoneal tissue revealed caseating granulomas and acid-fast bacilli (AFB) diagnostic for kidney and peritoneal tuberculosis (TB). She had no history of TB, and a TB skin test (purified protein derivative [PPD]) upon entering the United States from Cambodia a decade earlier was negative. Additionally, her pretransplantation PPD was negative.
Treatment with isoniazid, ethambutol, pyrazinamide, and levofloxacin was initiated immediately upon diagnosis, and all of her immunosuppressive medications—mycophenolate mofetil, tacrolimus, and prednisone—were discontinued. Her symptoms subsided within 1 week, and she was discharged from the hospital. Over the next 2 months, her immunosuppressive medications were restarted, and her TB medications were periodically discontinued by the Tuberculosis Control Program at the Department of Health (Philadelphia, Pennsylvania) due to severe thrombocytopenia. During this time, she was closely monitored twice weekly in the clinic with blood draws performed weekly.
Approximately 10 weeks after initiation of treatment, she noted recurrent subjective fever (temperature, up to 38.8°C) and painful lesions on the right side of the flank, left breast, and left arm of 3 days’ duration. Physical examination revealed a warm, dull red, tender nodule on the right side of the flank (Figure 1) and subcutaneous nodules with no overlying skin changes on the left breast and left arm. A biopsy of the lesion on the right side of the flank was performed, which resulted in substantial purulent drainage. Histologic analysis showed an inflammatory infiltrate within the deep dermis composed of neutrophils, macrophages, and giant cells, indicative of suppurative granulomatous dermatitis (Figure 2). Ziehl-Neelsen stain demonstrated rare AFB within the cytoplasm of macrophages, suggestive of Mycobacterium tuberculosis infection (Figure 3). A repeat chest radiograph was normal.



Based on the patient’s history and clinical presentation, she was continued on isoniazid, ethambutol, and levofloxacin, with complete resolution of symptoms and cutaneous lesions. Over the subsequent 2 months, the therapy was modified to rifabutin, pyrazinamide, and levofloxacin, and subsequently pyrazinamide was stopped. A subsequent biopsy of the left breast and histologic analysis indicated that the specimen was benign; stains for AFB were negative. Currently, both the fever and skin lesions have completely resolved, and she remains on anti-TB therapy.
Comment
Clinical Presentation
Cutaneous TB is an uncommon manifestation of TB that can occur either exogenously or endogenously.1 It tends to occur primarily in previously infected TB patients through hematogenous, lymphatic, or contiguous spread.2 Due to their immunocompromised state, solid organ transplant recipients have an increased incidence of primary and reactivated latent TB reported to be 20 to 74 times greater than the general population.3,4 One report stated the total incidence of posttransplant TB as 0.48% in the West and 11.8% in endemic regions such as India.5 The occurrence of cutaneous TB is rare among solid organ transplant recipients.1 On average, a diagnosis of latent TB is made 9 months after transplantation because of the opportunistic nature of M tuberculosis in an immunosuppressed environment.6
TB Subtypes
Cutaneous TB can be in the form of localized disease (eg, primary tuberculous chancre, TB verrucosa cutis, lupus vulgaris, smear-negative scrofuloderma), disseminated disease (eg, disseminated TB, TB gumma, orificial TB, miliary cutaneous TB), or tuberculids (eg, papulonecrotic tuberculid, lichen scrofulosorum, erythema induratum).7 Due to the pustular epithelioid cell granulomas and AFB positivity of the involved cutaneous lesions, our patient’s TB can be classified as a metastatic TB abscess or gummatous TB.7
Metastatic TB abscess, an uncommon subtype of cutaneous TB, generally is only seen in malnourished children and notably immunocompromised individuals.2,8,9 In these individuals, systemic failure of cell-mediated immunity enables M tuberculosis to hematogenously infect various organs of the body, resulting in alternative forms of TB, such as gummatous-type TB.10 One study reported that of the 0.1% of dermatology patients presenting with cutaneous TB, only 5.4% of these individuals had the rarer gummatous form.7 These metastatic TB abscesses begin as a single or multiple nontender subcutaneous nodule(s), which breaks down and softens to form a draining sinus abscess.2,8,9 Abscesses are most commonly seen on the trunk and extremities; however, they can be found nearly anywhere on the body.8 The pathology of cutaneous TB lesions demonstrates caseating necrosis with epithelioid and giant cells forming a surrounding rim.9
Diagnosis
Diagnosis may be difficult because of the vast number of dermatologic conditions that resemble cutaneous TB, including mycoses, sarcoidosis, leishmaniasis, leprosy, syphilis, other non-TB mycobacteria, and Wegener granulomatosis.9 Thus, confirmatory diagnosis is made via clinical presentation, detailed history and physical examination, and laboratory tests.11 These tests include the Mantoux tuberculin skin test (PPD or TST) or IFN-γ release assays (QuantiFERON-TB Gold test), identification of AFB on skin biopsy, and isolation of M tuberculosis from tissue culture or polymerase chain reaction.11
At-Risk Populations
The recommendation for the identification of at-risk populations for latent TB testing and treatment have been clearly defined by the World Health Organization (Table).12 Our patient met 2 of these criteria: she had been preparing for organ transplantation and was from a country with high TB burden. Such at-risk patients should be tested for a latent TB infection with either IFN-γ release assays or PPD.12

Treatment
The recommended treatment of active TB in transplant recipients is based on randomized trials in immunocompetent hosts, and thus the same as that used by the general population.16 This anti-TB regimen includes the use of 4 drugs—typically rifampicin, isoniazid, ethambutol, and pyrazinamide—for a 6-month duration.11 Unfortunately, the management of TB in an immunocompromised patient is more challenging due to the potential side effects and drug interactions.
Finally, thrombocytopenia is an infrequent, life-threatening complication that can be acquired by immunocompromised patients on anti-TB therapy.17 Drug-induced thrombocytopenia can be caused by a variety of medications, including rifampicin, isoniazid, ethambutol, and pyrazinamide. Diagnosis of drug-induced thrombocytopenia can be confirmed only after discontinuation of the suspected drug and subsequent resolution of the thrombocytopenia.17 Our patient initially became thrombocytopenic while taking isoniazid, ethambutol, pyrazinamide, and levofloxacin. However, her platelet levels improved once the pyrazinamide was discontinued, thereby suggesting pyrazinamide-induced thrombocytopenia.
Conclusion
The risk for infectious disease reactivation in an immunocompromised patient undergoing transplant surgery is notable. Our findings emphasize the value of a comprehensive pretransplant evaluation, vigilance even when test results appear negative, and treatment of latent TB within this population.16,18,19 Furthermore, this case illustrates a noteworthy example of a rare form of cutaneous TB, which should be considered and included in the differential for cutaneous lesions in an immunosuppressed patient.
- Sakhuja V, Jha V, Varma PP, et al. The high incidence of tuberculosis among renal transplant recipients in India. Transplantation. 1996;61:211-215.
- Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
- Schultz V, Marroni CA, Amorim CS, et al. Risk factors for hepatotoxicity in solid organ transplants recipients being treated for tuberculosis. Transplant Proc. 2014;46:3606-3610.
- Tabarsi P, Farshidpour M, Marjani M, et al. Mycobacterial infection and the impact of rifabutin treatment in organ transplant recipients: a single-center study. Saudi J Kidney Dis Transpl. 2015;26:6-11.
- Rathi M, Gundlapalli S, Ramachandran R, et al. A rare case of cytomegalovirus, scedosporium apiospermum and mycobacterium tuberculosis in a renal transplant recipient. BMC Infect Dis. 2014;14:259.
- Hickey MD, Quan DJ, Chin-Hong PV, et al. Use of rifabutin for the treatment of a latent tuberculosis infection in a patient after solid organ transplantation. Liver Transpl. 2013;19:457-461.
- Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty-year prospective study. Int J Tuberc Lung Dis. 1999;3:494-500.
- Dekeyzer S, Moerman F, Callens S, et al. Cutaneous metastatic tuberculous abscess in patient with cervico-mediastinal lymphatic tuberculosis. Acta Clin Belg. 2013;68:34-36.
- Ko M, Wu C, Chiu H. Tuberculous gumma (cutaneous metastatic tuberculous abscess). Dermatol Sinica. 2005;23:27-31.
- Steger JW, Barrett TL. Cutaneous tuberculosis. In: James WD, ed. Textbook of Military Medicine: Military Dermatology. Washington, DC: Borden Institute; 1994:355-389.
- Santos JB, Figueiredo AR, Ferraz CE, et al. Cutaneous tuberculosis: diagnosis, histopathology and treatment - part II. An Bras Dermatol. 2014;89:545-555.
- Guidelines on the Management of Latent Tuberculosis Infection. Geneva, Switzerland: World Health Organization; 2015.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 pt 2):S221-S247.
- Mycobacterium tuberculosis. Am J Transplant. 2004;4(suppl 10):37-41.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284.
- Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
- Kant S, Verma SK, Gupta V, et al. Pyrazinamide induced thrombocytopenia. Indian J Pharmacol. 2010;42:108-109.
- Screening for tuberculosis and tuberculosis infection in high-risk populations. recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:19-34.
- Fischer SA, Avery RK; AST Infectious Disease Community of Practice. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant. 2009;9(suppl 4):S7-S18.
- Sakhuja V, Jha V, Varma PP, et al. The high incidence of tuberculosis among renal transplant recipients in India. Transplantation. 1996;61:211-215.
- Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
- Schultz V, Marroni CA, Amorim CS, et al. Risk factors for hepatotoxicity in solid organ transplants recipients being treated for tuberculosis. Transplant Proc. 2014;46:3606-3610.
- Tabarsi P, Farshidpour M, Marjani M, et al. Mycobacterial infection and the impact of rifabutin treatment in organ transplant recipients: a single-center study. Saudi J Kidney Dis Transpl. 2015;26:6-11.
- Rathi M, Gundlapalli S, Ramachandran R, et al. A rare case of cytomegalovirus, scedosporium apiospermum and mycobacterium tuberculosis in a renal transplant recipient. BMC Infect Dis. 2014;14:259.
- Hickey MD, Quan DJ, Chin-Hong PV, et al. Use of rifabutin for the treatment of a latent tuberculosis infection in a patient after solid organ transplantation. Liver Transpl. 2013;19:457-461.
- Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty-year prospective study. Int J Tuberc Lung Dis. 1999;3:494-500.
- Dekeyzer S, Moerman F, Callens S, et al. Cutaneous metastatic tuberculous abscess in patient with cervico-mediastinal lymphatic tuberculosis. Acta Clin Belg. 2013;68:34-36.
- Ko M, Wu C, Chiu H. Tuberculous gumma (cutaneous metastatic tuberculous abscess). Dermatol Sinica. 2005;23:27-31.
- Steger JW, Barrett TL. Cutaneous tuberculosis. In: James WD, ed. Textbook of Military Medicine: Military Dermatology. Washington, DC: Borden Institute; 1994:355-389.
- Santos JB, Figueiredo AR, Ferraz CE, et al. Cutaneous tuberculosis: diagnosis, histopathology and treatment - part II. An Bras Dermatol. 2014;89:545-555.
- Guidelines on the Management of Latent Tuberculosis Infection. Geneva, Switzerland: World Health Organization; 2015.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 pt 2):S221-S247.
- Mycobacterium tuberculosis. Am J Transplant. 2004;4(suppl 10):37-41.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284.
- Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
- Kant S, Verma SK, Gupta V, et al. Pyrazinamide induced thrombocytopenia. Indian J Pharmacol. 2010;42:108-109.
- Screening for tuberculosis and tuberculosis infection in high-risk populations. recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:19-34.
- Fischer SA, Avery RK; AST Infectious Disease Community of Practice. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant. 2009;9(suppl 4):S7-S18.
Practice Points
- Transplant patients are at increased risk for infection given their immunosuppressed state.
- Although rare, cutaneous tuberculosis should be considered in the differential for cutaneous lesions in an immunosuppressed patient.