User login
IN THIS ARTICLE
- Presenting symptoms
- Diagnostic tests
- Differential diagnostic criteria
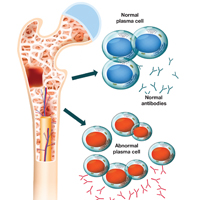
Multiple myeloma (MM) is a fatal, malignant neoplasm that originates in the plasma cells of bone marrow. A genetic mutation in the plasma cells creates myeloma cells, which replicate and produce monoclonal protein (M-protein). This accumulation of cells and abnormal protein can result in destruction and eventual marrow failure.1,2
MM’s insidious nature means it often goes undetected or misdiagnosed in its early stages; this delayed diagnosis can cause sequelae that limit quality of life. Furthermore, the five-year survival rate for myeloma varies by stage at which the disease is diagnosed: from 48% for distant (metastasized) myeloma to 71% for localized disease.3 It has also been noted that, in the past two decades, improvements in available treatment options and supportive care have contributed to a doubling of median survival time (from three years to six years).4 It is therefore paramount that providers be aware of MM and its signs to facilitate early diagnosis and treatment.
INCIDENCE AND EPIDEMIOLOGY
MM accounts for 1% of all cancers and about 10% of all hematologic malignancies.5 In 2017, the American Cancer Society estimated that more than 30,000 new cases of MM would be diagnosed in the United States.6 Additionally, MM was expected to cause more than 12,000 deaths last year.6
Median age at diagnosis is 69.3 In fact, 75% of men are older than 75 and 79% of women are older than 70 at diagnosis.1
Apart from age, other risk factors for MM have been identified but not fully explicated. For example, the disease is more common in men than in women (with men comprising two-thirds of new cases per year).3 MM is also two to three times more common in black than in white persons, making it the most common hematologic malignancy in this demographic group.3,7
The possibility of a genetic predisposition has also been studied. Several analyses have indicated an increased risk for MM in patients with a family history of the disease—as much as four times higher in those with an affected first-degree relative. This risk was further elevated in black compared with white patients (odds ratios, 17.4 and 1.5, respectively).7 However, many patients with MM have no relatives with this disorder.6,8
DISEASE PROGRESSION
Almost all patients who develop MM also experience an asymptomatic premalignant stage called monoclonal gammopathy of undetermined significance (MGUS). MGUS is present in 3% to 4% of the general population older than 50 and is often an incidental finding. This stage almost always precedes MM—but because it is asymptomatic, only 10% of individuals diagnosed with MM have a known history of MGUS.8
In some patients, an asymptomatic intermediate stage called smoldering multiple myeloma (SMM) can be identified. SMM progresses to MM at a rate of 10% per year for the first five years; the rate decreases to 3% per year over the following five years, and 1% per year after that.8
MM is not curable, but as noted, the survival rate is steadily increasing due to rapidly evolving treatment regimens. Discussion of treatment is outside the scope of this article, but early diagnosis can improve quality of life and clinical outcomes and prolong life expectancy.
SYMPTOMS
The initial symptoms of MM can be nonspecific and may lead the provider to suspect a host of other conditions.2,6 (Those for advanced disease are also vague but tend to be more pronounced.) These may include fatigue, weakness, easy bruising or bleeding, and bone pain. Other common clinical manifestations of MM are anemia, chronic infection, bone disease, and/or renal failure.1,4 Patients may also experience loss of appetite, nausea, vomiting, increased thirst, and increased urination.9
Recent studies have shown that patients with SMM and/or MGUS also exhibit early signs of bone disease and increased risk for fracture.10 Eighty percent of patients who progress to MM have evidence of pathologic bone fractures.10 It is also possible for bones in the spine to weaken and collapse, pressing on the spinal nerves. This is known as spinal cord compression, which can manifest with sudden, severe back pain or numbness and/or muscle weakness (most often in the legs).6
MM must be included in the differential diagnosis, particularly when symptoms do not point to one specific disease process. Without early diagnosis, disease progression can result in complications such as bone fracture and osteoporosis, reduced kidney function, peripheral neuropathy, chronic anemia, and ultimately, death.2,6 The presence of bone fractures increases mortality risk by 20%.10
DIAGNOSTIC WORKUP
Evidence of MM may be discovered during routine bloodwork and screening tests, while presenting symptoms or subtle changes in lab results can raise suspicion for the disease. Initial bloodwork abnormalities include anemia, elevated calcium levels, renal insufficiency, and/or elevated protein levels.8
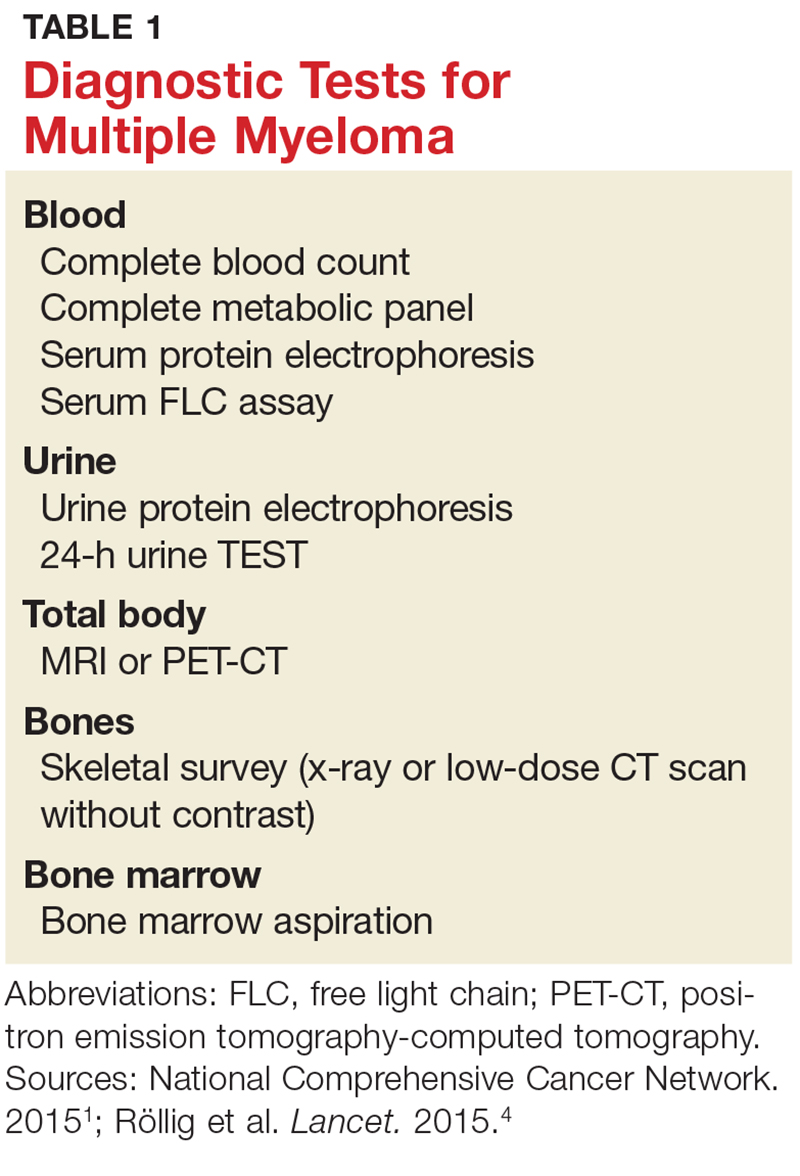
A combination of abnormalities in the complete blood count (CBC) and complete metabolic panel (CMP), along with symptoms, should alert the provider to the possibility of MM, prompting additional workup. Table 1 outlines suggested diagnostic tests; the possible findings are discussed below.
CBC. The CBC may reveal abnormalities including anemia (which occurs in 75% of patients with MM), thrombocytopenia, and leukopenia.1,8 These findings can contribute to fatigue, increased incidence of infection, and abnormal bruising of the skin.2,8
CMP. A CMP may show increases in serum calcium or protein. Hypercalcemia occurs in 15% of patients with MM, leading to symptoms such as loss of appetite, nausea, vomiting, increased urination, weakness, and confusion.8 An increase in protein may alter the albumin/globulin ratio, which should raise suspicion for MM. A decrease in albumin can signify disease severity. Also, the CMP may show worsening renal function and elevated serum creatinine, which occurs in 20% of patients with MM.8
Serum protein electrophoresis (SPEP). Suspicion of MM should prompt the clinician to evaluate proteins via SPEP. This test may be indicated for patients with anemia, hypercalcemia, bone pain, and unexplained neuropathy.9 The electrophoresis separates proteins based on their physical properties. This identifies the presence and amount of M-protein, which can determine the extent of the disease.1 M-protein is identified in approximately 82% of patients with MM using this test.8
Serum free light chain (FLC) assay. This diagnostic test can identify MM in individuals with high clinical suspicion for the disease but no discernible M-protein on SPEP; it increases sensitivity to 97%.8 The serum FLC assay evaluates for presence and ratio of free light chains—proteins produced by plasma cells. This test is also useful for monitoring treatment response and disease progression.1
Urine protein electrophoresis (UPEP). The UPEP separates proteins according to charge, which is helpful for classifying renal injury. Protein patterns are interpreted and may be reported as glomerular, tubular, or mixed. UPEP also tests for M-protein in the urine.1,11
24-hour urine. The 24-h urine test quantifies the amount and type of protein excreted in the urine and helps determine the extent of kidney disease.1
Skeletal survey. MM causes significant bone changes that can be identified with radiographic studies. The most common locations for fractures are the vertebral, pelvic, and clavicular areas.10 Currently, the skeletal survey is the gold standard for detecting fractures and osteolytic lesions associated with MM.10 Radiographic films ordered for other purposes may uncover abnormalities in bones.
Bone mineral density (BMD) test. Most often, BMD testing is used to evaluate treatment and progression of bone involvement. Because it can uncover osteopenia or osteoporosis, however, it can also be used to corroborate the diagnosis of MM.10
Once the presence of M-protein is identified, patients are referred for specialty care. At that time, further workup will include a bone marrow biopsy and imaging studies, such as additional radiographic films, CT scans (without contrast, as contrast dye can damage frail kidneys), and MRI.1,8 These diagnostic tests provide useful information for the classification of the disease and guide initiation of treatment.
CLASSIFICATION OF DISEASE
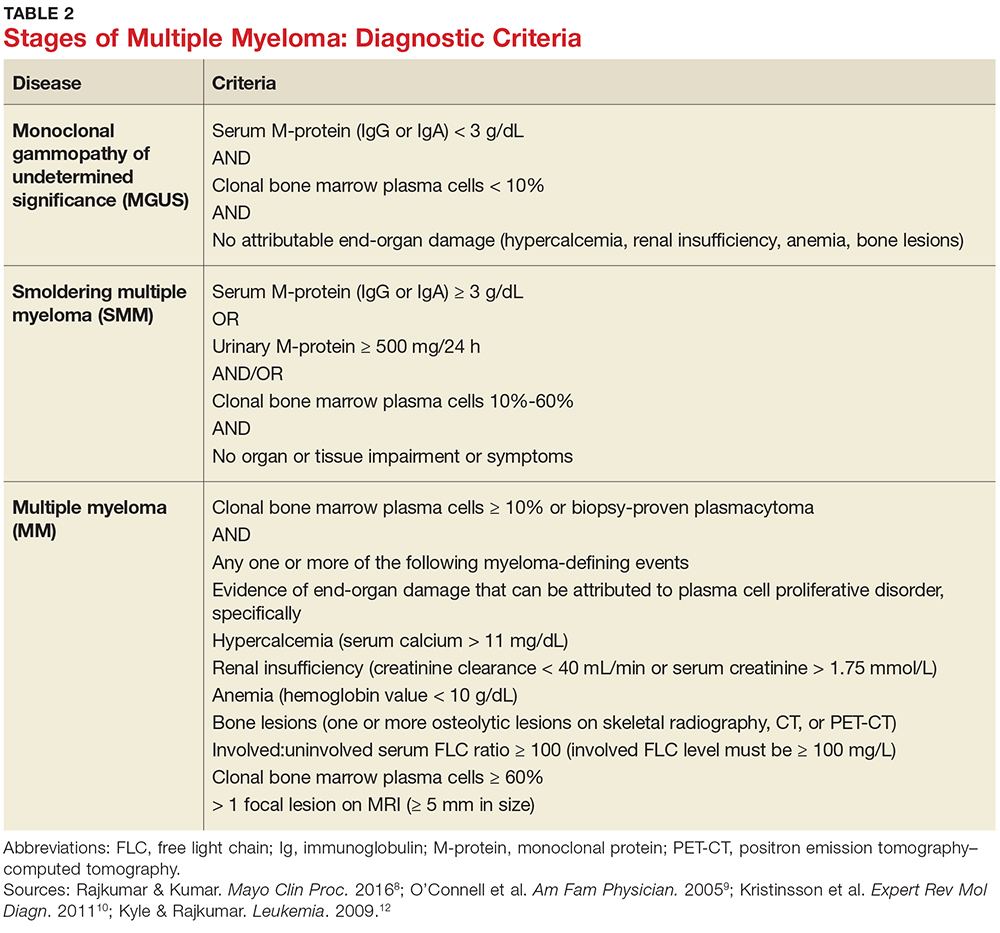
MM can be classified into three stages—MGUS, SMM, and MM—based on recommendations from the International Myeloma Working Group.12 Table 2 outlines the diagnostic criteria for each stage.
Individuals with MGUS and SMM are considered asymptomatic; guidelines do not recommend treatment for these patients. Those who are diagnosed with MM are referred to oncologists and treated based on current clinical practice guidelines.1
CONCLUSION
Multiple myeloma is a malignant neoplasm without a cure. Presenting symptoms may include anemia, bone pain, elevated creatinine or serum protein, fatigue, and hypercalcemia. Early diagnosis is key to early intervention and treatment, which can improve quality of life and clinical outcomes for those affected. Primary care providers play a major role in recognizing the subtle symptoms and ordering the appropriate diagnostic tests.
1. National Comprehensive Cancer Network. Multiple myeloma. NCCN clinical practice guidelines in oncology version 2.2015.
2. Rajkumar VS. Multiple myeloma symptoms, diagnosis, and staging. www.uptodate.com/contents/clinical-features-laboratory-manifestations-and-diagnosis-of-multiple-myeloma?source=machineLearning&search=multiple+myeloma&selectedTitle=1%7E150§ionRank=1&anchor=H25#H26. Accessed October 16, 2017.
3. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed October 26, 2017.
4. Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385(9983):2197-2208.
5. Moreau P, San Miguel J, Sonneveld M, et al. Multiple myeloma: ESMO clinical practice guidelines. Ann Oncol. 2017;28(4):iv52-iv61.
6. American Cancer Society. Multiple myeloma. www.cancer.org/cancer/multiplemyeloma/detailedguide. Accessed October 16, 2017.
7. Koura DT, Langston AA. Inherited predisposition to multiple myeloma. Ther Adv Hematol. 2013;4(4):291-297.
8. Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101-119.
9. O’Connell T, Horita TJ, Kasravi B. Understanding and interpreting serum electrophoresis. Am Fam Physician. 2005; 71(1):105-112.
10. Kristinsson SY, Minter AR, Korde N, et al. Bone disease in multiple myeloma and precursor disease; novel diagnostic approaches and implications on clinical management. Expert Rev Mol Diagn. 2011;11(6):593-603.
11. Jacobs D, DeMott W, Oxley D. Laboratory Test Handbook: Concise With Disease Index. Hudson, OH: Lexi-Comp; 2004.
12. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3-9.
IN THIS ARTICLE
- Presenting symptoms
- Diagnostic tests
- Differential diagnostic criteria
Multiple myeloma (MM) is a fatal, malignant neoplasm that originates in the plasma cells of bone marrow. A genetic mutation in the plasma cells creates myeloma cells, which replicate and produce monoclonal protein (M-protein). This accumulation of cells and abnormal protein can result in destruction and eventual marrow failure.1,2
MM’s insidious nature means it often goes undetected or misdiagnosed in its early stages; this delayed diagnosis can cause sequelae that limit quality of life. Furthermore, the five-year survival rate for myeloma varies by stage at which the disease is diagnosed: from 48% for distant (metastasized) myeloma to 71% for localized disease.3 It has also been noted that, in the past two decades, improvements in available treatment options and supportive care have contributed to a doubling of median survival time (from three years to six years).4 It is therefore paramount that providers be aware of MM and its signs to facilitate early diagnosis and treatment.
INCIDENCE AND EPIDEMIOLOGY
MM accounts for 1% of all cancers and about 10% of all hematologic malignancies.5 In 2017, the American Cancer Society estimated that more than 30,000 new cases of MM would be diagnosed in the United States.6 Additionally, MM was expected to cause more than 12,000 deaths last year.6
Median age at diagnosis is 69.3 In fact, 75% of men are older than 75 and 79% of women are older than 70 at diagnosis.1
Apart from age, other risk factors for MM have been identified but not fully explicated. For example, the disease is more common in men than in women (with men comprising two-thirds of new cases per year).3 MM is also two to three times more common in black than in white persons, making it the most common hematologic malignancy in this demographic group.3,7
The possibility of a genetic predisposition has also been studied. Several analyses have indicated an increased risk for MM in patients with a family history of the disease—as much as four times higher in those with an affected first-degree relative. This risk was further elevated in black compared with white patients (odds ratios, 17.4 and 1.5, respectively).7 However, many patients with MM have no relatives with this disorder.6,8
DISEASE PROGRESSION
Almost all patients who develop MM also experience an asymptomatic premalignant stage called monoclonal gammopathy of undetermined significance (MGUS). MGUS is present in 3% to 4% of the general population older than 50 and is often an incidental finding. This stage almost always precedes MM—but because it is asymptomatic, only 10% of individuals diagnosed with MM have a known history of MGUS.8
In some patients, an asymptomatic intermediate stage called smoldering multiple myeloma (SMM) can be identified. SMM progresses to MM at a rate of 10% per year for the first five years; the rate decreases to 3% per year over the following five years, and 1% per year after that.8
MM is not curable, but as noted, the survival rate is steadily increasing due to rapidly evolving treatment regimens. Discussion of treatment is outside the scope of this article, but early diagnosis can improve quality of life and clinical outcomes and prolong life expectancy.

SYMPTOMS
The initial symptoms of MM can be nonspecific and may lead the provider to suspect a host of other conditions.2,6 (Those for advanced disease are also vague but tend to be more pronounced.) These may include fatigue, weakness, easy bruising or bleeding, and bone pain. Other common clinical manifestations of MM are anemia, chronic infection, bone disease, and/or renal failure.1,4 Patients may also experience loss of appetite, nausea, vomiting, increased thirst, and increased urination.9
Recent studies have shown that patients with SMM and/or MGUS also exhibit early signs of bone disease and increased risk for fracture.10 Eighty percent of patients who progress to MM have evidence of pathologic bone fractures.10 It is also possible for bones in the spine to weaken and collapse, pressing on the spinal nerves. This is known as spinal cord compression, which can manifest with sudden, severe back pain or numbness and/or muscle weakness (most often in the legs).6
MM must be included in the differential diagnosis, particularly when symptoms do not point to one specific disease process. Without early diagnosis, disease progression can result in complications such as bone fracture and osteoporosis, reduced kidney function, peripheral neuropathy, chronic anemia, and ultimately, death.2,6 The presence of bone fractures increases mortality risk by 20%.10
DIAGNOSTIC WORKUP
Evidence of MM may be discovered during routine bloodwork and screening tests, while presenting symptoms or subtle changes in lab results can raise suspicion for the disease. Initial bloodwork abnormalities include anemia, elevated calcium levels, renal insufficiency, and/or elevated protein levels.8

A combination of abnormalities in the complete blood count (CBC) and complete metabolic panel (CMP), along with symptoms, should alert the provider to the possibility of MM, prompting additional workup. Table 1 outlines suggested diagnostic tests; the possible findings are discussed below.
CBC. The CBC may reveal abnormalities including anemia (which occurs in 75% of patients with MM), thrombocytopenia, and leukopenia.1,8 These findings can contribute to fatigue, increased incidence of infection, and abnormal bruising of the skin.2,8
CMP. A CMP may show increases in serum calcium or protein. Hypercalcemia occurs in 15% of patients with MM, leading to symptoms such as loss of appetite, nausea, vomiting, increased urination, weakness, and confusion.8 An increase in protein may alter the albumin/globulin ratio, which should raise suspicion for MM. A decrease in albumin can signify disease severity. Also, the CMP may show worsening renal function and elevated serum creatinine, which occurs in 20% of patients with MM.8
Serum protein electrophoresis (SPEP). Suspicion of MM should prompt the clinician to evaluate proteins via SPEP. This test may be indicated for patients with anemia, hypercalcemia, bone pain, and unexplained neuropathy.9 The electrophoresis separates proteins based on their physical properties. This identifies the presence and amount of M-protein, which can determine the extent of the disease.1 M-protein is identified in approximately 82% of patients with MM using this test.8
Serum free light chain (FLC) assay. This diagnostic test can identify MM in individuals with high clinical suspicion for the disease but no discernible M-protein on SPEP; it increases sensitivity to 97%.8 The serum FLC assay evaluates for presence and ratio of free light chains—proteins produced by plasma cells. This test is also useful for monitoring treatment response and disease progression.1
Urine protein electrophoresis (UPEP). The UPEP separates proteins according to charge, which is helpful for classifying renal injury. Protein patterns are interpreted and may be reported as glomerular, tubular, or mixed. UPEP also tests for M-protein in the urine.1,11
24-hour urine. The 24-h urine test quantifies the amount and type of protein excreted in the urine and helps determine the extent of kidney disease.1
Skeletal survey. MM causes significant bone changes that can be identified with radiographic studies. The most common locations for fractures are the vertebral, pelvic, and clavicular areas.10 Currently, the skeletal survey is the gold standard for detecting fractures and osteolytic lesions associated with MM.10 Radiographic films ordered for other purposes may uncover abnormalities in bones.
Bone mineral density (BMD) test. Most often, BMD testing is used to evaluate treatment and progression of bone involvement. Because it can uncover osteopenia or osteoporosis, however, it can also be used to corroborate the diagnosis of MM.10
Once the presence of M-protein is identified, patients are referred for specialty care. At that time, further workup will include a bone marrow biopsy and imaging studies, such as additional radiographic films, CT scans (without contrast, as contrast dye can damage frail kidneys), and MRI.1,8 These diagnostic tests provide useful information for the classification of the disease and guide initiation of treatment.
CLASSIFICATION OF DISEASE
MM can be classified into three stages—MGUS, SMM, and MM—based on recommendations from the International Myeloma Working Group.12 Table 2 outlines the diagnostic criteria for each stage.

Individuals with MGUS and SMM are considered asymptomatic; guidelines do not recommend treatment for these patients. Those who are diagnosed with MM are referred to oncologists and treated based on current clinical practice guidelines.1
CONCLUSION
Multiple myeloma is a malignant neoplasm without a cure. Presenting symptoms may include anemia, bone pain, elevated creatinine or serum protein, fatigue, and hypercalcemia. Early diagnosis is key to early intervention and treatment, which can improve quality of life and clinical outcomes for those affected. Primary care providers play a major role in recognizing the subtle symptoms and ordering the appropriate diagnostic tests.
IN THIS ARTICLE
- Presenting symptoms
- Diagnostic tests
- Differential diagnostic criteria
Multiple myeloma (MM) is a fatal, malignant neoplasm that originates in the plasma cells of bone marrow. A genetic mutation in the plasma cells creates myeloma cells, which replicate and produce monoclonal protein (M-protein). This accumulation of cells and abnormal protein can result in destruction and eventual marrow failure.1,2
MM’s insidious nature means it often goes undetected or misdiagnosed in its early stages; this delayed diagnosis can cause sequelae that limit quality of life. Furthermore, the five-year survival rate for myeloma varies by stage at which the disease is diagnosed: from 48% for distant (metastasized) myeloma to 71% for localized disease.3 It has also been noted that, in the past two decades, improvements in available treatment options and supportive care have contributed to a doubling of median survival time (from three years to six years).4 It is therefore paramount that providers be aware of MM and its signs to facilitate early diagnosis and treatment.
INCIDENCE AND EPIDEMIOLOGY
MM accounts for 1% of all cancers and about 10% of all hematologic malignancies.5 In 2017, the American Cancer Society estimated that more than 30,000 new cases of MM would be diagnosed in the United States.6 Additionally, MM was expected to cause more than 12,000 deaths last year.6
Median age at diagnosis is 69.3 In fact, 75% of men are older than 75 and 79% of women are older than 70 at diagnosis.1
Apart from age, other risk factors for MM have been identified but not fully explicated. For example, the disease is more common in men than in women (with men comprising two-thirds of new cases per year).3 MM is also two to three times more common in black than in white persons, making it the most common hematologic malignancy in this demographic group.3,7
The possibility of a genetic predisposition has also been studied. Several analyses have indicated an increased risk for MM in patients with a family history of the disease—as much as four times higher in those with an affected first-degree relative. This risk was further elevated in black compared with white patients (odds ratios, 17.4 and 1.5, respectively).7 However, many patients with MM have no relatives with this disorder.6,8
DISEASE PROGRESSION
Almost all patients who develop MM also experience an asymptomatic premalignant stage called monoclonal gammopathy of undetermined significance (MGUS). MGUS is present in 3% to 4% of the general population older than 50 and is often an incidental finding. This stage almost always precedes MM—but because it is asymptomatic, only 10% of individuals diagnosed with MM have a known history of MGUS.8
In some patients, an asymptomatic intermediate stage called smoldering multiple myeloma (SMM) can be identified. SMM progresses to MM at a rate of 10% per year for the first five years; the rate decreases to 3% per year over the following five years, and 1% per year after that.8
MM is not curable, but as noted, the survival rate is steadily increasing due to rapidly evolving treatment regimens. Discussion of treatment is outside the scope of this article, but early diagnosis can improve quality of life and clinical outcomes and prolong life expectancy.

SYMPTOMS
The initial symptoms of MM can be nonspecific and may lead the provider to suspect a host of other conditions.2,6 (Those for advanced disease are also vague but tend to be more pronounced.) These may include fatigue, weakness, easy bruising or bleeding, and bone pain. Other common clinical manifestations of MM are anemia, chronic infection, bone disease, and/or renal failure.1,4 Patients may also experience loss of appetite, nausea, vomiting, increased thirst, and increased urination.9
Recent studies have shown that patients with SMM and/or MGUS also exhibit early signs of bone disease and increased risk for fracture.10 Eighty percent of patients who progress to MM have evidence of pathologic bone fractures.10 It is also possible for bones in the spine to weaken and collapse, pressing on the spinal nerves. This is known as spinal cord compression, which can manifest with sudden, severe back pain or numbness and/or muscle weakness (most often in the legs).6
MM must be included in the differential diagnosis, particularly when symptoms do not point to one specific disease process. Without early diagnosis, disease progression can result in complications such as bone fracture and osteoporosis, reduced kidney function, peripheral neuropathy, chronic anemia, and ultimately, death.2,6 The presence of bone fractures increases mortality risk by 20%.10
DIAGNOSTIC WORKUP
Evidence of MM may be discovered during routine bloodwork and screening tests, while presenting symptoms or subtle changes in lab results can raise suspicion for the disease. Initial bloodwork abnormalities include anemia, elevated calcium levels, renal insufficiency, and/or elevated protein levels.8

A combination of abnormalities in the complete blood count (CBC) and complete metabolic panel (CMP), along with symptoms, should alert the provider to the possibility of MM, prompting additional workup. Table 1 outlines suggested diagnostic tests; the possible findings are discussed below.
CBC. The CBC may reveal abnormalities including anemia (which occurs in 75% of patients with MM), thrombocytopenia, and leukopenia.1,8 These findings can contribute to fatigue, increased incidence of infection, and abnormal bruising of the skin.2,8
CMP. A CMP may show increases in serum calcium or protein. Hypercalcemia occurs in 15% of patients with MM, leading to symptoms such as loss of appetite, nausea, vomiting, increased urination, weakness, and confusion.8 An increase in protein may alter the albumin/globulin ratio, which should raise suspicion for MM. A decrease in albumin can signify disease severity. Also, the CMP may show worsening renal function and elevated serum creatinine, which occurs in 20% of patients with MM.8
Serum protein electrophoresis (SPEP). Suspicion of MM should prompt the clinician to evaluate proteins via SPEP. This test may be indicated for patients with anemia, hypercalcemia, bone pain, and unexplained neuropathy.9 The electrophoresis separates proteins based on their physical properties. This identifies the presence and amount of M-protein, which can determine the extent of the disease.1 M-protein is identified in approximately 82% of patients with MM using this test.8
Serum free light chain (FLC) assay. This diagnostic test can identify MM in individuals with high clinical suspicion for the disease but no discernible M-protein on SPEP; it increases sensitivity to 97%.8 The serum FLC assay evaluates for presence and ratio of free light chains—proteins produced by plasma cells. This test is also useful for monitoring treatment response and disease progression.1
Urine protein electrophoresis (UPEP). The UPEP separates proteins according to charge, which is helpful for classifying renal injury. Protein patterns are interpreted and may be reported as glomerular, tubular, or mixed. UPEP also tests for M-protein in the urine.1,11
24-hour urine. The 24-h urine test quantifies the amount and type of protein excreted in the urine and helps determine the extent of kidney disease.1
Skeletal survey. MM causes significant bone changes that can be identified with radiographic studies. The most common locations for fractures are the vertebral, pelvic, and clavicular areas.10 Currently, the skeletal survey is the gold standard for detecting fractures and osteolytic lesions associated with MM.10 Radiographic films ordered for other purposes may uncover abnormalities in bones.
Bone mineral density (BMD) test. Most often, BMD testing is used to evaluate treatment and progression of bone involvement. Because it can uncover osteopenia or osteoporosis, however, it can also be used to corroborate the diagnosis of MM.10
Once the presence of M-protein is identified, patients are referred for specialty care. At that time, further workup will include a bone marrow biopsy and imaging studies, such as additional radiographic films, CT scans (without contrast, as contrast dye can damage frail kidneys), and MRI.1,8 These diagnostic tests provide useful information for the classification of the disease and guide initiation of treatment.
CLASSIFICATION OF DISEASE
MM can be classified into three stages—MGUS, SMM, and MM—based on recommendations from the International Myeloma Working Group.12 Table 2 outlines the diagnostic criteria for each stage.

Individuals with MGUS and SMM are considered asymptomatic; guidelines do not recommend treatment for these patients. Those who are diagnosed with MM are referred to oncologists and treated based on current clinical practice guidelines.1
CONCLUSION
Multiple myeloma is a malignant neoplasm without a cure. Presenting symptoms may include anemia, bone pain, elevated creatinine or serum protein, fatigue, and hypercalcemia. Early diagnosis is key to early intervention and treatment, which can improve quality of life and clinical outcomes for those affected. Primary care providers play a major role in recognizing the subtle symptoms and ordering the appropriate diagnostic tests.
1. National Comprehensive Cancer Network. Multiple myeloma. NCCN clinical practice guidelines in oncology version 2.2015.
2. Rajkumar VS. Multiple myeloma symptoms, diagnosis, and staging. www.uptodate.com/contents/clinical-features-laboratory-manifestations-and-diagnosis-of-multiple-myeloma?source=machineLearning&search=multiple+myeloma&selectedTitle=1%7E150§ionRank=1&anchor=H25#H26. Accessed October 16, 2017.
3. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed October 26, 2017.
4. Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385(9983):2197-2208.
5. Moreau P, San Miguel J, Sonneveld M, et al. Multiple myeloma: ESMO clinical practice guidelines. Ann Oncol. 2017;28(4):iv52-iv61.
6. American Cancer Society. Multiple myeloma. www.cancer.org/cancer/multiplemyeloma/detailedguide. Accessed October 16, 2017.
7. Koura DT, Langston AA. Inherited predisposition to multiple myeloma. Ther Adv Hematol. 2013;4(4):291-297.
8. Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101-119.
9. O’Connell T, Horita TJ, Kasravi B. Understanding and interpreting serum electrophoresis. Am Fam Physician. 2005; 71(1):105-112.
10. Kristinsson SY, Minter AR, Korde N, et al. Bone disease in multiple myeloma and precursor disease; novel diagnostic approaches and implications on clinical management. Expert Rev Mol Diagn. 2011;11(6):593-603.
11. Jacobs D, DeMott W, Oxley D. Laboratory Test Handbook: Concise With Disease Index. Hudson, OH: Lexi-Comp; 2004.
12. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3-9.
1. National Comprehensive Cancer Network. Multiple myeloma. NCCN clinical practice guidelines in oncology version 2.2015.
2. Rajkumar VS. Multiple myeloma symptoms, diagnosis, and staging. www.uptodate.com/contents/clinical-features-laboratory-manifestations-and-diagnosis-of-multiple-myeloma?source=machineLearning&search=multiple+myeloma&selectedTitle=1%7E150§ionRank=1&anchor=H25#H26. Accessed October 16, 2017.
3. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: myeloma. https://seer.cancer.gov/statfacts/html/mulmy.html. Accessed October 26, 2017.
4. Röllig C, Knop S, Bornhäuser M. Multiple myeloma. Lancet. 2015;385(9983):2197-2208.
5. Moreau P, San Miguel J, Sonneveld M, et al. Multiple myeloma: ESMO clinical practice guidelines. Ann Oncol. 2017;28(4):iv52-iv61.
6. American Cancer Society. Multiple myeloma. www.cancer.org/cancer/multiplemyeloma/detailedguide. Accessed October 16, 2017.
7. Koura DT, Langston AA. Inherited predisposition to multiple myeloma. Ther Adv Hematol. 2013;4(4):291-297.
8. Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101-119.
9. O’Connell T, Horita TJ, Kasravi B. Understanding and interpreting serum electrophoresis. Am Fam Physician. 2005; 71(1):105-112.
10. Kristinsson SY, Minter AR, Korde N, et al. Bone disease in multiple myeloma and precursor disease; novel diagnostic approaches and implications on clinical management. Expert Rev Mol Diagn. 2011;11(6):593-603.
11. Jacobs D, DeMott W, Oxley D. Laboratory Test Handbook: Concise With Disease Index. Hudson, OH: Lexi-Comp; 2004.
12. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3-9.