User login
One in seven women suffer with abnormal uterine bleeding during their reproductive years, according to Fraser et al. (Exp Rev Obstet Gynecol. 2009;4:179-89). Heavy menstrual bleeding (menorrhagia) is the most common pattern. Global endometrial ablation has become a very popular surgical technique for women complaining of menorrhagia, disinterested in either medical management or definitive therapy – hysterectomy – or where medical management has failed. With proper patient selection, endometrial ablation yields an 80%-90% success rate in reducing heavy menstrual flow and is associated with a 90% patient satisfaction rate (Cochrane Database Syst Rev. 2009 Oct 7;[4]:CD001501).
Literature is replete with conditions believed to increase risk of endometrial ablation failure. This list includes untreated uterine cornua, endometrial regrowth, the presence of submucous leiomyomas or polyps, abnormal uterine cavity, enlarged uterine cavity (width and/or length), endometrial ablation in a young patient, parity of five or greater, unsuspected adhesiolysis, postablation tubal sterilization syndrome, history of dysmenorrhea, smoking, obesity, prior cesarean section, previous gynecologic surgery, and procedure length. Interestingly, type of global endometrial ablation procedure or original bleeding pattern does not influence failure rate.
In this edition of the Master Class in Gynecologic Surgery, Dr. Morris Wortman discusses not only the prevention of endometrial ablation failure, but also how to treat the problem via conservative surgical management.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and is the director at the Center for Menstrual Disorders and Reproductive Choice, also in Rochester. Dr. Wortman has lectured extensively on endometrial ablation and has authored several scientific articles in peer reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported being a subinvestigator on a study sponsored by Channel Medsystems. Email him at [email protected].
Why failures occur and how to correct them
BY MORRIS WORTMAN, MD
Since the introduction almost 20 years ago of devices for nonresectoscopic – or “global” – endometrial ablation, the procedure has been widely adopted as the treatment of choice for abnormal uterine bleeding that is refractory to medical management.
Between 400,000 and 500,000 endometrial ablations are done in the United States every year in women who have completed childbearing, and it probably won’t be long before the procedure surpasses hysterectomy in prevalence for the management of abnormal bleeding.
In recent years, the literature has begun to address the incidence of these delayed complications and the requirement for subsequent hysterectomy. A 2007 practice bulletin issued by the American College of Obstetricians and Gynecologists stated that hysterectomy rates within 4 years of endometrial ablation are at least 24% (Obstet Gynecol. 2007 May;109[5]:1233-48). And a study published the following year reported that 26% of 3,681 women undergoing EA at Kaiser Permanente facilities in Northern California required hysterectomy within 8 years (Obstet Gynecol. 2008 Dec;112[6]:1214-20).
It appears that the vast majority of what we now refer to as late-onset EA failures – complications attributable to EA that occur beyond a perioperative period of 1 month – will occur within 5 years. Some EA failures have occurred over 5-10 years, however, and in my practice we have seen late-onset complications occurring 17 or more years after the initial ablation.
In our practice, we are successfully managing delayed complications after GEA using ultrasound-guided reoperative hysteroscopy to fully explore the uterine cavity and excise areas of endometrial growth and other disease. In 2014, we published a retrospective review of 50 women whom we treated for delayed complications after a variety of GEA techniques; almost 90% avoided hysterectomy during a mean follow-up period of 18 months (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:238-44).
Our experience since then has included reoperative surgery on more than 115 GEA failures. Additionally, we’ve managed 220 patients who have undergone various hysteroscopic and resectoscopic endometrial ablations, some of which date back to the use of the Nd:YAG laser in the late 1980s.
The fact that late-onset EA failures occur does not mean that hysterectomy should routinely be performed as a first-line treatment for intractable uterine bleeding. Overall, there is much more morbidity associated with hysterectomy than with EA.
What failures do suggest is that there are certain risk factors for late-onset EA complications. Our experience in treating women who have experienced late-onset EA failure has provided us with insight into who may be at greatest risk for late-onset EA failure and how patients can best be selected for the procedure. We’ve also learned more about the diagnosis of delayed complications.
Causes of EA failure
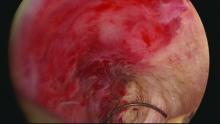
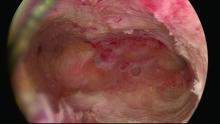
Untreated uterine cornua, and untreated submucous leiomyomas and endometrial polyps, are common causes of EA failure. Among the 50 women included in our retrospective review of ultrasound-guided reoperative hysteroscopy after GEA failure, 44% had intraoperative evidence of untreated cornua and nearly one-fourth had persistent or enlarging submucous leiomyomas.
Contrary to what some believe, most endometrial ablations will not adequately destroy submucous or intramural leiomyomas. Therefore, we recommend that these fibroids be entirely removed immediately before EA.
Moreover, GEA will not always provide adequate thermal destruction to the entire endometrial cavity. The cornua regions are particularly at risk; they are difficult to reach under ideal circumstances, and especially difficult to treat in patients who have a uterine septum or a T-shaped uterus (with the ostia and cornua deeply recessed). We have also seen late-onset EA failures in patients with an extended uterine transverse diameter. The limits of GEA are greatest when a device with a fixed configuration or geometry is used.
A history of abnormal hysteroscopy or other evidence of such anatomic distortions are therefore among the reported risk factors for GEA failure (J Minim Invasive Gynecol. 2015 Mar-Apr;22[3]:323-31). A history of tubal ligation also confers risk; the procedure further increases susceptibility for failure when functioning endometrial tissue remains or regrows at the cornua, because any retrograde menstrual bleeding that occurs will be constrained by the obstructed proximal portion of the fallopian tubes.
Obesity is another risk factor for GEA failure in that the condition increases the risk of endometrial cancer, making the need for reliable biopsies in the case of spotting or other signs or symptoms even more important. On the other hand, obesity may also worsen a patient’s status as a candidate for hysterectomy.
There is much to consider with these patients. For some obese patients, GEA may be less risky than hysterectomy while for others, such as those who also have polycystic ovarian syndrome (in whom the risk for developing endometrial cancer is further increased) the scale may tip in favor of hysterectomy.
Age at the time of the primary GEA may be the single most important risk factor for GEA failure and is an important predictor of success in patient selection. Numerous investigators have shown that women younger than 35 years of age at the time of their EA had a significantly increased risk for hysterectomy, compared with women who were at least 45 years old. The younger the patient, the longer the “bridge” to menopause and the greater the likelihood that bridge will fail.
While age is not necessarily a contraindication, it is worthy of serious consideration. We generally discourage GEA for patients younger than 35. We also advise ensuring that each patient undergoing initial EA is highly self-motivated to have a uterine-sparing procedure; if not, symptoms she may experience later will likely drive her toward hysterectomy anyway.
Additionally, we caution against performing GEA in patients who have chronic pelvic pain; these patients tend to have poorer outcomes with any type of hysteroscopic surgery.
Diagnosing failed EA
Delayed complications manifest in several ways: Renewed and increasing vaginal bleeding after a period of improvement, cyclic pelvic pain (unilateral, bilateral, or suprapubic), or both bleeding and pain. Some women – likely an underreported number of them – present with postmenopausal bleeding and proceed to have unsuccessful attempts at an endometrial biopsy due to EA-associated endometrial scarring.
The cyclic pelvic pain associated with endometrial persistence or regrowth tends to worsen over time and is often described as sharp or laborlike. In our experience, a description of “laborlike” pain and a history of EA is almost fully predictive of a finding of endometrial growth. Often a hematometra can be demonstrated on transvaginal ultrasound, but this isn’t always the case.
Pain typically precedes bleeding in patients who demonstrate both. In such cases, blood from functioning endometrial tissue or other sources becomes blocked from exiting the uterine cavity by EA-induced intrauterine scarring and contracture. Painful uterine contractions then aim to expel the pooled blood. In other cases of pain – mainly those without significant vaginal bleeding – the pain is often attributed to cornual and central hematometra.
For the majority of EA failures, the diagnosis lies in the history and current symptoms. Unfortunately, the traditional methods of assessing the endometrial cavity have little merit for women presenting with delayed-onset EA complications. A sonographically assisted pelvic examination can be useful in evaluating complications, but the interpretation of ultrasounds in women with a prior EA can be challenging and is often beyond the training of most radiologists and gynecologists.
It is not uncommon for images to be incorrectly interpreted in the emergency department or physicians’ offices as “normal” and for such readings to set off a chain of CT scans, MRIs, laparoscopies, ovarian cystectomies, and other procedures that miss the root causes of pain.
Unfortunately, there is little in the literature that describes and defines ultrasound findings after EA. We do know that sonography should be timed with episodes of pain, and that the absence of a demonstrable hematometra does not exclude a diagnosis of EA failure.
Correcting late-onset failures
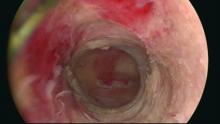
Our office-based operating room is fitted with side-by-side monitors that enable simultaneous sonographic and hysteroscopic views for correction of GEA failures; the rest of the set-up is similar to that of other operative hysteroscopies. However, we do employ a wide variety of resectoscopes with diameters ranging from 13 to 28 Fr. The smaller-diameter scopes are particularly useful for evaluating postmenopausal bleeding in women with a prior EA.
For those inexperienced with ultrasound-guided surgery, the initial resection is often the most challenging. The initial tissue removal is carried out on the thickest observed uterine wall – usually the posterior or anterior wall – and is done with near complete reliance on the ultrasound image. Hysteroscopic visualization is poor at this time because the outflow ports of the continuous flow resectoscope are obstructed by tissue in the narrow tubular cavity.
We then actually remove the resectoscope and clean the outflow ports of clots and debris that may have accumulated. When the scope is reinserted, there is typically sufficient room in the uterine cavity for continuous flow and excellent hysteroscopic visualization.
The sequence of resection from this point on will vary. If we’ve begun on the anterior wall, we’ll move to the posterior and then the two lateral walls to further restore the cavity. Areas of endometrial regrowth will typically be identified at this point and resected. The dissection then will extend upward, usually to within 10 mm of the fundus in the midline as measured by ultrasound. Reconfiguring the loop electrode to a 135- to 160-degree angle can be helpful in the delicate dissection that is required at the fundus.
Once all areas of endometrium have been identified and excised, we will deeply coagulate exposed myometrium with a ball-end electrode. Rarely, we will reach our maximum allowable fluid absorption limit prior to completing the case, a scenario seen in less than 1% of our patients.
In more than 330 reoperative hysteroscopic procedures, we’ve had only one uterine perforation that occurred when we switched ultrasound machines. Very likely, we were too aggressive in removing tissue at the fundus. The patient required a diagnostic laparoscopy but sustained no visceral injury.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and the director of the Center for Menstrual Disorders and Reproductive Choice in Rochester. He reported having no relevant financial disclosures.
One in seven women suffer with abnormal uterine bleeding during their reproductive years, according to Fraser et al. (Exp Rev Obstet Gynecol. 2009;4:179-89). Heavy menstrual bleeding (menorrhagia) is the most common pattern. Global endometrial ablation has become a very popular surgical technique for women complaining of menorrhagia, disinterested in either medical management or definitive therapy – hysterectomy – or where medical management has failed. With proper patient selection, endometrial ablation yields an 80%-90% success rate in reducing heavy menstrual flow and is associated with a 90% patient satisfaction rate (Cochrane Database Syst Rev. 2009 Oct 7;[4]:CD001501).
Literature is replete with conditions believed to increase risk of endometrial ablation failure. This list includes untreated uterine cornua, endometrial regrowth, the presence of submucous leiomyomas or polyps, abnormal uterine cavity, enlarged uterine cavity (width and/or length), endometrial ablation in a young patient, parity of five or greater, unsuspected adhesiolysis, postablation tubal sterilization syndrome, history of dysmenorrhea, smoking, obesity, prior cesarean section, previous gynecologic surgery, and procedure length. Interestingly, type of global endometrial ablation procedure or original bleeding pattern does not influence failure rate.
In this edition of the Master Class in Gynecologic Surgery, Dr. Morris Wortman discusses not only the prevention of endometrial ablation failure, but also how to treat the problem via conservative surgical management.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and is the director at the Center for Menstrual Disorders and Reproductive Choice, also in Rochester. Dr. Wortman has lectured extensively on endometrial ablation and has authored several scientific articles in peer reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported being a subinvestigator on a study sponsored by Channel Medsystems. Email him at [email protected].
Why failures occur and how to correct them
BY MORRIS WORTMAN, MD
Since the introduction almost 20 years ago of devices for nonresectoscopic – or “global” – endometrial ablation, the procedure has been widely adopted as the treatment of choice for abnormal uterine bleeding that is refractory to medical management.
Between 400,000 and 500,000 endometrial ablations are done in the United States every year in women who have completed childbearing, and it probably won’t be long before the procedure surpasses hysterectomy in prevalence for the management of abnormal bleeding.
In recent years, the literature has begun to address the incidence of these delayed complications and the requirement for subsequent hysterectomy. A 2007 practice bulletin issued by the American College of Obstetricians and Gynecologists stated that hysterectomy rates within 4 years of endometrial ablation are at least 24% (Obstet Gynecol. 2007 May;109[5]:1233-48). And a study published the following year reported that 26% of 3,681 women undergoing EA at Kaiser Permanente facilities in Northern California required hysterectomy within 8 years (Obstet Gynecol. 2008 Dec;112[6]:1214-20).
It appears that the vast majority of what we now refer to as late-onset EA failures – complications attributable to EA that occur beyond a perioperative period of 1 month – will occur within 5 years. Some EA failures have occurred over 5-10 years, however, and in my practice we have seen late-onset complications occurring 17 or more years after the initial ablation.
In our practice, we are successfully managing delayed complications after GEA using ultrasound-guided reoperative hysteroscopy to fully explore the uterine cavity and excise areas of endometrial growth and other disease. In 2014, we published a retrospective review of 50 women whom we treated for delayed complications after a variety of GEA techniques; almost 90% avoided hysterectomy during a mean follow-up period of 18 months (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:238-44).
Our experience since then has included reoperative surgery on more than 115 GEA failures. Additionally, we’ve managed 220 patients who have undergone various hysteroscopic and resectoscopic endometrial ablations, some of which date back to the use of the Nd:YAG laser in the late 1980s.
The fact that late-onset EA failures occur does not mean that hysterectomy should routinely be performed as a first-line treatment for intractable uterine bleeding. Overall, there is much more morbidity associated with hysterectomy than with EA.
What failures do suggest is that there are certain risk factors for late-onset EA complications. Our experience in treating women who have experienced late-onset EA failure has provided us with insight into who may be at greatest risk for late-onset EA failure and how patients can best be selected for the procedure. We’ve also learned more about the diagnosis of delayed complications.
Causes of EA failure
Untreated uterine cornua, and untreated submucous leiomyomas and endometrial polyps, are common causes of EA failure. Among the 50 women included in our retrospective review of ultrasound-guided reoperative hysteroscopy after GEA failure, 44% had intraoperative evidence of untreated cornua and nearly one-fourth had persistent or enlarging submucous leiomyomas.
Contrary to what some believe, most endometrial ablations will not adequately destroy submucous or intramural leiomyomas. Therefore, we recommend that these fibroids be entirely removed immediately before EA.
Moreover, GEA will not always provide adequate thermal destruction to the entire endometrial cavity. The cornua regions are particularly at risk; they are difficult to reach under ideal circumstances, and especially difficult to treat in patients who have a uterine septum or a T-shaped uterus (with the ostia and cornua deeply recessed). We have also seen late-onset EA failures in patients with an extended uterine transverse diameter. The limits of GEA are greatest when a device with a fixed configuration or geometry is used.
A history of abnormal hysteroscopy or other evidence of such anatomic distortions are therefore among the reported risk factors for GEA failure (J Minim Invasive Gynecol. 2015 Mar-Apr;22[3]:323-31). A history of tubal ligation also confers risk; the procedure further increases susceptibility for failure when functioning endometrial tissue remains or regrows at the cornua, because any retrograde menstrual bleeding that occurs will be constrained by the obstructed proximal portion of the fallopian tubes.
Obesity is another risk factor for GEA failure in that the condition increases the risk of endometrial cancer, making the need for reliable biopsies in the case of spotting or other signs or symptoms even more important. On the other hand, obesity may also worsen a patient’s status as a candidate for hysterectomy.
There is much to consider with these patients. For some obese patients, GEA may be less risky than hysterectomy while for others, such as those who also have polycystic ovarian syndrome (in whom the risk for developing endometrial cancer is further increased) the scale may tip in favor of hysterectomy.
Age at the time of the primary GEA may be the single most important risk factor for GEA failure and is an important predictor of success in patient selection. Numerous investigators have shown that women younger than 35 years of age at the time of their EA had a significantly increased risk for hysterectomy, compared with women who were at least 45 years old. The younger the patient, the longer the “bridge” to menopause and the greater the likelihood that bridge will fail.
While age is not necessarily a contraindication, it is worthy of serious consideration. We generally discourage GEA for patients younger than 35. We also advise ensuring that each patient undergoing initial EA is highly self-motivated to have a uterine-sparing procedure; if not, symptoms she may experience later will likely drive her toward hysterectomy anyway.
Additionally, we caution against performing GEA in patients who have chronic pelvic pain; these patients tend to have poorer outcomes with any type of hysteroscopic surgery.
Diagnosing failed EA
Delayed complications manifest in several ways: Renewed and increasing vaginal bleeding after a period of improvement, cyclic pelvic pain (unilateral, bilateral, or suprapubic), or both bleeding and pain. Some women – likely an underreported number of them – present with postmenopausal bleeding and proceed to have unsuccessful attempts at an endometrial biopsy due to EA-associated endometrial scarring.
The cyclic pelvic pain associated with endometrial persistence or regrowth tends to worsen over time and is often described as sharp or laborlike. In our experience, a description of “laborlike” pain and a history of EA is almost fully predictive of a finding of endometrial growth. Often a hematometra can be demonstrated on transvaginal ultrasound, but this isn’t always the case.
Pain typically precedes bleeding in patients who demonstrate both. In such cases, blood from functioning endometrial tissue or other sources becomes blocked from exiting the uterine cavity by EA-induced intrauterine scarring and contracture. Painful uterine contractions then aim to expel the pooled blood. In other cases of pain – mainly those without significant vaginal bleeding – the pain is often attributed to cornual and central hematometra.
For the majority of EA failures, the diagnosis lies in the history and current symptoms. Unfortunately, the traditional methods of assessing the endometrial cavity have little merit for women presenting with delayed-onset EA complications. A sonographically assisted pelvic examination can be useful in evaluating complications, but the interpretation of ultrasounds in women with a prior EA can be challenging and is often beyond the training of most radiologists and gynecologists.
It is not uncommon for images to be incorrectly interpreted in the emergency department or physicians’ offices as “normal” and for such readings to set off a chain of CT scans, MRIs, laparoscopies, ovarian cystectomies, and other procedures that miss the root causes of pain.
Unfortunately, there is little in the literature that describes and defines ultrasound findings after EA. We do know that sonography should be timed with episodes of pain, and that the absence of a demonstrable hematometra does not exclude a diagnosis of EA failure.
Correcting late-onset failures
Our office-based operating room is fitted with side-by-side monitors that enable simultaneous sonographic and hysteroscopic views for correction of GEA failures; the rest of the set-up is similar to that of other operative hysteroscopies. However, we do employ a wide variety of resectoscopes with diameters ranging from 13 to 28 Fr. The smaller-diameter scopes are particularly useful for evaluating postmenopausal bleeding in women with a prior EA.
For those inexperienced with ultrasound-guided surgery, the initial resection is often the most challenging. The initial tissue removal is carried out on the thickest observed uterine wall – usually the posterior or anterior wall – and is done with near complete reliance on the ultrasound image. Hysteroscopic visualization is poor at this time because the outflow ports of the continuous flow resectoscope are obstructed by tissue in the narrow tubular cavity.
We then actually remove the resectoscope and clean the outflow ports of clots and debris that may have accumulated. When the scope is reinserted, there is typically sufficient room in the uterine cavity for continuous flow and excellent hysteroscopic visualization.
The sequence of resection from this point on will vary. If we’ve begun on the anterior wall, we’ll move to the posterior and then the two lateral walls to further restore the cavity. Areas of endometrial regrowth will typically be identified at this point and resected. The dissection then will extend upward, usually to within 10 mm of the fundus in the midline as measured by ultrasound. Reconfiguring the loop electrode to a 135- to 160-degree angle can be helpful in the delicate dissection that is required at the fundus.
Once all areas of endometrium have been identified and excised, we will deeply coagulate exposed myometrium with a ball-end electrode. Rarely, we will reach our maximum allowable fluid absorption limit prior to completing the case, a scenario seen in less than 1% of our patients.
In more than 330 reoperative hysteroscopic procedures, we’ve had only one uterine perforation that occurred when we switched ultrasound machines. Very likely, we were too aggressive in removing tissue at the fundus. The patient required a diagnostic laparoscopy but sustained no visceral injury.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and the director of the Center for Menstrual Disorders and Reproductive Choice in Rochester. He reported having no relevant financial disclosures.
One in seven women suffer with abnormal uterine bleeding during their reproductive years, according to Fraser et al. (Exp Rev Obstet Gynecol. 2009;4:179-89). Heavy menstrual bleeding (menorrhagia) is the most common pattern. Global endometrial ablation has become a very popular surgical technique for women complaining of menorrhagia, disinterested in either medical management or definitive therapy – hysterectomy – or where medical management has failed. With proper patient selection, endometrial ablation yields an 80%-90% success rate in reducing heavy menstrual flow and is associated with a 90% patient satisfaction rate (Cochrane Database Syst Rev. 2009 Oct 7;[4]:CD001501).
Literature is replete with conditions believed to increase risk of endometrial ablation failure. This list includes untreated uterine cornua, endometrial regrowth, the presence of submucous leiomyomas or polyps, abnormal uterine cavity, enlarged uterine cavity (width and/or length), endometrial ablation in a young patient, parity of five or greater, unsuspected adhesiolysis, postablation tubal sterilization syndrome, history of dysmenorrhea, smoking, obesity, prior cesarean section, previous gynecologic surgery, and procedure length. Interestingly, type of global endometrial ablation procedure or original bleeding pattern does not influence failure rate.
In this edition of the Master Class in Gynecologic Surgery, Dr. Morris Wortman discusses not only the prevention of endometrial ablation failure, but also how to treat the problem via conservative surgical management.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and is the director at the Center for Menstrual Disorders and Reproductive Choice, also in Rochester. Dr. Wortman has lectured extensively on endometrial ablation and has authored several scientific articles in peer reviewed journals.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported being a subinvestigator on a study sponsored by Channel Medsystems. Email him at [email protected].
Why failures occur and how to correct them
BY MORRIS WORTMAN, MD
Since the introduction almost 20 years ago of devices for nonresectoscopic – or “global” – endometrial ablation, the procedure has been widely adopted as the treatment of choice for abnormal uterine bleeding that is refractory to medical management.
Between 400,000 and 500,000 endometrial ablations are done in the United States every year in women who have completed childbearing, and it probably won’t be long before the procedure surpasses hysterectomy in prevalence for the management of abnormal bleeding.
In recent years, the literature has begun to address the incidence of these delayed complications and the requirement for subsequent hysterectomy. A 2007 practice bulletin issued by the American College of Obstetricians and Gynecologists stated that hysterectomy rates within 4 years of endometrial ablation are at least 24% (Obstet Gynecol. 2007 May;109[5]:1233-48). And a study published the following year reported that 26% of 3,681 women undergoing EA at Kaiser Permanente facilities in Northern California required hysterectomy within 8 years (Obstet Gynecol. 2008 Dec;112[6]:1214-20).
It appears that the vast majority of what we now refer to as late-onset EA failures – complications attributable to EA that occur beyond a perioperative period of 1 month – will occur within 5 years. Some EA failures have occurred over 5-10 years, however, and in my practice we have seen late-onset complications occurring 17 or more years after the initial ablation.
In our practice, we are successfully managing delayed complications after GEA using ultrasound-guided reoperative hysteroscopy to fully explore the uterine cavity and excise areas of endometrial growth and other disease. In 2014, we published a retrospective review of 50 women whom we treated for delayed complications after a variety of GEA techniques; almost 90% avoided hysterectomy during a mean follow-up period of 18 months (J Minim Invasive Gynecol. 2014 Mar-Apr;21[2]:238-44).
Our experience since then has included reoperative surgery on more than 115 GEA failures. Additionally, we’ve managed 220 patients who have undergone various hysteroscopic and resectoscopic endometrial ablations, some of which date back to the use of the Nd:YAG laser in the late 1980s.
The fact that late-onset EA failures occur does not mean that hysterectomy should routinely be performed as a first-line treatment for intractable uterine bleeding. Overall, there is much more morbidity associated with hysterectomy than with EA.
What failures do suggest is that there are certain risk factors for late-onset EA complications. Our experience in treating women who have experienced late-onset EA failure has provided us with insight into who may be at greatest risk for late-onset EA failure and how patients can best be selected for the procedure. We’ve also learned more about the diagnosis of delayed complications.
Causes of EA failure
Untreated uterine cornua, and untreated submucous leiomyomas and endometrial polyps, are common causes of EA failure. Among the 50 women included in our retrospective review of ultrasound-guided reoperative hysteroscopy after GEA failure, 44% had intraoperative evidence of untreated cornua and nearly one-fourth had persistent or enlarging submucous leiomyomas.
Contrary to what some believe, most endometrial ablations will not adequately destroy submucous or intramural leiomyomas. Therefore, we recommend that these fibroids be entirely removed immediately before EA.
Moreover, GEA will not always provide adequate thermal destruction to the entire endometrial cavity. The cornua regions are particularly at risk; they are difficult to reach under ideal circumstances, and especially difficult to treat in patients who have a uterine septum or a T-shaped uterus (with the ostia and cornua deeply recessed). We have also seen late-onset EA failures in patients with an extended uterine transverse diameter. The limits of GEA are greatest when a device with a fixed configuration or geometry is used.
A history of abnormal hysteroscopy or other evidence of such anatomic distortions are therefore among the reported risk factors for GEA failure (J Minim Invasive Gynecol. 2015 Mar-Apr;22[3]:323-31). A history of tubal ligation also confers risk; the procedure further increases susceptibility for failure when functioning endometrial tissue remains or regrows at the cornua, because any retrograde menstrual bleeding that occurs will be constrained by the obstructed proximal portion of the fallopian tubes.
Obesity is another risk factor for GEA failure in that the condition increases the risk of endometrial cancer, making the need for reliable biopsies in the case of spotting or other signs or symptoms even more important. On the other hand, obesity may also worsen a patient’s status as a candidate for hysterectomy.
There is much to consider with these patients. For some obese patients, GEA may be less risky than hysterectomy while for others, such as those who also have polycystic ovarian syndrome (in whom the risk for developing endometrial cancer is further increased) the scale may tip in favor of hysterectomy.
Age at the time of the primary GEA may be the single most important risk factor for GEA failure and is an important predictor of success in patient selection. Numerous investigators have shown that women younger than 35 years of age at the time of their EA had a significantly increased risk for hysterectomy, compared with women who were at least 45 years old. The younger the patient, the longer the “bridge” to menopause and the greater the likelihood that bridge will fail.
While age is not necessarily a contraindication, it is worthy of serious consideration. We generally discourage GEA for patients younger than 35. We also advise ensuring that each patient undergoing initial EA is highly self-motivated to have a uterine-sparing procedure; if not, symptoms she may experience later will likely drive her toward hysterectomy anyway.
Additionally, we caution against performing GEA in patients who have chronic pelvic pain; these patients tend to have poorer outcomes with any type of hysteroscopic surgery.
Diagnosing failed EA
Delayed complications manifest in several ways: Renewed and increasing vaginal bleeding after a period of improvement, cyclic pelvic pain (unilateral, bilateral, or suprapubic), or both bleeding and pain. Some women – likely an underreported number of them – present with postmenopausal bleeding and proceed to have unsuccessful attempts at an endometrial biopsy due to EA-associated endometrial scarring.
The cyclic pelvic pain associated with endometrial persistence or regrowth tends to worsen over time and is often described as sharp or laborlike. In our experience, a description of “laborlike” pain and a history of EA is almost fully predictive of a finding of endometrial growth. Often a hematometra can be demonstrated on transvaginal ultrasound, but this isn’t always the case.
Pain typically precedes bleeding in patients who demonstrate both. In such cases, blood from functioning endometrial tissue or other sources becomes blocked from exiting the uterine cavity by EA-induced intrauterine scarring and contracture. Painful uterine contractions then aim to expel the pooled blood. In other cases of pain – mainly those without significant vaginal bleeding – the pain is often attributed to cornual and central hematometra.
For the majority of EA failures, the diagnosis lies in the history and current symptoms. Unfortunately, the traditional methods of assessing the endometrial cavity have little merit for women presenting with delayed-onset EA complications. A sonographically assisted pelvic examination can be useful in evaluating complications, but the interpretation of ultrasounds in women with a prior EA can be challenging and is often beyond the training of most radiologists and gynecologists.
It is not uncommon for images to be incorrectly interpreted in the emergency department or physicians’ offices as “normal” and for such readings to set off a chain of CT scans, MRIs, laparoscopies, ovarian cystectomies, and other procedures that miss the root causes of pain.
Unfortunately, there is little in the literature that describes and defines ultrasound findings after EA. We do know that sonography should be timed with episodes of pain, and that the absence of a demonstrable hematometra does not exclude a diagnosis of EA failure.
Correcting late-onset failures
Our office-based operating room is fitted with side-by-side monitors that enable simultaneous sonographic and hysteroscopic views for correction of GEA failures; the rest of the set-up is similar to that of other operative hysteroscopies. However, we do employ a wide variety of resectoscopes with diameters ranging from 13 to 28 Fr. The smaller-diameter scopes are particularly useful for evaluating postmenopausal bleeding in women with a prior EA.
For those inexperienced with ultrasound-guided surgery, the initial resection is often the most challenging. The initial tissue removal is carried out on the thickest observed uterine wall – usually the posterior or anterior wall – and is done with near complete reliance on the ultrasound image. Hysteroscopic visualization is poor at this time because the outflow ports of the continuous flow resectoscope are obstructed by tissue in the narrow tubular cavity.
We then actually remove the resectoscope and clean the outflow ports of clots and debris that may have accumulated. When the scope is reinserted, there is typically sufficient room in the uterine cavity for continuous flow and excellent hysteroscopic visualization.
The sequence of resection from this point on will vary. If we’ve begun on the anterior wall, we’ll move to the posterior and then the two lateral walls to further restore the cavity. Areas of endometrial regrowth will typically be identified at this point and resected. The dissection then will extend upward, usually to within 10 mm of the fundus in the midline as measured by ultrasound. Reconfiguring the loop electrode to a 135- to 160-degree angle can be helpful in the delicate dissection that is required at the fundus.
Once all areas of endometrium have been identified and excised, we will deeply coagulate exposed myometrium with a ball-end electrode. Rarely, we will reach our maximum allowable fluid absorption limit prior to completing the case, a scenario seen in less than 1% of our patients.
In more than 330 reoperative hysteroscopic procedures, we’ve had only one uterine perforation that occurred when we switched ultrasound machines. Very likely, we were too aggressive in removing tissue at the fundus. The patient required a diagnostic laparoscopy but sustained no visceral injury.
Dr. Wortman is a clinical associate professor of obstetrics and gynecology at the University of Rochester (N.Y.) and the director of the Center for Menstrual Disorders and Reproductive Choice in Rochester. He reported having no relevant financial disclosures.