User login
Skin conditions in cancer patients can lead to a decreased quality of life because of the psychosocial impact, financial burden, poor health, and disruption of anticancer treatments, said Dr. Mario E. Lacouture at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
Clinical trials are underway to address management, cost, and quality of life, but in the meantime, "an early and proactive approach toward toxicities is advisable," he said.
Dr. Lacouture, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York, discussed common cancer-associated dermatology at the seminar.
• Rashes. A RAF inhibitor–induced rash can occur within 1-2 weeks of starting treatment, Dr. Lacouture noted. This rash is associated with sensations of burning or itching in photo-exposed areas. In most cases, the rash can be managed with topical steroids and oral GABA agonists. Vemurafenib use also has been associated with rashes, as well as with photosensitivity, itching, and alopecia, he said.
In addition, mTOR inhibitor–induced rashes involving erythematous pruritic papules and ulcerlike lesions have been observed in patients on everolimus and temsirolimus.
• Skin cancers. Data from several studies suggest that the incidence of squamous cell carcinomas in patients on RAF inhibitors is 7%, and the incidence of actinic keratoses in these patients is 4%, according to Dr. Lacouture. These conditions usually develop after approximately 6 months of treatment. They can be managed by surgery or destruction; and there have been no reports of metastases.
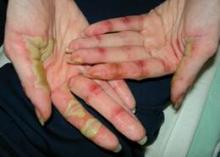
• Hand-foot syndrome. Hand-Foot Syndrome (HFSR) is a common side effect in cancer patients that usually occurs within 45 days of starting treatment, said Dr. Lacouture. HFSR is often associated with the use of sorafenib, sunitinib, and pazopanib, and the histology of these patients shows epidermal necrosis, he noted. Patients who develop HFSR may need to temporarily reduce their medications. Additional management of HFSR includes avoiding excess pressure on the affected areas and keeping them cool and moist to reduce patient discomfort.
• Hair and nail changes. Changes in hair can occur in patients being treated with epidermal growth factor receptor inhibitors (EGFRIs), including a slower hair growth rate and alopecia, Dr. Lacouture said. Changes in hair texture such as increased brittleness and hair curling also have been observed, he said. Other hair-related adverse events can include hirsutism on the face and eyelash trichomegaly. In addition, paronychia has been observed in patients on EGFRIs for more than 6 months.
• Radiation dermatitis. Approximately 50% of cancer patients develop radiation dermatitis, including 87% of breast cancer patients, reported Dr. Lacouture. However, the treatment of radiation dermatitis remains challenging, he said. In several studies, patients who used a nonsteroidal topical, including aloe vera and trolamine, showed no significant improvement in radiation dermatitis, compared with controls. However, studies are ongoing, and significant improvements in radiation dermatitis in breast cancer patients have been seen with topical corticosteroids including mometasone, beclomethasone, and betamethasone.
Dr. Lacouture disclosed financial relationships with multiple companies including Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Merck, and Roche. SDEF and this news organization are owned by Elsevier.
Skin conditions in cancer patients can lead to a decreased quality of life because of the psychosocial impact, financial burden, poor health, and disruption of anticancer treatments, said Dr. Mario E. Lacouture at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
Clinical trials are underway to address management, cost, and quality of life, but in the meantime, "an early and proactive approach toward toxicities is advisable," he said.
Dr. Lacouture, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York, discussed common cancer-associated dermatology at the seminar.
• Rashes. A RAF inhibitor–induced rash can occur within 1-2 weeks of starting treatment, Dr. Lacouture noted. This rash is associated with sensations of burning or itching in photo-exposed areas. In most cases, the rash can be managed with topical steroids and oral GABA agonists. Vemurafenib use also has been associated with rashes, as well as with photosensitivity, itching, and alopecia, he said.
In addition, mTOR inhibitor–induced rashes involving erythematous pruritic papules and ulcerlike lesions have been observed in patients on everolimus and temsirolimus.
• Skin cancers. Data from several studies suggest that the incidence of squamous cell carcinomas in patients on RAF inhibitors is 7%, and the incidence of actinic keratoses in these patients is 4%, according to Dr. Lacouture. These conditions usually develop after approximately 6 months of treatment. They can be managed by surgery or destruction; and there have been no reports of metastases.
• Hand-foot syndrome. Hand-Foot Syndrome (HFSR) is a common side effect in cancer patients that usually occurs within 45 days of starting treatment, said Dr. Lacouture. HFSR is often associated with the use of sorafenib, sunitinib, and pazopanib, and the histology of these patients shows epidermal necrosis, he noted. Patients who develop HFSR may need to temporarily reduce their medications. Additional management of HFSR includes avoiding excess pressure on the affected areas and keeping them cool and moist to reduce patient discomfort.
• Hair and nail changes. Changes in hair can occur in patients being treated with epidermal growth factor receptor inhibitors (EGFRIs), including a slower hair growth rate and alopecia, Dr. Lacouture said. Changes in hair texture such as increased brittleness and hair curling also have been observed, he said. Other hair-related adverse events can include hirsutism on the face and eyelash trichomegaly. In addition, paronychia has been observed in patients on EGFRIs for more than 6 months.
• Radiation dermatitis. Approximately 50% of cancer patients develop radiation dermatitis, including 87% of breast cancer patients, reported Dr. Lacouture. However, the treatment of radiation dermatitis remains challenging, he said. In several studies, patients who used a nonsteroidal topical, including aloe vera and trolamine, showed no significant improvement in radiation dermatitis, compared with controls. However, studies are ongoing, and significant improvements in radiation dermatitis in breast cancer patients have been seen with topical corticosteroids including mometasone, beclomethasone, and betamethasone.
Dr. Lacouture disclosed financial relationships with multiple companies including Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Merck, and Roche. SDEF and this news organization are owned by Elsevier.
Skin conditions in cancer patients can lead to a decreased quality of life because of the psychosocial impact, financial burden, poor health, and disruption of anticancer treatments, said Dr. Mario E. Lacouture at the annual Hawaii Dermatology Seminar sponsored by Skin Disease Education Foundation (SDEF).
Clinical trials are underway to address management, cost, and quality of life, but in the meantime, "an early and proactive approach toward toxicities is advisable," he said.
Dr. Lacouture, a dermatologist at Memorial Sloan-Kettering Cancer Center in New York, discussed common cancer-associated dermatology at the seminar.
• Rashes. A RAF inhibitor–induced rash can occur within 1-2 weeks of starting treatment, Dr. Lacouture noted. This rash is associated with sensations of burning or itching in photo-exposed areas. In most cases, the rash can be managed with topical steroids and oral GABA agonists. Vemurafenib use also has been associated with rashes, as well as with photosensitivity, itching, and alopecia, he said.
In addition, mTOR inhibitor–induced rashes involving erythematous pruritic papules and ulcerlike lesions have been observed in patients on everolimus and temsirolimus.
• Skin cancers. Data from several studies suggest that the incidence of squamous cell carcinomas in patients on RAF inhibitors is 7%, and the incidence of actinic keratoses in these patients is 4%, according to Dr. Lacouture. These conditions usually develop after approximately 6 months of treatment. They can be managed by surgery or destruction; and there have been no reports of metastases.
• Hand-foot syndrome. Hand-Foot Syndrome (HFSR) is a common side effect in cancer patients that usually occurs within 45 days of starting treatment, said Dr. Lacouture. HFSR is often associated with the use of sorafenib, sunitinib, and pazopanib, and the histology of these patients shows epidermal necrosis, he noted. Patients who develop HFSR may need to temporarily reduce their medications. Additional management of HFSR includes avoiding excess pressure on the affected areas and keeping them cool and moist to reduce patient discomfort.
• Hair and nail changes. Changes in hair can occur in patients being treated with epidermal growth factor receptor inhibitors (EGFRIs), including a slower hair growth rate and alopecia, Dr. Lacouture said. Changes in hair texture such as increased brittleness and hair curling also have been observed, he said. Other hair-related adverse events can include hirsutism on the face and eyelash trichomegaly. In addition, paronychia has been observed in patients on EGFRIs for more than 6 months.
• Radiation dermatitis. Approximately 50% of cancer patients develop radiation dermatitis, including 87% of breast cancer patients, reported Dr. Lacouture. However, the treatment of radiation dermatitis remains challenging, he said. In several studies, patients who used a nonsteroidal topical, including aloe vera and trolamine, showed no significant improvement in radiation dermatitis, compared with controls. However, studies are ongoing, and significant improvements in radiation dermatitis in breast cancer patients have been seen with topical corticosteroids including mometasone, beclomethasone, and betamethasone.
Dr. Lacouture disclosed financial relationships with multiple companies including Amgen, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Merck, and Roche. SDEF and this news organization are owned by Elsevier.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR