User login
CHICAGO – The number of skin lesions biopsied to rule out melanoma remains exceedingly high among U.S. dermatologists, compared with the MelaFind digital skin analysis device.
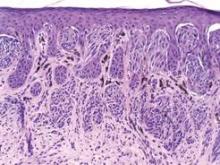
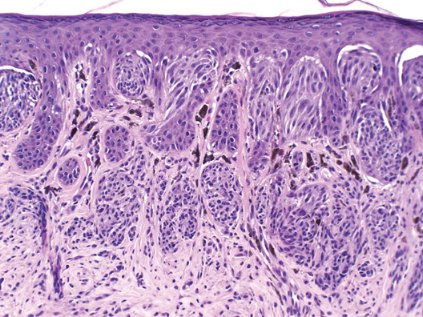
Dermatologists performed approximately 22 biopsies of histologically benign lesions for every one melanoma or severely dysplastic nevus removed, according to a dermatopathology review of 1,100 lesions from patients in approximately 500 practices nationwide.
This compares with a biopsy ratio of 7.6:1 reported in a pivotal prospective study of the MelaFind device (Arch. Dermatol. 2011;147:188-94), Dr. Clay J. Cockerell reported at the American Academy of Dermatology summer meeting.
The noninvasive MelaFind device was designed to aid dermatologists in diagnosing melanoma and uses multispectral light to characterize the morphologic disorganization of clinically atypical pigmented lesions.
It is approved in the European Union and gained U.S. approval in November 2011, with a long list of indications and caveats regarding how the device should be used and by whom.
The 22:1 biopsy rate among dermatologists is "pretty high" and likely reflects several factors, according to past AAD president Dr. Cockerell, who is director of the division of dermatopathology at the University of Texas Southwestern Medical Center and in group practice in Dallas.
Overall, clinicians are conservative regarding pigmented lesions, particularly in litigious areas of the country, and the device may have made some clinicians "more of a student of pigmented lesions, pushing them to look more carefully, and heightening awareness," he said in an interview.
Biopsies also may have been conducted by physician assistants and nurse practitioners, who may have a lower threshold for biopsy than dermatologists.
Lesion data were collected at Dr. Cockerell’s facility over a 3-week period for 1,400 lesions, but information was not available on how many practices had a MelaFind device, who performed the biopsy, the level of that clinician’s expertise in pigmented lesion management, or the proportion of high-risk patients, a group for whom biopsy ratios can reach 33:1 to 53:1, he said.
A total of 300 lesions were excluded from analysis because they were ineligible for use with the MelaFind device based on its indications.
Still, the MelaFind biopsy ratio is better than that observed in the real-world and was seen in lesions that were more clinically difficult, Dr. Cockerell reported in a poster.
The proportion of lesions that were melanoma or high-grade dysplastic nevi was higher in the pivotal study than in the real world data (11% vs. 4%), as was the proportion of nevi that were low-grade dysplastic nevi (79.3% vs. 41%).
Invasive melanomas, however, were thicker in the real life review (1.1 vs. 0.36 mm), raising a red flag that patients are still not fully educated on the need for early evaluation of suspicious lesions, he said.
"Biopsies, at least in our part of the country and I think in other parts as well, are performed in a seasonal fashion," Dr. Cockerell said. "There are people who don’t go to the dermatologist and don’t get a skin biopsy done for the first 5-6 months of the year because they’re waiting to get their deductible met. If they had a melanoma and put off a biopsy until June, it’s had 6 months of growth time. The doubling time can be very rapid."
Although the MelaFind-computed scores are subjective and do not state whether a biopsy is warranted, the data would be useful if sent along with a specimen to aid dermatopathologists in rendering a more definitive diagnosis, he said. Optimally, the device is "handcrafted" for practices and clinics that follow patients with hundreds of dysplastic nevi where repeated biopsies are not practical, an avenue Dr. Cockerell said he hopes to pursue in the future.
Mela Sciences sponsored the study. Dr. Cockerell and his coauthors disclosed no conflicting financial interests.
CHICAGO – The number of skin lesions biopsied to rule out melanoma remains exceedingly high among U.S. dermatologists, compared with the MelaFind digital skin analysis device.
Dermatologists performed approximately 22 biopsies of histologically benign lesions for every one melanoma or severely dysplastic nevus removed, according to a dermatopathology review of 1,100 lesions from patients in approximately 500 practices nationwide.
This compares with a biopsy ratio of 7.6:1 reported in a pivotal prospective study of the MelaFind device (Arch. Dermatol. 2011;147:188-94), Dr. Clay J. Cockerell reported at the American Academy of Dermatology summer meeting.
The noninvasive MelaFind device was designed to aid dermatologists in diagnosing melanoma and uses multispectral light to characterize the morphologic disorganization of clinically atypical pigmented lesions.
It is approved in the European Union and gained U.S. approval in November 2011, with a long list of indications and caveats regarding how the device should be used and by whom.
The 22:1 biopsy rate among dermatologists is "pretty high" and likely reflects several factors, according to past AAD president Dr. Cockerell, who is director of the division of dermatopathology at the University of Texas Southwestern Medical Center and in group practice in Dallas.
Overall, clinicians are conservative regarding pigmented lesions, particularly in litigious areas of the country, and the device may have made some clinicians "more of a student of pigmented lesions, pushing them to look more carefully, and heightening awareness," he said in an interview.
Biopsies also may have been conducted by physician assistants and nurse practitioners, who may have a lower threshold for biopsy than dermatologists.
Lesion data were collected at Dr. Cockerell’s facility over a 3-week period for 1,400 lesions, but information was not available on how many practices had a MelaFind device, who performed the biopsy, the level of that clinician’s expertise in pigmented lesion management, or the proportion of high-risk patients, a group for whom biopsy ratios can reach 33:1 to 53:1, he said.
A total of 300 lesions were excluded from analysis because they were ineligible for use with the MelaFind device based on its indications.
Still, the MelaFind biopsy ratio is better than that observed in the real-world and was seen in lesions that were more clinically difficult, Dr. Cockerell reported in a poster.
The proportion of lesions that were melanoma or high-grade dysplastic nevi was higher in the pivotal study than in the real world data (11% vs. 4%), as was the proportion of nevi that were low-grade dysplastic nevi (79.3% vs. 41%).
Invasive melanomas, however, were thicker in the real life review (1.1 vs. 0.36 mm), raising a red flag that patients are still not fully educated on the need for early evaluation of suspicious lesions, he said.
"Biopsies, at least in our part of the country and I think in other parts as well, are performed in a seasonal fashion," Dr. Cockerell said. "There are people who don’t go to the dermatologist and don’t get a skin biopsy done for the first 5-6 months of the year because they’re waiting to get their deductible met. If they had a melanoma and put off a biopsy until June, it’s had 6 months of growth time. The doubling time can be very rapid."
Although the MelaFind-computed scores are subjective and do not state whether a biopsy is warranted, the data would be useful if sent along with a specimen to aid dermatopathologists in rendering a more definitive diagnosis, he said. Optimally, the device is "handcrafted" for practices and clinics that follow patients with hundreds of dysplastic nevi where repeated biopsies are not practical, an avenue Dr. Cockerell said he hopes to pursue in the future.
Mela Sciences sponsored the study. Dr. Cockerell and his coauthors disclosed no conflicting financial interests.
CHICAGO – The number of skin lesions biopsied to rule out melanoma remains exceedingly high among U.S. dermatologists, compared with the MelaFind digital skin analysis device.
Dermatologists performed approximately 22 biopsies of histologically benign lesions for every one melanoma or severely dysplastic nevus removed, according to a dermatopathology review of 1,100 lesions from patients in approximately 500 practices nationwide.
This compares with a biopsy ratio of 7.6:1 reported in a pivotal prospective study of the MelaFind device (Arch. Dermatol. 2011;147:188-94), Dr. Clay J. Cockerell reported at the American Academy of Dermatology summer meeting.
The noninvasive MelaFind device was designed to aid dermatologists in diagnosing melanoma and uses multispectral light to characterize the morphologic disorganization of clinically atypical pigmented lesions.
It is approved in the European Union and gained U.S. approval in November 2011, with a long list of indications and caveats regarding how the device should be used and by whom.
The 22:1 biopsy rate among dermatologists is "pretty high" and likely reflects several factors, according to past AAD president Dr. Cockerell, who is director of the division of dermatopathology at the University of Texas Southwestern Medical Center and in group practice in Dallas.
Overall, clinicians are conservative regarding pigmented lesions, particularly in litigious areas of the country, and the device may have made some clinicians "more of a student of pigmented lesions, pushing them to look more carefully, and heightening awareness," he said in an interview.
Biopsies also may have been conducted by physician assistants and nurse practitioners, who may have a lower threshold for biopsy than dermatologists.
Lesion data were collected at Dr. Cockerell’s facility over a 3-week period for 1,400 lesions, but information was not available on how many practices had a MelaFind device, who performed the biopsy, the level of that clinician’s expertise in pigmented lesion management, or the proportion of high-risk patients, a group for whom biopsy ratios can reach 33:1 to 53:1, he said.
A total of 300 lesions were excluded from analysis because they were ineligible for use with the MelaFind device based on its indications.
Still, the MelaFind biopsy ratio is better than that observed in the real-world and was seen in lesions that were more clinically difficult, Dr. Cockerell reported in a poster.
The proportion of lesions that were melanoma or high-grade dysplastic nevi was higher in the pivotal study than in the real world data (11% vs. 4%), as was the proportion of nevi that were low-grade dysplastic nevi (79.3% vs. 41%).
Invasive melanomas, however, were thicker in the real life review (1.1 vs. 0.36 mm), raising a red flag that patients are still not fully educated on the need for early evaluation of suspicious lesions, he said.
"Biopsies, at least in our part of the country and I think in other parts as well, are performed in a seasonal fashion," Dr. Cockerell said. "There are people who don’t go to the dermatologist and don’t get a skin biopsy done for the first 5-6 months of the year because they’re waiting to get their deductible met. If they had a melanoma and put off a biopsy until June, it’s had 6 months of growth time. The doubling time can be very rapid."
Although the MelaFind-computed scores are subjective and do not state whether a biopsy is warranted, the data would be useful if sent along with a specimen to aid dermatopathologists in rendering a more definitive diagnosis, he said. Optimally, the device is "handcrafted" for practices and clinics that follow patients with hundreds of dysplastic nevi where repeated biopsies are not practical, an avenue Dr. Cockerell said he hopes to pursue in the future.
Mela Sciences sponsored the study. Dr. Cockerell and his coauthors disclosed no conflicting financial interests.
AT THE AAD SUMMER ACADEMY 2014
Key clinical point: The MelaFind device may be helpful to dermatologists when deciding which clinically ambiguous lesions may be early melanoma.
Major finding: The biopsy rate for melanoma or severely dysplastic nevi was 22:1 among clinicians in the review and 7.6:1 with the MelaFind in a prior prospective study.
Data source: A retrospective analysis of 1,100 biopsied skin lesions from patients in 500 U.S. practices.
Disclosures: Mela Sciences sponsored the study. Dr. Cockerell and his coauthors disclosed no conflicting financial interests.