User login
The discipline of family medicine is committed to providing patient-centered care through recommendations that are grounded both in evidence and in patients’ personal values.1,2 The current health care environment, however, often demands heavy reliance on outcome-based performance metrics that can be insensitive to patient preferences.3 This tension necessitates models of decision-making that maximize reliance on measured performance, yet fulfill the clinician’s fiduciary responsibility to prioritize patients’ interests. The philosophy and practice of shared decision-making (SDM) can facilitate these aims.
The 3 elements of shared decision-making
SDM provides a framework for offering everyday medical advice and facilitating informed consent.4 Its 3 elements are:
- discussing with patients relevant information about their health conditions, possible treatments, and likely outcomes,
- clarifying and understanding a patient’s unique values and priorities and how they relate to the treatment options, and
- enabling a patient to select a care plan that is in keeping with his or her personal goals.5
This model is significant not only from a theoretical perspective, but also from a practical one. Studies have shown that both health outcomes and patient satisfaction improve when patients participate more actively in health care decision-making.6,7
Unfortunately, there is evidence that some decision-making practices in primary care settings remain inadequate. For example, unlike the standard disclosure of procedure risks in surgical settings, the burdens of cancer screening are frequently omitted from primary care discussions.8 Moreover, agreement about what should be disclosed, as well as how to disclose it, is still not sufficient. The following 3 recommendations, one for each element of SDM, aim to help clinicians effectively engage patients in everyday decision-making.
1. Provide patients with relevant information
The first element of SDM requires discussing the health-related information that is relevant to the patient’s decision-making process. The literature about informed consent supports explaining the risks that are common, as well as those that are particularly dangerous, and the likely benefits of recommended treatment, nontreatment, and alternative treatments.9 Moreover, adequate informed consent requires identifying what a reasonable person in a particular patient’s position would want to know.10
Accounting for “a patient’s position” is significant because it signals that personal factors (eg, the individual’s beliefs, goals, and familial responsibilities) are as important as the patient’s external clinical situation and what can be known by reviewing medical evidence. Incorporating a patient’s particular circumstances distinguishes patient-centered care from the mechanical application of generic best practices. This is the standard for what information should be provided.
When evidence is lacking. Clinicians facilitating decisions for which data is lacking should convey the best available evidence, including the inherent uncertainties. Like evidence-based medicine (EBM), the principles of SDM should be at work in most clinical encounters. The extent to which one engages in SDM depends upon the seriousness of the proposed interventions, the degree to which the decision is preference-sensitive, and the availability of evidence.
Statistics: Explain absolute and baseline risk
It is generally better to provide absolute risk rather than relative risk, because people perceive absolute risk reductions more accurately.11 Presentation of risks, in terms of relative risk or relative risk reduction, typically exaggerates the benefits of treatment, especially when the risk is small. This exaggeration makes it more likely that patients will accept interventions they might otherwise have rejected after reviewing the data more fully. Furthermore, relative risk statistics can impact clinicians’ perceptions, leading them to recommend an intervention more often than they might when absolute risk statistics are discussed.12
But even absolute risk, if presented on its own, can be misleading. A reasonable person trying to determine the value of an intervention needs to know his or her baseline risk of an event, in addition to his or her absolute risk with the intervention. For example, a hypothetical absolute risk reduction associated with a breast cancer treatment of 10% has different meanings, depending upon whether the baseline risk is 10% or 80%. A reduction from 10% to 0% would be a miraculous cure, while going from 80% to 70% may be viewed as only a slight improvement. Try as we might to present a single neat statistic, presenting both baseline risk and absolute risk with intervention is often necessary.13
How to effectively communicate medical information
Many patients struggle with processing information that is expressed as a probability.14 Patients process frequencies (eg, 10 in 100) better than probabilities (eg, 10%), and there is evidence that they understand best when decision aids are used.15 Decision aids, such as pictographs (FIGURE16), are supplementary, evidence-based tools for effectively communicating with patients and their families in a way that facilitates comparison between available options. Such aids are readily available online for many conditions or can be created using various software tools.16,17
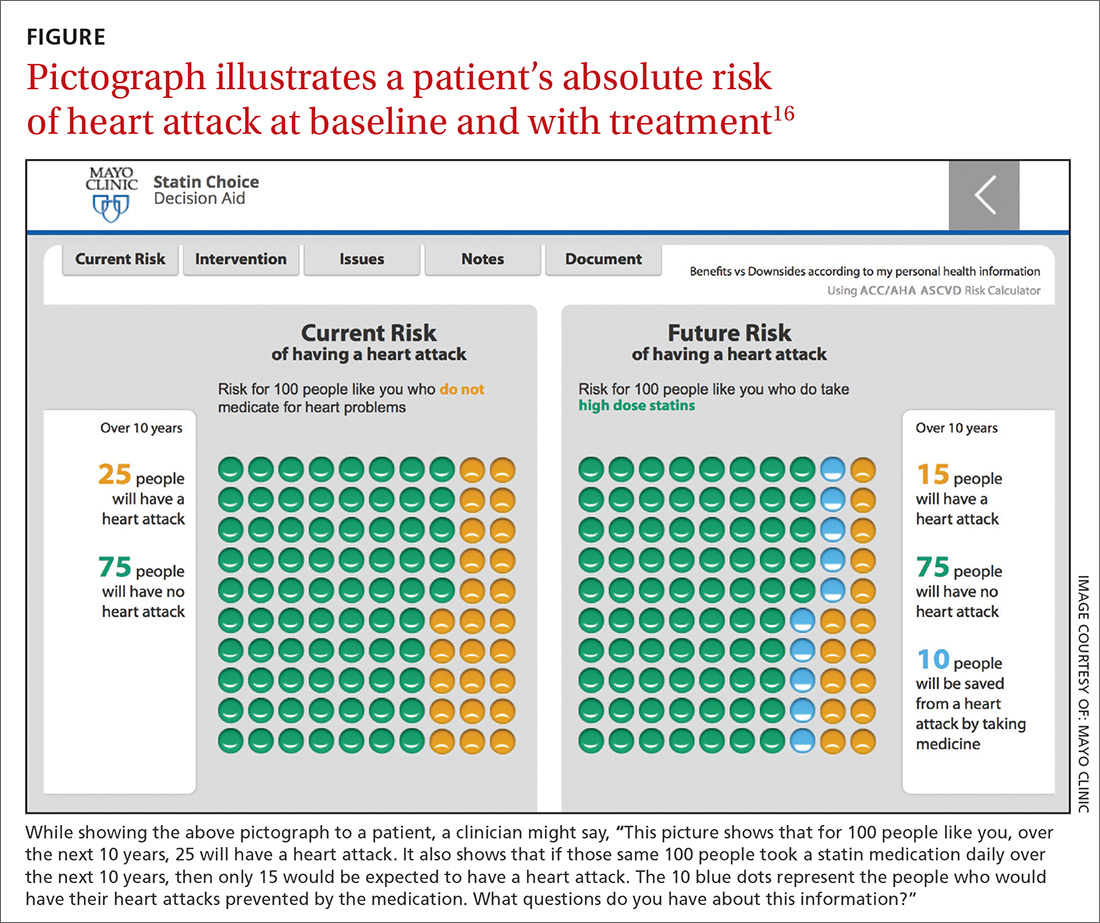
Pictographs reveal that there is a values-sensitive decision to be made and visually demonstrate the outcomes associated with each option. Both pictographs and bar graphs have been shown to improve patient understanding and satisfaction.11 The benefit of pictographs is their ability to effectively, and simultaneously, convey both the numerator and the denominator in frequency statistics.12,18
There is high-quality evidence demonstrating that decision aids enhance an individual’s knowledge about the treatment and screening options available to them. A 2014 Cochrane review of the effects of decision aids found that they increased average knowledge scores when compared to usual care.15 Decision aids also improved accurate perception of risk.15 It is our belief that one of the reasons pictographs work so well is that they combine the salience of absolute risks with and without intervention.12,13
Beyond increased understanding, the Cochrane review also found high-quality evidence indicating that people who make decisions using decision aids feel less decisional conflict when compared to usual care.15 Moreover, in the context of SDM, decisional conflict may contribute to patients passing the decision-making responsibility to their clinician.19 And finally, there is moderate-quality evidence that patients are more likely to participate in decision-making when given tools such as pictographs.15
A potential barrier to putting pictographs into practice concerns perceptions that decision aids increase the length of office visits. Indeed, previous studies have identified perceived time constraints as one of the major barriers to enacting SDM in clinical settings.20 On this topic, the Cochrane review offers variable yet potentially promising results: Studies of the effects on appointment length ranged from a decrease of 8 minutes to an increase of 23 minutes.15 These results suggest that, under the right circumstances, pictographs can be used to facilitate SDM within the constraints of current clinical practice. More research is needed to determine the optimal circumstances that promote efficient SDM.
2. Elicit the patient’s unique values and priorities
Formalized approaches to building rapport with patients have been popular for more than 2 decades,21,22 and they are now routinely part of medical training. Nevertheless, there is always room for improvement when it comes to aligning treatment and screening recommendations with patient values. Some decision aids are designed to offer the added benefit of clarifying individual values and, thus, increase the likelihood that patients will make decisions that are more in line with their goals.15
When decision aids are not available to elicit patient values, clinicians can integrate preference-clarifying questions as part of the standard patient encounter.23 These questions are aimed at surfacing the values underlying what the patient wants, what the patient does not want, and most importantly, why.
“Why” matters because it ultimately helps the clinician understand the patient’s mindset, enabling the clinician to help the patient make choices that serve his or her values.24 Eliciting values not only promotes patient well-being and self-determination, but also facilitates the development of empathic patient-clinician partnerships.
Categorizing decisions. Regardless of the particular method chosen to elicit patient values, the underlying questions faced by many patients often fit into one of 2 categories: 1) Do I prefer quality of life over length of life? or 2) Am I willing to be inconvenienced now to prevent more severe illness later? Clarifying the category into which a decision falls may open the conversation and help to explore patients’ values and priorities. Alternatively, asking questions such as, “Thinking about this decision, what is the most important aspect for you to consider?”25 may facilitate the conversation.
Much of the research on techniques geared to elicit values comes from the palliative care and oncology literature.26 Although this research generally focuses on decisions about serious illness or end-of-life preferences, preference-sensitive decisions in primary care settings create a need for clinicians who are effective in eliciting patient values.
The more serious and preference-sensitive the decision, the deeper the clinician needs to explore the patient’s personal goals. Despite scant literature about seemingly innocuous decisions, we recommend that clinicians elicit from their patients a brief, but overt, acknowledgement of the values guiding their choice for most preference-sensitive decisions.
3. Offer a professional recommendation
Once clinicians have a sense of an individual’s values and priorities, they are positioned to make a professional recommendation that aligns with these values and priorities, and leaves room for the patient to reach a decision. Historically, one of the clinician’s major roles was to provide advice and recommendations to patients. For a long time, this was done without the patient’s involvement in the decision-making.27
With an increasing emphasis on patient self-determination over the last 50 years, there has been some concern that the pendulum is swinging too far in the opposite direction, with clinicians shying away from providing specific recommendations.28 Although this line of thinking acknowledges the power of the clinician to influence patients, it falls short of distinguishing between a personal recommendation and a professional one. While personal recommendations have no place in medical decision-making, clinicians should offer patients a professional recommendation, along with their rationale.
How do personal and professional recommendations differ?
Personal recommendations arise from clinicians considering what they themselves might decide if they were in the patient’s position. Such recommendations are inappropriate because every person has unique values and priorities.
In contrast, professional recommendations stem from the clinician’s knowledge of the best available evidence, his or her understanding of the patient’s values, and his or her weaving of these pieces together in the context of the patient’s specific clinical presentation. Experienced clinicians bring all 3 elements of SDM to bear in making professional recommendations, even if these recommendations are at odds with what they might choose for themselves.
EBM and SDM: Not so different after all?
Another way to understand the legitimacy of a professional recommendation is to view the parallels between SDM and EBM. From the outset, EBM positioned itself as arising from the best available evidence, the patient’s values, and clinical expertise29—elements that are strikingly similar to the components of SDM.
Although commonly overlooked, the concept of EBM recognizes that established evidence alone is not sufficient for decision-making.30 Additionally, EBM allows for making a recommendation that may not appear to be guideline-based, because guidelines typically do not take into account individual patient preferences.30-32 What’s more, both EBM and SDM highlight the essential contribution of the clinician’s judgment about his or her patient’s unique presentation.
Thus, both EBM and SDM are dependent on the professional communicating a recommendation to the patient. This communication involves not only making clear what one recommends, but also why one recommends it. For example, a clinician might say the following to a patient with worsening asthma symptoms:
“The asthma guidelines give us 2 treatment options. We can either double the dose of your inhaled corticosteroid, or start a 5-day course of corticosteroid pills. Given your concerns about the adverse effects of the pills, and the moderate severity of this exacerbation, I recommend doubling the dose of your inhaled corticosteroid. We can reconsider the pills if your symptoms worsen or if you don’t improve within the next week. How does that sound to you?”
An informed choice. Explaining the evidence, articulating the patient’s values, and summarizing the clinical elements that went into the clinician’s recommendation clarifies and signals to the patient that this is a professional recommendation. Ultimately, the process of SDM concludes with the patient considering the clinician’s recommendation and making an informed choice from the available options.
CORRESPONDENCE
David J. Satin, MD, 2020 E 28th St., Minneapolis, MN 55407; [email protected].
1. Medalie JH. Family Medicine: Principles and Applications. Baltimore, MD: Williams and Wilkins; 1978.
2. Philips RL Jr, Brundgardt S, Lesko SE, et al. The future role of the family physician in the United States: a rigorous exercise in definition. Ann Fam Med. 2014;12:250-255.
3. Berwick DM. Era 3 for medicine and health care. JAMA. 2016;315:1329-1330.
4. Edwards A, Elwyn G. Shared decision making in health care: achieving evidence-based patient choice. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2009.
5. American Medical Association. Shared decision making H-373.997. Available at: https://policysearch.ama-assn.org/policyfinder/detail/H-373.997%20?uri=%2FAMADoc%2FHOD.xml-0-3162.xml. Accessed September 6, 2017.
6. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8:410-417.
8. Fowler FJ Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions? Results of a national survey. JAMA Intern Med. 2013;173:1215-1221.
9. Spatz ES, Krumhoiz HM, Moulton BW. The new era of informed consent: getting to a reasonable-patient standard through shared decision making. JAMA. 2016;315:2063-2064.
10. Faden RR, Becker C, Lewis C, et al. Disclosure of information to patients in medical care. Med Care. 1981;19:718-733.
11. Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270-280.
12. Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436-1443.
13. Stovitz SD, Shrier I. Medical decision making and the importance of baseline risk. Br J Gen Pract. 2013;63:e795-e797.
14. Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37-44.
15. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011:CD001431.
16. Mayo Clinic. Statin choice decision aid. Available at: https://statindecisionaid.mayoclinic.org/index.php/statin/index. Accessed September 6, 2017.
17. Risk Science Center and Center for Bioethics and Social Sciences in Medicine, University of Michigan. Icon Array. Available at: http://www.iconarray.com/. Accessed September 6, 2017.
18. Price M, Cameron R, Butow P. Communicating risk information: the influence of graphical display format on quantitative information perception–accuracy, comprehension and preferences. Patient Educ Couns. 2007;69:121-128.
19. Kon AA. The shared decision making continuum. JAMA. 2010;304:903-904.
20. Légaré F1, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535.
21. Haidet P, Paterniti DA. “Building” a history rather than “taking” one: a perspective on information sharing during the medical interview. Arch Intern Med. 2003;163:1134-1140.
22. Frankel RM, Stein R. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16:184-194.
23. Delbanco TL. Enriching the doctor-patient relationship by inviting the patient’s perspective. Ann Intern Med. 1992;116:414-418.
24. Doukas DJ, McCullough LB. The values history: the evaluation of the patient’s values and advance directives. J Fam Pract. 1991;32:145-153.
25. Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256.
26. Bernacki RE, Block SD, American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174:1994-2003.
27. Katz J. The Silent World of Doctor and Patient. Baltimore, MD, and London, England: The Johns Hopkins University Press; 1984.
28. Baylis F, Downie J. Professional recommendations: disclosing facts and values. J Med Ethics. 2001;27:20-24.
29. Sackett DL, Rosenburg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72.
30. Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310:2053-2054.
31. Mora S, Ames JM, Manson JE. Low-dose aspirin in the primary prevention of cardiovascular disease: shared decision making in clinical practice. JAMA. 2016;316:709-710.
32. Stovitz SD, Satin D, Shrier I. Shared decision making regarding aspirin in primary prevention of cardiovascular disease. JAMA. 2016;316:2276.
The discipline of family medicine is committed to providing patient-centered care through recommendations that are grounded both in evidence and in patients’ personal values.1,2 The current health care environment, however, often demands heavy reliance on outcome-based performance metrics that can be insensitive to patient preferences.3 This tension necessitates models of decision-making that maximize reliance on measured performance, yet fulfill the clinician’s fiduciary responsibility to prioritize patients’ interests. The philosophy and practice of shared decision-making (SDM) can facilitate these aims.
The 3 elements of shared decision-making
SDM provides a framework for offering everyday medical advice and facilitating informed consent.4 Its 3 elements are:
- discussing with patients relevant information about their health conditions, possible treatments, and likely outcomes,
- clarifying and understanding a patient’s unique values and priorities and how they relate to the treatment options, and
- enabling a patient to select a care plan that is in keeping with his or her personal goals.5
This model is significant not only from a theoretical perspective, but also from a practical one. Studies have shown that both health outcomes and patient satisfaction improve when patients participate more actively in health care decision-making.6,7
Unfortunately, there is evidence that some decision-making practices in primary care settings remain inadequate. For example, unlike the standard disclosure of procedure risks in surgical settings, the burdens of cancer screening are frequently omitted from primary care discussions.8 Moreover, agreement about what should be disclosed, as well as how to disclose it, is still not sufficient. The following 3 recommendations, one for each element of SDM, aim to help clinicians effectively engage patients in everyday decision-making.
1. Provide patients with relevant information
The first element of SDM requires discussing the health-related information that is relevant to the patient’s decision-making process. The literature about informed consent supports explaining the risks that are common, as well as those that are particularly dangerous, and the likely benefits of recommended treatment, nontreatment, and alternative treatments.9 Moreover, adequate informed consent requires identifying what a reasonable person in a particular patient’s position would want to know.10
Accounting for “a patient’s position” is significant because it signals that personal factors (eg, the individual’s beliefs, goals, and familial responsibilities) are as important as the patient’s external clinical situation and what can be known by reviewing medical evidence. Incorporating a patient’s particular circumstances distinguishes patient-centered care from the mechanical application of generic best practices. This is the standard for what information should be provided.
When evidence is lacking. Clinicians facilitating decisions for which data is lacking should convey the best available evidence, including the inherent uncertainties. Like evidence-based medicine (EBM), the principles of SDM should be at work in most clinical encounters. The extent to which one engages in SDM depends upon the seriousness of the proposed interventions, the degree to which the decision is preference-sensitive, and the availability of evidence.
Statistics: Explain absolute and baseline risk
It is generally better to provide absolute risk rather than relative risk, because people perceive absolute risk reductions more accurately.11 Presentation of risks, in terms of relative risk or relative risk reduction, typically exaggerates the benefits of treatment, especially when the risk is small. This exaggeration makes it more likely that patients will accept interventions they might otherwise have rejected after reviewing the data more fully. Furthermore, relative risk statistics can impact clinicians’ perceptions, leading them to recommend an intervention more often than they might when absolute risk statistics are discussed.12
But even absolute risk, if presented on its own, can be misleading. A reasonable person trying to determine the value of an intervention needs to know his or her baseline risk of an event, in addition to his or her absolute risk with the intervention. For example, a hypothetical absolute risk reduction associated with a breast cancer treatment of 10% has different meanings, depending upon whether the baseline risk is 10% or 80%. A reduction from 10% to 0% would be a miraculous cure, while going from 80% to 70% may be viewed as only a slight improvement. Try as we might to present a single neat statistic, presenting both baseline risk and absolute risk with intervention is often necessary.13
How to effectively communicate medical information
Many patients struggle with processing information that is expressed as a probability.14 Patients process frequencies (eg, 10 in 100) better than probabilities (eg, 10%), and there is evidence that they understand best when decision aids are used.15 Decision aids, such as pictographs (FIGURE16), are supplementary, evidence-based tools for effectively communicating with patients and their families in a way that facilitates comparison between available options. Such aids are readily available online for many conditions or can be created using various software tools.16,17

Pictographs reveal that there is a values-sensitive decision to be made and visually demonstrate the outcomes associated with each option. Both pictographs and bar graphs have been shown to improve patient understanding and satisfaction.11 The benefit of pictographs is their ability to effectively, and simultaneously, convey both the numerator and the denominator in frequency statistics.12,18
There is high-quality evidence demonstrating that decision aids enhance an individual’s knowledge about the treatment and screening options available to them. A 2014 Cochrane review of the effects of decision aids found that they increased average knowledge scores when compared to usual care.15 Decision aids also improved accurate perception of risk.15 It is our belief that one of the reasons pictographs work so well is that they combine the salience of absolute risks with and without intervention.12,13
Beyond increased understanding, the Cochrane review also found high-quality evidence indicating that people who make decisions using decision aids feel less decisional conflict when compared to usual care.15 Moreover, in the context of SDM, decisional conflict may contribute to patients passing the decision-making responsibility to their clinician.19 And finally, there is moderate-quality evidence that patients are more likely to participate in decision-making when given tools such as pictographs.15
A potential barrier to putting pictographs into practice concerns perceptions that decision aids increase the length of office visits. Indeed, previous studies have identified perceived time constraints as one of the major barriers to enacting SDM in clinical settings.20 On this topic, the Cochrane review offers variable yet potentially promising results: Studies of the effects on appointment length ranged from a decrease of 8 minutes to an increase of 23 minutes.15 These results suggest that, under the right circumstances, pictographs can be used to facilitate SDM within the constraints of current clinical practice. More research is needed to determine the optimal circumstances that promote efficient SDM.
2. Elicit the patient’s unique values and priorities
Formalized approaches to building rapport with patients have been popular for more than 2 decades,21,22 and they are now routinely part of medical training. Nevertheless, there is always room for improvement when it comes to aligning treatment and screening recommendations with patient values. Some decision aids are designed to offer the added benefit of clarifying individual values and, thus, increase the likelihood that patients will make decisions that are more in line with their goals.15
When decision aids are not available to elicit patient values, clinicians can integrate preference-clarifying questions as part of the standard patient encounter.23 These questions are aimed at surfacing the values underlying what the patient wants, what the patient does not want, and most importantly, why.
“Why” matters because it ultimately helps the clinician understand the patient’s mindset, enabling the clinician to help the patient make choices that serve his or her values.24 Eliciting values not only promotes patient well-being and self-determination, but also facilitates the development of empathic patient-clinician partnerships.
Categorizing decisions. Regardless of the particular method chosen to elicit patient values, the underlying questions faced by many patients often fit into one of 2 categories: 1) Do I prefer quality of life over length of life? or 2) Am I willing to be inconvenienced now to prevent more severe illness later? Clarifying the category into which a decision falls may open the conversation and help to explore patients’ values and priorities. Alternatively, asking questions such as, “Thinking about this decision, what is the most important aspect for you to consider?”25 may facilitate the conversation.
Much of the research on techniques geared to elicit values comes from the palliative care and oncology literature.26 Although this research generally focuses on decisions about serious illness or end-of-life preferences, preference-sensitive decisions in primary care settings create a need for clinicians who are effective in eliciting patient values.
The more serious and preference-sensitive the decision, the deeper the clinician needs to explore the patient’s personal goals. Despite scant literature about seemingly innocuous decisions, we recommend that clinicians elicit from their patients a brief, but overt, acknowledgement of the values guiding their choice for most preference-sensitive decisions.
3. Offer a professional recommendation
Once clinicians have a sense of an individual’s values and priorities, they are positioned to make a professional recommendation that aligns with these values and priorities, and leaves room for the patient to reach a decision. Historically, one of the clinician’s major roles was to provide advice and recommendations to patients. For a long time, this was done without the patient’s involvement in the decision-making.27
With an increasing emphasis on patient self-determination over the last 50 years, there has been some concern that the pendulum is swinging too far in the opposite direction, with clinicians shying away from providing specific recommendations.28 Although this line of thinking acknowledges the power of the clinician to influence patients, it falls short of distinguishing between a personal recommendation and a professional one. While personal recommendations have no place in medical decision-making, clinicians should offer patients a professional recommendation, along with their rationale.
How do personal and professional recommendations differ?
Personal recommendations arise from clinicians considering what they themselves might decide if they were in the patient’s position. Such recommendations are inappropriate because every person has unique values and priorities.
In contrast, professional recommendations stem from the clinician’s knowledge of the best available evidence, his or her understanding of the patient’s values, and his or her weaving of these pieces together in the context of the patient’s specific clinical presentation. Experienced clinicians bring all 3 elements of SDM to bear in making professional recommendations, even if these recommendations are at odds with what they might choose for themselves.
EBM and SDM: Not so different after all?
Another way to understand the legitimacy of a professional recommendation is to view the parallels between SDM and EBM. From the outset, EBM positioned itself as arising from the best available evidence, the patient’s values, and clinical expertise29—elements that are strikingly similar to the components of SDM.
Although commonly overlooked, the concept of EBM recognizes that established evidence alone is not sufficient for decision-making.30 Additionally, EBM allows for making a recommendation that may not appear to be guideline-based, because guidelines typically do not take into account individual patient preferences.30-32 What’s more, both EBM and SDM highlight the essential contribution of the clinician’s judgment about his or her patient’s unique presentation.
Thus, both EBM and SDM are dependent on the professional communicating a recommendation to the patient. This communication involves not only making clear what one recommends, but also why one recommends it. For example, a clinician might say the following to a patient with worsening asthma symptoms:
“The asthma guidelines give us 2 treatment options. We can either double the dose of your inhaled corticosteroid, or start a 5-day course of corticosteroid pills. Given your concerns about the adverse effects of the pills, and the moderate severity of this exacerbation, I recommend doubling the dose of your inhaled corticosteroid. We can reconsider the pills if your symptoms worsen or if you don’t improve within the next week. How does that sound to you?”
An informed choice. Explaining the evidence, articulating the patient’s values, and summarizing the clinical elements that went into the clinician’s recommendation clarifies and signals to the patient that this is a professional recommendation. Ultimately, the process of SDM concludes with the patient considering the clinician’s recommendation and making an informed choice from the available options.
CORRESPONDENCE
David J. Satin, MD, 2020 E 28th St., Minneapolis, MN 55407; [email protected].
The discipline of family medicine is committed to providing patient-centered care through recommendations that are grounded both in evidence and in patients’ personal values.1,2 The current health care environment, however, often demands heavy reliance on outcome-based performance metrics that can be insensitive to patient preferences.3 This tension necessitates models of decision-making that maximize reliance on measured performance, yet fulfill the clinician’s fiduciary responsibility to prioritize patients’ interests. The philosophy and practice of shared decision-making (SDM) can facilitate these aims.
The 3 elements of shared decision-making
SDM provides a framework for offering everyday medical advice and facilitating informed consent.4 Its 3 elements are:
- discussing with patients relevant information about their health conditions, possible treatments, and likely outcomes,
- clarifying and understanding a patient’s unique values and priorities and how they relate to the treatment options, and
- enabling a patient to select a care plan that is in keeping with his or her personal goals.5
This model is significant not only from a theoretical perspective, but also from a practical one. Studies have shown that both health outcomes and patient satisfaction improve when patients participate more actively in health care decision-making.6,7
Unfortunately, there is evidence that some decision-making practices in primary care settings remain inadequate. For example, unlike the standard disclosure of procedure risks in surgical settings, the burdens of cancer screening are frequently omitted from primary care discussions.8 Moreover, agreement about what should be disclosed, as well as how to disclose it, is still not sufficient. The following 3 recommendations, one for each element of SDM, aim to help clinicians effectively engage patients in everyday decision-making.
1. Provide patients with relevant information
The first element of SDM requires discussing the health-related information that is relevant to the patient’s decision-making process. The literature about informed consent supports explaining the risks that are common, as well as those that are particularly dangerous, and the likely benefits of recommended treatment, nontreatment, and alternative treatments.9 Moreover, adequate informed consent requires identifying what a reasonable person in a particular patient’s position would want to know.10
Accounting for “a patient’s position” is significant because it signals that personal factors (eg, the individual’s beliefs, goals, and familial responsibilities) are as important as the patient’s external clinical situation and what can be known by reviewing medical evidence. Incorporating a patient’s particular circumstances distinguishes patient-centered care from the mechanical application of generic best practices. This is the standard for what information should be provided.
When evidence is lacking. Clinicians facilitating decisions for which data is lacking should convey the best available evidence, including the inherent uncertainties. Like evidence-based medicine (EBM), the principles of SDM should be at work in most clinical encounters. The extent to which one engages in SDM depends upon the seriousness of the proposed interventions, the degree to which the decision is preference-sensitive, and the availability of evidence.
Statistics: Explain absolute and baseline risk
It is generally better to provide absolute risk rather than relative risk, because people perceive absolute risk reductions more accurately.11 Presentation of risks, in terms of relative risk or relative risk reduction, typically exaggerates the benefits of treatment, especially when the risk is small. This exaggeration makes it more likely that patients will accept interventions they might otherwise have rejected after reviewing the data more fully. Furthermore, relative risk statistics can impact clinicians’ perceptions, leading them to recommend an intervention more often than they might when absolute risk statistics are discussed.12
But even absolute risk, if presented on its own, can be misleading. A reasonable person trying to determine the value of an intervention needs to know his or her baseline risk of an event, in addition to his or her absolute risk with the intervention. For example, a hypothetical absolute risk reduction associated with a breast cancer treatment of 10% has different meanings, depending upon whether the baseline risk is 10% or 80%. A reduction from 10% to 0% would be a miraculous cure, while going from 80% to 70% may be viewed as only a slight improvement. Try as we might to present a single neat statistic, presenting both baseline risk and absolute risk with intervention is often necessary.13
How to effectively communicate medical information
Many patients struggle with processing information that is expressed as a probability.14 Patients process frequencies (eg, 10 in 100) better than probabilities (eg, 10%), and there is evidence that they understand best when decision aids are used.15 Decision aids, such as pictographs (FIGURE16), are supplementary, evidence-based tools for effectively communicating with patients and their families in a way that facilitates comparison between available options. Such aids are readily available online for many conditions or can be created using various software tools.16,17

Pictographs reveal that there is a values-sensitive decision to be made and visually demonstrate the outcomes associated with each option. Both pictographs and bar graphs have been shown to improve patient understanding and satisfaction.11 The benefit of pictographs is their ability to effectively, and simultaneously, convey both the numerator and the denominator in frequency statistics.12,18
There is high-quality evidence demonstrating that decision aids enhance an individual’s knowledge about the treatment and screening options available to them. A 2014 Cochrane review of the effects of decision aids found that they increased average knowledge scores when compared to usual care.15 Decision aids also improved accurate perception of risk.15 It is our belief that one of the reasons pictographs work so well is that they combine the salience of absolute risks with and without intervention.12,13
Beyond increased understanding, the Cochrane review also found high-quality evidence indicating that people who make decisions using decision aids feel less decisional conflict when compared to usual care.15 Moreover, in the context of SDM, decisional conflict may contribute to patients passing the decision-making responsibility to their clinician.19 And finally, there is moderate-quality evidence that patients are more likely to participate in decision-making when given tools such as pictographs.15
A potential barrier to putting pictographs into practice concerns perceptions that decision aids increase the length of office visits. Indeed, previous studies have identified perceived time constraints as one of the major barriers to enacting SDM in clinical settings.20 On this topic, the Cochrane review offers variable yet potentially promising results: Studies of the effects on appointment length ranged from a decrease of 8 minutes to an increase of 23 minutes.15 These results suggest that, under the right circumstances, pictographs can be used to facilitate SDM within the constraints of current clinical practice. More research is needed to determine the optimal circumstances that promote efficient SDM.
2. Elicit the patient’s unique values and priorities
Formalized approaches to building rapport with patients have been popular for more than 2 decades,21,22 and they are now routinely part of medical training. Nevertheless, there is always room for improvement when it comes to aligning treatment and screening recommendations with patient values. Some decision aids are designed to offer the added benefit of clarifying individual values and, thus, increase the likelihood that patients will make decisions that are more in line with their goals.15
When decision aids are not available to elicit patient values, clinicians can integrate preference-clarifying questions as part of the standard patient encounter.23 These questions are aimed at surfacing the values underlying what the patient wants, what the patient does not want, and most importantly, why.
“Why” matters because it ultimately helps the clinician understand the patient’s mindset, enabling the clinician to help the patient make choices that serve his or her values.24 Eliciting values not only promotes patient well-being and self-determination, but also facilitates the development of empathic patient-clinician partnerships.
Categorizing decisions. Regardless of the particular method chosen to elicit patient values, the underlying questions faced by many patients often fit into one of 2 categories: 1) Do I prefer quality of life over length of life? or 2) Am I willing to be inconvenienced now to prevent more severe illness later? Clarifying the category into which a decision falls may open the conversation and help to explore patients’ values and priorities. Alternatively, asking questions such as, “Thinking about this decision, what is the most important aspect for you to consider?”25 may facilitate the conversation.
Much of the research on techniques geared to elicit values comes from the palliative care and oncology literature.26 Although this research generally focuses on decisions about serious illness or end-of-life preferences, preference-sensitive decisions in primary care settings create a need for clinicians who are effective in eliciting patient values.
The more serious and preference-sensitive the decision, the deeper the clinician needs to explore the patient’s personal goals. Despite scant literature about seemingly innocuous decisions, we recommend that clinicians elicit from their patients a brief, but overt, acknowledgement of the values guiding their choice for most preference-sensitive decisions.
3. Offer a professional recommendation
Once clinicians have a sense of an individual’s values and priorities, they are positioned to make a professional recommendation that aligns with these values and priorities, and leaves room for the patient to reach a decision. Historically, one of the clinician’s major roles was to provide advice and recommendations to patients. For a long time, this was done without the patient’s involvement in the decision-making.27
With an increasing emphasis on patient self-determination over the last 50 years, there has been some concern that the pendulum is swinging too far in the opposite direction, with clinicians shying away from providing specific recommendations.28 Although this line of thinking acknowledges the power of the clinician to influence patients, it falls short of distinguishing between a personal recommendation and a professional one. While personal recommendations have no place in medical decision-making, clinicians should offer patients a professional recommendation, along with their rationale.
How do personal and professional recommendations differ?
Personal recommendations arise from clinicians considering what they themselves might decide if they were in the patient’s position. Such recommendations are inappropriate because every person has unique values and priorities.
In contrast, professional recommendations stem from the clinician’s knowledge of the best available evidence, his or her understanding of the patient’s values, and his or her weaving of these pieces together in the context of the patient’s specific clinical presentation. Experienced clinicians bring all 3 elements of SDM to bear in making professional recommendations, even if these recommendations are at odds with what they might choose for themselves.
EBM and SDM: Not so different after all?
Another way to understand the legitimacy of a professional recommendation is to view the parallels between SDM and EBM. From the outset, EBM positioned itself as arising from the best available evidence, the patient’s values, and clinical expertise29—elements that are strikingly similar to the components of SDM.
Although commonly overlooked, the concept of EBM recognizes that established evidence alone is not sufficient for decision-making.30 Additionally, EBM allows for making a recommendation that may not appear to be guideline-based, because guidelines typically do not take into account individual patient preferences.30-32 What’s more, both EBM and SDM highlight the essential contribution of the clinician’s judgment about his or her patient’s unique presentation.
Thus, both EBM and SDM are dependent on the professional communicating a recommendation to the patient. This communication involves not only making clear what one recommends, but also why one recommends it. For example, a clinician might say the following to a patient with worsening asthma symptoms:
“The asthma guidelines give us 2 treatment options. We can either double the dose of your inhaled corticosteroid, or start a 5-day course of corticosteroid pills. Given your concerns about the adverse effects of the pills, and the moderate severity of this exacerbation, I recommend doubling the dose of your inhaled corticosteroid. We can reconsider the pills if your symptoms worsen or if you don’t improve within the next week. How does that sound to you?”
An informed choice. Explaining the evidence, articulating the patient’s values, and summarizing the clinical elements that went into the clinician’s recommendation clarifies and signals to the patient that this is a professional recommendation. Ultimately, the process of SDM concludes with the patient considering the clinician’s recommendation and making an informed choice from the available options.
CORRESPONDENCE
David J. Satin, MD, 2020 E 28th St., Minneapolis, MN 55407; [email protected].
1. Medalie JH. Family Medicine: Principles and Applications. Baltimore, MD: Williams and Wilkins; 1978.
2. Philips RL Jr, Brundgardt S, Lesko SE, et al. The future role of the family physician in the United States: a rigorous exercise in definition. Ann Fam Med. 2014;12:250-255.
3. Berwick DM. Era 3 for medicine and health care. JAMA. 2016;315:1329-1330.
4. Edwards A, Elwyn G. Shared decision making in health care: achieving evidence-based patient choice. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2009.
5. American Medical Association. Shared decision making H-373.997. Available at: https://policysearch.ama-assn.org/policyfinder/detail/H-373.997%20?uri=%2FAMADoc%2FHOD.xml-0-3162.xml. Accessed September 6, 2017.
6. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8:410-417.
8. Fowler FJ Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions? Results of a national survey. JAMA Intern Med. 2013;173:1215-1221.
9. Spatz ES, Krumhoiz HM, Moulton BW. The new era of informed consent: getting to a reasonable-patient standard through shared decision making. JAMA. 2016;315:2063-2064.
10. Faden RR, Becker C, Lewis C, et al. Disclosure of information to patients in medical care. Med Care. 1981;19:718-733.
11. Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270-280.
12. Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436-1443.
13. Stovitz SD, Shrier I. Medical decision making and the importance of baseline risk. Br J Gen Pract. 2013;63:e795-e797.
14. Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37-44.
15. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011:CD001431.
16. Mayo Clinic. Statin choice decision aid. Available at: https://statindecisionaid.mayoclinic.org/index.php/statin/index. Accessed September 6, 2017.
17. Risk Science Center and Center for Bioethics and Social Sciences in Medicine, University of Michigan. Icon Array. Available at: http://www.iconarray.com/. Accessed September 6, 2017.
18. Price M, Cameron R, Butow P. Communicating risk information: the influence of graphical display format on quantitative information perception–accuracy, comprehension and preferences. Patient Educ Couns. 2007;69:121-128.
19. Kon AA. The shared decision making continuum. JAMA. 2010;304:903-904.
20. Légaré F1, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535.
21. Haidet P, Paterniti DA. “Building” a history rather than “taking” one: a perspective on information sharing during the medical interview. Arch Intern Med. 2003;163:1134-1140.
22. Frankel RM, Stein R. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16:184-194.
23. Delbanco TL. Enriching the doctor-patient relationship by inviting the patient’s perspective. Ann Intern Med. 1992;116:414-418.
24. Doukas DJ, McCullough LB. The values history: the evaluation of the patient’s values and advance directives. J Fam Pract. 1991;32:145-153.
25. Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256.
26. Bernacki RE, Block SD, American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174:1994-2003.
27. Katz J. The Silent World of Doctor and Patient. Baltimore, MD, and London, England: The Johns Hopkins University Press; 1984.
28. Baylis F, Downie J. Professional recommendations: disclosing facts and values. J Med Ethics. 2001;27:20-24.
29. Sackett DL, Rosenburg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72.
30. Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310:2053-2054.
31. Mora S, Ames JM, Manson JE. Low-dose aspirin in the primary prevention of cardiovascular disease: shared decision making in clinical practice. JAMA. 2016;316:709-710.
32. Stovitz SD, Satin D, Shrier I. Shared decision making regarding aspirin in primary prevention of cardiovascular disease. JAMA. 2016;316:2276.
1. Medalie JH. Family Medicine: Principles and Applications. Baltimore, MD: Williams and Wilkins; 1978.
2. Philips RL Jr, Brundgardt S, Lesko SE, et al. The future role of the family physician in the United States: a rigorous exercise in definition. Ann Fam Med. 2014;12:250-255.
3. Berwick DM. Era 3 for medicine and health care. JAMA. 2016;315:1329-1330.
4. Edwards A, Elwyn G. Shared decision making in health care: achieving evidence-based patient choice. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2009.
5. American Medical Association. Shared decision making H-373.997. Available at: https://policysearch.ama-assn.org/policyfinder/detail/H-373.997%20?uri=%2FAMADoc%2FHOD.xml-0-3162.xml. Accessed September 6, 2017.
6. Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32:207-214.
7. Parchman ML, Zeber JE, Palmer RF. Participatory decision making, patient activation, medication adherence, and intermediate clinical outcomes in type 2 diabetes: a STARNet study. Ann Fam Med. 2010;8:410-417.
8. Fowler FJ Jr, Gerstein BS, Barry MJ. How patient centered are medical decisions? Results of a national survey. JAMA Intern Med. 2013;173:1215-1221.
9. Spatz ES, Krumhoiz HM, Moulton BW. The new era of informed consent: getting to a reasonable-patient standard through shared decision making. JAMA. 2016;315:2063-2064.
10. Faden RR, Becker C, Lewis C, et al. Disclosure of information to patients in medical care. Med Care. 1981;19:718-733.
11. Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161:270-280.
12. Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436-1443.
13. Stovitz SD, Shrier I. Medical decision making and the importance of baseline risk. Br J Gen Pract. 2013;63:e795-e797.
14. Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37-44.
15. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011:CD001431.
16. Mayo Clinic. Statin choice decision aid. Available at: https://statindecisionaid.mayoclinic.org/index.php/statin/index. Accessed September 6, 2017.
17. Risk Science Center and Center for Bioethics and Social Sciences in Medicine, University of Michigan. Icon Array. Available at: http://www.iconarray.com/. Accessed September 6, 2017.
18. Price M, Cameron R, Butow P. Communicating risk information: the influence of graphical display format on quantitative information perception–accuracy, comprehension and preferences. Patient Educ Couns. 2007;69:121-128.
19. Kon AA. The shared decision making continuum. JAMA. 2010;304:903-904.
20. Légaré F1, Ratté S, Gravel K, et al. Barriers and facilitators to implementing shared decision making in clinical practice: update of a systematic review of health professionals’ perceptions. Patient Educ Couns. 2008;73:526-535.
21. Haidet P, Paterniti DA. “Building” a history rather than “taking” one: a perspective on information sharing during the medical interview. Arch Intern Med. 2003;163:1134-1140.
22. Frankel RM, Stein R. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16:184-194.
23. Delbanco TL. Enriching the doctor-patient relationship by inviting the patient’s perspective. Ann Intern Med. 1992;116:414-418.
24. Doukas DJ, McCullough LB. The values history: the evaluation of the patient’s values and advance directives. J Fam Pract. 1991;32:145-153.
25. Stiggelbout AM, Van der Weijden T, De Wit MP, et al. Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256.
26. Bernacki RE, Block SD, American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174:1994-2003.
27. Katz J. The Silent World of Doctor and Patient. Baltimore, MD, and London, England: The Johns Hopkins University Press; 1984.
28. Baylis F, Downie J. Professional recommendations: disclosing facts and values. J Med Ethics. 2001;27:20-24.
29. Sackett DL, Rosenburg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72.
30. Montori VM, Brito JP, Murad MH. The optimal practice of evidence-based medicine: incorporating patient preferences in practice guidelines. JAMA. 2013;310:2053-2054.
31. Mora S, Ames JM, Manson JE. Low-dose aspirin in the primary prevention of cardiovascular disease: shared decision making in clinical practice. JAMA. 2016;316:709-710.
32. Stovitz SD, Satin D, Shrier I. Shared decision making regarding aspirin in primary prevention of cardiovascular disease. JAMA. 2016;316:2276.
PRACTICE RECOMMENDATIONS
› Provide patients with information in terms of absolute and baseline risks, ideally using pictograph decision aids. A
› Elicit the patient’s values and priorities by categorizing decisions and asking broad open-ended questions. C
› Offer patients a professional (not a personal) recommendation, including your rationale. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series