User login
Recognizing and treating trigger finger
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
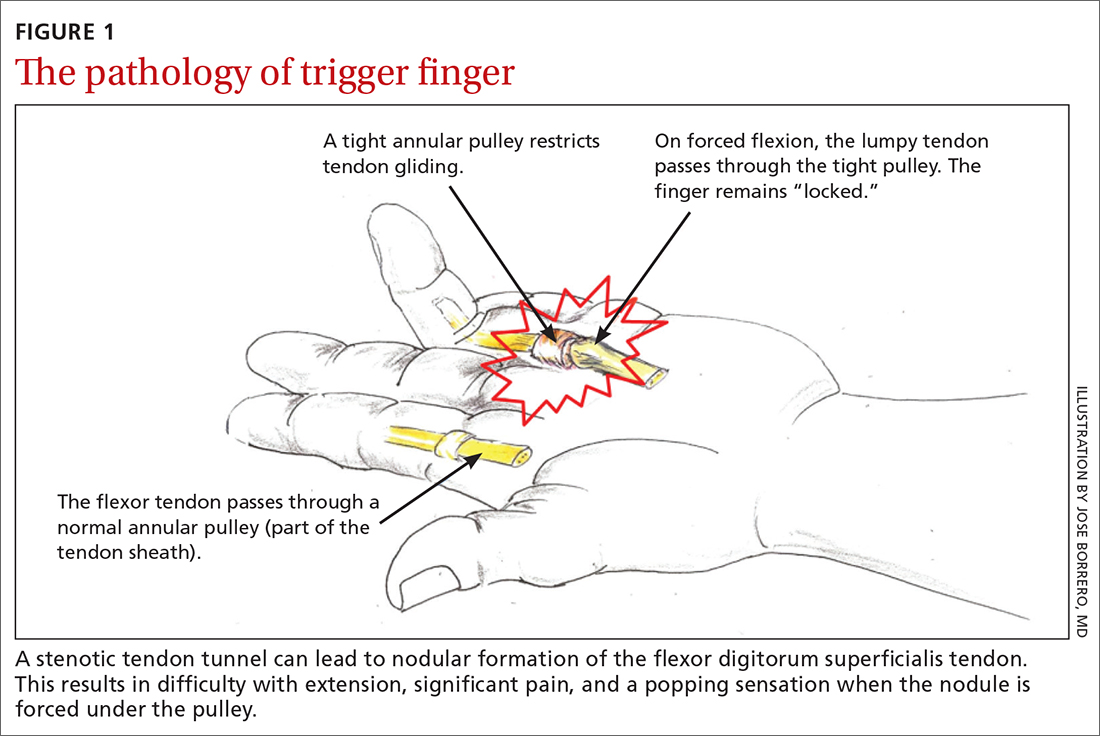
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.
What you’ll see
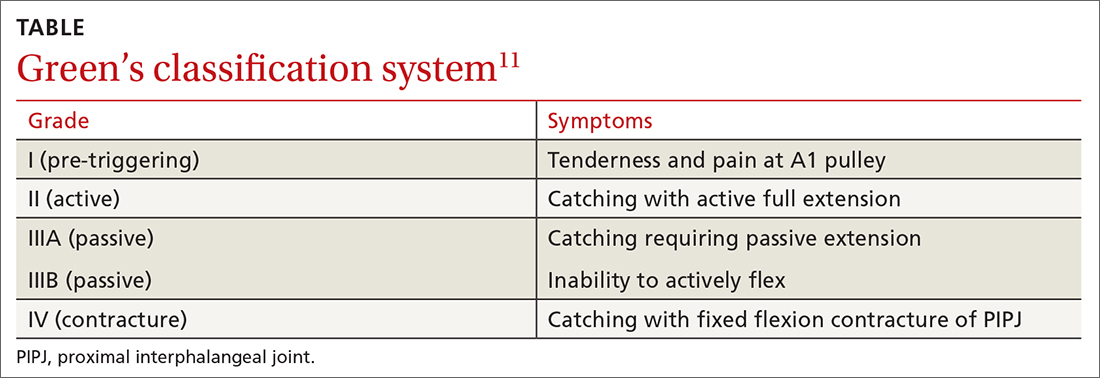
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).
Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
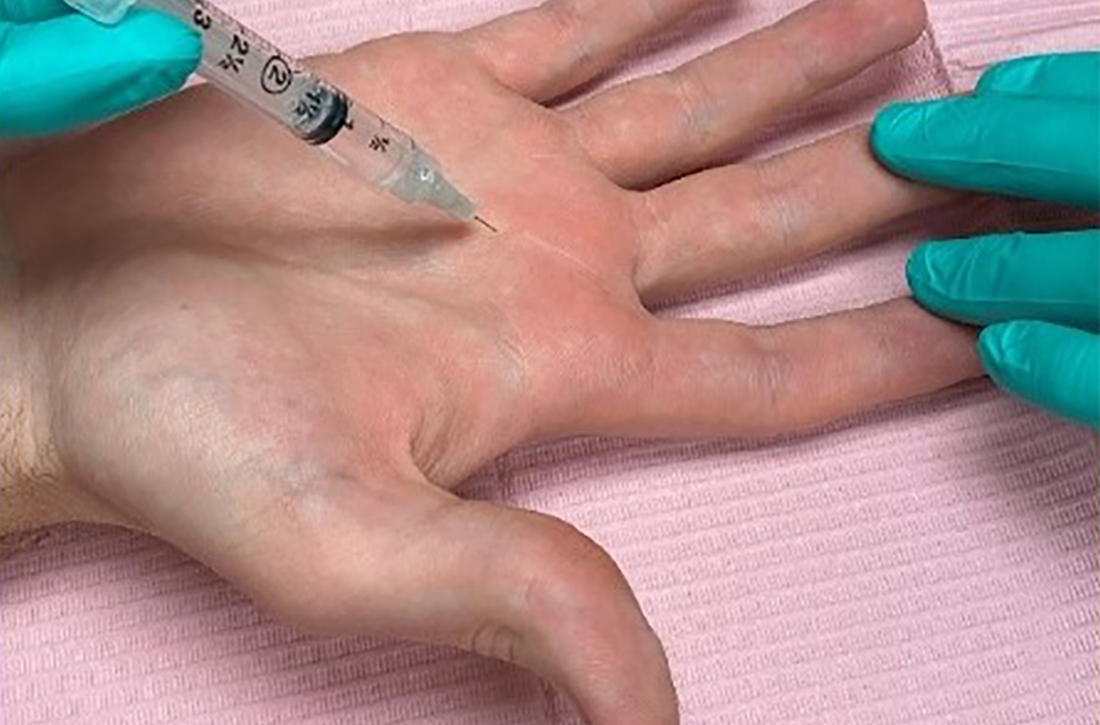
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
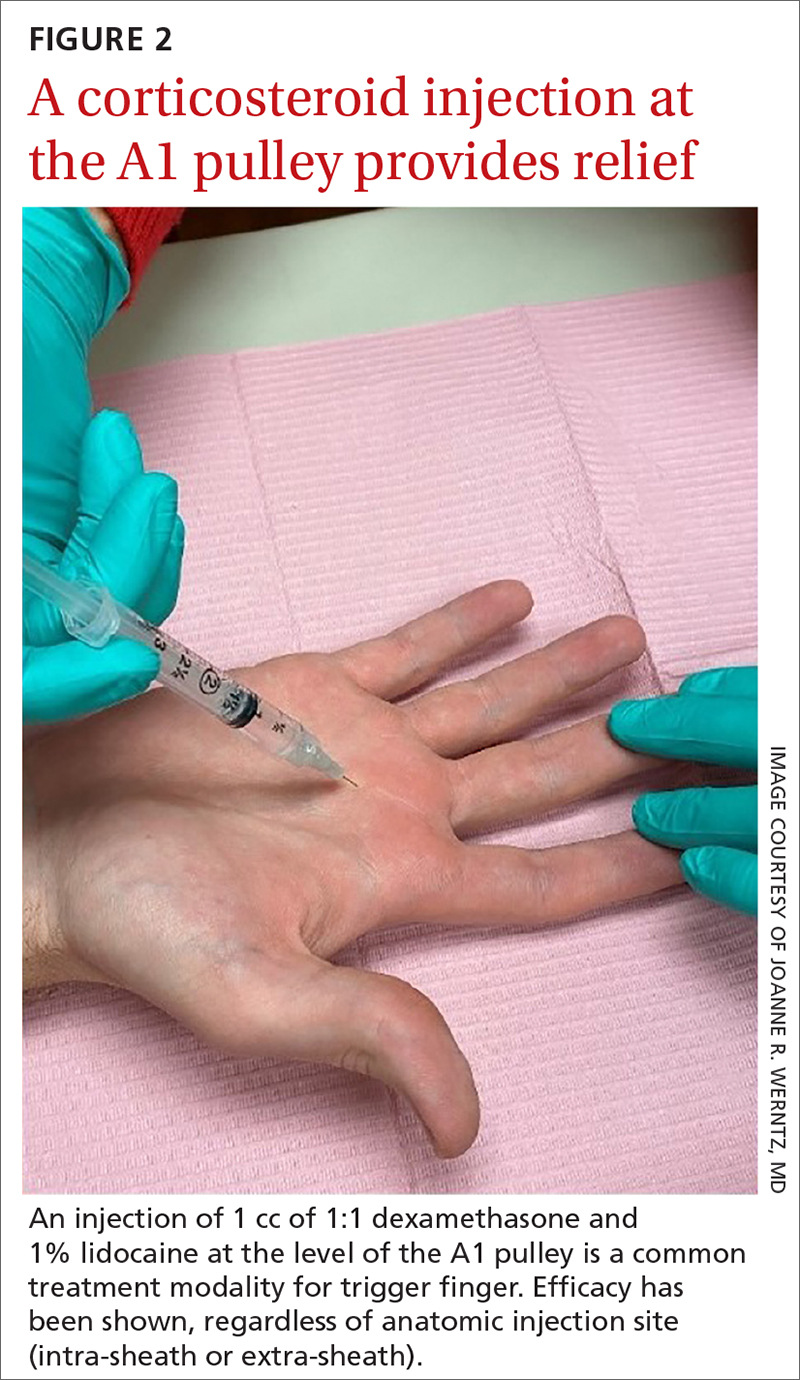
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20
A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
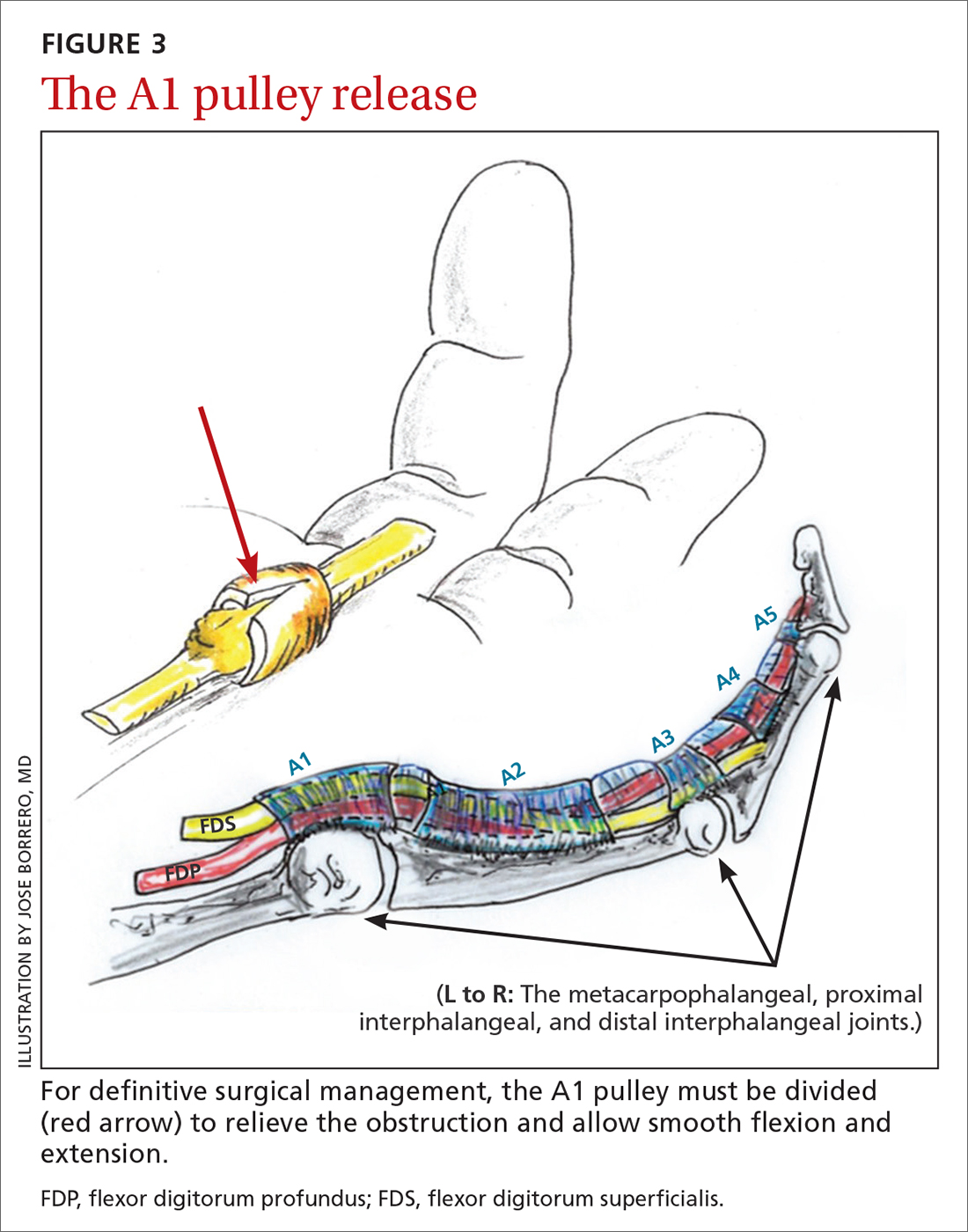
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.
Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.

What you’ll see
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).

Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20

A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.

Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
CASE
A 55-year-old right-hand-dominant woman presented to the clinic with a chief complaint of right ring finger pain and stiffness. There was no history of trauma or prior surgery. She had no tingling or numbness. She had a history of type 2 diabetes that was well controlled. She worked as a clerk for a government office for many years, and her painful, limited finger motion interfered with keyboarding and picking up items. Physical examination revealed tenderness to palpation over the palmar aspect of the metacarpophalangeal joint (MCPJ) of the ring finger with no other joint tenderness or swelling. When she made a fist, her ring finger MCPJ, proximal interphalangeal joint (PIPJ), and distal interphalangeal joint (DIPJ) locked in a flexed position that required manipulation to extend the finger. A firm mass was palpated in the palm with finger flexion that moved into the finger with extension.
Stenosing tenosynovitis, also known as trigger finger (TF), is an inflammatory condition that causes pain in the distal palm and proximal digit with associated limited motion. The most commonly affected digits are the middle and ring fingers of the dominant hand.1 The disorder is particularly noticeable when it inhibits day-to-day functioning.
TF affects 2% to 3% of the general population and up to 20% of patients with diabetes.2,3 Patient age and duration of diabetes are commonly cited as contributing factors, although the effect of well-controlled blood glucose and A1C on the frequency and cure rate of TF has not been established.3,4 TF is most commonly seen in individuals ages 40 to 60 years, with a 6 times’ greater frequency in females than males.5
In the United States, there are an estimated 200,000 cases of TF each year, with initial presentation typically being to a primary care physician.6 For this reason, it is essential for primary care physicians to recognize this common pathology and treat symptoms early to prevent progression and the need for surgical intervention.
An impaired gliding motion of the flexor tendons
In each finger, a tendon sheath, consisting of 5 annular pulleys and 3 cruciate pulleys, forms a tunnel around the flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS). The tendon sheath allows for maximum force by eliminating bowstringing of the tendons when the digit is flexed. Deep to the tendons and surrounding the tendons is a synovial membrane that provides nutrition and reduces friction between the tendons and the tendon sheath.7
The FDP is longer and assists in flexion of the MCPJ and the PIPJ. It is the sole flexor of the DIPJ.
In the thumb, the flexor pollicis longus (FPL) is the only flexor within its tendon sheath. The FPL assists in flexion of the MCPJ and flexes the thumb interphalangeal joint (IPJ). The intrinsic muscles (lumbricals and interossei) do not extend into the tendon sheath and do not contribute to TF.
Continue to: TF occurs when
TF occurs when the tendon sheath, most commonly at the first annular pulley (A1), or the flexor tendons thicken due to fibrocartilaginous metaplasia. This results in impaired gliding motion of the flexor tendons.8 The stenosed A1 pulley can lead to pinching of the flexor tendons and cause the formation of a nodule on the FDS tendon at its bifurcation.9 The nodule of the FDS bifurcation moves proximal to the A1 pulley when the finger is flexed. Upon extension, the tendon nodule may get caught on the A1 pulley. This prevents smooth extension and is the source of pain and triggering (FIGURE 1). In a similar manner, thumb triggering is the result of a stenosed A1 pulley creating a nodule on the FPL tendon, which prevents smooth gliding of the FPL.

What you’ll see
TF is characterized by locking, popping, or clicking at the base of the finger or thumb.7,10 A small nodule may be palpated on the palmar aspect of the MCPJ when the finger is flexed. This nodule will then move distally when the finger is extended. Patients will present with the affected digit in a flexed position and will have difficulty extending the digit. In some cases, the patient may have to use the other hand to straighten the affected digit. In more severe cases, the digit may be fixed in a position of flexion or extension. The severity of triggering is commonly graded by the Green’s classification system (see TABLE11).

Is it Dupuytren contracture, trigger finger, or something else?
The differential diagnosis for TF includes Dupuytren contracture, MCPJ sprain, calcific peritendinitis, flexor tenosynovitis, diabetic cheiroarthropathy (DCA), rheumatoid arthritis (RA), osteoarthritis (OA), and crystalline arthropathy (gout).5
Dupuytren contracture is usually nonpainful and manifests with a palpable cord in the palm and a fixed flexion contracture that has progressed over time, with no history of catching.
MCPJ sprain is diagnosed with tenderness of the MCPJ and a history of trauma.
Continue to: Calcific peritendinitis
Calcific peritendinitis is characterized by pain, tenderness, and edema near a joint with calcified deposits seen on radiographs.
Flexor tenosynovitis manifests with fusiform swelling of the digit, tenderness over the flexor tendon sheath, and pain with passive extension of the digit; it is more commonly associated with RA.
DCA, RA, OA, and gout usually affect more than 1 digit. DCA is associated with both type 1 and type 2 diabetes and is characterized by thickened, waxy skin and painless, limited extension of the digits. RA and OA are diagnosed by medical history, lab work, and radiographs. Gout is diagnosed with lab work and aspiration of joint fluid.
A thorough history, physical exam, and review of radiographs must be performed to rule out these other disorders. Once the diagnosis of TF is made, available treatment options should be pursued.
Treatment: A conservative or surgical approach?
Current treatment options include both nonsurgical (conservative) and surgical interventions. Nonsurgical interventions include activity modification, splinting, and corticosteroid injections. While nonsteroidal anti-inflammatory drugs are commonly recommended to resolve the local inflammation secondary to triggering, there is no scientific evidence to support their use at this time.7 Surgical interventions, utilized in more severe cases or after conservative treatment has failed, include percutaneous and open release of the tendon sheath.2,7
Continue to: Conservative treatments
Conservative treatments
Splinting is only an option for digits that retain flexibility (Green’s classification grades I, II, and III). The goal of splinting is to keep the affected digit in extension to avoid repeated friction between the tendon and the tendon sheath.12 This ideally allows any cartilaginous metaplasia or inflammation to resolve, subsequently alleviating symptoms. The recommended length of treatment with splinting ranges from 3 to 12 weeks, with an average of 6 weeks.1
Multiple studies have shown long-term alleviation of symptoms with the use of orthotic devices. A retrospective analysis found that 87% of patients who wore their PIPJ orthotic device both day and night for a minimum of 6 weeks required no further treatment at 1-year follow-up.13 In contrast, MCPJ splinting only at night has been shown to resolve symptoms in just 55% of patients after 6 weeks.14 From a practical standpoint, however, patients are more likely to be compliant with night-only splinting, making it a reasonable option. Splinting does remain efficacious for patients even after 6 months of symptomatology.15
Day and night splinting for approximately 8 weeks using a PIPJ orthotic could be considered as an effective first-line intervention.16 Notably, PIPJ splinting is more functional, as it allows motion of the MCPJ and DIPJ.
An adjunct treatment to splinting is tendon-gliding exercises, including passive IPJ flexion, full finger flexion and extension, and hooking.13 Patients may remove the orthotic device to perform these exercises 3 times a day for 5 repetitions, as well as for activities that are not conducive to splinting.13
Corticosteroid injections. Injections of a corticosteroid and 1% lidocaine in a 1:1 mixture for a total volume of 1 cc can be inserted into the tendon sheath, A1 pulley, or adjacent tissue.17 Steroid injections help to decrease inflammation and pain in the affected area, giving symptom relief lasting a few months in as many as 57% to 87% of patients.18
Continue to: While the location of the injection...
While the location of the injection has been debated, recent literature suggests that symptoms can be effectively alleviated regardless of the specific anatomic injection site, such as intra-sheath or extra-sheath (FIGURE 2).19 This allows flexibility for the clinician, as the injection does not have to be placed within the tendon sheath. Corticosteroids should not be injected into the tendon itself, and the needle tip should be slightly withdrawn if there is resistance while injecting. Patients who are averse to injections have been shown to benefit from needle-free jet lidocaine (J-tip) administration prior to the actual steroid injection.20

A randomized controlled trial comparing dexamethasone to triamcinolone injections found no difference in outcome at the 3-month follow-up (n = 84).17 This may suggest that the choice of corticosteroid is at the clinician’s discretion. In terms of long-term efficacy of steroid injections, it has been shown that 70% of trigger digits had complete resolution of symptoms at a mean follow-up of 8 years after just 1 injection (n = 43).21
Some patients, though, may require additional corticosteroid injections to maintain symptom control. If multiple injections are performed, they should not be given in intervals shorter than 4 months between treatments.5 Furthermore, steroids can be administered safely up to 3 times in the same digit before surgery is recommended.22
A patient’s options should be reconsidered if efficacy is not demonstrated with prior injections. Notably, a lower success rate has been shown in patients with type 2 diabetes (66%) compared to those without diabetes (90%).4,23 This difference in success rates is not well understood, as there is no causal relationship between well-controlled diabetes and TF.4 Complications of corticosteroid injections include local pain, fat atrophy, and hypopigmentation at the site of the injection, as well as short-term elevations in blood glucose levels in patients with diabetes.5,24
Surgical correction (to be discussed) remains superior to steroid injections in terms of cure rate and resolution of symptoms. A randomized controlled trial (n = 165) found that an injection-only group reported 86% and 49% success at 3-month and 12-month follow-up, respectively, compared to 99% success at both 3- and 12-month follow-up for the surgical group. Further, at 12-month follow-up, the median pain scores were significantly higher in the injection group (3; range, 1-9) than in the surgery group (1; range, 1-7).25 If conservative treatment modalities lead to unresolved symptoms or recurrence, referral to a hand specialist for surgery is recommended.
Continue to: Surgical treatments in an office setting
Surgical treatments in an office setting
Procedures for TF can be safely performed under conscious sedation or local anesthesia, with or without a tourniquet.26 Wide-awake procedures with local anesthesia and no tourniquet (WALANT) can be performed in an office-based procedure room rather than the operating room. This increases efficiency for the surgeon, reduces the amount of preparation and recovery time for the patient, and helps to keep costs down.
Percutaneous release involves the insertion of a 16-gauge hypodermic needle into the affected A1 pulley. The needle is used to fray and disrupt the pulley by moving the needle tip over the fibrotic A1 pulley.
However, it is not without possible complications.27 Inadvertent A2 pulley damage is particularly troublesome, as it leads to “bowstringing” or protrusion of the flexor tendon into the palm upon flexion. This can cause pain and failure to fully extend or flex the finger.10 Because the anatomy is not well visualized during the percutaneous approach, incomplete release, neurovascular injury, and iatrogenic injury to the A2 pulley or deep tendon may occur.28 Ultrasound-guided percutaneous release techniques have shown effective clinical outcomes with minimal complications compared to nonguided percutaneous release techniques.29,30
Open release is the gold standard surgical treatment for trigger finger (FIGURE 3). A small incision (1-2 cm) is made directly over or proximal to the A1 pulley in the distal palmar crease at the base of the affected digit. After blunt dissection through the subcutaneous tissue, the A1 pulley is sharply incised. An open approach has the clear benefit of avoiding the digital neurovascular bundles, as well as visualizing the resolution of triggering upon flexion and extension prior to closure. The WALANT procedure has the advantage of allowing the awake patient to actively flex and extend the digit to determine if the A1 release has been successful prior to closure of the incision.

Outcomes and complications of surgery. A recent systematic review and meta-analysis has shown percutaneous techniques to be successful in 94% of cases.27 The success rate of open surgery has been reported at 99% to 100% at varying follow-up intervals up to 1 year.25,30,31 The complication rate for percutaneous release (guided and nonguided) was calculated at 2.2% (n = 2114).27 In another study, the overall complication rate of open releases was calculated at 1% (n = 999).32 When comparing percutaneous release (guided and nonguided) and open release, a meta-analysis found no significant difference in complication rate (RR = 0.84) or failure rate (RR = 0.94).32
Continue to: Several risk factors...
Several risk factors have been associated with postoperative surgical infection, including recent steroid injection (< 80 d), smoking status, increasing age, and pre-operative use of lidocaine with epinephrine.33 Open release has been shown to be an effective and safe treatment modality for patients with and without diabetes alike.34 Overall, definitive surgical correction has been demonstrated to be superior to conservative measures due to a significantly lower rate of recurrence.35
CASE
Given the patient’s presentation with triggering of the digit, tenderness over the A1 pulley, and lack of trauma history, we diagnosed trigger finger in this patient. Potential treatments included splinting, corticosteroid injections, and surgery. After discussion of the risks and benefits of each treatment option, the patient elected to undergo a corticosteroid injection. She was also given a neoprene finger sleeve to wear every night, and in the daytime when possible.
At 12-week follow-up, she noted early improvement in her triggering, which had since recurred. Due to her history of diabetes, the patient was then referred for surgery. She had an open release under local anesthesia. The surgery was uncomplicated, and the abnormality was corrected. At the patient’s 1-year postoperative follow-up visit, there was no evidence of recurrence, and she had regained full active and passive range of motion of her finger.
Acknowledgements
The authors wish to thank Jose Borrero, MD, for contributing his time and creative talents to produce the illustrations in this article.
CORRESPONDENCE
Evan P. Johnson, MD; 506 South Greer Street, Memphis, TN 38111; [email protected]
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
1. Lunsford D, Valdes K, Hengy S. Conservative management of trigger finger: a systematic review. J Hand Ther. 2019;32:212-221. doi: 10.1016/j.jht.2017.10.016
2. Makkouk AH, Oetgen ME, Swigart CR, et al. Trigger finger: etiology, evaluation, and treatment. Curr Rev Musculoskelet Med. 2008;1:92-96. doi: 10.1007/s12178-007-9012-1
3. Fitzgibbons PG, Weiss AP. Hand manifestations of diabetes mellitus. J Hand Surg Am. 2008;33:771-775. doi: 10.1016/j.jhsa.2008.01.038
4. Junot HSN, Anderson Hertz AFL, Gustavo Vasconcelos GR, et al. Epidemiology of trigger finger: metabolic syndrome as a new perspective of associated disease. Hand (N Y). 2019:1558944719867135. doi: 10.1177/1558944719867135.
5. Matthews A, Smith K, Read L, et al. Trigger finger: an overview of the treatment options. JAAPA. 2019;32:17-21. doi: 10.1097/01.Jaa.0000550281.42592.97
6. Pencle FJ, Waheed A, Molnar JA. Trigger thumb. StatPearls [Internet]. StatPearls Publishing; 2020. www.ncbi.nlm.nih.gov/books/NBK441854/
7. Giugale JM, Fowler JR. Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 2015;46:561-569. doi: 10.1016/j.ocl.2015.06.014
8. Bianchi S, Gitto S, Draghi F. Ultrasound features of trigger finger: review of the literature. J Ultrasound Med. 2019;38:3141-3154. doi: 10.1002/jum.15025
9. Chuang XL, Ooi CC, Chin ST, et al. What triggers in trigger finger? The flexor tendons at the flexor digitorum superficialis bifurcation. J Plast Reconstr Aesthet Surg. 2017;70:1411-1419. doi: 10.1016/j.bjps.2017.05.037
10. Ryzewicz M, Wolf JM. Trigger digits: principles, management, and complications. J Hand Surg Am. 2006;31:135-146. doi: 10.1016/j.jhsa.2005.10.013
11. Chapter 56: Tendinoapthy. In: Wolfe SW, Peterson WC, Kozin SH, Cohen MS. Green’s Operative Hand Surgery. Vol 2. 7th ed. Elsevier; 2017: 1904-1925.
12. Tarbhai K, Hannah S, von Schroeder HP. Trigger finger treatment: a comparison of 2 splint designs. J Hand Surg Am. 2012;37:243-249, 249.e241. doi: 10.1016/j.jhsa.2011.10.038
13. Valdes K. A retrospective review to determine the long-term efficacy of orthotic devices for trigger finger. J Hand Ther. 2012;25:89-95. doi: 10.1016/j.jht.2011.09.005
14. Drijkoningen T, van Berckel M, Becker SJE, et al. Night splinting for idiopathic trigger digits. Hand (N Y). 2018;13:558-562. doi: 10.1177/1558944717725374
15. Colbourn J, Heath N, Manary S, et al. Effectiveness of splinting for the treatment of trigger finger. J Hand Ther. 2008;21:336-343. doi: 10.1197/j.jht.2008.05.001
16. Teo SH, Ng DCL, Wong YKY. Effectiveness of proximal interphalangeal joint-blocking orthosis vs metacarpophalangeal joint-blocking orthosis in trigger digit management: A randomized clinical trial. J Hand Ther. 2018;32:444-451. doi: 10.1016/j.jht.2018.02.007
17. Ring D, Lozano-Calderon S, Shin R, et al. A prospective randomized controlled trial of injection of dexamethasone versus triamcinolone for idiopathic trigger finger. J Hand Surg Am. 2008;33:516-522; discussion 523-514. doi: 10.1016/j.jhsa.2008.01.001
18. Fleisch SB, Spindler KP, Lee DH. Corticosteroid injections in the treatment of trigger finger: A level I and II systematic review. J Am Acad Orthop Surg. 2007;15:166-171. doi: 10.5435/00124635-200703000-00006
19. Shinomiya R, Sunagawa T, Nakashima Y, et al. Impact of corticosteroid injection site on the treatment success rate of trigger finger: a prospective study comparing ultrasound-guided true intra-sheath and true extra-sheath injections. Ultrasound Med Biol. 2016;42:2203-2208. doi: 10.1016/j.ultrasmedbio.2016.05.015
20. Earp BE, Stanbury SJ, Mora AN, et al. Needle-free jet lidocaine administration for preinjection anesthesia in trigger finger injection: a randomized controlled trial. J Hand Surg Am. 2017;42:618-622. doi: 10.1016/j.jhsa.2017.05.001
21. Castellanos J, Munoz-Mahamud E, Dominguez E, et al. Long-term effectiveness of corticosteroid injections for trigger finger and thumb. J Hand Surg Am. 2015;40:121-126. doi: 10.1016/j.jhsa.2014.09.006
22. Dala-Ali BM, Nakhdjevani A, Lloyd MA, et al. The efficacy of steroid injection in the treatment of trigger finger. Clin Orthop Surg. 2012;4:263-268. doi: 10.4055/cios.2012.4.4.263
23. Griggs SM, Weiss AP, Lane LB, et al. Treatment of trigger finger in patients with diabetes mellitus. J Hand Surg Am. 1995;20:787-789. doi: 10.1016/s0363-5023(05)80432-0
24. Stepan JG, London DA, Boyer MI, et al. Blood glucose levels in diabetic patients following corticosteroid injections into the hand and wrist. J Hand Surg Am. 2014;39:706-712. doi: 10.1016/j.jhsa.2014.01.014
25. Hansen RL, Sondergaard M, Lange J. Open surgery versus ultrasound-guided corticosteroid injection for trigger finger: a randomized controlled trial with 1-year follow-up. J Hand Surg Am. 2017;42:359-366. doi: 10.1016/j.jhsa.2017.02.011
26. Mohd Rashid MZ, Sapuan J, Abdullah S. A randomized controlled trial of trigger finger release under digital anesthesia with (WALANT) and without adrenaline. J Orthop Surg (Hong Kong). 2019;27:2309499019833002. doi: 10.1177/2309499019833002
27. Zhao J-G, Kan S-L, Zhao L, et al. Percutaneous first annular pulley release for trigger digits: a systematic review and meta-analysis of current evidence. J Hand Surg Am. 2014;39:2192-2202. doi: 10.1016/j.jhsa.2014.07.044
28. Guler F, Kose O, Ercan EC, et al. Open versus percutaneous release for the treatment of trigger thumb. Orthopedics. 2013;36:e1290-1294. doi: 10.3928/01477447-20130920-22
29. Wu KC, Chern TC, Jou IM. Ultrasound-assisted percutaneous trigger finger release: it is safe [letter]. Hand (N Y). 2009;4:339. doi: 10.1007/s11552-009-9179-6
30. Nikolaou VS, Malahias M-A, Kaseta M-K, et al. Comparative clinical study of ultrasound-guided A1 pulley release vs open surgical intervention in the treatment of trigger finger. World J Orthop. 2017;8:163-169. doi: 10.5312/wjo.v8.i2.163
31. Lim M-H, Lim K-K, Rasheed MZ, et al. Outcome of open trigger digit release. J Hand Surg Eur Vol. 2007;32:457-459. doi: 10.1016/j.Jhsb.2007.02.016
32. Wang J, Zhao J-G, Liang C-C. Percutaneous release, open surgery, or corticosteroid injection, which is the best treatment method for trigger digits? Clin Orthop Relat Res. 2013;471:1879-1886. doi: 10.1007/s11999-012-2716-6
33. Ng WKY, Olmscheid N, Worhacz K, et al. Steroid injection and open trigger finger release outcomes: a retrospective review of 999 digits. Hand (N Y). 2018;15:399-406. doi: 10.1177/1558944718796559
34. Ho SWL, Chia CY, Rajaratnam V. Characteristics and clinical outcomes of open surgery for trigger digits in diabetes. J Hand Microsurg. 2019;11:80-83. doi: 10.1055/s-0038-1670927
35. Sato ES, dos Santos JB, Belloti JC, et al. Percutaneous release of trigger fingers. Hand Clin. 2014;30:39-45. doi: 10.1016/j.hcl.2013.08.017
PRACTICE RECOMMENDATIONS
› Recommend splinting as a first-line conservative treatment for trigger finger if there is not a fixed contracture. B
› Prescribe corticosteroids, which may completely resolve trigger finger in the majority of patients without diabetes. A
› Refer patients for surgical release if they do not respond to conservative management. The surgical success rate is as high as 99%. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Dupuytren’s disease: How to recognize its early signs
CASE › A 52-year-old right-hand-dominant white man arrived at our clinic complaining that he was unable to straighten his right ring finger. He had no associated pain or numbness, and had not injured his hand. The patient had type 2 diabetes that was controlled with metformin. He had no history of surgery or drug allergies, did not smoke, and said he drank 2 to 3 alcoholic beverages per day. He was a car salesman and was self-conscious when shaking hands with customers. On physical examination, we noted that he held his right ring finger at roughly 45 degrees of flexion at the metacarpophalangeal joint; a painless cord-like structure was palpable on the palmar surface of that joint. His left hand had no abnormalities.
If this were your patient, how would you proceed?
Dupuytren’s disease (DD) is a disabling fibroproliferative disorder of the hand for which there is no cure. While the exact cause of DD is unknown, it has been linked to a number of risk factors, including smoking, alcohol consumption, and diabetes. It affects about 5% of the US population, and up to 70% of affected individuals may initially seek treatment from a primary care physician.1 The disease is also referred to as Dupuytren’s contracture, which describes the flexion contractures of fingers at the end stage of the disease. Palmar fibromatosis is yet another name for the disorder.
DD refers to a spectrum of presentations ranging from nodules to cords to discernible contractures, and it is not known which patients with early Dupuytren changes will progress to severe contracture. With recognition of early changes, nonsurgical intervention is possible, such as collagenase injection or percutaneous fasciotomy, and can slow the progression of DD, restore function, and avoid or delay surgical intervention. DD is a clinically challenging disorder. Treatment for an affected area may resolve symptoms, only to have them recur in that location or another.
How underlying pathology correlates with clinical findings
DD affects the palmar fascia, a thick triangular-shaped sheet of dense fibrous collagenous connective tissue that lies deep to the dermis and superficial to the flexor tendons of the hand with fibers extending both into the skin and into the deep tissue. The palmar fascia secures the skin during gripping and twisting motions, and it bifurcates into distal extensions, called pretendinous bands, that overlay and mimic the flexor tendons.
Clinical findings reflect the progression of underlying pathology. The earliest manifestation of DD is painless dimpling of the skin on the palmar surface of the hand.2 Over time, the underlying fibrosis with increased collagen deposition can progress, leading to development of nodules and eventually, cords, which are sometimes mistaken for flexor tendons. Dupuytren-like fibrotic tissue can occur on the sole of the foot (Ledderhose disease) and penis (Peyronie’s disease).2,3 In patients with these coexisting conditions, prognosis is generally worse.4 With the hands, bilateral involvement is common, although it is usually more severe in one hand. The ring finger is the digit most frequently involved, followed by the little finger, middle finger, and index finger. The thumb is rarely affected.3
As the disease progresses and cords contract, the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints develop flexion contractures. The distal interphalangeal (DIP) joint, rarely involved, instead exhibits a hyperextension contracture. Digital flexion contractures are often disabling, interfering with daily activities such as picking up a glass, shaking hands, or putting one's hand in a pants pocket. Many patients seek medical attention only after a palpable nodule, cord, or flexion contracture becomes apparent (FIGURE 1).
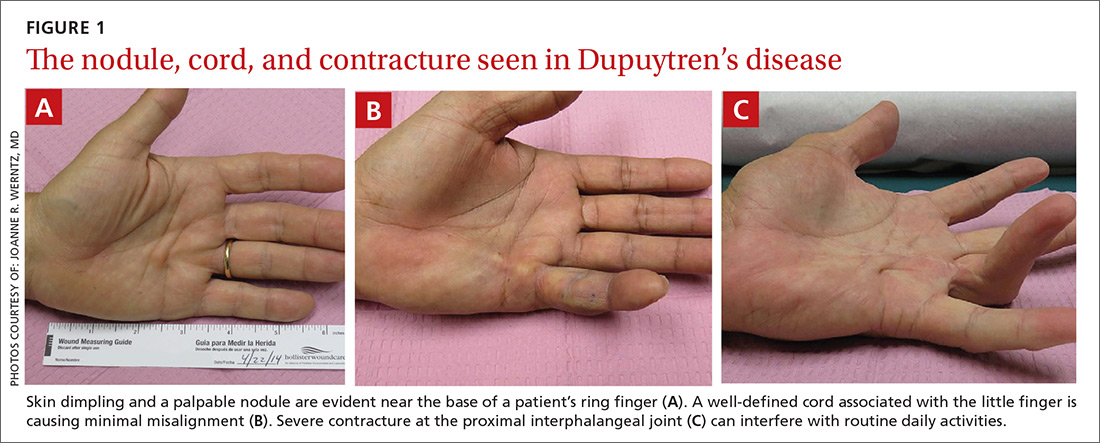
In a study of 326 patients who reported Dupuytren’s symptoms, the most common symptoms that led them to seek treatment were, in descending order, a hard bump (48%), a ropelike growth (12%), dimpling (11%), and finger contractures (10%). Only 9% of patients seeking treatment for hand symptoms associated with DD had received a diagnosis of DD from their initial medical encounter, causing an unnecessary delay in treatment.1
Who is at increased risk for DD?
DD is most often seen in elderly white men of European descent.5 In the United States, the prevalence of the disease is roughly 5%,1 compared with 4% to 39% in northwestern Europe (eg, Iceland).6 The male-to-female ratio of DD ranges from 6:1 to 15:1.7 The prevalence of DD appears to increase with age. The prevalence in men and women is similar up to age 45 years, after which the rate is much greater in males.7
DD is associated with many risk factors including smoking, alcohol consumption, vascular insufficiency, epilepsy, hyperlipidemia, manual labor, occupations with exposure to vibration, hand trauma, and even hand surgery such as carpal tunnel release or trigger finger release.8-11 It is also associated with diabetes; particularly type 1 insulin-dependent diabetes.12 There may also be an association with frozen shoulder.12,13
The need for surgical treatment of DD becomes more likely with a history of cigarette smoking and heavy alcohol consumption. There seems to be an association with epilepsy, most likely from anti-epileptic drugs.14 Rheumatoid arthritis is the only condition that has been associated with a lower incidence of DD, possibly because of the use of anti-inflammatory drugs.15 There are genetic differences between patients with and without DD, although a “Dupuytren’s gene” has not been identified.
Rule out possible DD mimics
The differential diagnosis for flexion contracture of the MCP or PIP joints seen in Dupuytren’s disease includes stenosing flexor tenosynovitis, or trigger finger. Other conditions that can mimic DD are ulnar claw hand, trauma scars, intrinsic joint diseases such as degenerative or rheumatoid arthritis (RA), diabetic cheiroarthropathy, camptodactyly, and Volkmann’s contracture. Trauma scars—especially longitudinal scars—have a tendency to contract and develop keloid formation.
Stenosing flexor tenosynovitis and ulnar claw hand are distinguishable from DD by full or nearly full active or passive extension of the affected digit, whereas DD is a true contracture of the joint that does not allow full extension. A careful history can rule out previous injury to the area. Although intrinsic joint disease, such as RA, can cause finger contractures, the joints are usually enlarged, painful, and associated with characteristic radiologic findings.
Unlike DD, diabetic cheiroarthropathy often involves all of the digits except for the thumb, and is often associated with a waxy appearance of the skin. Camptodactyly is an autosomal dominant disorder that more often presents in childhood and can be caused by a number of congenital syndromes. Volkmann’s contracture can manifest as a claw-like deformity of the hand caused by undiagnosed compartment syndrome of the forearm.
Assess the severity of DD
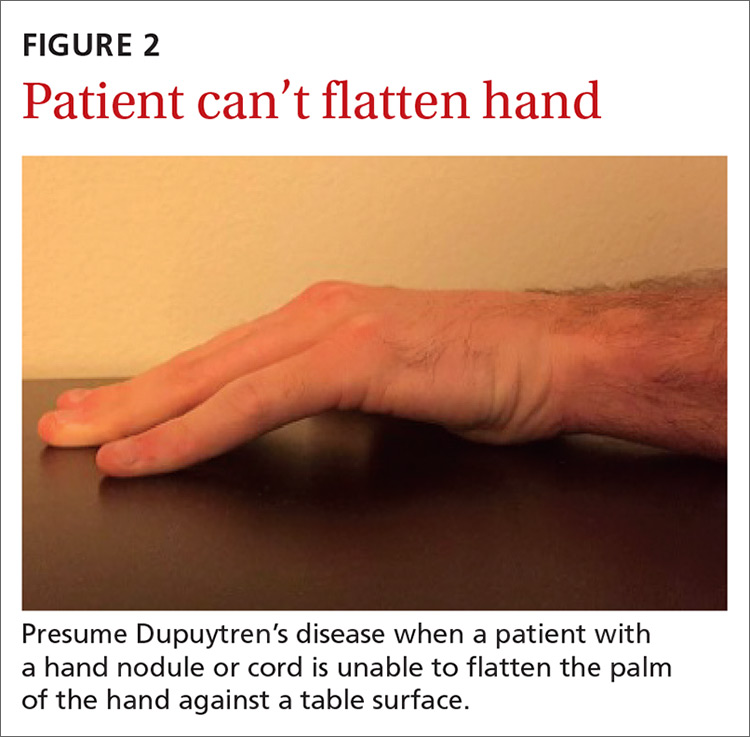
In your evaluation, first identify palmar or digital fibromatosis presenting as a nodule or a cord. Second, estimate the degree of MCP and PIP joint contractures. A common measure of contracture is the Hueston tabletop test. Ask the patient to place the palm of the hand on a flat surface. If the patient is unable to completely flatten the hand against the surface, presume a positive result (FIGURE 2).16

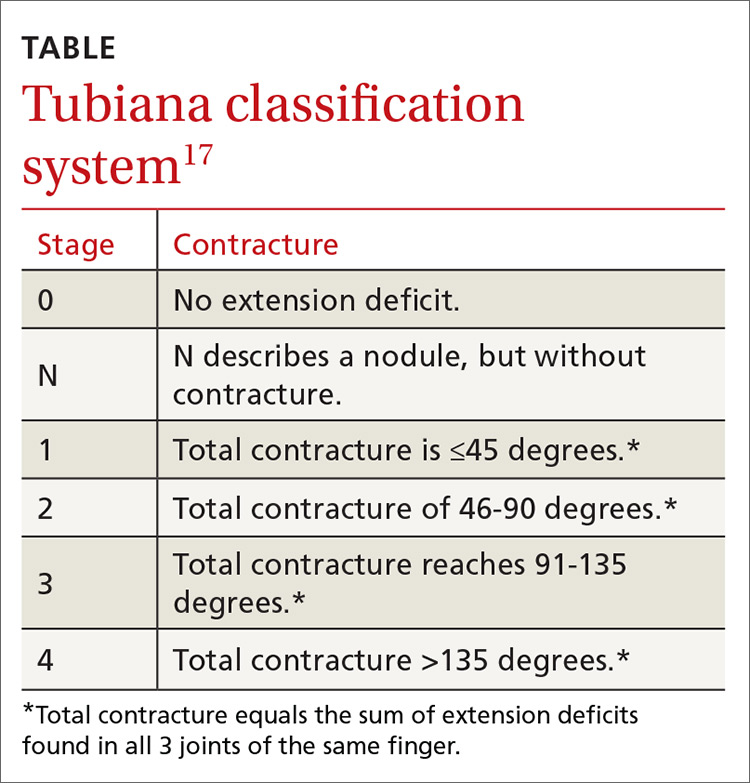
An accurate measure of the degree of flexion can be accomplished with a goniometer. For a simple assessment of severity, have the patient place each affected finger along the convexity of a spoon. If adjoining surfaces are flush, assume that the contracture is at least 30 degrees (FIGURE 3). The severity of DD can also be graded according to the Tubiana classification system (TABLE17), wherein the total deformity or contracture score is the sum of the angles of all 3 digital joints of the finger.
A look at the treatment options
Once the diagnosis of DD is made, reassure the patient that the disease is not cancerous, although it may be progressive. Advise patients, too, that several treatment options (detailed below) are available, but that surgery is the mainstay of treatment. (Of note: Despite what would seem logical, stretching a cord to straighten the finger is not recommended, as it may actually worsen the condition.18)
Options for early DD. Corticosteroids such as triamcinolone may have a role as an adjunct for early DD by reducing the size and firmness of the Dupuytren’s nodules and possibly slowing the progression of the disease. However, in one study of 63 patients, half of the individuals experienced reactivation of disease between one and 3 years.19
Radiotherapy has been studied as a potential treatment for early DD and for patients unable to undergo surgery. Radiation does have a biologic effect on nodular DD, but has no effect on the cords that cause the joint contracture. In a long-term follow-up study of 135 patients treated with radiotherapy, disease remained stable in 59% of patients, improved in 10%, and progressed in 31%.20 Results were better for early stage disease. This modality is not used for more advanced cases.
Surgery is the primary method by which to restore function and minimize complications. In the past, referral was recommended for MCP joint contractures >30 degrees or for any involvment of the PIP joint, because involvement of the latter carries a worse prognosis and may not be fully correctable, even with surgery.21 However, treatment may also be warranted in cases of functional disability—regardless of the degree of contracture (eg, for pain associated with a prominent nodule or cord when gripping objects).
Depending on disease progression and the surgeon’s preference, the most popular procedures are fasciotomies and fasciectomies. Surgical options for contractures—in increasing order of invasiveness and amounts of fascia removed—are fasciotomy (needle, open), fasciectomy (partial, radical), and dermofasciectomy.
Needle fasciotomy is an outpatient procedure performed under local block. The surgeon uses a needle bevel to transect the diseased cord at multiple puncture sites.
Open fasciotomy is also an outpatient procedure involving an incision that allows direct visualization of the cord and internal structures before dividing the fascia. Percutaneous and open fasciotomies are more successful for MCP joint contractures and less successful for PIP joint contractures.
With partial fasciectomy, only the grossly affected fascia is removed.
With radical fasciectomy, the entire palmar fascia is removed, regardless of which areas are grossly diseased.
Dermofasciectomy removes diseased fascia and the overlying skin, with a skin graft applied to heal the wound.
Another option: Collagenase injection. In February 2010, the US Food and Drug Administration approved collagenase clostridium histolyticum (Xiaflex) as the first drug specifically indicated for the treatment of Dupuytren’s contracture. Collagenase is injected directly into the fascial cord, cleaving its collagen component and progressively softening and breaking down the cord. If not properly injected, collagenase may injure neurovascular structures and may rupture tendons.
So how do the treatment options compare?
We found no randomized prospective trials comparing treatment options for DD, but there are a number of trials that shed light on a variety of the available options.
Treatment success. In general, partial fasciectomies have shown the greatest success in reducing contractures and maintaining the lowest recurrence rates. Correction of contracture to ≤5 degrees was seen in 94% of MCP joint contractures treated with partial fasciectomy, in 77% of MCP joints treated with collagenase, and in 55% of MCP joints treated with percutaneous needle fasciotomies.22,23 PIP joint contracture correction was not as successful: Correction of contracture to ≤5 degrees was seen in 47% of those treated with partial fasciectomy, 40% treated with collagenase, and 26% treated with needle fasciotomy.22,23
Recurrence rates. When recurrence was defined as loss of passive extension >30 degrees, the recurrence rate for MCP joint contractures with partial fasciectomy was 21%, compared with 85% for needle fasciotomy.22 In a similar review, recurrence rates for partial fasciectomy ranged from 12% to 39% compared with 50% to 58% for needle fasciotomy.24 With collagenase injection, 5-year data have shown an overall recurrence rate of 32%, with a recurrence of 26% at the MCP joint and 46% at the PIP joint.25 In this trial, recurrence was
Complications. Although fasciectomies have shown the best efficacy and lowest recurrence rates, complications such as infection, neurapraxia, and digital nerve injury are more likely with these procedures.26,27
Early recognition and referral to a hand specialist for the treatment of DD may allow the use of less invasive techniques and improved functional results.
CASE › Absent a history of trauma and features typical of other hand/digit disorders, we diagnosed Dupuytren’s disease in this patient. Potential treatments included partial fasciectomy and collagenase injection. After discussing the risks and benefits of each of these procedures, the patient elected to undergo a partial fasciectomy. The surgery was uncomplicated and the abnormality was corrected to within 5 degrees of full extension. At the patient’s one-year follow-up visit, there was no evidence of recurrence.
1. DiBenedetti DB, Nguyen D, Zografos L, et al. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand. 2011;6:149-158.
2. Townley WA, Baker R, Sheppard N, et al. Dupuytren’s contracture unfolded. BMJ. 2006;332:397-400.
3. Rayan GM. Clinical presentation and types of Dupuytren’s disease. Hand Clin. 1999;15:87-96.
4. Hindocha S, Stanley JK, Watson S, et al. Dupuytren’s diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31:1626-1634.
5. Bayat A, McGrouther DA. Management of Dupuytren’s disease—clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88:3-8.
6. Gudmundsson KG, Arngrimsson R, Sigfússon M, et al. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53:291-296.
7. Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg Br. 1999;24:456-459.
8. Noble J, Arafa M, Royle SG, et al. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg Br. 1992;17:71–74.
9. Mikkelsen OA. Dupuytren’s disease—initial symptoms, age of onset and spontaneous course. Hand. 1977;9:11-15.
10. Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24:153-159.
11. Liss GM, Stock SR. Can Dupuytren’s contracture be work related? review of the evidence. Am J Ind Med. 1996;29:521-532.
12. Geoghegan JM, Forbes J, Clark DI, et al. Dupuytren’s disease risk factors. J Hand Surg Br. 2004;29:423-426.
13. Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg. 2001;10:149-151.
14. Nunn AC, Schreuder FB. Dupuytren’s contracture: emerging insight into a Viking disease. J Hand Surg. 2014;19:481-490.
15. Arafa M, Steingold R F, Noble J. The incidence of Dupuytren’s disease in patients with rheumatoid arthritis. J Hand Surg Br. 1984;9:165-166.
16. Hueston J. Lessons in Dupuytren’s disease. Ann Chir Main Memb Super. 1992;11:349-354.
17. Tubiana R. Prognosis and treatment of Dupuytren’s contracture. J Bone and Joint Surg AM. 1955;37:1155-1168.
18. Howard JC, Varallo VM, Ross DC, et al. Elevated levels of ß-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16.
19. Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25:1157-1162.
20. Betz N, Ott OJ, Adamietz B, et al. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186:82-90.
21. Smith AC. Diagnosis and indications for surgical treatment. Hand Clin. 1991;7:635-642.
22. van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469-477.
23. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968-979.
24. Chen NC, Srinivasan RC, Shauver MJ, et al. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand. 2011;6:250-255.
25. Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015;40:1597-1605.
26. Toppi JT, Trompf L, Smoll NR, et al. Dupuytren’s contracture: an analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy. ANZ J Surg. 2015;85:639-643.
27. Cheung K, Walley KC, Rozental TD. Management of complications of Dupuytren contracture. Hand Clini. 2015;31:345-354.
CASE › A 52-year-old right-hand-dominant white man arrived at our clinic complaining that he was unable to straighten his right ring finger. He had no associated pain or numbness, and had not injured his hand. The patient had type 2 diabetes that was controlled with metformin. He had no history of surgery or drug allergies, did not smoke, and said he drank 2 to 3 alcoholic beverages per day. He was a car salesman and was self-conscious when shaking hands with customers. On physical examination, we noted that he held his right ring finger at roughly 45 degrees of flexion at the metacarpophalangeal joint; a painless cord-like structure was palpable on the palmar surface of that joint. His left hand had no abnormalities.
If this were your patient, how would you proceed?
Dupuytren’s disease (DD) is a disabling fibroproliferative disorder of the hand for which there is no cure. While the exact cause of DD is unknown, it has been linked to a number of risk factors, including smoking, alcohol consumption, and diabetes. It affects about 5% of the US population, and up to 70% of affected individuals may initially seek treatment from a primary care physician.1 The disease is also referred to as Dupuytren’s contracture, which describes the flexion contractures of fingers at the end stage of the disease. Palmar fibromatosis is yet another name for the disorder.
DD refers to a spectrum of presentations ranging from nodules to cords to discernible contractures, and it is not known which patients with early Dupuytren changes will progress to severe contracture. With recognition of early changes, nonsurgical intervention is possible, such as collagenase injection or percutaneous fasciotomy, and can slow the progression of DD, restore function, and avoid or delay surgical intervention. DD is a clinically challenging disorder. Treatment for an affected area may resolve symptoms, only to have them recur in that location or another.
How underlying pathology correlates with clinical findings
DD affects the palmar fascia, a thick triangular-shaped sheet of dense fibrous collagenous connective tissue that lies deep to the dermis and superficial to the flexor tendons of the hand with fibers extending both into the skin and into the deep tissue. The palmar fascia secures the skin during gripping and twisting motions, and it bifurcates into distal extensions, called pretendinous bands, that overlay and mimic the flexor tendons.
Clinical findings reflect the progression of underlying pathology. The earliest manifestation of DD is painless dimpling of the skin on the palmar surface of the hand.2 Over time, the underlying fibrosis with increased collagen deposition can progress, leading to development of nodules and eventually, cords, which are sometimes mistaken for flexor tendons. Dupuytren-like fibrotic tissue can occur on the sole of the foot (Ledderhose disease) and penis (Peyronie’s disease).2,3 In patients with these coexisting conditions, prognosis is generally worse.4 With the hands, bilateral involvement is common, although it is usually more severe in one hand. The ring finger is the digit most frequently involved, followed by the little finger, middle finger, and index finger. The thumb is rarely affected.3
As the disease progresses and cords contract, the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints develop flexion contractures. The distal interphalangeal (DIP) joint, rarely involved, instead exhibits a hyperextension contracture. Digital flexion contractures are often disabling, interfering with daily activities such as picking up a glass, shaking hands, or putting one's hand in a pants pocket. Many patients seek medical attention only after a palpable nodule, cord, or flexion contracture becomes apparent (FIGURE 1).

In a study of 326 patients who reported Dupuytren’s symptoms, the most common symptoms that led them to seek treatment were, in descending order, a hard bump (48%), a ropelike growth (12%), dimpling (11%), and finger contractures (10%). Only 9% of patients seeking treatment for hand symptoms associated with DD had received a diagnosis of DD from their initial medical encounter, causing an unnecessary delay in treatment.1
Who is at increased risk for DD?
DD is most often seen in elderly white men of European descent.5 In the United States, the prevalence of the disease is roughly 5%,1 compared with 4% to 39% in northwestern Europe (eg, Iceland).6 The male-to-female ratio of DD ranges from 6:1 to 15:1.7 The prevalence of DD appears to increase with age. The prevalence in men and women is similar up to age 45 years, after which the rate is much greater in males.7
DD is associated with many risk factors including smoking, alcohol consumption, vascular insufficiency, epilepsy, hyperlipidemia, manual labor, occupations with exposure to vibration, hand trauma, and even hand surgery such as carpal tunnel release or trigger finger release.8-11 It is also associated with diabetes; particularly type 1 insulin-dependent diabetes.12 There may also be an association with frozen shoulder.12,13
The need for surgical treatment of DD becomes more likely with a history of cigarette smoking and heavy alcohol consumption. There seems to be an association with epilepsy, most likely from anti-epileptic drugs.14 Rheumatoid arthritis is the only condition that has been associated with a lower incidence of DD, possibly because of the use of anti-inflammatory drugs.15 There are genetic differences between patients with and without DD, although a “Dupuytren’s gene” has not been identified.
Rule out possible DD mimics
The differential diagnosis for flexion contracture of the MCP or PIP joints seen in Dupuytren’s disease includes stenosing flexor tenosynovitis, or trigger finger. Other conditions that can mimic DD are ulnar claw hand, trauma scars, intrinsic joint diseases such as degenerative or rheumatoid arthritis (RA), diabetic cheiroarthropathy, camptodactyly, and Volkmann’s contracture. Trauma scars—especially longitudinal scars—have a tendency to contract and develop keloid formation.
Stenosing flexor tenosynovitis and ulnar claw hand are distinguishable from DD by full or nearly full active or passive extension of the affected digit, whereas DD is a true contracture of the joint that does not allow full extension. A careful history can rule out previous injury to the area. Although intrinsic joint disease, such as RA, can cause finger contractures, the joints are usually enlarged, painful, and associated with characteristic radiologic findings.
Unlike DD, diabetic cheiroarthropathy often involves all of the digits except for the thumb, and is often associated with a waxy appearance of the skin. Camptodactyly is an autosomal dominant disorder that more often presents in childhood and can be caused by a number of congenital syndromes. Volkmann’s contracture can manifest as a claw-like deformity of the hand caused by undiagnosed compartment syndrome of the forearm.

Assess the severity of DD
In your evaluation, first identify palmar or digital fibromatosis presenting as a nodule or a cord. Second, estimate the degree of MCP and PIP joint contractures. A common measure of contracture is the Hueston tabletop test. Ask the patient to place the palm of the hand on a flat surface. If the patient is unable to completely flatten the hand against the surface, presume a positive result (FIGURE 2).16

An accurate measure of the degree of flexion can be accomplished with a goniometer. For a simple assessment of severity, have the patient place each affected finger along the convexity of a spoon. If adjoining surfaces are flush, assume that the contracture is at least 30 degrees (FIGURE 3). The severity of DD can also be graded according to the Tubiana classification system (TABLE17), wherein the total deformity or contracture score is the sum of the angles of all 3 digital joints of the finger.

A look at the treatment options
Once the diagnosis of DD is made, reassure the patient that the disease is not cancerous, although it may be progressive. Advise patients, too, that several treatment options (detailed below) are available, but that surgery is the mainstay of treatment. (Of note: Despite what would seem logical, stretching a cord to straighten the finger is not recommended, as it may actually worsen the condition.18)
Options for early DD. Corticosteroids such as triamcinolone may have a role as an adjunct for early DD by reducing the size and firmness of the Dupuytren’s nodules and possibly slowing the progression of the disease. However, in one study of 63 patients, half of the individuals experienced reactivation of disease between one and 3 years.19
Radiotherapy has been studied as a potential treatment for early DD and for patients unable to undergo surgery. Radiation does have a biologic effect on nodular DD, but has no effect on the cords that cause the joint contracture. In a long-term follow-up study of 135 patients treated with radiotherapy, disease remained stable in 59% of patients, improved in 10%, and progressed in 31%.20 Results were better for early stage disease. This modality is not used for more advanced cases.
Surgery is the primary method by which to restore function and minimize complications. In the past, referral was recommended for MCP joint contractures >30 degrees or for any involvment of the PIP joint, because involvement of the latter carries a worse prognosis and may not be fully correctable, even with surgery.21 However, treatment may also be warranted in cases of functional disability—regardless of the degree of contracture (eg, for pain associated with a prominent nodule or cord when gripping objects).
Depending on disease progression and the surgeon’s preference, the most popular procedures are fasciotomies and fasciectomies. Surgical options for contractures—in increasing order of invasiveness and amounts of fascia removed—are fasciotomy (needle, open), fasciectomy (partial, radical), and dermofasciectomy.
Needle fasciotomy is an outpatient procedure performed under local block. The surgeon uses a needle bevel to transect the diseased cord at multiple puncture sites.
Open fasciotomy is also an outpatient procedure involving an incision that allows direct visualization of the cord and internal structures before dividing the fascia. Percutaneous and open fasciotomies are more successful for MCP joint contractures and less successful for PIP joint contractures.
With partial fasciectomy, only the grossly affected fascia is removed.
With radical fasciectomy, the entire palmar fascia is removed, regardless of which areas are grossly diseased.
Dermofasciectomy removes diseased fascia and the overlying skin, with a skin graft applied to heal the wound.
Another option: Collagenase injection. In February 2010, the US Food and Drug Administration approved collagenase clostridium histolyticum (Xiaflex) as the first drug specifically indicated for the treatment of Dupuytren’s contracture. Collagenase is injected directly into the fascial cord, cleaving its collagen component and progressively softening and breaking down the cord. If not properly injected, collagenase may injure neurovascular structures and may rupture tendons.
So how do the treatment options compare?
We found no randomized prospective trials comparing treatment options for DD, but there are a number of trials that shed light on a variety of the available options.
Treatment success. In general, partial fasciectomies have shown the greatest success in reducing contractures and maintaining the lowest recurrence rates. Correction of contracture to ≤5 degrees was seen in 94% of MCP joint contractures treated with partial fasciectomy, in 77% of MCP joints treated with collagenase, and in 55% of MCP joints treated with percutaneous needle fasciotomies.22,23 PIP joint contracture correction was not as successful: Correction of contracture to ≤5 degrees was seen in 47% of those treated with partial fasciectomy, 40% treated with collagenase, and 26% treated with needle fasciotomy.22,23
Recurrence rates. When recurrence was defined as loss of passive extension >30 degrees, the recurrence rate for MCP joint contractures with partial fasciectomy was 21%, compared with 85% for needle fasciotomy.22 In a similar review, recurrence rates for partial fasciectomy ranged from 12% to 39% compared with 50% to 58% for needle fasciotomy.24 With collagenase injection, 5-year data have shown an overall recurrence rate of 32%, with a recurrence of 26% at the MCP joint and 46% at the PIP joint.25 In this trial, recurrence was
Complications. Although fasciectomies have shown the best efficacy and lowest recurrence rates, complications such as infection, neurapraxia, and digital nerve injury are more likely with these procedures.26,27
Early recognition and referral to a hand specialist for the treatment of DD may allow the use of less invasive techniques and improved functional results.
CASE › Absent a history of trauma and features typical of other hand/digit disorders, we diagnosed Dupuytren’s disease in this patient. Potential treatments included partial fasciectomy and collagenase injection. After discussing the risks and benefits of each of these procedures, the patient elected to undergo a partial fasciectomy. The surgery was uncomplicated and the abnormality was corrected to within 5 degrees of full extension. At the patient’s one-year follow-up visit, there was no evidence of recurrence.
CASE › A 52-year-old right-hand-dominant white man arrived at our clinic complaining that he was unable to straighten his right ring finger. He had no associated pain or numbness, and had not injured his hand. The patient had type 2 diabetes that was controlled with metformin. He had no history of surgery or drug allergies, did not smoke, and said he drank 2 to 3 alcoholic beverages per day. He was a car salesman and was self-conscious when shaking hands with customers. On physical examination, we noted that he held his right ring finger at roughly 45 degrees of flexion at the metacarpophalangeal joint; a painless cord-like structure was palpable on the palmar surface of that joint. His left hand had no abnormalities.
If this were your patient, how would you proceed?
Dupuytren’s disease (DD) is a disabling fibroproliferative disorder of the hand for which there is no cure. While the exact cause of DD is unknown, it has been linked to a number of risk factors, including smoking, alcohol consumption, and diabetes. It affects about 5% of the US population, and up to 70% of affected individuals may initially seek treatment from a primary care physician.1 The disease is also referred to as Dupuytren’s contracture, which describes the flexion contractures of fingers at the end stage of the disease. Palmar fibromatosis is yet another name for the disorder.
DD refers to a spectrum of presentations ranging from nodules to cords to discernible contractures, and it is not known which patients with early Dupuytren changes will progress to severe contracture. With recognition of early changes, nonsurgical intervention is possible, such as collagenase injection or percutaneous fasciotomy, and can slow the progression of DD, restore function, and avoid or delay surgical intervention. DD is a clinically challenging disorder. Treatment for an affected area may resolve symptoms, only to have them recur in that location or another.
How underlying pathology correlates with clinical findings
DD affects the palmar fascia, a thick triangular-shaped sheet of dense fibrous collagenous connective tissue that lies deep to the dermis and superficial to the flexor tendons of the hand with fibers extending both into the skin and into the deep tissue. The palmar fascia secures the skin during gripping and twisting motions, and it bifurcates into distal extensions, called pretendinous bands, that overlay and mimic the flexor tendons.
Clinical findings reflect the progression of underlying pathology. The earliest manifestation of DD is painless dimpling of the skin on the palmar surface of the hand.2 Over time, the underlying fibrosis with increased collagen deposition can progress, leading to development of nodules and eventually, cords, which are sometimes mistaken for flexor tendons. Dupuytren-like fibrotic tissue can occur on the sole of the foot (Ledderhose disease) and penis (Peyronie’s disease).2,3 In patients with these coexisting conditions, prognosis is generally worse.4 With the hands, bilateral involvement is common, although it is usually more severe in one hand. The ring finger is the digit most frequently involved, followed by the little finger, middle finger, and index finger. The thumb is rarely affected.3
As the disease progresses and cords contract, the metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints develop flexion contractures. The distal interphalangeal (DIP) joint, rarely involved, instead exhibits a hyperextension contracture. Digital flexion contractures are often disabling, interfering with daily activities such as picking up a glass, shaking hands, or putting one's hand in a pants pocket. Many patients seek medical attention only after a palpable nodule, cord, or flexion contracture becomes apparent (FIGURE 1).

In a study of 326 patients who reported Dupuytren’s symptoms, the most common symptoms that led them to seek treatment were, in descending order, a hard bump (48%), a ropelike growth (12%), dimpling (11%), and finger contractures (10%). Only 9% of patients seeking treatment for hand symptoms associated with DD had received a diagnosis of DD from their initial medical encounter, causing an unnecessary delay in treatment.1
Who is at increased risk for DD?
DD is most often seen in elderly white men of European descent.5 In the United States, the prevalence of the disease is roughly 5%,1 compared with 4% to 39% in northwestern Europe (eg, Iceland).6 The male-to-female ratio of DD ranges from 6:1 to 15:1.7 The prevalence of DD appears to increase with age. The prevalence in men and women is similar up to age 45 years, after which the rate is much greater in males.7
DD is associated with many risk factors including smoking, alcohol consumption, vascular insufficiency, epilepsy, hyperlipidemia, manual labor, occupations with exposure to vibration, hand trauma, and even hand surgery such as carpal tunnel release or trigger finger release.8-11 It is also associated with diabetes; particularly type 1 insulin-dependent diabetes.12 There may also be an association with frozen shoulder.12,13
The need for surgical treatment of DD becomes more likely with a history of cigarette smoking and heavy alcohol consumption. There seems to be an association with epilepsy, most likely from anti-epileptic drugs.14 Rheumatoid arthritis is the only condition that has been associated with a lower incidence of DD, possibly because of the use of anti-inflammatory drugs.15 There are genetic differences between patients with and without DD, although a “Dupuytren’s gene” has not been identified.
Rule out possible DD mimics
The differential diagnosis for flexion contracture of the MCP or PIP joints seen in Dupuytren’s disease includes stenosing flexor tenosynovitis, or trigger finger. Other conditions that can mimic DD are ulnar claw hand, trauma scars, intrinsic joint diseases such as degenerative or rheumatoid arthritis (RA), diabetic cheiroarthropathy, camptodactyly, and Volkmann’s contracture. Trauma scars—especially longitudinal scars—have a tendency to contract and develop keloid formation.
Stenosing flexor tenosynovitis and ulnar claw hand are distinguishable from DD by full or nearly full active or passive extension of the affected digit, whereas DD is a true contracture of the joint that does not allow full extension. A careful history can rule out previous injury to the area. Although intrinsic joint disease, such as RA, can cause finger contractures, the joints are usually enlarged, painful, and associated with characteristic radiologic findings.
Unlike DD, diabetic cheiroarthropathy often involves all of the digits except for the thumb, and is often associated with a waxy appearance of the skin. Camptodactyly is an autosomal dominant disorder that more often presents in childhood and can be caused by a number of congenital syndromes. Volkmann’s contracture can manifest as a claw-like deformity of the hand caused by undiagnosed compartment syndrome of the forearm.

Assess the severity of DD
In your evaluation, first identify palmar or digital fibromatosis presenting as a nodule or a cord. Second, estimate the degree of MCP and PIP joint contractures. A common measure of contracture is the Hueston tabletop test. Ask the patient to place the palm of the hand on a flat surface. If the patient is unable to completely flatten the hand against the surface, presume a positive result (FIGURE 2).16

An accurate measure of the degree of flexion can be accomplished with a goniometer. For a simple assessment of severity, have the patient place each affected finger along the convexity of a spoon. If adjoining surfaces are flush, assume that the contracture is at least 30 degrees (FIGURE 3). The severity of DD can also be graded according to the Tubiana classification system (TABLE17), wherein the total deformity or contracture score is the sum of the angles of all 3 digital joints of the finger.

A look at the treatment options
Once the diagnosis of DD is made, reassure the patient that the disease is not cancerous, although it may be progressive. Advise patients, too, that several treatment options (detailed below) are available, but that surgery is the mainstay of treatment. (Of note: Despite what would seem logical, stretching a cord to straighten the finger is not recommended, as it may actually worsen the condition.18)
Options for early DD. Corticosteroids such as triamcinolone may have a role as an adjunct for early DD by reducing the size and firmness of the Dupuytren’s nodules and possibly slowing the progression of the disease. However, in one study of 63 patients, half of the individuals experienced reactivation of disease between one and 3 years.19
Radiotherapy has been studied as a potential treatment for early DD and for patients unable to undergo surgery. Radiation does have a biologic effect on nodular DD, but has no effect on the cords that cause the joint contracture. In a long-term follow-up study of 135 patients treated with radiotherapy, disease remained stable in 59% of patients, improved in 10%, and progressed in 31%.20 Results were better for early stage disease. This modality is not used for more advanced cases.
Surgery is the primary method by which to restore function and minimize complications. In the past, referral was recommended for MCP joint contractures >30 degrees or for any involvment of the PIP joint, because involvement of the latter carries a worse prognosis and may not be fully correctable, even with surgery.21 However, treatment may also be warranted in cases of functional disability—regardless of the degree of contracture (eg, for pain associated with a prominent nodule or cord when gripping objects).
Depending on disease progression and the surgeon’s preference, the most popular procedures are fasciotomies and fasciectomies. Surgical options for contractures—in increasing order of invasiveness and amounts of fascia removed—are fasciotomy (needle, open), fasciectomy (partial, radical), and dermofasciectomy.
Needle fasciotomy is an outpatient procedure performed under local block. The surgeon uses a needle bevel to transect the diseased cord at multiple puncture sites.
Open fasciotomy is also an outpatient procedure involving an incision that allows direct visualization of the cord and internal structures before dividing the fascia. Percutaneous and open fasciotomies are more successful for MCP joint contractures and less successful for PIP joint contractures.
With partial fasciectomy, only the grossly affected fascia is removed.
With radical fasciectomy, the entire palmar fascia is removed, regardless of which areas are grossly diseased.
Dermofasciectomy removes diseased fascia and the overlying skin, with a skin graft applied to heal the wound.
Another option: Collagenase injection. In February 2010, the US Food and Drug Administration approved collagenase clostridium histolyticum (Xiaflex) as the first drug specifically indicated for the treatment of Dupuytren’s contracture. Collagenase is injected directly into the fascial cord, cleaving its collagen component and progressively softening and breaking down the cord. If not properly injected, collagenase may injure neurovascular structures and may rupture tendons.
So how do the treatment options compare?
We found no randomized prospective trials comparing treatment options for DD, but there are a number of trials that shed light on a variety of the available options.
Treatment success. In general, partial fasciectomies have shown the greatest success in reducing contractures and maintaining the lowest recurrence rates. Correction of contracture to ≤5 degrees was seen in 94% of MCP joint contractures treated with partial fasciectomy, in 77% of MCP joints treated with collagenase, and in 55% of MCP joints treated with percutaneous needle fasciotomies.22,23 PIP joint contracture correction was not as successful: Correction of contracture to ≤5 degrees was seen in 47% of those treated with partial fasciectomy, 40% treated with collagenase, and 26% treated with needle fasciotomy.22,23
Recurrence rates. When recurrence was defined as loss of passive extension >30 degrees, the recurrence rate for MCP joint contractures with partial fasciectomy was 21%, compared with 85% for needle fasciotomy.22 In a similar review, recurrence rates for partial fasciectomy ranged from 12% to 39% compared with 50% to 58% for needle fasciotomy.24 With collagenase injection, 5-year data have shown an overall recurrence rate of 32%, with a recurrence of 26% at the MCP joint and 46% at the PIP joint.25 In this trial, recurrence was
Complications. Although fasciectomies have shown the best efficacy and lowest recurrence rates, complications such as infection, neurapraxia, and digital nerve injury are more likely with these procedures.26,27
Early recognition and referral to a hand specialist for the treatment of DD may allow the use of less invasive techniques and improved functional results.
CASE › Absent a history of trauma and features typical of other hand/digit disorders, we diagnosed Dupuytren’s disease in this patient. Potential treatments included partial fasciectomy and collagenase injection. After discussing the risks and benefits of each of these procedures, the patient elected to undergo a partial fasciectomy. The surgery was uncomplicated and the abnormality was corrected to within 5 degrees of full extension. At the patient’s one-year follow-up visit, there was no evidence of recurrence.
1. DiBenedetti DB, Nguyen D, Zografos L, et al. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand. 2011;6:149-158.
2. Townley WA, Baker R, Sheppard N, et al. Dupuytren’s contracture unfolded. BMJ. 2006;332:397-400.
3. Rayan GM. Clinical presentation and types of Dupuytren’s disease. Hand Clin. 1999;15:87-96.
4. Hindocha S, Stanley JK, Watson S, et al. Dupuytren’s diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31:1626-1634.
5. Bayat A, McGrouther DA. Management of Dupuytren’s disease—clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88:3-8.
6. Gudmundsson KG, Arngrimsson R, Sigfússon M, et al. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53:291-296.
7. Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg Br. 1999;24:456-459.
8. Noble J, Arafa M, Royle SG, et al. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg Br. 1992;17:71–74.
9. Mikkelsen OA. Dupuytren’s disease—initial symptoms, age of onset and spontaneous course. Hand. 1977;9:11-15.
10. Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24:153-159.
11. Liss GM, Stock SR. Can Dupuytren’s contracture be work related? review of the evidence. Am J Ind Med. 1996;29:521-532.
12. Geoghegan JM, Forbes J, Clark DI, et al. Dupuytren’s disease risk factors. J Hand Surg Br. 2004;29:423-426.
13. Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg. 2001;10:149-151.
14. Nunn AC, Schreuder FB. Dupuytren’s contracture: emerging insight into a Viking disease. J Hand Surg. 2014;19:481-490.
15. Arafa M, Steingold R F, Noble J. The incidence of Dupuytren’s disease in patients with rheumatoid arthritis. J Hand Surg Br. 1984;9:165-166.
16. Hueston J. Lessons in Dupuytren’s disease. Ann Chir Main Memb Super. 1992;11:349-354.
17. Tubiana R. Prognosis and treatment of Dupuytren’s contracture. J Bone and Joint Surg AM. 1955;37:1155-1168.
18. Howard JC, Varallo VM, Ross DC, et al. Elevated levels of ß-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16.
19. Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25:1157-1162.
20. Betz N, Ott OJ, Adamietz B, et al. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186:82-90.
21. Smith AC. Diagnosis and indications for surgical treatment. Hand Clin. 1991;7:635-642.
22. van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469-477.
23. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968-979.
24. Chen NC, Srinivasan RC, Shauver MJ, et al. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand. 2011;6:250-255.
25. Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015;40:1597-1605.
26. Toppi JT, Trompf L, Smoll NR, et al. Dupuytren’s contracture: an analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy. ANZ J Surg. 2015;85:639-643.
27. Cheung K, Walley KC, Rozental TD. Management of complications of Dupuytren contracture. Hand Clini. 2015;31:345-354.
1. DiBenedetti DB, Nguyen D, Zografos L, et al. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand. 2011;6:149-158.
2. Townley WA, Baker R, Sheppard N, et al. Dupuytren’s contracture unfolded. BMJ. 2006;332:397-400.
3. Rayan GM. Clinical presentation and types of Dupuytren’s disease. Hand Clin. 1999;15:87-96.
4. Hindocha S, Stanley JK, Watson S, et al. Dupuytren’s diathesis revisited: Evaluation of prognostic indicators for risk of disease recurrence. J Hand Surg Am. 2006;31:1626-1634.
5. Bayat A, McGrouther DA. Management of Dupuytren’s disease—clear advice for an elusive condition. Ann R Coll Surg Engl. 2006;88:3-8.
6. Gudmundsson KG, Arngrimsson R, Sigfússon M, et al. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53:291-296.
7. Wilbrand S, Ekbom A, Gerdin B. The sex ratio and rate of reoperation for Dupuytren’s contracture in men and women. J Hand Surg Br. 1999;24:456-459.
8. Noble J, Arafa M, Royle SG, et al. The association between alcohol, hepatic pathology and Dupuytren’s disease. J Hand Surg Br. 1992;17:71–74.
9. Mikkelsen OA. Dupuytren’s disease—initial symptoms, age of onset and spontaneous course. Hand. 1977;9:11-15.
10. Arkkila PE, Kantola IM, Viikari JS. Dupuytren’s disease: association with chronic diabetic complications. J Rheumatol. 1997;24:153-159.
11. Liss GM, Stock SR. Can Dupuytren’s contracture be work related? review of the evidence. Am J Ind Med. 1996;29:521-532.
12. Geoghegan JM, Forbes J, Clark DI, et al. Dupuytren’s disease risk factors. J Hand Surg Br. 2004;29:423-426.
13. Smith SP, Devaraj VS, Bunker TD. The association between frozen shoulder and Dupuytren’s disease. J Shoulder Elbow Surg. 2001;10:149-151.
14. Nunn AC, Schreuder FB. Dupuytren’s contracture: emerging insight into a Viking disease. J Hand Surg. 2014;19:481-490.
15. Arafa M, Steingold R F, Noble J. The incidence of Dupuytren’s disease in patients with rheumatoid arthritis. J Hand Surg Br. 1984;9:165-166.
16. Hueston J. Lessons in Dupuytren’s disease. Ann Chir Main Memb Super. 1992;11:349-354.
17. Tubiana R. Prognosis and treatment of Dupuytren’s contracture. J Bone and Joint Surg AM. 1955;37:1155-1168.
18. Howard JC, Varallo VM, Ross DC, et al. Elevated levels of ß-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16.
19. Ketchum LD, Donahue TK. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg Am. 2000;25:1157-1162.
20. Betz N, Ott OJ, Adamietz B, et al. Radiotherapy in early-stage Dupuytren’s contracture. Long-term results after 13 years. Strahlenther Onkol. 2010;186:82-90.
21. Smith AC. Diagnosis and indications for surgical treatment. Hand Clin. 1991;7:635-642.
22. van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469-477.
23. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968-979.
24. Chen NC, Srinivasan RC, Shauver MJ, et al. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand. 2011;6:250-255.
25. Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg Am. 2015;40:1597-1605.
26. Toppi JT, Trompf L, Smoll NR, et al. Dupuytren’s contracture: an analysis of outcomes of percutaneous needle fasciotomy versus open fasciectomy. ANZ J Surg. 2015;85:639-643.
27. Cheung K, Walley KC, Rozental TD. Management of complications of Dupuytren contracture. Hand Clini. 2015;31:345-354.
PRACTICE RECOMMENDATIONS
› Evaluate patients with suspected Dupuytren’s disease (DD) for coexisting conditions such as Ledderhose disease and Peyronie’s disease. C
› Use the Hueston tabletop test to assess severity of DD. C
› Do not recommend stretching exercises for DD; they can hasten disease progression. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series