User login
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
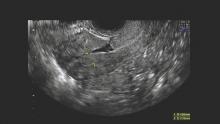
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
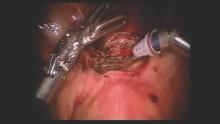
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
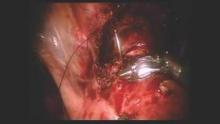
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.
In recent years, uterine isthmocele has increasingly been included as part of the differential in women with a history of a cesarean section who present with postmenstrual bleeding, pelvic pain, or secondary infertility.
The defect appears as a fluid-filled, pouch-like abnormality in the anterior uterine wall at the site of a prior cesarean section. The best method for diagnosis is usually a saline-infused sonogram. It can be treated in various ways, depending on the patient’s symptoms and desire for future fertility. Although we have treated isthmoceles with hysteroscopic desiccation, or resection, our best success has occurred with laparoscopic resection and reapproximation of normal tissue in a small series of patients.
There is no standard definition of the defect that fully describes its size, depth, and other characteristics. Many words and phrases have been used to describe the defect: It is commonly referred to as an isthmocele, because of its usual location at the uterine isthmus, but others have referred to it as a cesarean scar defect or niche, as the defect may be found at the endocervical canal or in the lower uterine segment. In any case, while diagnoses appear to be increasing, the incidence of the defect is unknown.
More research on risk factors and treatment is needed, but the literature, as well as our own experience, has demonstrated that this treatable defect should be considered in the differential diagnosis for women who have undergone cesarean section and subsequently have abnormal bleeding or staining, pelvic pain, or secondary infertility, especially when fluid is clearly visible in the cesarean section defect.
Diagnosis, symptoms
An isthmocele forms in the first place, it is thought, after an incision scar forms and causes retraction and dilation in the thinner, lower segment of the anterior wall and a thickening in the upper portion. There is a deficient scar, in other words, with disparate wound healing on the sides of the incision site.
The defect and its consequences were described in 1995 by Dr. Hugh Morris, who studied hysterectomy specimens in 51 women with a history of cesarean section (in most cases, more than one). Dr. Morris concluded that scar tissue in these patients contributed to significant pathological changes and anatomical abnormalities that, in turn, gave rise to a variety of clinical symptoms including menorrhagia, dysmenorrhea, dyspareunia, and lower abdominal pain refractory to medical management.
Distortion and widening of the lower uterine segment and “free” red blood cells in endometrial stroma of the scar were the most frequently identified pathological changes, followed by fragmentation and breakdown of the endometrium of the scar, and iatrogenic adenomyosis (Int. J. Gynecol. Pathol.1995;14:16-20).
Several small reports and case series published in the late 1990s offered additional support for a cause-and-effect correlation between cesarean scar defects and abnormal vaginal bleeding. Several years later, the link was strengthened as more investigators reported connections between the defects and various symptoms. These reports were followed by published comparisons of imaging techniques for the diagnosis of isthmoceles.
Diagnosis of the defects can be made with transvaginal ultrasound (TVUS), saline infused sonohysterogram (SIS), hysterosalpingogram, hysteroscopy, and magnetic resonance imaging (MRI). With any modality, imaging is best performed in the early proliferative phase, right after the menstrual cycle has ended.
Comparisons of unenhanced TVUS and SIS – both of which may be easily performed in the office and at a much lower cost than MRI – have shown the latter technique to be superior for evaluating isthmoceles. Distension of the endometrial cavity makes the borders of the defects easier to delineate, which enables detection of more subtle defects and improves our ability to measure the size of defects.
This advantage was described by in 2010 by Dr. O. Vikhareva Osser and colleagues, who performed both TVUS and SIS in 108 women with a history of one or more cesarean sections. They identified more scar defects with SIS than with TVUS (Ultrasound Obstet. Gynecol. 2010;35:75-83).
Another benefit of SIS over TVUS and hysterosalpingogram is that one can measure the thickness of the remaining myometrium overlying the isthmocele, which is especially important knowledge for patients considering another pregnancy. As a result, we have relied on this technique to diagnose every case within our practice. I will perform SIS in a patient who has a history of one or multiple cesarean sections and symptoms of abnormal bleeding, pelvic pain, or secondary infertility as part of the basic work-up.
Similarly, an observational prospective cohort study of 225 women who had undergone a cesarean section 6-12 months prior compared TVUS and gel-infused sonohysterogram (GIS), and found that the prevalence of a niche – defined as an anechoic area at the site of the cesarean scar, with a depth of at least 1 mm on GIS – was 24% with TVUS and 56% with GIS (Ultrasound Obstet. Gynecol. 2011;37:93-9).
The abnormal bleeding is often described by patients as spotting or bleeding that continues for days or weeks after menstrual flow has ended; it is believed to result from an accumulation of blood in the defect and a lack of coordinated muscle contractions, which leads to continued accumulation of blood and menstrual debris. Dysmenorrhea and chronic pelvic pain are thought to be associated with iatrogenic adenomyosis and/or a chronic inflammatory state created when accumulated blood and mucus are intermittently expelled. Secondary infertility can occur, it is believed, as accumulated fluid and blood interfere with the endocervical and even the endometrial environment and disrupt sperm transport, sperm quality, and embryo implantation. Difficulty in embryo transfer may also occur because of the distortion caused to the endometrial cavity. Many of the isthmoceles that we and others have diagnosed have been in patients undergoing invitro fertilization. The patients are often found to have an accumulation of fluid in the endometrial canal and isthmocele during stimulation for either a fresh or frozen embryo transfer, thus necessitating the cancellation of their cycle.
Treatment
The choice of treatment depends upon the patient’s symptoms and desire for future fertility, but it can include hormonal treatment, hysteroscopic resection, transvaginal repair, a laparoscopic or robot-assisted approach, and hysterectomy.
Little has been published on nonsurgical treatment, but this may be considered for patients whose primary symptoms are bleeding or pain and who desire the least invasive option. In a small observational study of women with an isthmocele and bleeding, symptoms were eliminated with several cycles of oral contraceptive pills (Fertil. Steril. 2006;86: 477-9).
Hysteroscopic isthmocele correction or resection are the surgical techniques most frequently described in the literature, but, as with other surgical approaches, studies are small. Hysteroscopic repair has typically involved the use of electrical energy to desiccate or cauterize abnormal tissue and eliminate the outpouching in which blood and fluid accumulate. Hysteroscopic resection is another technique that has also been championed.
However, for patients who desire future pregnancy, we do not recommend a hysteroscopic approach because it does not reinforce the often-thinning myometrium covering the defect. We are concerned that if this area is simply desiccated or resected, and not reapproximated, the patient will be at greater risk of pregnancy-related complications, including cesarean scar ectopic pregnancy with potential uterine dehiscence.
Laparoscopic repair was first described by Dr. Olivier Donnez, who rightly pointed out that the laparoscopic approach offers an optimal view from above during dissection of the vesico-vaginal space. Dr. Donnez used a CO2 laser to excise fibrotic tissue, followed by laparoscopic closure (Fertil. Steril. 2008;89:974-80).
We have had success with a laparoscopic approach that uses concomitant hysteroscopy. The vesico-uterine peritoneum is incised over the anterior uterine wall, and the bladder is backfilled so that its boundaries may be identified prior to further dissection. With the area exposed, we perform a hysteroscopy to determine the exact location of the isthmocele. As the hysteroscope enters the thinned out isthmocele, the light will be more visible via laparoscopic visualization.
When performing conventional laparoscopy, the isthmocele is excised with an ultrasonic curved blade. We use this instrument because it has no opposing arm and because it enables precise tissue dissection in multiple planes. With harmonic energy, we can limit tissue dessication and destruction, lowering the risk of future pregnancy-related complications. Monopolar scissors are best when a robotic approach is used.
Once the isthmocele is resected, the clean edges are sutured together in two layers. The first layer is sutured in an interrupted mattress-style fashion, to prevent tissue strangulation and necrosis. We use a monofilament nonbarbed delayed-absorbable 3-0 PDS suture on a CT-1 needle – a choice that limits tissue trauma and postoperative inflammation.
Sutures are initially placed at each angle with one or two sutures placed between. These sutures must be placed deep to close the bottom of the defect. A second layer of suture is then placed to imbricate over the initial layer of closure. We utilize 3-0 PDS in a running or mattress style, or a running 3-0 V-Loc suture. Our patients return after 1-3 months for a postoperative image, and are instructed to wait at least 3 months after surgery before attempting conception.
In our experience, of more than 10 patients, symptoms ceased in all patients whose surgery was performed for the indication of abnormal uterine bleeding. The follow-up on our series of patients who underwent the procedure for secondary infertility is ongoing, but the preliminary results are very positive, with resolution of intrauterine fluid in all of the patients, as well as several successful pregnancy outcomes.
A recent systematic review of minimally invasive therapy for symptoms related to an isthmocele shows good outcomes across the 12 included studies but does not offer evidence to favor one treatment over another. The studies show significant reductions in abnormal uterine bleeding and pain, as well as a high rate of satisfaction in most patients after hysteroscopic niche resection or vaginal or laparoscopic niche repair, with a low complication rate (BJOG 2014;121:145-6).
Pregnancies were reported after treatment, but sample sizes and follow-up were insufficient to draw conclusions on pregnancy and delivery outcomes, according to the review. As the reviewers wrote, following patients through their next delivery in larger, higher-quality studies will help provide more guidance for selecting the best isthmocele treatments and implementing these treatments into practice.
Dr. Sasaki reported having no financial disclosures relevant to this Master Class.