User login
Geriatric Dermatology: Q&A With Daniel C. Butler, MD
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
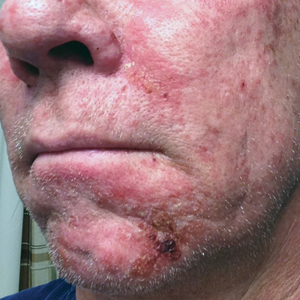
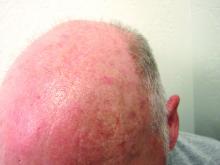
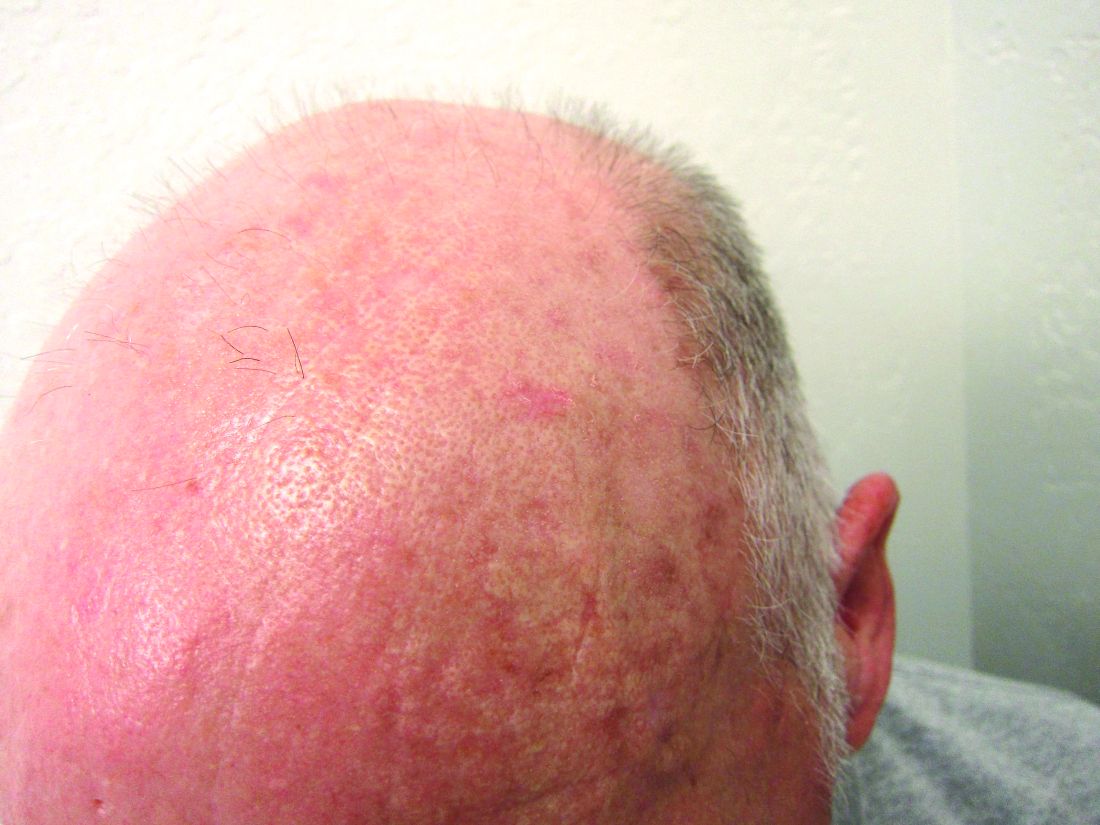
Expanded Surface Area Safe, Well-Tolerated for AK treatment
TOPLINE:
METHODOLOGY:
- This phase 3 multicenter, single-arm trial evaluated the safety and tolerability of tirbanibulin ointment 1% in 105 adults with 4-12 clinically typical, visible, and discrete AKs on the face or balding scalp from June to December 2022 in the United States. (In June 2024, the Food and Drug Administration approved a supplemental new drug application for tirbanibulin 1%, a microtubule inhibitor, allowing the expansion of the surface area treated for AKs of the face or scalp from 25 cm2 to 100 cm2.)
- Participants applied tirbanibulin ointment 1% once daily for 5 days over a treatment field of about 100 cm2 on the face or balding scalp. A total of 102 patients completed the study.
- Safety and tolerability were evaluated with reports of treatment-emergent adverse events (TEAEs) and a composite score of six local tolerability signs on days 5, 8, 15, and 29, and on completion of the evaluation period on day 57.
TAKEAWAY:
- The most common local effects of treatment were erythema (96.1% of patients) and flaking or scaling (84.4%), with severe cases reported in 5.8% and 8.7% of the patients, respectively.
- The mean maximum local tolerability composite score was 4.1 out of 18, which peaked around day 8 and returned to baseline by day 29.
- TEAEs considered related to the treatment were reported in 18.1% of patients; the most frequent were application site pruritus (10.5%) and application site pain (8.6%). No adverse events led to the discontinuation of treatment.
- The mean percent reduction in the lesion count from baseline was 77.8% at day 57, with a mean lesion count of 1.8 at the end of the study.
IN PRACTICE:
In this study, “local tolerability and safety profiles were well characterized in patients with 4-12 clinically typical, visible, and discrete AK lesions in a field of 100 cm2 and were consistent with those previously reported in patients with AK treated in pivotal trials with tirbanibulin over a smaller field (25 cm2),” the authors wrote.
SOURCE:
The study, led by Neal Bhatia, MD, of Therapeutics Clinical Research, San Diego, was published online in JAAD International.
LIMITATIONS:
The study was limited by the lack of a placebo group and the absence of long-term follow-up.
DISCLOSURES:
This study was funded by Almirall. Five authors reported being employees of Almirall. Other authors declared having ties with various other sources, including Almirall.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This phase 3 multicenter, single-arm trial evaluated the safety and tolerability of tirbanibulin ointment 1% in 105 adults with 4-12 clinically typical, visible, and discrete AKs on the face or balding scalp from June to December 2022 in the United States. (In June 2024, the Food and Drug Administration approved a supplemental new drug application for tirbanibulin 1%, a microtubule inhibitor, allowing the expansion of the surface area treated for AKs of the face or scalp from 25 cm2 to 100 cm2.)
- Participants applied tirbanibulin ointment 1% once daily for 5 days over a treatment field of about 100 cm2 on the face or balding scalp. A total of 102 patients completed the study.
- Safety and tolerability were evaluated with reports of treatment-emergent adverse events (TEAEs) and a composite score of six local tolerability signs on days 5, 8, 15, and 29, and on completion of the evaluation period on day 57.
TAKEAWAY:
- The most common local effects of treatment were erythema (96.1% of patients) and flaking or scaling (84.4%), with severe cases reported in 5.8% and 8.7% of the patients, respectively.
- The mean maximum local tolerability composite score was 4.1 out of 18, which peaked around day 8 and returned to baseline by day 29.
- TEAEs considered related to the treatment were reported in 18.1% of patients; the most frequent were application site pruritus (10.5%) and application site pain (8.6%). No adverse events led to the discontinuation of treatment.
- The mean percent reduction in the lesion count from baseline was 77.8% at day 57, with a mean lesion count of 1.8 at the end of the study.
IN PRACTICE:
In this study, “local tolerability and safety profiles were well characterized in patients with 4-12 clinically typical, visible, and discrete AK lesions in a field of 100 cm2 and were consistent with those previously reported in patients with AK treated in pivotal trials with tirbanibulin over a smaller field (25 cm2),” the authors wrote.
SOURCE:
The study, led by Neal Bhatia, MD, of Therapeutics Clinical Research, San Diego, was published online in JAAD International.
LIMITATIONS:
The study was limited by the lack of a placebo group and the absence of long-term follow-up.
DISCLOSURES:
This study was funded by Almirall. Five authors reported being employees of Almirall. Other authors declared having ties with various other sources, including Almirall.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- This phase 3 multicenter, single-arm trial evaluated the safety and tolerability of tirbanibulin ointment 1% in 105 adults with 4-12 clinically typical, visible, and discrete AKs on the face or balding scalp from June to December 2022 in the United States. (In June 2024, the Food and Drug Administration approved a supplemental new drug application for tirbanibulin 1%, a microtubule inhibitor, allowing the expansion of the surface area treated for AKs of the face or scalp from 25 cm2 to 100 cm2.)
- Participants applied tirbanibulin ointment 1% once daily for 5 days over a treatment field of about 100 cm2 on the face or balding scalp. A total of 102 patients completed the study.
- Safety and tolerability were evaluated with reports of treatment-emergent adverse events (TEAEs) and a composite score of six local tolerability signs on days 5, 8, 15, and 29, and on completion of the evaluation period on day 57.
TAKEAWAY:
- The most common local effects of treatment were erythema (96.1% of patients) and flaking or scaling (84.4%), with severe cases reported in 5.8% and 8.7% of the patients, respectively.
- The mean maximum local tolerability composite score was 4.1 out of 18, which peaked around day 8 and returned to baseline by day 29.
- TEAEs considered related to the treatment were reported in 18.1% of patients; the most frequent were application site pruritus (10.5%) and application site pain (8.6%). No adverse events led to the discontinuation of treatment.
- The mean percent reduction in the lesion count from baseline was 77.8% at day 57, with a mean lesion count of 1.8 at the end of the study.
IN PRACTICE:
In this study, “local tolerability and safety profiles were well characterized in patients with 4-12 clinically typical, visible, and discrete AK lesions in a field of 100 cm2 and were consistent with those previously reported in patients with AK treated in pivotal trials with tirbanibulin over a smaller field (25 cm2),” the authors wrote.
SOURCE:
The study, led by Neal Bhatia, MD, of Therapeutics Clinical Research, San Diego, was published online in JAAD International.
LIMITATIONS:
The study was limited by the lack of a placebo group and the absence of long-term follow-up.
DISCLOSURES:
This study was funded by Almirall. Five authors reported being employees of Almirall. Other authors declared having ties with various other sources, including Almirall.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Actinic keratoses may predict skin cancers in older adults
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- AKs have been associated with a small risk for cutaneous SCC, but associations with risk for other skin cancers have not been well studied.
- AKs may be a marker of overall skin cancer risk, but guidelines for AK management lack recommendations for follow-up cancer surveillance.
- The researchers reviewed data from a random sample of 5 million fee-for-service Medicare beneficiaries treated for AKs from 2009 through 2018 in the United States. Patients with seborrheic keratoses (SKs) were included as comparators, and patients with a history of skin cancer were excluded.
- The primary outcome was the first surgically treated skin cancer, including SCC, BCC, and melanoma.
TAKEAWAY:
- A total of 555,945 adults with AKs and 481,024 with SKs were included. The mean age was approximately 74.0 years. More than half were female. Most were non-Hispanic White.
- Among patients with AKs, the absolute risk for any skin cancer after the first AK was 6.3%, 18.4%, and 28.5% at 1, 3, and 5 years, respectively.
- Patients with AKs had a significantly increased relative risk for any skin cancer compared with those with SKs (adjusted hazard ratio [aHR], 2.17) and separately for keratinocyte carcinoma (aHR, 2.20), SCC (aHR, 2.63), BCC (aHR, 1.85), and melanoma (aHR, 1.67).
- Although AKs are not considered a biological precursor of melanoma or BCC, the results suggest that AKs may be clinical indicators of increased UV exposure that subsequently increases the risk for skin cancer.
IN PRACTICE:
“The present results highlight the importance of developing evidence-based guidelines for follow-up skin cancer surveillance in patients with AKs, optimally including measures of AK burden,” the researchers wrote.
SOURCE:
The lead author on the study was Cassandra Mohr, BS, with corresponding author Mackenzie R. Wehner, MD, MPhil, of The University of Texas MD Anderson Cancer Center, Houston. The study was published online in JAMA Dermatology .
LIMITATIONS:
The study population of Medicare beneficiaries aged 65 years or older may not be a nationally representative sample, and surveillance bias may contribute to the increased risk for skin cancer in patients with AKs. The use of both ICD and CPT codes may underestimate the number of skin cancers because of cases that were treated nonsurgically.
DISCLOSURES:
The study was supported by the National Cancer Institute of the National Institutes of Health, the Cancer Prevention and Research Institute of Texas, and The University of Texas Rising STARS program. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Polypodium leucotomos found to reverse AK skin damage
MILAN – Application of topical or both treated over 12 months, in a randomized, blinded study presented at the annual congress of the European Academy of Dermatology and Venereology.
At 12 months, the percentage of patients with a normal or almost normal honeycomb pattern when evaluated blindly with reflectance confocal microscopy (RCM) was about twice as great in either of the two groups that received PLE relative to those treated with topical photoprotection alone, according to Giovanni Pellacani, MD, PhD, chair of dermatology, University of Sapienza, Rome.
“In patients with severe actinic keratosis, the 12-month use of a PLE-based topical or oral photoprotection is associated with positive clinical and anatomical outcomes,” Dr. Pellacani said.
PLE, which is already commonly used in sun protection products, is derived from a South American species of fern and has been proposed for a broad array of dermatologic diseases. According to Dr. Pellacani, in vivo studies associating PLE with immune photoprotection make this agent particularly promising for severe AKs.
In this study involving two clinical research centers in Italy, 131 patients with photoaging and at least three AKs were randomized to one of three treatment arms. The control arm received topical photoprotection with an SPF of 100 or higher applied twice daily to all sun-exposed areas. The two treatment arms received the same topical photoprotection plus either a PLE-containing topical cream alone or a PLE-containing topical cream plus PLE in an oral form (240 mg) once daily
Patients were evaluated at 3 months, 6 months, and 1 year with several measures, including the Actinic Keratosis Area Score Index (AKASI) and the AK Field Assessment Scale Area (AK-FAS). They were also assessed with RCM. All clinical assessments and RCM evaluations, which assessed seven different parameters, such as honeycomb pattern, mottled pigmentation, and reticulated collagen, were performed by dermatologists blinded to the treatment assignment.
Complete data were available for 116 patients who completed all three evaluations over the 12 months of follow-up. On RCM, 50% of those receiving the oral and topical forms of PLE and 45% of those receiving topical PLE had normalization of the honeycomb pattern. These responses were significantly greater (P = .04 for both) than the 26% with normalization in the control group.
Although there were no significant differences in any of the other parameters evaluated by RCM, the improvement in the honeycomb pattern was accompanied by a 7% improvement in the AKASI score in patients taking PLE, either topically or orally and topically, while there was a 6% worsening (P < .001) among controls.
The AK-FAS score improved at 12 months by 26% in the group on oral/topical PLE and by 4% in the group on topical PLE. The score worsened by 13% among controls.
Over the course of the study, patients were permitted to take an appropriate therapy, such as imiquimod, cryotherapy, or 5-flourouracil if there was worsening of the AK-FAS score or if new lesions appeared.
On this measure, 38% of controls and 11% of those randomized to topical PLE had progressive disease versus only 2% of those randomized to take both topical and oral PLE, Dr. Pellacani reported.
The lower rate of new lesions or a start of a new drug over the course of the study in the group receiving both the topical and the oral formulations of PLE relative to those receiving topical PLE alone did not reach statistical significance, but Dr. Pellacani concluded that the addition of PLE to topical photoprotection without PLE seemed to provide a potentially clinically meaningful advantage.
Larger studies and longer term studies are needed, according to Dr. Pellacani, who noted that the substantial body of clinical studies associating PLE with benefit in a variety of dermatologic disorders has been weakened by the absence of well-designed studies that are adequately powered to guide clinical use.
Salvador González, MD, PhD, a dermatology specialist at Alcalá University, Madrid, also believes that PLE deserves further evaluation not just for photoprotection but for reinvigorating damaged skin due to its antioxidant and anti-inflammatory properties. He was the senior author of a 2020 paper in Photochemical and Photobiological Sciences that summarized the potential benefits of PLE in preventing damage related to sun exposure.
Among its mechanism, PLE generates reactive oxygen species (ROS) and prevents depletion of Langerhans cells induced by ultraviolet (UV) light, Dr. González explained in an interview. “At the cellular level, PLE activates tumor suppression p53, inhibits UV-induced COX-2 expression, reduces inflammation, and preventions immunosuppression,” he continued. In addition, he said PLE also prevents UV-A-induced common deletions related to mitochondrial damage and MMP1 expression induced by various UV wavelengths.
“These molecular and cellular effects may translate into long-term inhibition of carcinogenesis including actinic keratosis,” he said, noting that all of these findings “justify the work by Pellacani and collaborators.”
Dr. Pellacani reports no potential conflicts of interest. Dr. González has a financial relationship with Cantabria Laboratories.
MILAN – Application of topical or both treated over 12 months, in a randomized, blinded study presented at the annual congress of the European Academy of Dermatology and Venereology.
At 12 months, the percentage of patients with a normal or almost normal honeycomb pattern when evaluated blindly with reflectance confocal microscopy (RCM) was about twice as great in either of the two groups that received PLE relative to those treated with topical photoprotection alone, according to Giovanni Pellacani, MD, PhD, chair of dermatology, University of Sapienza, Rome.
“In patients with severe actinic keratosis, the 12-month use of a PLE-based topical or oral photoprotection is associated with positive clinical and anatomical outcomes,” Dr. Pellacani said.
PLE, which is already commonly used in sun protection products, is derived from a South American species of fern and has been proposed for a broad array of dermatologic diseases. According to Dr. Pellacani, in vivo studies associating PLE with immune photoprotection make this agent particularly promising for severe AKs.
In this study involving two clinical research centers in Italy, 131 patients with photoaging and at least three AKs were randomized to one of three treatment arms. The control arm received topical photoprotection with an SPF of 100 or higher applied twice daily to all sun-exposed areas. The two treatment arms received the same topical photoprotection plus either a PLE-containing topical cream alone or a PLE-containing topical cream plus PLE in an oral form (240 mg) once daily
Patients were evaluated at 3 months, 6 months, and 1 year with several measures, including the Actinic Keratosis Area Score Index (AKASI) and the AK Field Assessment Scale Area (AK-FAS). They were also assessed with RCM. All clinical assessments and RCM evaluations, which assessed seven different parameters, such as honeycomb pattern, mottled pigmentation, and reticulated collagen, were performed by dermatologists blinded to the treatment assignment.
Complete data were available for 116 patients who completed all three evaluations over the 12 months of follow-up. On RCM, 50% of those receiving the oral and topical forms of PLE and 45% of those receiving topical PLE had normalization of the honeycomb pattern. These responses were significantly greater (P = .04 for both) than the 26% with normalization in the control group.
Although there were no significant differences in any of the other parameters evaluated by RCM, the improvement in the honeycomb pattern was accompanied by a 7% improvement in the AKASI score in patients taking PLE, either topically or orally and topically, while there was a 6% worsening (P < .001) among controls.
The AK-FAS score improved at 12 months by 26% in the group on oral/topical PLE and by 4% in the group on topical PLE. The score worsened by 13% among controls.
Over the course of the study, patients were permitted to take an appropriate therapy, such as imiquimod, cryotherapy, or 5-flourouracil if there was worsening of the AK-FAS score or if new lesions appeared.
On this measure, 38% of controls and 11% of those randomized to topical PLE had progressive disease versus only 2% of those randomized to take both topical and oral PLE, Dr. Pellacani reported.
The lower rate of new lesions or a start of a new drug over the course of the study in the group receiving both the topical and the oral formulations of PLE relative to those receiving topical PLE alone did not reach statistical significance, but Dr. Pellacani concluded that the addition of PLE to topical photoprotection without PLE seemed to provide a potentially clinically meaningful advantage.
Larger studies and longer term studies are needed, according to Dr. Pellacani, who noted that the substantial body of clinical studies associating PLE with benefit in a variety of dermatologic disorders has been weakened by the absence of well-designed studies that are adequately powered to guide clinical use.
Salvador González, MD, PhD, a dermatology specialist at Alcalá University, Madrid, also believes that PLE deserves further evaluation not just for photoprotection but for reinvigorating damaged skin due to its antioxidant and anti-inflammatory properties. He was the senior author of a 2020 paper in Photochemical and Photobiological Sciences that summarized the potential benefits of PLE in preventing damage related to sun exposure.
Among its mechanism, PLE generates reactive oxygen species (ROS) and prevents depletion of Langerhans cells induced by ultraviolet (UV) light, Dr. González explained in an interview. “At the cellular level, PLE activates tumor suppression p53, inhibits UV-induced COX-2 expression, reduces inflammation, and preventions immunosuppression,” he continued. In addition, he said PLE also prevents UV-A-induced common deletions related to mitochondrial damage and MMP1 expression induced by various UV wavelengths.
“These molecular and cellular effects may translate into long-term inhibition of carcinogenesis including actinic keratosis,” he said, noting that all of these findings “justify the work by Pellacani and collaborators.”
Dr. Pellacani reports no potential conflicts of interest. Dr. González has a financial relationship with Cantabria Laboratories.
MILAN – Application of topical or both treated over 12 months, in a randomized, blinded study presented at the annual congress of the European Academy of Dermatology and Venereology.
At 12 months, the percentage of patients with a normal or almost normal honeycomb pattern when evaluated blindly with reflectance confocal microscopy (RCM) was about twice as great in either of the two groups that received PLE relative to those treated with topical photoprotection alone, according to Giovanni Pellacani, MD, PhD, chair of dermatology, University of Sapienza, Rome.
“In patients with severe actinic keratosis, the 12-month use of a PLE-based topical or oral photoprotection is associated with positive clinical and anatomical outcomes,” Dr. Pellacani said.
PLE, which is already commonly used in sun protection products, is derived from a South American species of fern and has been proposed for a broad array of dermatologic diseases. According to Dr. Pellacani, in vivo studies associating PLE with immune photoprotection make this agent particularly promising for severe AKs.
In this study involving two clinical research centers in Italy, 131 patients with photoaging and at least three AKs were randomized to one of three treatment arms. The control arm received topical photoprotection with an SPF of 100 or higher applied twice daily to all sun-exposed areas. The two treatment arms received the same topical photoprotection plus either a PLE-containing topical cream alone or a PLE-containing topical cream plus PLE in an oral form (240 mg) once daily
Patients were evaluated at 3 months, 6 months, and 1 year with several measures, including the Actinic Keratosis Area Score Index (AKASI) and the AK Field Assessment Scale Area (AK-FAS). They were also assessed with RCM. All clinical assessments and RCM evaluations, which assessed seven different parameters, such as honeycomb pattern, mottled pigmentation, and reticulated collagen, were performed by dermatologists blinded to the treatment assignment.
Complete data were available for 116 patients who completed all three evaluations over the 12 months of follow-up. On RCM, 50% of those receiving the oral and topical forms of PLE and 45% of those receiving topical PLE had normalization of the honeycomb pattern. These responses were significantly greater (P = .04 for both) than the 26% with normalization in the control group.
Although there were no significant differences in any of the other parameters evaluated by RCM, the improvement in the honeycomb pattern was accompanied by a 7% improvement in the AKASI score in patients taking PLE, either topically or orally and topically, while there was a 6% worsening (P < .001) among controls.
The AK-FAS score improved at 12 months by 26% in the group on oral/topical PLE and by 4% in the group on topical PLE. The score worsened by 13% among controls.
Over the course of the study, patients were permitted to take an appropriate therapy, such as imiquimod, cryotherapy, or 5-flourouracil if there was worsening of the AK-FAS score or if new lesions appeared.
On this measure, 38% of controls and 11% of those randomized to topical PLE had progressive disease versus only 2% of those randomized to take both topical and oral PLE, Dr. Pellacani reported.
The lower rate of new lesions or a start of a new drug over the course of the study in the group receiving both the topical and the oral formulations of PLE relative to those receiving topical PLE alone did not reach statistical significance, but Dr. Pellacani concluded that the addition of PLE to topical photoprotection without PLE seemed to provide a potentially clinically meaningful advantage.
Larger studies and longer term studies are needed, according to Dr. Pellacani, who noted that the substantial body of clinical studies associating PLE with benefit in a variety of dermatologic disorders has been weakened by the absence of well-designed studies that are adequately powered to guide clinical use.
Salvador González, MD, PhD, a dermatology specialist at Alcalá University, Madrid, also believes that PLE deserves further evaluation not just for photoprotection but for reinvigorating damaged skin due to its antioxidant and anti-inflammatory properties. He was the senior author of a 2020 paper in Photochemical and Photobiological Sciences that summarized the potential benefits of PLE in preventing damage related to sun exposure.
Among its mechanism, PLE generates reactive oxygen species (ROS) and prevents depletion of Langerhans cells induced by ultraviolet (UV) light, Dr. González explained in an interview. “At the cellular level, PLE activates tumor suppression p53, inhibits UV-induced COX-2 expression, reduces inflammation, and preventions immunosuppression,” he continued. In addition, he said PLE also prevents UV-A-induced common deletions related to mitochondrial damage and MMP1 expression induced by various UV wavelengths.
“These molecular and cellular effects may translate into long-term inhibition of carcinogenesis including actinic keratosis,” he said, noting that all of these findings “justify the work by Pellacani and collaborators.”
Dr. Pellacani reports no potential conflicts of interest. Dr. González has a financial relationship with Cantabria Laboratories.
AT THE EADV CONGRESS
Field Cancerization in Dermatology: Updates on Treatment Considerations and Emerging Therapies
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
There has been increasing awareness of field cancerization in dermatology and how it relates to actinic damage, actinic keratoses (AKs), and the development of cutaneous squamous cell carcinomas (SCCs). The concept of field cancerization, which was first described in the context of oropharyngeal SCCs, attempted to explain the repeated observation of local recurrences that were instead multiple primary oropharyngeal SCCs occurring within a specific region of tissue. It was hypothesized that the tissue surrounding a malignancy also harbors irreversible oncogenic damage and therefore predisposes the surrounding tissue to developing further malignancy.1 The development of additional malignant lesions would be considered distinct from a true recurrence of the original malignancy.
Field cancerization may be partially explained by a genetic basis, as mutations in the tumor suppressor gene, TP53—the most frequently observed mutation in cutaneous SCCs—also is found in sun-exposed but clinically normal skin.2,3 The finding of oncogenic mutations in nonlesional skin supports the theory of field cancerization, in which a region contains multiple genetically altered populations, some of which may progress to cancer. Because there currently is no widely accepted clinical definition or validated clinical measurement of field cancerization in dermatology, it may be difficult for dermatologists to recognize which patients may be at risk for developing further malignancy in a potential area of field cancerization. Willenbrink et al4 updated the definition of field cancerization in dermatology as “multifocal clinical atypia characterized by AKs or SCCs in situ with or without invasive disease occurring in a field exposed to chronic UV radiation.” Managing patients with field cancerization can be challenging. Herein, we discuss updates to nonsurgical field-directed and lesion-directed therapies as well as other emerging therapies.
Field-Directed Therapies
Topical 5-fluorouracil (5-FU) and imiquimod cream 5% used as field-directed therapies help reduce the extent of AKs and actinic damage in areas of possible field cancerization.5 The addition of calcipotriol to topical 5-FU, which theoretically augments the skin’s T-cell antitumor response via the cytokine thymic stromal lymphopoietin, recently has been studied using short treatment courses resulting in an 87.8% reduction in AKs compared to a 26.3% reduction with topical 5-FU alone (when used twice daily for 4 days) and conferred a reduced risk of cutaneous SCCs 3 years after treatment (hazard ratio, 0.215 [95% CI, 0.048-0.972]; P=.032).6,7 Chemowraps using topical 5-FU may be considered in more difficult-to-treat areas of field cancerization with multiple AKs or keratinocyte carcinomas of the lower extremities.8 The routine use of chemowraps—weekly application of 5-FU covered with an occlusive dressing—may be limited by the inability to control the extent of epidermal damage and subsequent systemic absorption. Ingenol mebutate, which was approved for treatment of AKs in 2012, was removed from both the European and US markets in 2020 because the medication may paradoxically increase the long-term incidence of skin cancer.9
Meta-analysis has shown that photodynamic therapy (PDT) with aminolevulinic acid demonstrated complete AK clearance in 75.8% of patients (N=156)(95% CI, 55.4%-96.2%).10 A more recent method of PDT using natural sunlight as the activation source demonstrated AK clearance of 95.5%, and it appeared to be a less painful alternative to traditional PDT.11 Tacalcitol, another form of vitamin D, also has been shown to enhance the efficacy of PDT for AKs.12
Field-directed treatment with erbium:YAG and CO2 lasers, which physically remove the actinically damaged epidermis, have been shown to possibly be as efficacious as topical 5-FU and 30% trichloroacetic acid (TCA) but possibly inferior to PDT.13 There has been growing interest in laser-assisted therapy, in which an ablative fractional laser is used to generate microscopic channels to theoretically enhance the absorption of a topical medication. A meta-analysis of the use of laser-assisted therapy for photosensitizing agents in PDT demonstrated a 33% increased chance of AK clearance compared to PDT alone (P<.01).14
Lesion-Directed Therapies
Multiple KAs or cutaneous SCCs may develop in an area of field cancerization, and surgically treating these multiple lesions in a concentrated area may be challenging. Intralesional agents, including methotrexate, 5-FU, bleomycin, and interferon, are known treatments for KAs.15 Intralesional 5-FU (25 mg once weekly for 3–4 weeks) in particular produced complete resolution in 92% of cutaneous SCCs and may be optimal for multiple or rapidly growing lesions, especially on the extremities.16
Oral Therapies
Oral therapies are considered in high-risk patients with multiple or recurrent cutaneous SCCs or in those who are immunosuppressed. Two trials demonstrated that nicotinamide 500 mg twice daily for 4 and 12 months decreased AKs by 29% to 35% and 13% (average of 3–5 fewer AKs as compared to baseline), respectively.17,18 A meta-analysis found a reduction of cutaneous SCCs (rate ratio, 0.48 [95% CI, 0.26-0.88]; I2=67%; 552 patients, 5 trials), and given the favorable safety profile, nicotinamide can be considered for chemoprevention.19
Acitretin, shown to reduce AKs by 13.4% to 50%, is the primary oral chemoprevention recommended in transplant recipients.20 Interestingly, a recent meta-analysis failed to find significant differences between the efficacy of acitretin and nicotinamide.21 The tolerability of acitretin requires serious consideration, as 52.2% of patients withdrew due to adverse effects in one trial.22
Capecitabine (250–1150 mg twice daily), the oral form of 5-FU, decreased the incidence of AKs and cutaneous SCCs in 53% and 72% of transplant recipients, respectively.23 Although several reports observed paradoxical eruptions of AKs following capecitabine for other malignancies, this actually underscores the efficacy of capecitabine, as the newly emerged AKs resolved thereafter.24 Still, the evidence supporting capecitabine does not include any controlled studies.
Novel Therapies
In 2021, tirbanibulin ointment 1%, a Src tyrosine kinase inhibitor of tubulin polymerization that induces p53 expression and subsequent cell death, was approved by the US Food and Drug Administration for the treatment of AKs.25 Two trials reported AK clearance rates of 44% and 54% with application of tirbanibulin once daily for 5 days (vs 5% and 13%, respectively, with placebo, each with P<.001) at 2 months and a sustained clearance rate of 27% at 1 year. The predominant adverse effects were local skin reactions, including application-site pain, pruritus, mild erythema, or scaling. Unlike in other treatments such as 5-FU or cryotherapy, erosions, dyspigmentation, or scarring were not notably observed.
Intralesional talimogene laherparepvec (T-VEC), an oncolytic, genetically modified herpes simplex virus type 1 that incites antitumor immune responses, received US Food and Drug Administration approval in 2015 for the treatment of cutaneous and lymph node metastases of melanoma that are unable to be surgically resected. More recently, T-VEC has been investigated for oropharyngeal SCC. A phase 1 and phase 2 trial of 17 stage III/IV SCC patients receiving T-VEC and cisplatin demonstrated pathologic remission in 14 of 15 (93%) patients, with 82.4% survival at 29 months.26 A multicenter phase 1b trial of 36 patients with recurrent or metastatic head and neck SCCs treated with T-VEC and pembrolizumab exhibited a tolerable safety profile, and 5 cases had a partial response.27 However, phase 3 trials of T-VEC have yet to be pursued. Regarding its potential use for cutaneous SCCs, it has been reportedly used in a liver transplant recipient with metastatic cutaneous SCCs who received 2 doses of T-VEC (1 month apart) and attained remission of disease.28 There currently is a phase 2 trial examining the effectiveness of T-VEC in patients with cutaneous SCCs (ClinicalTrials.gov identifier NCT03714828).
Final Thoughts
It is important for dermatologists to bear in mind the possible role of field cancerization in their comprehensive care of patients at risk for multiple skin cancers. Management of areas of field cancerization can be challenging, particularly in patients who develop multiple KAs or cutaneous SCCs in a concentrated area and may need to involve different levels of treatment options, including field-directed therapies and lesion-directed therapies, as well as systemic chemoprevention.
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
- Braakhuis BJM, Tabor MP, Kummer JA, et al. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730.
- Ashford BG, Clark J, Gupta R, et al. Reviewing the genetic alterations in high-risk cutaneous squamous cell carcinoma: a search for prognostic markers and therapeutic targets. Head Neck. 2017;39:1462-1469. doi:10.1002/hed.24765
- Albibas AA, Rose-Zerilli MJJ, Lai C, et al. Subclonal evolution of cancer-related gene mutations in p53 immunopositive patches in human skin. J Invest Dermatol. 2018;138:189-198. doi:10.1016/j.jid.2017.07.844
- Willenbrink TJ, Ruiz ES, Cornejo CM, et al. Field cancerization: definition, epidemiology, risk factors, and outcomes. J Am Acad Dermatol. 2020;83:709-717. doi:10.1016/j.jaad.2020.03.126
- Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi:10.1056/NEJMoa1811850
- Cunningham TJ, Tabacchi M, Eliane JP, et al. Randomized trial of calcipotriol combined with 5-fluorouracil for skin cancer precursor immunotherapy. J Clin Invest. 2017;127:106-116. doi:10.1172/JCI89820
- Rosenberg AR, Tabacchi M, Ngo KH, et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight. 2019;4:125476. doi:10.1172/jci.insight.125476
- Peuvrel L, Saint-Jean M, Quereux G, et al. 5-fluorouracil chemowraps for the treatment of multiple actinic keratoses. Eur J Dermatol. 2017;27:635-640. doi:10.1684/ejd.2017.3128
- Eisen DB, Asgari MM, Bennett DD, et al. Guidelines of care for the management of actinic keratosis. J Am Acad Dermatol. 2021;85:E209-E233. doi:10.1016/j.jaad.2021.02.082
- Vegter S, Tolley K. A network meta-analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One. 2014;9:E96829. doi:10.1371/journal.pone.0096829
- Zhu L, Wang P, Zhang G, et al. Conventional versus daylight photodynamic therapy for actinic keratosis: a randomized and prospective study in China. Photodiagnosis Photodyn Ther. 2018;24:366-371. doi:10.1016/j.pdpdt.2018.10.010
- Borgia F, Riso G, Catalano F, et al. Topical tacalcitol as neoadjuvant for photodynamic therapy of acral actinic keratoses: an intra-patient randomized study. Photodiagnosis Photodyn Ther. 2020;31:101803. doi:10.1016/j.pdpdt.2020.101803
- Tai F, Shah M, Pon K, et al. Laser resurfacing monotherapy for the treatment of actinic keratosis. J Cutan Med Surg. 2021;25:634-642. doi:10.1177/12034754211027515
- Steeb T, Schlager JG, Kohl C, et al. Laser-assisted photodynamic therapy for actinic keratosis: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:947-956. doi:10.1016/j.jaad.2018.09.021
- Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702. doi:10.1016/j.jaad.2009.09.048
- Maxfield L, Shah M, Schwartz C, et al. Intralesional 5-fluorouracil for the treatment of squamous cell carcinomas. J Am Acad Dermatol. 2021;84:1696-1697. doi:10.1016/j.jaad.2020.12.049
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi:10.1056/NEJMoa1506197
- Surjana D, Halliday GM, Martin AJ, et al. Oral nicotinamide reduces actinic keratoses in phase II double-blinded randomized controlled trials. J Invest Dermatol. 2012;132:1497-1500. doi:10.1038/jid.2011.459
- Mainville L, Smilga AS, Fortin PR. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: a systematic review and meta-analysis [published online February 8, 2022]. J Cutan Med Surg. doi:10.1177/12034754221078201
- Massey PR, Schmults CD, Li SJ, et al. Consensus-based recommendations on the prevention of squamous cell carcinoma in solid organ transplant recipients: a Delphi Consensus Statement. JAMA Dermatol. 2021;157:1219-1226. doi:10.1001/jamadermatol.2021.3180
- Tee LY, Sultana R, Tam SYC, et al. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid-organ transplant recipients: systematic review and meta-analyses. J Am Acad Dermatol. 2021;84:528-530. doi:10.1016/j.jaad.2020.04.160
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273. doi:10.1046/j.1440-0960.2002.00613.x
- Schauder DM, Kim J, Nijhawan RI. Evaluation of the use of capecitabine for the treatment and prevention of actinic keratoses, squamous cell carcinoma, and basal cell carcinoma: a systematic review. JAMA Dermatol. 2020;156:1117-1124. doi:10.1001/jamadermatol.2020.2327
- Antoniolli LP, Escobar GF, Peruzzo J. Inflammatory actinic keratosis following capecitabine therapy. Dermatol Ther. 2020;33:E14082. doi:10.1111/dth.14082
- Blauvelt A, Kempers S, Lain E, et al. Phase 3 trials of tirbanibulin ointment for actinic keratosis. N Engl J Med. 2021;384:512-520. doi:10.1056/NEJMoa2024040
- Harrington KJ, Hingorani M, Tanay MA, et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin Cancer Res. 2010;16:4005-4015. doi:10.1158/1078-0432.CCR-10-0196
- Harrington KJ, Kong A, Mach N, et al. Talimogene laherparepvec and pembrolizumab in recurrent or metastatic squamous cell carcinoma of the head and neck (MASTERKEY-232): a multicenter, phase 1b study. Clin Cancer Res. 2020;26:5153-5161. doi:10.1158/1078-0432.CCR-20-1170
- Nguyen TA, Offner M, Hamid O, et al. Complete and sustained remission of metastatic cutaneous squamous cell carcinoma in a liver transplant patient treated with talimogene laherparepvec. Dermatol Surg. 2021;47:820-822. doi:10.1097/DSS.0000000000002739
Pink or red actinic keratoses signal more inflammation
lesions.
Data suggest that up to 65% of squamous cell carcinomas (SCCs) were at some point diagnosed as AKs, wrote Jessica G. Labadie, MD, of the department of dermatology at Northwestern University, Chicago, and colleagues. Early identification of AKs is important to prevent progression to SCCs; previous studies have used histology or morphology, but not color, to characterize AK lesions, they said. In the study published in Dermatologic Surgery, the researchers analyzed images and histopathology slides to characterize AKs by color and to examine associations with inflammation and vasculature. They identified AKs from patients diagnosed between January 2018 and October 2019. The lesions were classified as white (4), brown (9), red (15), or pink (21).
Overall, white AKs had an absence of erythema and were significantly less likely to show inflammation on histopathology, compared with other colors. Brown AKs showed no significant increase in vascularity, but were significantly associated with pigment incontinence, basilar pigment presence, and absence of inflammation.
Notably, dermoscopy of red AKs revealed a distinctive polymorphous vessel pattern in most samples, as well as erythema in all. Similarly, all pink AKs showed erythema, and all showed inflammatory infiltrate on histology, although most did not show increased vascularity.
For all colors of AKs, there was a significant association between the presence of erythema on dermoscopy and the presence of inflammation on histology, while the absence of erythema on dermoscopy corresponded to a significant absence of inflammation on histology, the researchers noted.
“This report adds to the armamentarium of a dermatologist by proposing a novel way to characterize AK lesions,” the researchers wrote.
The study findings were limited by several factors including the inability to confirm which AKs would transform into SCCs based on color, and the inclusion of a study population with advanced AKs that may not generalize to typical AKs, the researchers noted. More research is needed to explore the impact of AK color on SCC development, they emphasized.
However, the results represent a novel way to characterize AK lesions, and triage them in a way that may spare some patients from unneeded biopsies, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
lesions.
Data suggest that up to 65% of squamous cell carcinomas (SCCs) were at some point diagnosed as AKs, wrote Jessica G. Labadie, MD, of the department of dermatology at Northwestern University, Chicago, and colleagues. Early identification of AKs is important to prevent progression to SCCs; previous studies have used histology or morphology, but not color, to characterize AK lesions, they said. In the study published in Dermatologic Surgery, the researchers analyzed images and histopathology slides to characterize AKs by color and to examine associations with inflammation and vasculature. They identified AKs from patients diagnosed between January 2018 and October 2019. The lesions were classified as white (4), brown (9), red (15), or pink (21).
Overall, white AKs had an absence of erythema and were significantly less likely to show inflammation on histopathology, compared with other colors. Brown AKs showed no significant increase in vascularity, but were significantly associated with pigment incontinence, basilar pigment presence, and absence of inflammation.
Notably, dermoscopy of red AKs revealed a distinctive polymorphous vessel pattern in most samples, as well as erythema in all. Similarly, all pink AKs showed erythema, and all showed inflammatory infiltrate on histology, although most did not show increased vascularity.
For all colors of AKs, there was a significant association between the presence of erythema on dermoscopy and the presence of inflammation on histology, while the absence of erythema on dermoscopy corresponded to a significant absence of inflammation on histology, the researchers noted.
“This report adds to the armamentarium of a dermatologist by proposing a novel way to characterize AK lesions,” the researchers wrote.
The study findings were limited by several factors including the inability to confirm which AKs would transform into SCCs based on color, and the inclusion of a study population with advanced AKs that may not generalize to typical AKs, the researchers noted. More research is needed to explore the impact of AK color on SCC development, they emphasized.
However, the results represent a novel way to characterize AK lesions, and triage them in a way that may spare some patients from unneeded biopsies, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
lesions.
Data suggest that up to 65% of squamous cell carcinomas (SCCs) were at some point diagnosed as AKs, wrote Jessica G. Labadie, MD, of the department of dermatology at Northwestern University, Chicago, and colleagues. Early identification of AKs is important to prevent progression to SCCs; previous studies have used histology or morphology, but not color, to characterize AK lesions, they said. In the study published in Dermatologic Surgery, the researchers analyzed images and histopathology slides to characterize AKs by color and to examine associations with inflammation and vasculature. They identified AKs from patients diagnosed between January 2018 and October 2019. The lesions were classified as white (4), brown (9), red (15), or pink (21).
Overall, white AKs had an absence of erythema and were significantly less likely to show inflammation on histopathology, compared with other colors. Brown AKs showed no significant increase in vascularity, but were significantly associated with pigment incontinence, basilar pigment presence, and absence of inflammation.
Notably, dermoscopy of red AKs revealed a distinctive polymorphous vessel pattern in most samples, as well as erythema in all. Similarly, all pink AKs showed erythema, and all showed inflammatory infiltrate on histology, although most did not show increased vascularity.
For all colors of AKs, there was a significant association between the presence of erythema on dermoscopy and the presence of inflammation on histology, while the absence of erythema on dermoscopy corresponded to a significant absence of inflammation on histology, the researchers noted.
“This report adds to the armamentarium of a dermatologist by proposing a novel way to characterize AK lesions,” the researchers wrote.
The study findings were limited by several factors including the inability to confirm which AKs would transform into SCCs based on color, and the inclusion of a study population with advanced AKs that may not generalize to typical AKs, the researchers noted. More research is needed to explore the impact of AK color on SCC development, they emphasized.
However, the results represent a novel way to characterize AK lesions, and triage them in a way that may spare some patients from unneeded biopsies, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM DERMATOLOGIC SURGERY
Literature review highlights benefits of chemical peels for field AK treatment
including 88 patients.
AKs remain an ongoing health concern because of their potential to become malignant, and chemical peels are among the recommended options for field therapy, wrote Angela J. Jiang, MD, from the department of dermatology at the Henry Ford Health System, Detroit, and colleagues. “Although most dermatologists agree on the importance of field treatment, cryotherapy still remains the standard of care for treatment of AKs,” they noted, adding that the safety and efficacy of chemical peels for AK field therapy have not been well studied.
Chemical peels offer the benefit of a single treatment for patients, which eliminates the patient compliance issue needed for successful topical therapy, the researchers said. In fact, “patients report preference for the tolerability of treatment with chemical peels and the shorter downtime, compared with other field treatments,” they added.
In the study published in Dermatologic Surgery, they reviewed data from five prospective studies on the safety and efficacy of chemical peels as AK field treatments published from 1946 to March 2020 in the National Library of Medicine’s PubMed database. Of the 151 articles on the use of chemical peels for AKs, the 5 studies met the criteria for their review.
One split-face study evaluated glycolic acid peels (published in 1998), two split-face studies evaluated a combination of Jessner’s and 35% trichloroacetic acid (TCA) peels (published in 1995 and 1997), and two randomized studies evaluated TCA peels alone (published in 2006 and 2016).
Overall, the studies showed efficacy of peels in reducing AK counts, with minimal adverse events. In the glycolic acid study, 70% glycolic acid plus 5-fluorouracil (5-FU) yielded a 91.9% mean reduction in AKs at 6 months’ follow-up. A combination of Jessner’s solution and 35% TCA showed a significant reduction in AKs at 12 and at 32 months post treatment – a 75% reduction at 12 months in one study and 78% at 32 months in the other – similar to results achieved with 5-FU.
In studies of TCA alone, 30% TCA peels were similar in AK reduction (89%) to 5-FU (83%) and carbon dioxide laser resurfacing (92%). In another TCA study, 35% TCA was less effective at AK reduction at 12 months, compared with aminolevulinic acid photodynamic therapy (ALA-PDT), but the 35% peel was applied at a more superficial level than in the study of 30% TCA, the authors wrote.
Chemical peels also demonstrated effectiveness in preventing keratinocytic carcinomas, the researchers wrote. In the 30% TCA study, the rate of keratinocyte carcinoma development was 3.75-5.25 times lower in patients treated with 30% TCA peels, compared with 5-FU and carbon dioxide laser resurfacing (CO2) after 5 years.
Chemical peels were well tolerated overall, although side effects varied among the studies. Patients in one study reported no side effects, while patients in other studies reported transient erythema and discomfort. In the study comparing TCA with PDT treatment, PDT was associated with greater pain, erythema, and pustules, the researchers wrote; however, patients treated with 35% TCA reported scarring.
From patients’ perspectives, chemical peels were preferable because of the single application, brief downtime, and minimal adverse effects. From the provider perspective, chemical peels are a more cost-effective way to treat large surface areas for AKs, compared with 5-FU or lasers, the researchers said.
The study findings were limited by several factors including the small number of prospective studies and relatively small number of patients, they noted. “The small number of included studies is partially due to the lack of studies that performed AK counts before and after treatments,” they said. The dearth of literature on chemical peels for AKs may stem from lack of residency training on the use of peels, they added.
However, the results support the use of chemical peels as an effective option for field treatment of AKs, with the added benefits of convenience and cost-effectiveness for patients, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
including 88 patients.
AKs remain an ongoing health concern because of their potential to become malignant, and chemical peels are among the recommended options for field therapy, wrote Angela J. Jiang, MD, from the department of dermatology at the Henry Ford Health System, Detroit, and colleagues. “Although most dermatologists agree on the importance of field treatment, cryotherapy still remains the standard of care for treatment of AKs,” they noted, adding that the safety and efficacy of chemical peels for AK field therapy have not been well studied.
Chemical peels offer the benefit of a single treatment for patients, which eliminates the patient compliance issue needed for successful topical therapy, the researchers said. In fact, “patients report preference for the tolerability of treatment with chemical peels and the shorter downtime, compared with other field treatments,” they added.
In the study published in Dermatologic Surgery, they reviewed data from five prospective studies on the safety and efficacy of chemical peels as AK field treatments published from 1946 to March 2020 in the National Library of Medicine’s PubMed database. Of the 151 articles on the use of chemical peels for AKs, the 5 studies met the criteria for their review.
One split-face study evaluated glycolic acid peels (published in 1998), two split-face studies evaluated a combination of Jessner’s and 35% trichloroacetic acid (TCA) peels (published in 1995 and 1997), and two randomized studies evaluated TCA peels alone (published in 2006 and 2016).
Overall, the studies showed efficacy of peels in reducing AK counts, with minimal adverse events. In the glycolic acid study, 70% glycolic acid plus 5-fluorouracil (5-FU) yielded a 91.9% mean reduction in AKs at 6 months’ follow-up. A combination of Jessner’s solution and 35% TCA showed a significant reduction in AKs at 12 and at 32 months post treatment – a 75% reduction at 12 months in one study and 78% at 32 months in the other – similar to results achieved with 5-FU.
In studies of TCA alone, 30% TCA peels were similar in AK reduction (89%) to 5-FU (83%) and carbon dioxide laser resurfacing (92%). In another TCA study, 35% TCA was less effective at AK reduction at 12 months, compared with aminolevulinic acid photodynamic therapy (ALA-PDT), but the 35% peel was applied at a more superficial level than in the study of 30% TCA, the authors wrote.
Chemical peels also demonstrated effectiveness in preventing keratinocytic carcinomas, the researchers wrote. In the 30% TCA study, the rate of keratinocyte carcinoma development was 3.75-5.25 times lower in patients treated with 30% TCA peels, compared with 5-FU and carbon dioxide laser resurfacing (CO2) after 5 years.
Chemical peels were well tolerated overall, although side effects varied among the studies. Patients in one study reported no side effects, while patients in other studies reported transient erythema and discomfort. In the study comparing TCA with PDT treatment, PDT was associated with greater pain, erythema, and pustules, the researchers wrote; however, patients treated with 35% TCA reported scarring.
From patients’ perspectives, chemical peels were preferable because of the single application, brief downtime, and minimal adverse effects. From the provider perspective, chemical peels are a more cost-effective way to treat large surface areas for AKs, compared with 5-FU or lasers, the researchers said.
The study findings were limited by several factors including the small number of prospective studies and relatively small number of patients, they noted. “The small number of included studies is partially due to the lack of studies that performed AK counts before and after treatments,” they said. The dearth of literature on chemical peels for AKs may stem from lack of residency training on the use of peels, they added.
However, the results support the use of chemical peels as an effective option for field treatment of AKs, with the added benefits of convenience and cost-effectiveness for patients, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
including 88 patients.
AKs remain an ongoing health concern because of their potential to become malignant, and chemical peels are among the recommended options for field therapy, wrote Angela J. Jiang, MD, from the department of dermatology at the Henry Ford Health System, Detroit, and colleagues. “Although most dermatologists agree on the importance of field treatment, cryotherapy still remains the standard of care for treatment of AKs,” they noted, adding that the safety and efficacy of chemical peels for AK field therapy have not been well studied.
Chemical peels offer the benefit of a single treatment for patients, which eliminates the patient compliance issue needed for successful topical therapy, the researchers said. In fact, “patients report preference for the tolerability of treatment with chemical peels and the shorter downtime, compared with other field treatments,” they added.
In the study published in Dermatologic Surgery, they reviewed data from five prospective studies on the safety and efficacy of chemical peels as AK field treatments published from 1946 to March 2020 in the National Library of Medicine’s PubMed database. Of the 151 articles on the use of chemical peels for AKs, the 5 studies met the criteria for their review.
One split-face study evaluated glycolic acid peels (published in 1998), two split-face studies evaluated a combination of Jessner’s and 35% trichloroacetic acid (TCA) peels (published in 1995 and 1997), and two randomized studies evaluated TCA peels alone (published in 2006 and 2016).
Overall, the studies showed efficacy of peels in reducing AK counts, with minimal adverse events. In the glycolic acid study, 70% glycolic acid plus 5-fluorouracil (5-FU) yielded a 91.9% mean reduction in AKs at 6 months’ follow-up. A combination of Jessner’s solution and 35% TCA showed a significant reduction in AKs at 12 and at 32 months post treatment – a 75% reduction at 12 months in one study and 78% at 32 months in the other – similar to results achieved with 5-FU.
In studies of TCA alone, 30% TCA peels were similar in AK reduction (89%) to 5-FU (83%) and carbon dioxide laser resurfacing (92%). In another TCA study, 35% TCA was less effective at AK reduction at 12 months, compared with aminolevulinic acid photodynamic therapy (ALA-PDT), but the 35% peel was applied at a more superficial level than in the study of 30% TCA, the authors wrote.
Chemical peels also demonstrated effectiveness in preventing keratinocytic carcinomas, the researchers wrote. In the 30% TCA study, the rate of keratinocyte carcinoma development was 3.75-5.25 times lower in patients treated with 30% TCA peels, compared with 5-FU and carbon dioxide laser resurfacing (CO2) after 5 years.
Chemical peels were well tolerated overall, although side effects varied among the studies. Patients in one study reported no side effects, while patients in other studies reported transient erythema and discomfort. In the study comparing TCA with PDT treatment, PDT was associated with greater pain, erythema, and pustules, the researchers wrote; however, patients treated with 35% TCA reported scarring.
From patients’ perspectives, chemical peels were preferable because of the single application, brief downtime, and minimal adverse effects. From the provider perspective, chemical peels are a more cost-effective way to treat large surface areas for AKs, compared with 5-FU or lasers, the researchers said.
The study findings were limited by several factors including the small number of prospective studies and relatively small number of patients, they noted. “The small number of included studies is partially due to the lack of studies that performed AK counts before and after treatments,” they said. The dearth of literature on chemical peels for AKs may stem from lack of residency training on the use of peels, they added.
However, the results support the use of chemical peels as an effective option for field treatment of AKs, with the added benefits of convenience and cost-effectiveness for patients, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM DERMATOLOGIC SURGERY
Phototoxicity Secondary to Home Fireplace Exposure After Photodynamic Therapy for Actinic Keratosis
To the Editor:
Photodynamic therapy (PDT) is a US Food and Drug Administration–approved treatment for actinic keratosis (AK). It also commonly is administered off label for basal cell carcinoma, Bowen disease, photoaging, and acne vulgaris and is being investigated for other applications.1,2 In the context of treating AK, the mechanism employed in PDT most commonly involves the application of exogenous aminolevulinic acid (ALA), which is metabolized to the endogenous photosensitizer protoporphyrin IX (PpIX) in skin cells by enzymes in the heme biosynthetic pathway.3 The preferential uptake of ALA and conversion to PpIX is due to the altered and increased permeability of abnormal keratin layers of aging, sun-damaged cells, and skin tumors. Selectivity of ALA also occurs due to the preferential intracellular accumulation of PpIX in proliferating, relatively iron–deficient, precancerous and cancerous cells. The therapeutic effect is achieved with light exposure to blue light wavelength at 417 nm and corresponds to the excitation peak of PpIX,4 which activates PpIX and forms reactive oxygen species in the presence of oxygen that ultimately cause cell necrosis and apoptosis.5 Because it takes approximately 24 hours for PpIX to be completely metabolized from the skin, patients are counseled to avoid sun or artificial light exposure in the first 24 hours post-PDT, regardless of the indication, to avoid a severe phototoxic reaction.3,6,7 Although it is normal and desirable for patients to experience some form of a phototoxic reaction, which may include erythema, edema, crusting, vesiculation, or erosion in most patients, these types of reactions most often are secondary to the intended exposure and incidental natural or artificial light exposures.6 We report a case of a severe phototoxic reaction in which a patient experienced painful erythema and purulence on the left side of the chin after being within an arm’s length of a flame in a fireplace following PDT treatment.
A 59-year-old man presented to our dermatology clinic for his second of 3 PDT sessions to treat AKs on the face. He had a history of a basal cell carcinoma on the left nasolabial fold that previously was treated with Mohs micrographic surgery and melanoma on the left ear that was previously treated with excision. The patient received the initial PDT session 1 month prior and experienced a mild reaction with minimal redness and peeling that resolved in 4 to 5 days. For the second treatment, per standard protocol at our clinic, ALA was applied to the face, after which the patient incubated for 1 hour prior to blue light exposure (mean [SD] peak output of 417 [5] nm for 1000 seconds and 10 J/cm2).
After blue light exposure, broad-spectrum sunscreen (sun protection factor 47) was applied to our patient’s face, and he wore a wide-brimmed hat upon leaving the clinic and walking to his car. Similar to the first PDT session 1 month prior, he experienced minimal pain immediately after treatment. Once home and approximately 4 to 5 hours after PDT, he tended to a fire using his left hand and leaned into the fireplace with the left side of his face, which was within an arm’s length of the flames. Although his skin did not come in direct contact with the flames, the brief 2- to 3-minute exposure to the flame’s light and heat produced an immediate intense burning pain that the patient likened to the pain of blue light exposure. Within 24 hours, he developed a severe inflammatory reaction that included erythema, edema, desquamation, and pustules on the left side of the chin and cheek that produced a purulent discharge (Figure). The purulence resolved the next day; however, the other clinical manifestations persisted for 1 week. Despite the discomfort and symptoms, our patient did not seek medical attention and instead managed his symptoms conservatively with cold compresses. Although he noticed an overall subjective improvement in the appearance of his face after this second treatment, he received a third treatment with PDT approximately 1 month later, which resulted in a response that was similar to his first visit.
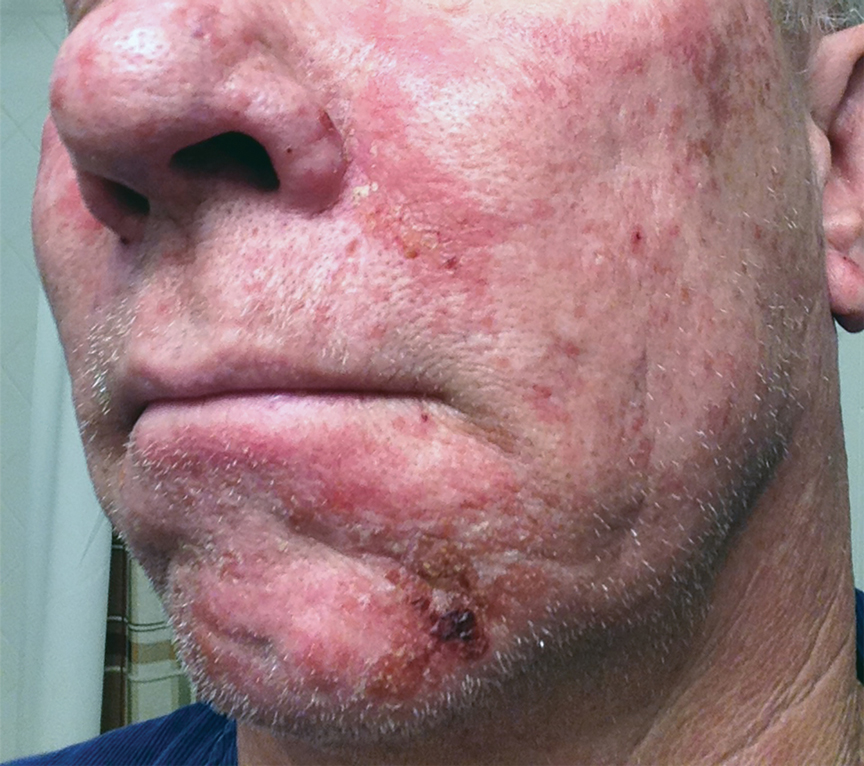
Photodynamic therapy is an increasingly accepted treatment modality for a plethora of benign and malignant dermatologic conditions. Although blue and red light are the most common light sources utilized with PDT because their wavelengths (404–420 nm and 635 nm, respectively) correspond to the excitation peaks of photosensitizers, alternative light sources increasingly are being explored. There is increasing interest in utilizing infrared (IR) light sources (700–1,000,000 nm) to penetrate deeper into the skin in the treatment of precancerous and cancerous lesions. Exposure to IR radiation is known to raise skin temperature via inside-out dermal water absorption and is thought to be useful in PDT-ALA by promoting ALA penetration and its conversion to PpIX.8 In a randomized controlled trial by Giehl et al,9 visible light plus water-filtered IR-A light was shown to produce considerably less pain in ALA-PDT compared to placebo, though efficacy was not statistically affected. There are burgeoning trials examining the role of IR in treating dermatologic conditions such as acne, but research is still needed on ALA-PDT activated by IR radiation to target AKs.
Although the PDT side-effect profile of phototoxicity, dyspigmentation, and hypersensitivity is well documented, phototoxicity secondary to flame exposure is rare. In our patient, the synergistic effect of light and heat produced an exuberant phototoxic reaction. As the applications for PDT continue to broaden, this case may represent the importance of addressing additional precautions, such as avoiding open flames in the house or while camping, in the PDT aftercare instructions to maximize patient safety.
- Fritsch C, Ruzicka T. Fluorescence diagnosis and photodynamic therapy in dermatology from experimental state to clinic standard methods. J Environ Pathol Toxicol Oncol. 2006;25:425-439.
- Lang K, Schulte KW, Ruzicka T, et al. Aminolevulinic acid (Levulan)in photodynamic therapy of actinic keratoses. Skin Therapy Lett. 2001;6:1-2, 5.
- Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992;14:275-292.
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163.
- Gad F, Viau G, Boushira M, et al. Photodynamic therapy with 5-aminolevulinic acid induces apoptosis and caspase activation in malignant T cells. J Cutan Med Surg. 2001;5:8-13.
- Piacquadio DJ, Chen DM, Farber HF, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol. 2004;140:41-46.
- Rhodes LE, Tsoukas MM, Anderson RR, et al. Iontophoretic delivery of ALA provides a quantitative model for ALA pharmacokinetics and PpIX phototoxicity in human skin. J Invest Dermatol. 1997;108:87-91.
- Dover JS, Phillips TJ, Arndt KA. Cutaneous effects and therapeutic uses of heat with emphasis on infrared radiation. J Am Acad Dermatol. 1989;20(2, pt 1):278-286.
- Giehl KA, Kriz M, Grahovac M, et al. A controlled trial of photodynamic therapy of actinic keratosis comparing different red light sources. Eur J Dermatol. 2014;24:335-341.
To the Editor:
Photodynamic therapy (PDT) is a US Food and Drug Administration–approved treatment for actinic keratosis (AK). It also commonly is administered off label for basal cell carcinoma, Bowen disease, photoaging, and acne vulgaris and is being investigated for other applications.1,2 In the context of treating AK, the mechanism employed in PDT most commonly involves the application of exogenous aminolevulinic acid (ALA), which is metabolized to the endogenous photosensitizer protoporphyrin IX (PpIX) in skin cells by enzymes in the heme biosynthetic pathway.3 The preferential uptake of ALA and conversion to PpIX is due to the altered and increased permeability of abnormal keratin layers of aging, sun-damaged cells, and skin tumors. Selectivity of ALA also occurs due to the preferential intracellular accumulation of PpIX in proliferating, relatively iron–deficient, precancerous and cancerous cells. The therapeutic effect is achieved with light exposure to blue light wavelength at 417 nm and corresponds to the excitation peak of PpIX,4 which activates PpIX and forms reactive oxygen species in the presence of oxygen that ultimately cause cell necrosis and apoptosis.5 Because it takes approximately 24 hours for PpIX to be completely metabolized from the skin, patients are counseled to avoid sun or artificial light exposure in the first 24 hours post-PDT, regardless of the indication, to avoid a severe phototoxic reaction.3,6,7 Although it is normal and desirable for patients to experience some form of a phototoxic reaction, which may include erythema, edema, crusting, vesiculation, or erosion in most patients, these types of reactions most often are secondary to the intended exposure and incidental natural or artificial light exposures.6 We report a case of a severe phototoxic reaction in which a patient experienced painful erythema and purulence on the left side of the chin after being within an arm’s length of a flame in a fireplace following PDT treatment.
A 59-year-old man presented to our dermatology clinic for his second of 3 PDT sessions to treat AKs on the face. He had a history of a basal cell carcinoma on the left nasolabial fold that previously was treated with Mohs micrographic surgery and melanoma on the left ear that was previously treated with excision. The patient received the initial PDT session 1 month prior and experienced a mild reaction with minimal redness and peeling that resolved in 4 to 5 days. For the second treatment, per standard protocol at our clinic, ALA was applied to the face, after which the patient incubated for 1 hour prior to blue light exposure (mean [SD] peak output of 417 [5] nm for 1000 seconds and 10 J/cm2).
After blue light exposure, broad-spectrum sunscreen (sun protection factor 47) was applied to our patient’s face, and he wore a wide-brimmed hat upon leaving the clinic and walking to his car. Similar to the first PDT session 1 month prior, he experienced minimal pain immediately after treatment. Once home and approximately 4 to 5 hours after PDT, he tended to a fire using his left hand and leaned into the fireplace with the left side of his face, which was within an arm’s length of the flames. Although his skin did not come in direct contact with the flames, the brief 2- to 3-minute exposure to the flame’s light and heat produced an immediate intense burning pain that the patient likened to the pain of blue light exposure. Within 24 hours, he developed a severe inflammatory reaction that included erythema, edema, desquamation, and pustules on the left side of the chin and cheek that produced a purulent discharge (Figure). The purulence resolved the next day; however, the other clinical manifestations persisted for 1 week. Despite the discomfort and symptoms, our patient did not seek medical attention and instead managed his symptoms conservatively with cold compresses. Although he noticed an overall subjective improvement in the appearance of his face after this second treatment, he received a third treatment with PDT approximately 1 month later, which resulted in a response that was similar to his first visit.

Photodynamic therapy is an increasingly accepted treatment modality for a plethora of benign and malignant dermatologic conditions. Although blue and red light are the most common light sources utilized with PDT because their wavelengths (404–420 nm and 635 nm, respectively) correspond to the excitation peaks of photosensitizers, alternative light sources increasingly are being explored. There is increasing interest in utilizing infrared (IR) light sources (700–1,000,000 nm) to penetrate deeper into the skin in the treatment of precancerous and cancerous lesions. Exposure to IR radiation is known to raise skin temperature via inside-out dermal water absorption and is thought to be useful in PDT-ALA by promoting ALA penetration and its conversion to PpIX.8 In a randomized controlled trial by Giehl et al,9 visible light plus water-filtered IR-A light was shown to produce considerably less pain in ALA-PDT compared to placebo, though efficacy was not statistically affected. There are burgeoning trials examining the role of IR in treating dermatologic conditions such as acne, but research is still needed on ALA-PDT activated by IR radiation to target AKs.
Although the PDT side-effect profile of phototoxicity, dyspigmentation, and hypersensitivity is well documented, phototoxicity secondary to flame exposure is rare. In our patient, the synergistic effect of light and heat produced an exuberant phototoxic reaction. As the applications for PDT continue to broaden, this case may represent the importance of addressing additional precautions, such as avoiding open flames in the house or while camping, in the PDT aftercare instructions to maximize patient safety.
To the Editor:
Photodynamic therapy (PDT) is a US Food and Drug Administration–approved treatment for actinic keratosis (AK). It also commonly is administered off label for basal cell carcinoma, Bowen disease, photoaging, and acne vulgaris and is being investigated for other applications.1,2 In the context of treating AK, the mechanism employed in PDT most commonly involves the application of exogenous aminolevulinic acid (ALA), which is metabolized to the endogenous photosensitizer protoporphyrin IX (PpIX) in skin cells by enzymes in the heme biosynthetic pathway.3 The preferential uptake of ALA and conversion to PpIX is due to the altered and increased permeability of abnormal keratin layers of aging, sun-damaged cells, and skin tumors. Selectivity of ALA also occurs due to the preferential intracellular accumulation of PpIX in proliferating, relatively iron–deficient, precancerous and cancerous cells. The therapeutic effect is achieved with light exposure to blue light wavelength at 417 nm and corresponds to the excitation peak of PpIX,4 which activates PpIX and forms reactive oxygen species in the presence of oxygen that ultimately cause cell necrosis and apoptosis.5 Because it takes approximately 24 hours for PpIX to be completely metabolized from the skin, patients are counseled to avoid sun or artificial light exposure in the first 24 hours post-PDT, regardless of the indication, to avoid a severe phototoxic reaction.3,6,7 Although it is normal and desirable for patients to experience some form of a phototoxic reaction, which may include erythema, edema, crusting, vesiculation, or erosion in most patients, these types of reactions most often are secondary to the intended exposure and incidental natural or artificial light exposures.6 We report a case of a severe phototoxic reaction in which a patient experienced painful erythema and purulence on the left side of the chin after being within an arm’s length of a flame in a fireplace following PDT treatment.
A 59-year-old man presented to our dermatology clinic for his second of 3 PDT sessions to treat AKs on the face. He had a history of a basal cell carcinoma on the left nasolabial fold that previously was treated with Mohs micrographic surgery and melanoma on the left ear that was previously treated with excision. The patient received the initial PDT session 1 month prior and experienced a mild reaction with minimal redness and peeling that resolved in 4 to 5 days. For the second treatment, per standard protocol at our clinic, ALA was applied to the face, after which the patient incubated for 1 hour prior to blue light exposure (mean [SD] peak output of 417 [5] nm for 1000 seconds and 10 J/cm2).
After blue light exposure, broad-spectrum sunscreen (sun protection factor 47) was applied to our patient’s face, and he wore a wide-brimmed hat upon leaving the clinic and walking to his car. Similar to the first PDT session 1 month prior, he experienced minimal pain immediately after treatment. Once home and approximately 4 to 5 hours after PDT, he tended to a fire using his left hand and leaned into the fireplace with the left side of his face, which was within an arm’s length of the flames. Although his skin did not come in direct contact with the flames, the brief 2- to 3-minute exposure to the flame’s light and heat produced an immediate intense burning pain that the patient likened to the pain of blue light exposure. Within 24 hours, he developed a severe inflammatory reaction that included erythema, edema, desquamation, and pustules on the left side of the chin and cheek that produced a purulent discharge (Figure). The purulence resolved the next day; however, the other clinical manifestations persisted for 1 week. Despite the discomfort and symptoms, our patient did not seek medical attention and instead managed his symptoms conservatively with cold compresses. Although he noticed an overall subjective improvement in the appearance of his face after this second treatment, he received a third treatment with PDT approximately 1 month later, which resulted in a response that was similar to his first visit.

Photodynamic therapy is an increasingly accepted treatment modality for a plethora of benign and malignant dermatologic conditions. Although blue and red light are the most common light sources utilized with PDT because their wavelengths (404–420 nm and 635 nm, respectively) correspond to the excitation peaks of photosensitizers, alternative light sources increasingly are being explored. There is increasing interest in utilizing infrared (IR) light sources (700–1,000,000 nm) to penetrate deeper into the skin in the treatment of precancerous and cancerous lesions. Exposure to IR radiation is known to raise skin temperature via inside-out dermal water absorption and is thought to be useful in PDT-ALA by promoting ALA penetration and its conversion to PpIX.8 In a randomized controlled trial by Giehl et al,9 visible light plus water-filtered IR-A light was shown to produce considerably less pain in ALA-PDT compared to placebo, though efficacy was not statistically affected. There are burgeoning trials examining the role of IR in treating dermatologic conditions such as acne, but research is still needed on ALA-PDT activated by IR radiation to target AKs.
Although the PDT side-effect profile of phototoxicity, dyspigmentation, and hypersensitivity is well documented, phototoxicity secondary to flame exposure is rare. In our patient, the synergistic effect of light and heat produced an exuberant phototoxic reaction. As the applications for PDT continue to broaden, this case may represent the importance of addressing additional precautions, such as avoiding open flames in the house or while camping, in the PDT aftercare instructions to maximize patient safety.
- Fritsch C, Ruzicka T. Fluorescence diagnosis and photodynamic therapy in dermatology from experimental state to clinic standard methods. J Environ Pathol Toxicol Oncol. 2006;25:425-439.
- Lang K, Schulte KW, Ruzicka T, et al. Aminolevulinic acid (Levulan)in photodynamic therapy of actinic keratoses. Skin Therapy Lett. 2001;6:1-2, 5.
- Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992;14:275-292.
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163.
- Gad F, Viau G, Boushira M, et al. Photodynamic therapy with 5-aminolevulinic acid induces apoptosis and caspase activation in malignant T cells. J Cutan Med Surg. 2001;5:8-13.
- Piacquadio DJ, Chen DM, Farber HF, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol. 2004;140:41-46.
- Rhodes LE, Tsoukas MM, Anderson RR, et al. Iontophoretic delivery of ALA provides a quantitative model for ALA pharmacokinetics and PpIX phototoxicity in human skin. J Invest Dermatol. 1997;108:87-91.
- Dover JS, Phillips TJ, Arndt KA. Cutaneous effects and therapeutic uses of heat with emphasis on infrared radiation. J Am Acad Dermatol. 1989;20(2, pt 1):278-286.
- Giehl KA, Kriz M, Grahovac M, et al. A controlled trial of photodynamic therapy of actinic keratosis comparing different red light sources. Eur J Dermatol. 2014;24:335-341.
- Fritsch C, Ruzicka T. Fluorescence diagnosis and photodynamic therapy in dermatology from experimental state to clinic standard methods. J Environ Pathol Toxicol Oncol. 2006;25:425-439.
- Lang K, Schulte KW, Ruzicka T, et al. Aminolevulinic acid (Levulan)in photodynamic therapy of actinic keratoses. Skin Therapy Lett. 2001;6:1-2, 5.
- Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol B. 1992;14:275-292.
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163.
- Gad F, Viau G, Boushira M, et al. Photodynamic therapy with 5-aminolevulinic acid induces apoptosis and caspase activation in malignant T cells. J Cutan Med Surg. 2001;5:8-13.
- Piacquadio DJ, Chen DM, Farber HF, et al. Photodynamic therapy with aminolevulinic acid topical solution and visible blue light in the treatment of multiple actinic keratoses of the face and scalp: investigator-blinded, phase 3, multicenter trials. Arch Dermatol. 2004;140:41-46.
- Rhodes LE, Tsoukas MM, Anderson RR, et al. Iontophoretic delivery of ALA provides a quantitative model for ALA pharmacokinetics and PpIX phototoxicity in human skin. J Invest Dermatol. 1997;108:87-91.
- Dover JS, Phillips TJ, Arndt KA. Cutaneous effects and therapeutic uses of heat with emphasis on infrared radiation. J Am Acad Dermatol. 1989;20(2, pt 1):278-286.
- Giehl KA, Kriz M, Grahovac M, et al. A controlled trial of photodynamic therapy of actinic keratosis comparing different red light sources. Eur J Dermatol. 2014;24:335-341.
Practice Points
- As the applications of photodynamic therapy (PDT) in dermatology continue to expand, it is imperative for providers and patients alike to be knowledgeable with aftercare instructions and potential adverse effects.
- Avoid open flames in the house or while camping following PDT to maximize patient safety and prevent phototoxicity.
PDT for actinic keratoses continues to be refined
.

“We have conventional PDT, daylight PDT; we can combine with a range of topicals, and we can combine a range of different physical treatment procedures in order to provide better and individualized treatment regimens for our patients,” Merete Haedersdal, MD, PhD, DMSc, professor of dermatology at the University of Copenhagen, said during a course on laser and aesthetic skin therapy.
In Europe, PDT consists of a three-step procedure: curettage of AKs, application of photosensitizer on the skin (typically methyl aminolevulinate, versus aminolevulinic acid, used more often in the United States), and illumination with red light (versus blue light, used more often in the United States), which causes a photochemical reaction.
“It’s a photochemical-reaction concept with which we can achieve up to 90% cure rates of AKs at 3 months,” Dr. Haedersdal said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
PDT is also used in Europe for select patients with Bowen’s disease (yielding a 90% cure rate at 3 months, 70% at 2 years); superficial basal cell carcinoma (yielding a 90% cure rate at 3 months, 75% at 5 years), and nodular BCC (yielding a 90% cure rate at 3 months, 75% at 5 years.
“With conventional PDT, whether it’s blue light, red light, MAL, or ALA, we have beautiful cosmesis, but we also have a challenge, which is pain,” she said. This is behind the motivation to look at other ways to provide PDT.
Daylight PDT, which was pioneered by Dr. Haedersdal’s mentor, Hans Christian Wulf, MD, DMSc, PharmD, professor of dermatology at the University of Copenhagen, provides 80%-90% clearance of thin AKs, lower clearance of thick AKs – and is a nearly pain-free procedure because of continuous photoactivation of protoporphyrin IX, with a Visual Analog Scale in the range of 1-3. “Globally, thousands of patients have been treated [with daylight PDT], which is backed up in the literature with more than 150 publications,” she said.
According to Dr. Haedersdal, MAL cream with daylight activation for treatment of AK was approved in Colombia and Mexico in 2013; in Australia, Brazil, and Costa Rica in 2014; and in Chile, Europe, and New Zealand in 2015. “I do hope that one day you will have daylight PDT approved in the United States,” she said.
The suggested protocol for daylight PDT starts by applying a sunscreen with an organic filter. After about 15 minutes, the lesion or lesions are prepared, and MAL is applied with no occlusion. Patients should start their exposure to daylight within 30 minutes of application, remaining outdoors for 2 hours of continuous exposure, either at a dedicated space located on the ground of the hospital or clinic or at their home. After 2 hours, patients wipe off the remaining cream and are advised to stay indoors for the rest of the day.
“Ideal candidates are those who have large skin areas that can be easily exposed to sunlight,” such as the scalp and lower legs, said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston. “If patients are treated in areas covered by clothing, it can be greasy and sticky with the cream. In these cases, you can cover the area with Tegaderm, which allows for 99% light transmission. Daylight can shine through and the Tegaderm can be removed after the procedure.”
On rainy days between April 1 and October 1 in Copenhagen, she said, daylight PDT is provided in a greenhouse in the hospital garden, with a heater and blankets for patients when the temperature falls below 10° C.
The amount of light required for a treatment effect is 5,000-10,000 lux, and the number of lux on a sunny day in Denmark is about 100,000, she said. “That means that all year round in countries south of latitude 45 degrees N, patients can be treated with daylight PDT.”
To intensify the treatment efficacy of conventional PDT and daylight PDT, especially in those with severely photodamaged skin, combining treatment with a physical pretreatment technique such as curettage, ablative fractional laser, microdermabrasion, microneedling, and nonablative fractional laser is another strategy. A small randomized controlled trial found that ablative fractional laser treatment extended notable relative effectiveness, compared with other physical enhancement techniques.
Dr. Haedersdal and colleagues published a study that compared pretreatment with ablative fractional laser and microdermabrasion pads before daylight PDT for AKs in field-cancerized skin. They found that with a single treatment, combination therapy with ablative fractional laser before daylight PDT led to significantly greater efficacious AK clearance and skin rejuvenation, compared with treatment with microdermabrasion.
“We don’t know why this is, but we believe it may be due to the fact that with the laser, we have a photothermal response, which in combination with the photochemical response from the photodynamic therapy induces a synergistic effect,” she said.
A range of topical treatments can also be given in combination with PDT. In a meta-analysis of 10 randomized controlled trials, German researchers evaluated the efficacy of PDT combined with imiquimod cream, 5-fluorouracil cream, tazarotene gel, and calcipotriol ointment. They concluded that the combination of PDT with another topical drug intervention improves AK clearance rates, compared with monotherapy.
More recently, the same authors summarized the current knowledge on the efficacy and safety of local combination therapies for the treatment of patients with AK in a review article, which Dr. Haedersdal said provides a nice overview of this topic.
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
.

“We have conventional PDT, daylight PDT; we can combine with a range of topicals, and we can combine a range of different physical treatment procedures in order to provide better and individualized treatment regimens for our patients,” Merete Haedersdal, MD, PhD, DMSc, professor of dermatology at the University of Copenhagen, said during a course on laser and aesthetic skin therapy.
In Europe, PDT consists of a three-step procedure: curettage of AKs, application of photosensitizer on the skin (typically methyl aminolevulinate, versus aminolevulinic acid, used more often in the United States), and illumination with red light (versus blue light, used more often in the United States), which causes a photochemical reaction.
“It’s a photochemical-reaction concept with which we can achieve up to 90% cure rates of AKs at 3 months,” Dr. Haedersdal said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
PDT is also used in Europe for select patients with Bowen’s disease (yielding a 90% cure rate at 3 months, 70% at 2 years); superficial basal cell carcinoma (yielding a 90% cure rate at 3 months, 75% at 5 years), and nodular BCC (yielding a 90% cure rate at 3 months, 75% at 5 years.
“With conventional PDT, whether it’s blue light, red light, MAL, or ALA, we have beautiful cosmesis, but we also have a challenge, which is pain,” she said. This is behind the motivation to look at other ways to provide PDT.
Daylight PDT, which was pioneered by Dr. Haedersdal’s mentor, Hans Christian Wulf, MD, DMSc, PharmD, professor of dermatology at the University of Copenhagen, provides 80%-90% clearance of thin AKs, lower clearance of thick AKs – and is a nearly pain-free procedure because of continuous photoactivation of protoporphyrin IX, with a Visual Analog Scale in the range of 1-3. “Globally, thousands of patients have been treated [with daylight PDT], which is backed up in the literature with more than 150 publications,” she said.
According to Dr. Haedersdal, MAL cream with daylight activation for treatment of AK was approved in Colombia and Mexico in 2013; in Australia, Brazil, and Costa Rica in 2014; and in Chile, Europe, and New Zealand in 2015. “I do hope that one day you will have daylight PDT approved in the United States,” she said.
The suggested protocol for daylight PDT starts by applying a sunscreen with an organic filter. After about 15 minutes, the lesion or lesions are prepared, and MAL is applied with no occlusion. Patients should start their exposure to daylight within 30 minutes of application, remaining outdoors for 2 hours of continuous exposure, either at a dedicated space located on the ground of the hospital or clinic or at their home. After 2 hours, patients wipe off the remaining cream and are advised to stay indoors for the rest of the day.
“Ideal candidates are those who have large skin areas that can be easily exposed to sunlight,” such as the scalp and lower legs, said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston. “If patients are treated in areas covered by clothing, it can be greasy and sticky with the cream. In these cases, you can cover the area with Tegaderm, which allows for 99% light transmission. Daylight can shine through and the Tegaderm can be removed after the procedure.”
On rainy days between April 1 and October 1 in Copenhagen, she said, daylight PDT is provided in a greenhouse in the hospital garden, with a heater and blankets for patients when the temperature falls below 10° C.
The amount of light required for a treatment effect is 5,000-10,000 lux, and the number of lux on a sunny day in Denmark is about 100,000, she said. “That means that all year round in countries south of latitude 45 degrees N, patients can be treated with daylight PDT.”
To intensify the treatment efficacy of conventional PDT and daylight PDT, especially in those with severely photodamaged skin, combining treatment with a physical pretreatment technique such as curettage, ablative fractional laser, microdermabrasion, microneedling, and nonablative fractional laser is another strategy. A small randomized controlled trial found that ablative fractional laser treatment extended notable relative effectiveness, compared with other physical enhancement techniques.
Dr. Haedersdal and colleagues published a study that compared pretreatment with ablative fractional laser and microdermabrasion pads before daylight PDT for AKs in field-cancerized skin. They found that with a single treatment, combination therapy with ablative fractional laser before daylight PDT led to significantly greater efficacious AK clearance and skin rejuvenation, compared with treatment with microdermabrasion.
“We don’t know why this is, but we believe it may be due to the fact that with the laser, we have a photothermal response, which in combination with the photochemical response from the photodynamic therapy induces a synergistic effect,” she said.
A range of topical treatments can also be given in combination with PDT. In a meta-analysis of 10 randomized controlled trials, German researchers evaluated the efficacy of PDT combined with imiquimod cream, 5-fluorouracil cream, tazarotene gel, and calcipotriol ointment. They concluded that the combination of PDT with another topical drug intervention improves AK clearance rates, compared with monotherapy.
More recently, the same authors summarized the current knowledge on the efficacy and safety of local combination therapies for the treatment of patients with AK in a review article, which Dr. Haedersdal said provides a nice overview of this topic.
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
.

“We have conventional PDT, daylight PDT; we can combine with a range of topicals, and we can combine a range of different physical treatment procedures in order to provide better and individualized treatment regimens for our patients,” Merete Haedersdal, MD, PhD, DMSc, professor of dermatology at the University of Copenhagen, said during a course on laser and aesthetic skin therapy.
In Europe, PDT consists of a three-step procedure: curettage of AKs, application of photosensitizer on the skin (typically methyl aminolevulinate, versus aminolevulinic acid, used more often in the United States), and illumination with red light (versus blue light, used more often in the United States), which causes a photochemical reaction.
“It’s a photochemical-reaction concept with which we can achieve up to 90% cure rates of AKs at 3 months,” Dr. Haedersdal said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine.
PDT is also used in Europe for select patients with Bowen’s disease (yielding a 90% cure rate at 3 months, 70% at 2 years); superficial basal cell carcinoma (yielding a 90% cure rate at 3 months, 75% at 5 years), and nodular BCC (yielding a 90% cure rate at 3 months, 75% at 5 years.
“With conventional PDT, whether it’s blue light, red light, MAL, or ALA, we have beautiful cosmesis, but we also have a challenge, which is pain,” she said. This is behind the motivation to look at other ways to provide PDT.
Daylight PDT, which was pioneered by Dr. Haedersdal’s mentor, Hans Christian Wulf, MD, DMSc, PharmD, professor of dermatology at the University of Copenhagen, provides 80%-90% clearance of thin AKs, lower clearance of thick AKs – and is a nearly pain-free procedure because of continuous photoactivation of protoporphyrin IX, with a Visual Analog Scale in the range of 1-3. “Globally, thousands of patients have been treated [with daylight PDT], which is backed up in the literature with more than 150 publications,” she said.
According to Dr. Haedersdal, MAL cream with daylight activation for treatment of AK was approved in Colombia and Mexico in 2013; in Australia, Brazil, and Costa Rica in 2014; and in Chile, Europe, and New Zealand in 2015. “I do hope that one day you will have daylight PDT approved in the United States,” she said.
The suggested protocol for daylight PDT starts by applying a sunscreen with an organic filter. After about 15 minutes, the lesion or lesions are prepared, and MAL is applied with no occlusion. Patients should start their exposure to daylight within 30 minutes of application, remaining outdoors for 2 hours of continuous exposure, either at a dedicated space located on the ground of the hospital or clinic or at their home. After 2 hours, patients wipe off the remaining cream and are advised to stay indoors for the rest of the day.
“Ideal candidates are those who have large skin areas that can be easily exposed to sunlight,” such as the scalp and lower legs, said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston. “If patients are treated in areas covered by clothing, it can be greasy and sticky with the cream. In these cases, you can cover the area with Tegaderm, which allows for 99% light transmission. Daylight can shine through and the Tegaderm can be removed after the procedure.”
On rainy days between April 1 and October 1 in Copenhagen, she said, daylight PDT is provided in a greenhouse in the hospital garden, with a heater and blankets for patients when the temperature falls below 10° C.
The amount of light required for a treatment effect is 5,000-10,000 lux, and the number of lux on a sunny day in Denmark is about 100,000, she said. “That means that all year round in countries south of latitude 45 degrees N, patients can be treated with daylight PDT.”
To intensify the treatment efficacy of conventional PDT and daylight PDT, especially in those with severely photodamaged skin, combining treatment with a physical pretreatment technique such as curettage, ablative fractional laser, microdermabrasion, microneedling, and nonablative fractional laser is another strategy. A small randomized controlled trial found that ablative fractional laser treatment extended notable relative effectiveness, compared with other physical enhancement techniques.
Dr. Haedersdal and colleagues published a study that compared pretreatment with ablative fractional laser and microdermabrasion pads before daylight PDT for AKs in field-cancerized skin. They found that with a single treatment, combination therapy with ablative fractional laser before daylight PDT led to significantly greater efficacious AK clearance and skin rejuvenation, compared with treatment with microdermabrasion.
“We don’t know why this is, but we believe it may be due to the fact that with the laser, we have a photothermal response, which in combination with the photochemical response from the photodynamic therapy induces a synergistic effect,” she said.
A range of topical treatments can also be given in combination with PDT. In a meta-analysis of 10 randomized controlled trials, German researchers evaluated the efficacy of PDT combined with imiquimod cream, 5-fluorouracil cream, tazarotene gel, and calcipotriol ointment. They concluded that the combination of PDT with another topical drug intervention improves AK clearance rates, compared with monotherapy.
More recently, the same authors summarized the current knowledge on the efficacy and safety of local combination therapies for the treatment of patients with AK in a review article, which Dr. Haedersdal said provides a nice overview of this topic.
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Which AK treatment has the best long-term efficacy? A study reviews the data
The four results from a systemic review and meta-analysis suggest.
To date, many studies have reported that “most interventions are superior to placebo in terms of lesion clearance and improving the cosmetic image,” corresponding author Markus V. Heppt, MD, MSc, and colleagues wrote in a study published online Aug. 4, 2021, in JAMA Dermatology.
“However, most randomized clinical trials (RCTs) and meta-analyses focused on short-term outcomes that are evaluated within 3-6 months after treatment, although AK is increasingly being considered a chronic condition and reducing the incidence of cSCC [cutaneous squamous cell carcinoma] should be the ultimate goal of treatment,” they said. In addition, most treatments have been compared with placebo “and head-to-head comparisons are widely lacking, limiting the possibility to cross compare distinct active treatments. To this end, no evidence-based recommendation regarding the long-term efficacy of interventions for AK exists.”
To determine the long-term clearance rates of treatments used in adults with AK, a precursor of cSCC, Dr. Heppt, of the department of dermatology at University Hospital Erlangen (Germany), and colleagues drew from 15 randomized clinical trials that reported sustained clearance rates after at least 12 months of treatment and were published up to April 6, 2020. They conducted the review by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline and its extension for network meta-analyses (PRIMSA-NMA) and using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process to determine the certainty of the evidence for network meta-analyses.
The study population included 4,252 patients. Among 10 studies included in a network meta-analysis for the outcome of participant complete clearance, ALA-PDT showed the most favorable risk ratio profile, compared with placebo (RR, 8.06; moderate-quality evidence on GRADE), followed by imiquimod, 5% (RR, 5.98; very-low-quality evidence on GRADE); MAL-PDT (RR, 5.95; low-quality evidence on GRADE); and cryosurgery (RR, 4.76; very-low-quality evidence on GRADE).
ALA-PDT had the highest RR in the network meta-analyses for lesion-specific clearance (RR, 5.08; moderate-quality evidence on GRADE).
“Although ALA-PDT showed the most favorable RR and was ranked best among all interventions, the relative efficacy values and treatment rankings must be interpreted with caution,” because of the low certainty of evidence and few direct, head-to-head comparisons, the authors emphasized. “In particular, it remains elusive how to translate the distinct RR values into clinical relevance. We are hesitant to derive hierarchical or algorithmic treatment recommendations from our results.”
“The current meta-analysis notes that there are conflicting results in different studies,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn. who was asked to comment on the study. “Sustained participant complete clearance of actinic keratoses at 12 months is used as an outcome measure, although the authors comment that prevention/reduction of squamous cell carcinoma might be the more valid outcome measure.”
In her clinical experience, Dr. Ko said that patients often have good, sustained clearance of AKs with field treatment using a topical medication like 5-fluorouracil. “Patients can also have a good result with photodynamic therapy,” she said. “The paper’s results therefore do reflect what I have seen in my own practice. I also agree with the authors that, while it is difficult to measure, a meaningful outcome for patients is reduction/prevention of squamous cell carcinoma. It would be useful to have data on which treatment of actinic keratosis is best to reduce/prevent squamous cell carcinoma.”
The authors acknowledged limitations of the study, including the fact that field-directed treatments such as imiquimod, PDT, and fluorouracil were compared with lesion-directed approaches such as cryosurgery, “which may limit the generalizability of our results.” They concluded that their analysis “provides data that might contribute to an evidence-based framework to guide the selection of interventions for AK with proven long-term efficacy and sustained AK clearance.”
The analysis did not include data on tirbanibulin, a first-in-class dual Src kinase and tubulin polymerization inhibitor that was approved by the FDA for the topical treatment of AKs on the face or scalp in December 2020.
Dr. Heppt disclosed that he has been a member of the advisory boards of Almirall Hermal and Sanofi-Aventis and has received speaker’s honoraria from Galderma and Biofrontera. Many of his coauthors also reported having relevant financial disclosures. Dr. Ko reported having no relevant disclosures.
The four results from a systemic review and meta-analysis suggest.
To date, many studies have reported that “most interventions are superior to placebo in terms of lesion clearance and improving the cosmetic image,” corresponding author Markus V. Heppt, MD, MSc, and colleagues wrote in a study published online Aug. 4, 2021, in JAMA Dermatology.
“However, most randomized clinical trials (RCTs) and meta-analyses focused on short-term outcomes that are evaluated within 3-6 months after treatment, although AK is increasingly being considered a chronic condition and reducing the incidence of cSCC [cutaneous squamous cell carcinoma] should be the ultimate goal of treatment,” they said. In addition, most treatments have been compared with placebo “and head-to-head comparisons are widely lacking, limiting the possibility to cross compare distinct active treatments. To this end, no evidence-based recommendation regarding the long-term efficacy of interventions for AK exists.”
To determine the long-term clearance rates of treatments used in adults with AK, a precursor of cSCC, Dr. Heppt, of the department of dermatology at University Hospital Erlangen (Germany), and colleagues drew from 15 randomized clinical trials that reported sustained clearance rates after at least 12 months of treatment and were published up to April 6, 2020. They conducted the review by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline and its extension for network meta-analyses (PRIMSA-NMA) and using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process to determine the certainty of the evidence for network meta-analyses.
The study population included 4,252 patients. Among 10 studies included in a network meta-analysis for the outcome of participant complete clearance, ALA-PDT showed the most favorable risk ratio profile, compared with placebo (RR, 8.06; moderate-quality evidence on GRADE), followed by imiquimod, 5% (RR, 5.98; very-low-quality evidence on GRADE); MAL-PDT (RR, 5.95; low-quality evidence on GRADE); and cryosurgery (RR, 4.76; very-low-quality evidence on GRADE).
ALA-PDT had the highest RR in the network meta-analyses for lesion-specific clearance (RR, 5.08; moderate-quality evidence on GRADE).
“Although ALA-PDT showed the most favorable RR and was ranked best among all interventions, the relative efficacy values and treatment rankings must be interpreted with caution,” because of the low certainty of evidence and few direct, head-to-head comparisons, the authors emphasized. “In particular, it remains elusive how to translate the distinct RR values into clinical relevance. We are hesitant to derive hierarchical or algorithmic treatment recommendations from our results.”
“The current meta-analysis notes that there are conflicting results in different studies,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn. who was asked to comment on the study. “Sustained participant complete clearance of actinic keratoses at 12 months is used as an outcome measure, although the authors comment that prevention/reduction of squamous cell carcinoma might be the more valid outcome measure.”
In her clinical experience, Dr. Ko said that patients often have good, sustained clearance of AKs with field treatment using a topical medication like 5-fluorouracil. “Patients can also have a good result with photodynamic therapy,” she said. “The paper’s results therefore do reflect what I have seen in my own practice. I also agree with the authors that, while it is difficult to measure, a meaningful outcome for patients is reduction/prevention of squamous cell carcinoma. It would be useful to have data on which treatment of actinic keratosis is best to reduce/prevent squamous cell carcinoma.”
The authors acknowledged limitations of the study, including the fact that field-directed treatments such as imiquimod, PDT, and fluorouracil were compared with lesion-directed approaches such as cryosurgery, “which may limit the generalizability of our results.” They concluded that their analysis “provides data that might contribute to an evidence-based framework to guide the selection of interventions for AK with proven long-term efficacy and sustained AK clearance.”
The analysis did not include data on tirbanibulin, a first-in-class dual Src kinase and tubulin polymerization inhibitor that was approved by the FDA for the topical treatment of AKs on the face or scalp in December 2020.
Dr. Heppt disclosed that he has been a member of the advisory boards of Almirall Hermal and Sanofi-Aventis and has received speaker’s honoraria from Galderma and Biofrontera. Many of his coauthors also reported having relevant financial disclosures. Dr. Ko reported having no relevant disclosures.
The four results from a systemic review and meta-analysis suggest.
To date, many studies have reported that “most interventions are superior to placebo in terms of lesion clearance and improving the cosmetic image,” corresponding author Markus V. Heppt, MD, MSc, and colleagues wrote in a study published online Aug. 4, 2021, in JAMA Dermatology.
“However, most randomized clinical trials (RCTs) and meta-analyses focused on short-term outcomes that are evaluated within 3-6 months after treatment, although AK is increasingly being considered a chronic condition and reducing the incidence of cSCC [cutaneous squamous cell carcinoma] should be the ultimate goal of treatment,” they said. In addition, most treatments have been compared with placebo “and head-to-head comparisons are widely lacking, limiting the possibility to cross compare distinct active treatments. To this end, no evidence-based recommendation regarding the long-term efficacy of interventions for AK exists.”
To determine the long-term clearance rates of treatments used in adults with AK, a precursor of cSCC, Dr. Heppt, of the department of dermatology at University Hospital Erlangen (Germany), and colleagues drew from 15 randomized clinical trials that reported sustained clearance rates after at least 12 months of treatment and were published up to April 6, 2020. They conducted the review by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline and its extension for network meta-analyses (PRIMSA-NMA) and using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process to determine the certainty of the evidence for network meta-analyses.
The study population included 4,252 patients. Among 10 studies included in a network meta-analysis for the outcome of participant complete clearance, ALA-PDT showed the most favorable risk ratio profile, compared with placebo (RR, 8.06; moderate-quality evidence on GRADE), followed by imiquimod, 5% (RR, 5.98; very-low-quality evidence on GRADE); MAL-PDT (RR, 5.95; low-quality evidence on GRADE); and cryosurgery (RR, 4.76; very-low-quality evidence on GRADE).
ALA-PDT had the highest RR in the network meta-analyses for lesion-specific clearance (RR, 5.08; moderate-quality evidence on GRADE).
“Although ALA-PDT showed the most favorable RR and was ranked best among all interventions, the relative efficacy values and treatment rankings must be interpreted with caution,” because of the low certainty of evidence and few direct, head-to-head comparisons, the authors emphasized. “In particular, it remains elusive how to translate the distinct RR values into clinical relevance. We are hesitant to derive hierarchical or algorithmic treatment recommendations from our results.”
“The current meta-analysis notes that there are conflicting results in different studies,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn. who was asked to comment on the study. “Sustained participant complete clearance of actinic keratoses at 12 months is used as an outcome measure, although the authors comment that prevention/reduction of squamous cell carcinoma might be the more valid outcome measure.”
In her clinical experience, Dr. Ko said that patients often have good, sustained clearance of AKs with field treatment using a topical medication like 5-fluorouracil. “Patients can also have a good result with photodynamic therapy,” she said. “The paper’s results therefore do reflect what I have seen in my own practice. I also agree with the authors that, while it is difficult to measure, a meaningful outcome for patients is reduction/prevention of squamous cell carcinoma. It would be useful to have data on which treatment of actinic keratosis is best to reduce/prevent squamous cell carcinoma.”
The authors acknowledged limitations of the study, including the fact that field-directed treatments such as imiquimod, PDT, and fluorouracil were compared with lesion-directed approaches such as cryosurgery, “which may limit the generalizability of our results.” They concluded that their analysis “provides data that might contribute to an evidence-based framework to guide the selection of interventions for AK with proven long-term efficacy and sustained AK clearance.”
The analysis did not include data on tirbanibulin, a first-in-class dual Src kinase and tubulin polymerization inhibitor that was approved by the FDA for the topical treatment of AKs on the face or scalp in December 2020.
Dr. Heppt disclosed that he has been a member of the advisory boards of Almirall Hermal and Sanofi-Aventis and has received speaker’s honoraria from Galderma and Biofrontera. Many of his coauthors also reported having relevant financial disclosures. Dr. Ko reported having no relevant disclosures.
FROM JAMA DERMATOLOGY