User login
CASE › A 52-year-old right-hand-dominant white man arrived at our clinic complaining that he was unable to straighten his right ring finger. He had no associated pain or numbness, and had not injured his hand. The patient had type 2 diabetes that was controlled with metformin. He had no history of surgery or drug allergies, did not smoke, and said he drank 2 to 3 alcoholic beverages per day. He was a car salesman and was self-conscious when shaking hands with customers. On physical examination, we noted that he held his right ring finger at roughly 45 degrees of flexion at the metacarpophalangeal joint; a painless cord-like structure was palpable on the palmar surface of that joint. His left hand had no abnormalities.
If this were your patient, how would you proceed?
Dupuytren’s disease (DD) is a disabling fibroproliferative disorder of the hand for which there is no cure. While the exact cause of DD is unknown, it has been linked to a number of risk factors, including smoking, alcohol consumption, and diabetes. It affects about 5% of the US population, and up to 70% of affected individuals may initially seek treatment from a primary care physician.1 The disease is also referred to as Dupuytren’s contracture, which describes the flexion contractures of fingers at the end stage of the disease. Palmar fibromatosis is yet another name for the disorder.
DD refers to a spectrum of presentations ranging from nodules to cords to discernible contractures, and it is not known which patients with early Dupuytren changes will progress to severe contracture. With recognition of early changes, nonsurgical intervention is possible, such as collagenase injection or percutaneous fasciotomy, and can slow the progression of DD, restore function, and avoid or delay surgical intervention. DD is a clinically challenging disorder. Treatment for an affected area may resolve symptoms, only to have them recur in that location or another.
How underlying pathology correlates with clinical findings
DD affects the palmar fascia, a thick triangular-shaped sheet of dense fibrous collagenous connective tissue that lies deep to the dermis and superficial to the flexor tendons of the hand with fibers extending both into the skin and into the deep tissue. The palmar fascia secures the skin during gripping and twisting motions, and it bifurcates into distal extensions, called pretendinous bands, that overlay and mimic the flexor tendons.
Clinical findings reflect the progression of underlying pathology. The earliest manifestation of DD is painless dimpling of the skin on the palmar surface of the hand.2 Over time, the underlying fibrosis with increased collagen deposition can progress, leading to development of nodules and eventually, cords, which are sometimes mistaken for flexor tendons. Dupuytren-like fibrotic tissue can occur on the sole of the foot (Ledderhose disease) and penis (Peyronie’s disease).2,3 In patients with these coexisting conditions, prognosis is generally worse.4 With the hands, bilateral involvement is common, although it is usually more severe in one hand. The ring finger is the digit most frequently involved, followed by the little finger, middle finger, and index finger. The thumb is rarely affected.3
As the disease progresses and cords contract, the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints develop flexion contractures. The distal interphalangeal (DIP) joint, rarely involved, instead exhibits a hyperextension contracture. Digital flexion contractures are often disabling, interfering with daily activities such as picking up a glass, shaking hands, or putting one's hand in a pants pocket. Many patients seek medical attention only after a palpable nodule, cord, or flexion contracture becomes apparent (FIGURE 1).
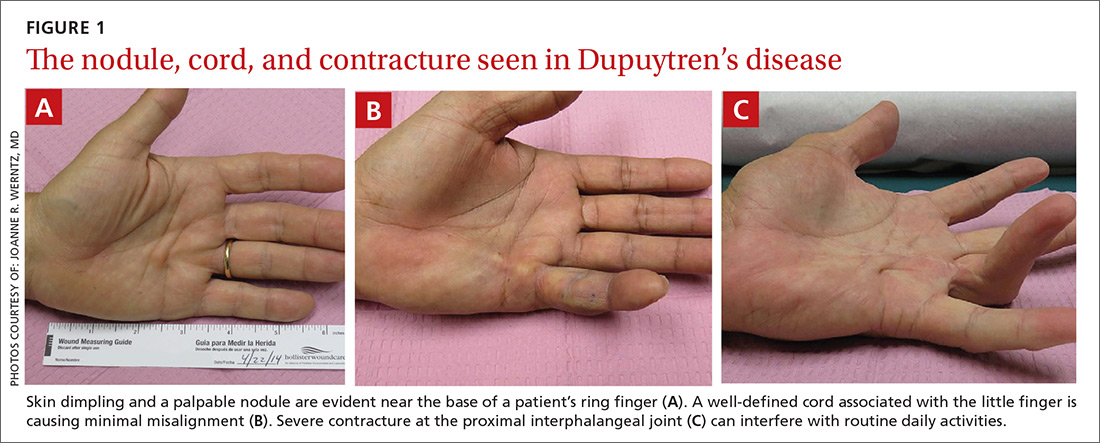
In a study of 326 patients who reported Dupuytren’s symptoms, the most common symptoms that led them to seek treatment were, in descending order, a hard bump (48%), a ropelike growth (12%), dimpling (11%), and finger contractures (10%). Only 9% of patients seeking treatment for hand symptoms associated with DD had received a diagnosis of DD from their initial medical encounter, causing an unnecessary delay in treatment.1
Who is at increased risk for DD?
DD is most often seen in elderly white men of European descent.5 In the United States, the prevalence of the disease is roughly 5%,1 compared with 4% to 39% in northwestern Europe (eg, Iceland).6 The male-to-female ratio of DD ranges from 6:1 to 15:1.7 The prevalence of DD appears to increase with age. The prevalence in men and women is similar up to age 45 years, after which the rate is much greater in males.7
DD is associated with many risk factors including smoking, alcohol consumption, vascular insufficiency, epilepsy, hyperlipidemia, manual labor, occupations with exposure to vibration, hand trauma, and even hand surgery such as carpal tunnel release or trigger finger release.8-11 It is also associated with diabetes; particularly type 1 insulin-dependent diabetes.12 There may also be an association with frozen shoulder.12,13
The need for surgical treatment of DD becomes more likely with a history of cigarette smoking and heavy alcohol consumption. There seems to be an association with epilepsy, most likely from anti-epileptic drugs.14 Rheumatoid arthritis is the only condition that has been associated with a lower incidence of DD, possibly because of the use of anti-inflammatory drugs.15 There are genetic differences between patients with and without DD, although a “Dupuytren’s gene” has not been identified.
Rule out possible DD mimics
The differential diagnosis for flexion contracture of the MCP or PIP joints seen in Dupuytren’s disease includes stenosing flexor tenosynovitis, or trigger finger. Other conditions that can mimic DD are ulnar claw hand, trauma scars, intrinsic joint diseases such as degenerative or rheumatoid arthritis (RA), diabetic cheiroarthropathy, camptodactyly, and Volkmann’s contracture. Trauma scars—especially longitudinal scars—have a tendency to contract and develop keloid formation.
Stenosing flexor tenosynovitis and ulnar claw hand are distinguishable from DD by full or nearly full active or passive extension of the affected digit, whereas DD is a true contracture of the joint that does not allow full extension. A careful history can rule out previous injury to the area. Although intrinsic joint disease, such as RA, can cause finger contractures, the joints are usually enlarged, painful, and associated with characteristic radiologic findings.
Unlike DD, diabetic cheiroarthropathy often involves all of the digits except for the thumb, and is often associated with a waxy appearance of the skin. Camptodactyly is an autosomal dominant disorder that more often presents in childhood and can be caused by a number of congenital syndromes. Volkmann’s contracture can manifest as a claw-like deformity of the hand caused by undiagnosed compartment syndrome of the forearm.
Assess the severity of DD
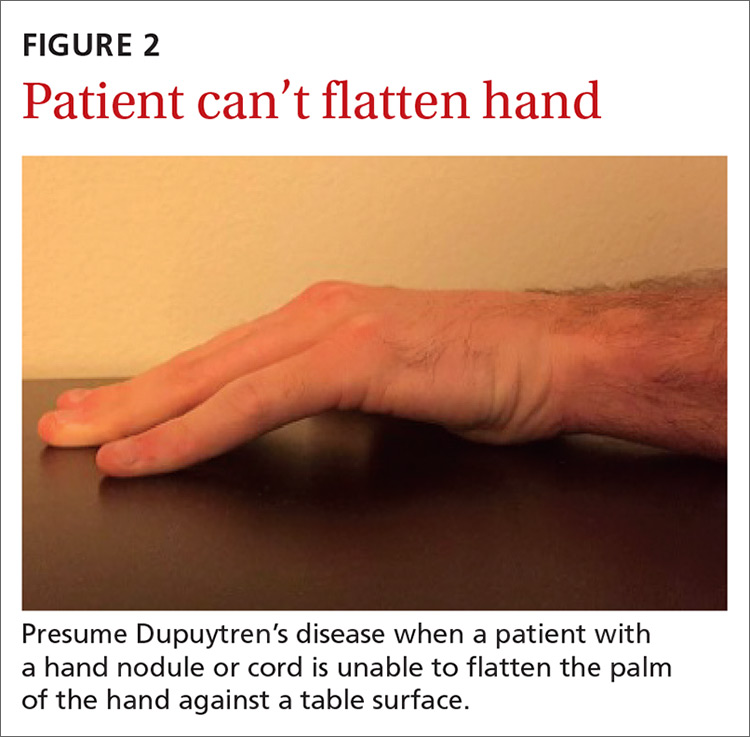
In your evaluation, first identify palmar or digital fibromatosis presenting as a nodule or a cord. Second, estimate the degree of MCP and PIP joint contractures. A common measure of contracture is the Hueston tabletop test. Ask the patient to place the palm of the hand on a flat surface. If the patient is unable to completely flatten the hand against the surface, presume a positive result (FIGURE 2).16

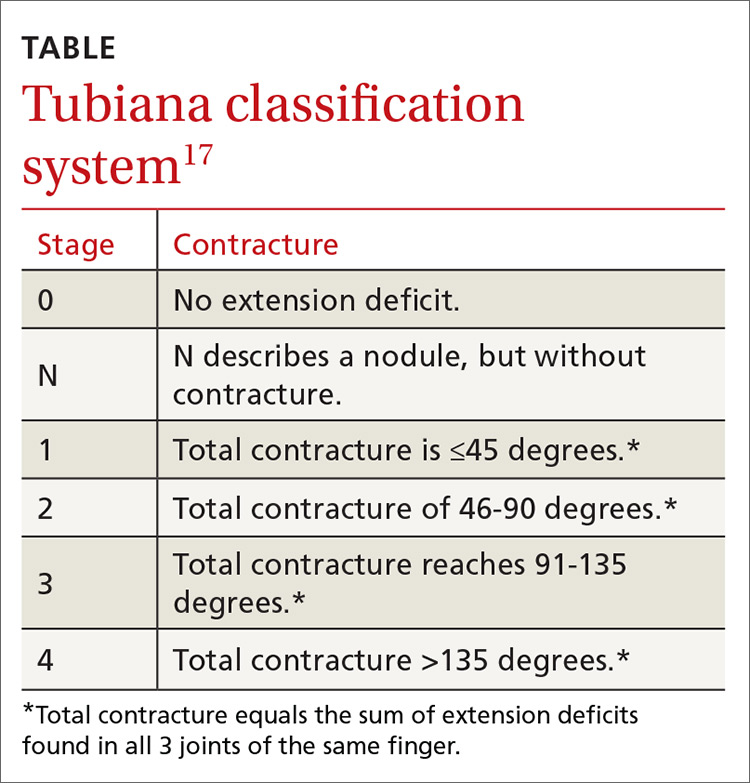
An accurate measure of the degree of flexion can be accomplished with a goniometer. For a simple assessment of severity, have the patient place each affected finger along the convexity of a spoon. If adjoining surfaces are flush, assume that the contracture is at least 30 degrees (FIGURE 3). The severity of DD can also be graded according to the Tubiana classification system (TABLE17), wherein the total deformity or contracture score is the sum of the angles of all 3 digital joints of the finger.
A look at the treatment options
Once the diagnosis of DD is made, reassure the patient that the disease is not cancerous, although it may be progressive. Advise patients, too, that several treatment options (detailed below) are available, but that surgery is the mainstay of treatment. (Of note: Despite what would seem logical, stretching a cord to straighten the finger is not recommended, as it may actually worsen the condition.18)
Options for early DD. Corticosteroids such as triamcinolone may have a role as an adjunct for early DD by reducing the size and firmness of the Dupuytren’s nodules and possibly slowing the progression of the disease. However, in one study of 63 patients, half of the individuals experienced reactivation of disease between one and 3 years.19
Radiotherapy has been studied as a potential treatment for early DD and for patients unable to undergo surgery. Radiation does have a biologic effect on nodular DD, but has no effect on the cords that cause the joint contracture. In a long-term follow-up study of 135 patients treated with radiotherapy, disease remained stable in 59% of patients, improved in 10%, and progressed in 31%.20 Results were better for early stage disease. This modality is not used for more advanced cases.
Surgery is the primary method by which to restore function and minimize complications. In the past, referral was recommended for MCP joint contractures >30 degrees or for any involvment of the PIP joint, because involvement of the latter carries a worse prognosis and may not be fully correctable, even with surgery.21 However, treatment may also be warranted in cases of functional disability—regardless of the degree of contracture (eg, for pain associated with a prominent nodule or cord when gripping objects).
Depending on disease progression and the surgeon’s preference, the most popular procedures are fasciotomies and fasciectomies. Surgical options for contractures—in increasing order of invasiveness and amounts of fascia removed—are fasciotomy (needle, open), fasciectomy (partial, radical), and dermofasciectomy.
Needle fasciotomy is an outpatient procedure performed under local block. The surgeon uses a needle bevel to transect the diseased cord at multiple puncture sites.
Open fasciotomy is also an outpatient procedure involving an incision that allows direct visualization of the cord and internal structures before dividing the fascia. Percutaneous and open fasciotomies are more successful for MCP joint contractures and less successful for PIP joint contractures.
With partial fasciectomy, only the grossly affected fascia is removed.
With radical fasciectomy, the entire palmar fascia is removed, regardless of which areas are grossly diseased.
Dermofasciectomy removes diseased fascia and the overlying skin, with a skin graft applied to heal the wound.
Another option: Collagenase injection. In February 2010, the US Food and Drug Administration approved collagenase clostridium histolyticum (Xiaflex) as the first drug specifically indicated for the treatment of Dupuytren’s contracture. Collagenase is injected directly into the fascial cord, cleaving its collagen component and progressively softening and breaking down the cord. If not properly injected, collagenase may injure neurovascular structures and may rupture tendons.
So how do the treatment options compare?
We found no randomized prospective trials comparing treatment options for DD, but there are a number of trials that shed light on a variety of the available options.
Treatment success. In general, partial fasciectomies have shown the greatest success in reducing contractures and maintaining the lowest recurrence rates. Correction of contracture to ≤5 degrees was seen in 94% of MCP joint contractures treated with partial fasciectomy, in 77% of MCP joints treated with collagenase, and in 55% of MCP joints treated with percutaneous needle fasciotomies.22,23 PIP joint contracture correction was not as successful: Correction of contracture to ≤5 degrees was seen in 47% of those treated with partial fasciectomy, 40% treated with collagenase, and 26% treated with needle fasciotomy.22,23
Recurrence rates. When recurrence was defined as loss of passive extension >30 degrees, the recurrence rate for MCP joint contractures with partial fasciectomy was 21%, compared with 85% for needle fasciotomy.22 In a similar review, recurrence rates for partial fasciectomy ranged from 12% to 39% compared with 50% to 58% for needle fasciotomy.24 With collagenase injection, 5-year data have shown an overall recurrence rate of 32%, with a recurrence of 26% at the MCP joint and 46% at the PIP joint.25 In this trial, recurrence was
Complications. Although fasciectomies have shown the best efficacy and lowest recurrence rates, complications such as infection, neurapraxia, and digital nerve injury are more likely with these procedures.26,27
Early recognition and referral to a hand specialist for the treatment of DD may allow the use of less invasive techniques and improved functional results.
CASE › Absent a history of trauma and features typical of other hand/digit disorders, we diagnosed Dupuytren’s disease in this patient. Potential treatments included partial fasciectomy and collagenase injection. After discussing the risks and benefits of each of these procedures, the patient elected to undergo a partial fasciectomy. The surgery was uncomplicated and the abnormality was corrected to within 5 degrees of full extension. At the patient’s one-year follow-up visit, there was no evidence of recurrence.
1. DiBenedetti DB, Nguyen D, Zografos L, et al. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand. 2011;6:149-158.
2. Townley WA, Baker R, Sheppard N, et al. Dupuytren’s contracture unfolded. BMJ. 2006;332:397-400.
3. Rayan GM. Clinical presentation and types of Dupuytren’s disease. Hand Clin. 1999;15:87-96.
4. Hindocha S, Stanley JK, Watson S, et al. Dupuytren’s diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31:1626-1634.
5. Bayat A, McGrouther DA. Management of Dupuytren’s disease—clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88:3-8.
6. Gudmundsson KG, Arngrimsson R, Sigfússon M, et al. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53:291-296.
7. Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg Br. 1999;24:456-459.
8. Noble J, Arafa M, Royle SG, et al. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg Br. 1992;17:71–74.
9. Mikkelsen OA. Dupuytren’s disease—initial symptoms, age of onset and spontaneous course. Hand. 1977;9:11-15.
10. Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24:153-159.
11. Liss GM, Stock SR. Can Dupuytren’s contracture be work related? review of the evidence. Am J Ind Med. 1996;29:521-532.
12. Geoghegan JM, Forbes J, Clark DI, et al. Dupuytren’s disease risk factors. J Hand Surg Br. 2004;29:423-426.
13. Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg. 2001;10:149-151.
14. Nunn AC, Schreuder FB. Dupuytren’s contracture: emerging insight into a Viking disease. J Hand Surg. 2014;19:481-490.
15. Arafa M, Steingold R F, Noble J. The incidence of Dupuytren’s disease in patients with rheumatoid arthritis. J Hand Surg Br. 1984;9:165-166.
16. Hueston J. Lessons in Dupuytren’s disease. Ann Chir Main Memb Super. 1992;11:349-354.
17. Tubiana R. Prognosis and treatment of Dupuytren’s contracture. J Bone and Joint Surg AM. 1955;37:1155-1168.
18. Howard JC, Varallo VM, Ross DC, et al. Elevated levels of ß-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16.
19. Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25:1157-1162.
20. Betz N, Ott OJ, Adamietz B, et al. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186:82-90.
21. Smith AC. Diagnosis and indications for surgical treatment. Hand Clin. 1991;7:635-642.
22. van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469-477.
23. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968-979.
24. Chen NC, Srinivasan RC, Shauver MJ, et al. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand. 2011;6:250-255.
25. Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015;40:1597-1605.
26. Toppi JT, Trompf L, Smoll NR, et al. Dupuytren’s contracture: an analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy. ANZ J Surg. 2015;85:639-643.
27. Cheung K, Walley KC, Rozental TD. Management of complications of Dupuytren contracture. Hand Clini. 2015;31:345-354.
CASE › A 52-year-old right-hand-dominant white man arrived at our clinic complaining that he was unable to straighten his right ring finger. He had no associated pain or numbness, and had not injured his hand. The patient had type 2 diabetes that was controlled with metformin. He had no history of surgery or drug allergies, did not smoke, and said he drank 2 to 3 alcoholic beverages per day. He was a car salesman and was self-conscious when shaking hands with customers. On physical examination, we noted that he held his right ring finger at roughly 45 degrees of flexion at the metacarpophalangeal joint; a painless cord-like structure was palpable on the palmar surface of that joint. His left hand had no abnormalities.
If this were your patient, how would you proceed?
Dupuytren’s disease (DD) is a disabling fibroproliferative disorder of the hand for which there is no cure. While the exact cause of DD is unknown, it has been linked to a number of risk factors, including smoking, alcohol consumption, and diabetes. It affects about 5% of the US population, and up to 70% of affected individuals may initially seek treatment from a primary care physician.1 The disease is also referred to as Dupuytren’s contracture, which describes the flexion contractures of fingers at the end stage of the disease. Palmar fibromatosis is yet another name for the disorder.
DD refers to a spectrum of presentations ranging from nodules to cords to discernible contractures, and it is not known which patients with early Dupuytren changes will progress to severe contracture. With recognition of early changes, nonsurgical intervention is possible, such as collagenase injection or percutaneous fasciotomy, and can slow the progression of DD, restore function, and avoid or delay surgical intervention. DD is a clinically challenging disorder. Treatment for an affected area may resolve symptoms, only to have them recur in that location or another.
How underlying pathology correlates with clinical findings
DD affects the palmar fascia, a thick triangular-shaped sheet of dense fibrous collagenous connective tissue that lies deep to the dermis and superficial to the flexor tendons of the hand with fibers extending both into the skin and into the deep tissue. The palmar fascia secures the skin during gripping and twisting motions, and it bifurcates into distal extensions, called pretendinous bands, that overlay and mimic the flexor tendons.
Clinical findings reflect the progression of underlying pathology. The earliest manifestation of DD is painless dimpling of the skin on the palmar surface of the hand.2 Over time, the underlying fibrosis with increased collagen deposition can progress, leading to development of nodules and eventually, cords, which are sometimes mistaken for flexor tendons. Dupuytren-like fibrotic tissue can occur on the sole of the foot (Ledderhose disease) and penis (Peyronie’s disease).2,3 In patients with these coexisting conditions, prognosis is generally worse.4 With the hands, bilateral involvement is common, although it is usually more severe in one hand. The ring finger is the digit most frequently involved, followed by the little finger, middle finger, and index finger. The thumb is rarely affected.3
As the disease progresses and cords contract, the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints develop flexion contractures. The distal interphalangeal (DIP) joint, rarely involved, instead exhibits a hyperextension contracture. Digital flexion contractures are often disabling, interfering with daily activities such as picking up a glass, shaking hands, or putting one's hand in a pants pocket. Many patients seek medical attention only after a palpable nodule, cord, or flexion contracture becomes apparent (FIGURE 1).

In a study of 326 patients who reported Dupuytren’s symptoms, the most common symptoms that led them to seek treatment were, in descending order, a hard bump (48%), a ropelike growth (12%), dimpling (11%), and finger contractures (10%). Only 9% of patients seeking treatment for hand symptoms associated with DD had received a diagnosis of DD from their initial medical encounter, causing an unnecessary delay in treatment.1
Who is at increased risk for DD?
DD is most often seen in elderly white men of European descent.5 In the United States, the prevalence of the disease is roughly 5%,1 compared with 4% to 39% in northwestern Europe (eg, Iceland).6 The male-to-female ratio of DD ranges from 6:1 to 15:1.7 The prevalence of DD appears to increase with age. The prevalence in men and women is similar up to age 45 years, after which the rate is much greater in males.7
DD is associated with many risk factors including smoking, alcohol consumption, vascular insufficiency, epilepsy, hyperlipidemia, manual labor, occupations with exposure to vibration, hand trauma, and even hand surgery such as carpal tunnel release or trigger finger release.8-11 It is also associated with diabetes; particularly type 1 insulin-dependent diabetes.12 There may also be an association with frozen shoulder.12,13
The need for surgical treatment of DD becomes more likely with a history of cigarette smoking and heavy alcohol consumption. There seems to be an association with epilepsy, most likely from anti-epileptic drugs.14 Rheumatoid arthritis is the only condition that has been associated with a lower incidence of DD, possibly because of the use of anti-inflammatory drugs.15 There are genetic differences between patients with and without DD, although a “Dupuytren’s gene” has not been identified.
Rule out possible DD mimics
The differential diagnosis for flexion contracture of the MCP or PIP joints seen in Dupuytren’s disease includes stenosing flexor tenosynovitis, or trigger finger. Other conditions that can mimic DD are ulnar claw hand, trauma scars, intrinsic joint diseases such as degenerative or rheumatoid arthritis (RA), diabetic cheiroarthropathy, camptodactyly, and Volkmann’s contracture. Trauma scars—especially longitudinal scars—have a tendency to contract and develop keloid formation.
Stenosing flexor tenosynovitis and ulnar claw hand are distinguishable from DD by full or nearly full active or passive extension of the affected digit, whereas DD is a true contracture of the joint that does not allow full extension. A careful history can rule out previous injury to the area. Although intrinsic joint disease, such as RA, can cause finger contractures, the joints are usually enlarged, painful, and associated with characteristic radiologic findings.
Unlike DD, diabetic cheiroarthropathy often involves all of the digits except for the thumb, and is often associated with a waxy appearance of the skin. Camptodactyly is an autosomal dominant disorder that more often presents in childhood and can be caused by a number of congenital syndromes. Volkmann’s contracture can manifest as a claw-like deformity of the hand caused by undiagnosed compartment syndrome of the forearm.

Assess the severity of DD
In your evaluation, first identify palmar or digital fibromatosis presenting as a nodule or a cord. Second, estimate the degree of MCP and PIP joint contractures. A common measure of contracture is the Hueston tabletop test. Ask the patient to place the palm of the hand on a flat surface. If the patient is unable to completely flatten the hand against the surface, presume a positive result (FIGURE 2).16

An accurate measure of the degree of flexion can be accomplished with a goniometer. For a simple assessment of severity, have the patient place each affected finger along the convexity of a spoon. If adjoining surfaces are flush, assume that the contracture is at least 30 degrees (FIGURE 3). The severity of DD can also be graded according to the Tubiana classification system (TABLE17), wherein the total deformity or contracture score is the sum of the angles of all 3 digital joints of the finger.

A look at the treatment options
Once the diagnosis of DD is made, reassure the patient that the disease is not cancerous, although it may be progressive. Advise patients, too, that several treatment options (detailed below) are available, but that surgery is the mainstay of treatment. (Of note: Despite what would seem logical, stretching a cord to straighten the finger is not recommended, as it may actually worsen the condition.18)
Options for early DD. Corticosteroids such as triamcinolone may have a role as an adjunct for early DD by reducing the size and firmness of the Dupuytren’s nodules and possibly slowing the progression of the disease. However, in one study of 63 patients, half of the individuals experienced reactivation of disease between one and 3 years.19
Radiotherapy has been studied as a potential treatment for early DD and for patients unable to undergo surgery. Radiation does have a biologic effect on nodular DD, but has no effect on the cords that cause the joint contracture. In a long-term follow-up study of 135 patients treated with radiotherapy, disease remained stable in 59% of patients, improved in 10%, and progressed in 31%.20 Results were better for early stage disease. This modality is not used for more advanced cases.
Surgery is the primary method by which to restore function and minimize complications. In the past, referral was recommended for MCP joint contractures >30 degrees or for any involvment of the PIP joint, because involvement of the latter carries a worse prognosis and may not be fully correctable, even with surgery.21 However, treatment may also be warranted in cases of functional disability—regardless of the degree of contracture (eg, for pain associated with a prominent nodule or cord when gripping objects).
Depending on disease progression and the surgeon’s preference, the most popular procedures are fasciotomies and fasciectomies. Surgical options for contractures—in increasing order of invasiveness and amounts of fascia removed—are fasciotomy (needle, open), fasciectomy (partial, radical), and dermofasciectomy.
Needle fasciotomy is an outpatient procedure performed under local block. The surgeon uses a needle bevel to transect the diseased cord at multiple puncture sites.
Open fasciotomy is also an outpatient procedure involving an incision that allows direct visualization of the cord and internal structures before dividing the fascia. Percutaneous and open fasciotomies are more successful for MCP joint contractures and less successful for PIP joint contractures.
With partial fasciectomy, only the grossly affected fascia is removed.
With radical fasciectomy, the entire palmar fascia is removed, regardless of which areas are grossly diseased.
Dermofasciectomy removes diseased fascia and the overlying skin, with a skin graft applied to heal the wound.
Another option: Collagenase injection. In February 2010, the US Food and Drug Administration approved collagenase clostridium histolyticum (Xiaflex) as the first drug specifically indicated for the treatment of Dupuytren’s contracture. Collagenase is injected directly into the fascial cord, cleaving its collagen component and progressively softening and breaking down the cord. If not properly injected, collagenase may injure neurovascular structures and may rupture tendons.
So how do the treatment options compare?
We found no randomized prospective trials comparing treatment options for DD, but there are a number of trials that shed light on a variety of the available options.
Treatment success. In general, partial fasciectomies have shown the greatest success in reducing contractures and maintaining the lowest recurrence rates. Correction of contracture to ≤5 degrees was seen in 94% of MCP joint contractures treated with partial fasciectomy, in 77% of MCP joints treated with collagenase, and in 55% of MCP joints treated with percutaneous needle fasciotomies.22,23 PIP joint contracture correction was not as successful: Correction of contracture to ≤5 degrees was seen in 47% of those treated with partial fasciectomy, 40% treated with collagenase, and 26% treated with needle fasciotomy.22,23
Recurrence rates. When recurrence was defined as loss of passive extension >30 degrees, the recurrence rate for MCP joint contractures with partial fasciectomy was 21%, compared with 85% for needle fasciotomy.22 In a similar review, recurrence rates for partial fasciectomy ranged from 12% to 39% compared with 50% to 58% for needle fasciotomy.24 With collagenase injection, 5-year data have shown an overall recurrence rate of 32%, with a recurrence of 26% at the MCP joint and 46% at the PIP joint.25 In this trial, recurrence was
Complications. Although fasciectomies have shown the best efficacy and lowest recurrence rates, complications such as infection, neurapraxia, and digital nerve injury are more likely with these procedures.26,27
Early recognition and referral to a hand specialist for the treatment of DD may allow the use of less invasive techniques and improved functional results.
CASE › Absent a history of trauma and features typical of other hand/digit disorders, we diagnosed Dupuytren’s disease in this patient. Potential treatments included partial fasciectomy and collagenase injection. After discussing the risks and benefits of each of these procedures, the patient elected to undergo a partial fasciectomy. The surgery was uncomplicated and the abnormality was corrected to within 5 degrees of full extension. At the patient’s one-year follow-up visit, there was no evidence of recurrence.
CASE › A 52-year-old right-hand-dominant white man arrived at our clinic complaining that he was unable to straighten his right ring finger. He had no associated pain or numbness, and had not injured his hand. The patient had type 2 diabetes that was controlled with metformin. He had no history of surgery or drug allergies, did not smoke, and said he drank 2 to 3 alcoholic beverages per day. He was a car salesman and was self-conscious when shaking hands with customers. On physical examination, we noted that he held his right ring finger at roughly 45 degrees of flexion at the metacarpophalangeal joint; a painless cord-like structure was palpable on the palmar surface of that joint. His left hand had no abnormalities.
If this were your patient, how would you proceed?
Dupuytren’s disease (DD) is a disabling fibroproliferative disorder of the hand for which there is no cure. While the exact cause of DD is unknown, it has been linked to a number of risk factors, including smoking, alcohol consumption, and diabetes. It affects about 5% of the US population, and up to 70% of affected individuals may initially seek treatment from a primary care physician.1 The disease is also referred to as Dupuytren’s contracture, which describes the flexion contractures of fingers at the end stage of the disease. Palmar fibromatosis is yet another name for the disorder.
DD refers to a spectrum of presentations ranging from nodules to cords to discernible contractures, and it is not known which patients with early Dupuytren changes will progress to severe contracture. With recognition of early changes, nonsurgical intervention is possible, such as collagenase injection or percutaneous fasciotomy, and can slow the progression of DD, restore function, and avoid or delay surgical intervention. DD is a clinically challenging disorder. Treatment for an affected area may resolve symptoms, only to have them recur in that location or another.
How underlying pathology correlates with clinical findings
DD affects the palmar fascia, a thick triangular-shaped sheet of dense fibrous collagenous connective tissue that lies deep to the dermis and superficial to the flexor tendons of the hand with fibers extending both into the skin and into the deep tissue. The palmar fascia secures the skin during gripping and twisting motions, and it bifurcates into distal extensions, called pretendinous bands, that overlay and mimic the flexor tendons.
Clinical findings reflect the progression of underlying pathology. The earliest manifestation of DD is painless dimpling of the skin on the palmar surface of the hand.2 Over time, the underlying fibrosis with increased collagen deposition can progress, leading to development of nodules and eventually, cords, which are sometimes mistaken for flexor tendons. Dupuytren-like fibrotic tissue can occur on the sole of the foot (Ledderhose disease) and penis (Peyronie’s disease).2,3 In patients with these coexisting conditions, prognosis is generally worse.4 With the hands, bilateral involvement is common, although it is usually more severe in one hand. The ring finger is the digit most frequently involved, followed by the little finger, middle finger, and index finger. The thumb is rarely affected.3
As the disease progresses and cords contract, the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints develop flexion contractures. The distal interphalangeal (DIP) joint, rarely involved, instead exhibits a hyperextension contracture. Digital flexion contractures are often disabling, interfering with daily activities such as picking up a glass, shaking hands, or putting one's hand in a pants pocket. Many patients seek medical attention only after a palpable nodule, cord, or flexion contracture becomes apparent (FIGURE 1).

In a study of 326 patients who reported Dupuytren’s symptoms, the most common symptoms that led them to seek treatment were, in descending order, a hard bump (48%), a ropelike growth (12%), dimpling (11%), and finger contractures (10%). Only 9% of patients seeking treatment for hand symptoms associated with DD had received a diagnosis of DD from their initial medical encounter, causing an unnecessary delay in treatment.1
Who is at increased risk for DD?
DD is most often seen in elderly white men of European descent.5 In the United States, the prevalence of the disease is roughly 5%,1 compared with 4% to 39% in northwestern Europe (eg, Iceland).6 The male-to-female ratio of DD ranges from 6:1 to 15:1.7 The prevalence of DD appears to increase with age. The prevalence in men and women is similar up to age 45 years, after which the rate is much greater in males.7
DD is associated with many risk factors including smoking, alcohol consumption, vascular insufficiency, epilepsy, hyperlipidemia, manual labor, occupations with exposure to vibration, hand trauma, and even hand surgery such as carpal tunnel release or trigger finger release.8-11 It is also associated with diabetes; particularly type 1 insulin-dependent diabetes.12 There may also be an association with frozen shoulder.12,13
The need for surgical treatment of DD becomes more likely with a history of cigarette smoking and heavy alcohol consumption. There seems to be an association with epilepsy, most likely from anti-epileptic drugs.14 Rheumatoid arthritis is the only condition that has been associated with a lower incidence of DD, possibly because of the use of anti-inflammatory drugs.15 There are genetic differences between patients with and without DD, although a “Dupuytren’s gene” has not been identified.
Rule out possible DD mimics
The differential diagnosis for flexion contracture of the MCP or PIP joints seen in Dupuytren’s disease includes stenosing flexor tenosynovitis, or trigger finger. Other conditions that can mimic DD are ulnar claw hand, trauma scars, intrinsic joint diseases such as degenerative or rheumatoid arthritis (RA), diabetic cheiroarthropathy, camptodactyly, and Volkmann’s contracture. Trauma scars—especially longitudinal scars—have a tendency to contract and develop keloid formation.
Stenosing flexor tenosynovitis and ulnar claw hand are distinguishable from DD by full or nearly full active or passive extension of the affected digit, whereas DD is a true contracture of the joint that does not allow full extension. A careful history can rule out previous injury to the area. Although intrinsic joint disease, such as RA, can cause finger contractures, the joints are usually enlarged, painful, and associated with characteristic radiologic findings.
Unlike DD, diabetic cheiroarthropathy often involves all of the digits except for the thumb, and is often associated with a waxy appearance of the skin. Camptodactyly is an autosomal dominant disorder that more often presents in childhood and can be caused by a number of congenital syndromes. Volkmann’s contracture can manifest as a claw-like deformity of the hand caused by undiagnosed compartment syndrome of the forearm.

Assess the severity of DD
In your evaluation, first identify palmar or digital fibromatosis presenting as a nodule or a cord. Second, estimate the degree of MCP and PIP joint contractures. A common measure of contracture is the Hueston tabletop test. Ask the patient to place the palm of the hand on a flat surface. If the patient is unable to completely flatten the hand against the surface, presume a positive result (FIGURE 2).16

An accurate measure of the degree of flexion can be accomplished with a goniometer. For a simple assessment of severity, have the patient place each affected finger along the convexity of a spoon. If adjoining surfaces are flush, assume that the contracture is at least 30 degrees (FIGURE 3). The severity of DD can also be graded according to the Tubiana classification system (TABLE17), wherein the total deformity or contracture score is the sum of the angles of all 3 digital joints of the finger.

A look at the treatment options
Once the diagnosis of DD is made, reassure the patient that the disease is not cancerous, although it may be progressive. Advise patients, too, that several treatment options (detailed below) are available, but that surgery is the mainstay of treatment. (Of note: Despite what would seem logical, stretching a cord to straighten the finger is not recommended, as it may actually worsen the condition.18)
Options for early DD. Corticosteroids such as triamcinolone may have a role as an adjunct for early DD by reducing the size and firmness of the Dupuytren’s nodules and possibly slowing the progression of the disease. However, in one study of 63 patients, half of the individuals experienced reactivation of disease between one and 3 years.19
Radiotherapy has been studied as a potential treatment for early DD and for patients unable to undergo surgery. Radiation does have a biologic effect on nodular DD, but has no effect on the cords that cause the joint contracture. In a long-term follow-up study of 135 patients treated with radiotherapy, disease remained stable in 59% of patients, improved in 10%, and progressed in 31%.20 Results were better for early stage disease. This modality is not used for more advanced cases.
Surgery is the primary method by which to restore function and minimize complications. In the past, referral was recommended for MCP joint contractures >30 degrees or for any involvment of the PIP joint, because involvement of the latter carries a worse prognosis and may not be fully correctable, even with surgery.21 However, treatment may also be warranted in cases of functional disability—regardless of the degree of contracture (eg, for pain associated with a prominent nodule or cord when gripping objects).
Depending on disease progression and the surgeon’s preference, the most popular procedures are fasciotomies and fasciectomies. Surgical options for contractures—in increasing order of invasiveness and amounts of fascia removed—are fasciotomy (needle, open), fasciectomy (partial, radical), and dermofasciectomy.
Needle fasciotomy is an outpatient procedure performed under local block. The surgeon uses a needle bevel to transect the diseased cord at multiple puncture sites.
Open fasciotomy is also an outpatient procedure involving an incision that allows direct visualization of the cord and internal structures before dividing the fascia. Percutaneous and open fasciotomies are more successful for MCP joint contractures and less successful for PIP joint contractures.
With partial fasciectomy, only the grossly affected fascia is removed.
With radical fasciectomy, the entire palmar fascia is removed, regardless of which areas are grossly diseased.
Dermofasciectomy removes diseased fascia and the overlying skin, with a skin graft applied to heal the wound.
Another option: Collagenase injection. In February 2010, the US Food and Drug Administration approved collagenase clostridium histolyticum (Xiaflex) as the first drug specifically indicated for the treatment of Dupuytren’s contracture. Collagenase is injected directly into the fascial cord, cleaving its collagen component and progressively softening and breaking down the cord. If not properly injected, collagenase may injure neurovascular structures and may rupture tendons.
So how do the treatment options compare?
We found no randomized prospective trials comparing treatment options for DD, but there are a number of trials that shed light on a variety of the available options.
Treatment success. In general, partial fasciectomies have shown the greatest success in reducing contractures and maintaining the lowest recurrence rates. Correction of contracture to ≤5 degrees was seen in 94% of MCP joint contractures treated with partial fasciectomy, in 77% of MCP joints treated with collagenase, and in 55% of MCP joints treated with percutaneous needle fasciotomies.22,23 PIP joint contracture correction was not as successful: Correction of contracture to ≤5 degrees was seen in 47% of those treated with partial fasciectomy, 40% treated with collagenase, and 26% treated with needle fasciotomy.22,23
Recurrence rates. When recurrence was defined as loss of passive extension >30 degrees, the recurrence rate for MCP joint contractures with partial fasciectomy was 21%, compared with 85% for needle fasciotomy.22 In a similar review, recurrence rates for partial fasciectomy ranged from 12% to 39% compared with 50% to 58% for needle fasciotomy.24 With collagenase injection, 5-year data have shown an overall recurrence rate of 32%, with a recurrence of 26% at the MCP joint and 46% at the PIP joint.25 In this trial, recurrence was
Complications. Although fasciectomies have shown the best efficacy and lowest recurrence rates, complications such as infection, neurapraxia, and digital nerve injury are more likely with these procedures.26,27
Early recognition and referral to a hand specialist for the treatment of DD may allow the use of less invasive techniques and improved functional results.
CASE › Absent a history of trauma and features typical of other hand/digit disorders, we diagnosed Dupuytren’s disease in this patient. Potential treatments included partial fasciectomy and collagenase injection. After discussing the risks and benefits of each of these procedures, the patient elected to undergo a partial fasciectomy. The surgery was uncomplicated and the abnormality was corrected to within 5 degrees of full extension. At the patient’s one-year follow-up visit, there was no evidence of recurrence.
1. DiBenedetti DB, Nguyen D, Zografos L, et al. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand. 2011;6:149-158.
2. Townley WA, Baker R, Sheppard N, et al. Dupuytren’s contracture unfolded. BMJ. 2006;332:397-400.
3. Rayan GM. Clinical presentation and types of Dupuytren’s disease. Hand Clin. 1999;15:87-96.
4. Hindocha S, Stanley JK, Watson S, et al. Dupuytren’s diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31:1626-1634.
5. Bayat A, McGrouther DA. Management of Dupuytren’s disease—clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88:3-8.
6. Gudmundsson KG, Arngrimsson R, Sigfússon M, et al. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53:291-296.
7. Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg Br. 1999;24:456-459.
8. Noble J, Arafa M, Royle SG, et al. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg Br. 1992;17:71–74.
9. Mikkelsen OA. Dupuytren’s disease—initial symptoms, age of onset and spontaneous course. Hand. 1977;9:11-15.
10. Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24:153-159.
11. Liss GM, Stock SR. Can Dupuytren’s contracture be work related? review of the evidence. Am J Ind Med. 1996;29:521-532.
12. Geoghegan JM, Forbes J, Clark DI, et al. Dupuytren’s disease risk factors. J Hand Surg Br. 2004;29:423-426.
13. Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg. 2001;10:149-151.
14. Nunn AC, Schreuder FB. Dupuytren’s contracture: emerging insight into a Viking disease. J Hand Surg. 2014;19:481-490.
15. Arafa M, Steingold R F, Noble J. The incidence of Dupuytren’s disease in patients with rheumatoid arthritis. J Hand Surg Br. 1984;9:165-166.
16. Hueston J. Lessons in Dupuytren’s disease. Ann Chir Main Memb Super. 1992;11:349-354.
17. Tubiana R. Prognosis and treatment of Dupuytren’s contracture. J Bone and Joint Surg AM. 1955;37:1155-1168.
18. Howard JC, Varallo VM, Ross DC, et al. Elevated levels of ß-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16.
19. Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25:1157-1162.
20. Betz N, Ott OJ, Adamietz B, et al. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186:82-90.
21. Smith AC. Diagnosis and indications for surgical treatment. Hand Clin. 1991;7:635-642.
22. van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469-477.
23. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968-979.
24. Chen NC, Srinivasan RC, Shauver MJ, et al. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand. 2011;6:250-255.
25. Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015;40:1597-1605.
26. Toppi JT, Trompf L, Smoll NR, et al. Dupuytren’s contracture: an analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy. ANZ J Surg. 2015;85:639-643.
27. Cheung K, Walley KC, Rozental TD. Management of complications of Dupuytren contracture. Hand Clini. 2015;31:345-354.
1. DiBenedetti DB, Nguyen D, Zografos L, et al. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand. 2011;6:149-158.
2. Townley WA, Baker R, Sheppard N, et al. Dupuytren’s contracture unfolded. BMJ. 2006;332:397-400.
3. Rayan GM. Clinical presentation and types of Dupuytren’s disease. Hand Clin. 1999;15:87-96.
4. Hindocha S, Stanley JK, Watson S, et al. Dupuytren’s diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31:1626-1634.
5. Bayat A, McGrouther DA. Management of Dupuytren’s disease—clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88:3-8.
6. Gudmundsson KG, Arngrimsson R, Sigfússon M, et al. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53:291-296.
7. Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg Br. 1999;24:456-459.
8. Noble J, Arafa M, Royle SG, et al. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg Br. 1992;17:71–74.
9. Mikkelsen OA. Dupuytren’s disease—initial symptoms, age of onset and spontaneous course. Hand. 1977;9:11-15.
10. Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24:153-159.
11. Liss GM, Stock SR. Can Dupuytren’s contracture be work related? review of the evidence. Am J Ind Med. 1996;29:521-532.
12. Geoghegan JM, Forbes J, Clark DI, et al. Dupuytren’s disease risk factors. J Hand Surg Br. 2004;29:423-426.
13. Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg. 2001;10:149-151.
14. Nunn AC, Schreuder FB. Dupuytren’s contracture: emerging insight into a Viking disease. J Hand Surg. 2014;19:481-490.
15. Arafa M, Steingold R F, Noble J. The incidence of Dupuytren’s disease in patients with rheumatoid arthritis. J Hand Surg Br. 1984;9:165-166.
16. Hueston J. Lessons in Dupuytren’s disease. Ann Chir Main Memb Super. 1992;11:349-354.
17. Tubiana R. Prognosis and treatment of Dupuytren’s contracture. J Bone and Joint Surg AM. 1955;37:1155-1168.
18. Howard JC, Varallo VM, Ross DC, et al. Elevated levels of ß-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16.
19. Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25:1157-1162.
20. Betz N, Ott OJ, Adamietz B, et al. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186:82-90.
21. Smith AC. Diagnosis and indications for surgical treatment. Hand Clin. 1991;7:635-642.
22. van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469-477.
23. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968-979.
24. Chen NC, Srinivasan RC, Shauver MJ, et al. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand. 2011;6:250-255.
25. Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015;40:1597-1605.
26. Toppi JT, Trompf L, Smoll NR, et al. Dupuytren’s contracture: an analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy. ANZ J Surg. 2015;85:639-643.
27. Cheung K, Walley KC, Rozental TD. Management of complications of Dupuytren contracture. Hand Clini. 2015;31:345-354.
PRACTICE RECOMMENDATIONS
› Evaluate patients with suspected Dupuytren’s disease (DD) for coexisting conditions such as Ledderhose disease and Peyronie’s disease. C
› Use the Hueston tabletop test to assess severity of DD. C
› Do not recommend stretching exercises for DD; they can hasten disease progression. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
