User login
• Assess the ejection fraction (EF) of all heart failure patients, and treat those with reduced EF according to established guidelines. A
• Reassess EF only when the clinical situation demands it; there is no need for routine EF surveillance. B
• Continue to treat patients with heart failure medications even after their EF has normalized. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Joe H is a 64-year-old African American man with a history of heart failure, hypertension, coronary artery disease (CAD), type 2 diabetes, chronic kidney disease, atrial fibrillation, and gout. His ejection fraction (EF), measured several years ago by echocardiography, was 20%, and he has New York Heart Association class II–III symptoms. Joe is taking an angiotensin-converting enzyme (ACE) inhibitor, a beta-blocker, an aldosterone antagonist, a loop diuretic, a nitrate, and digoxin, and he has an implantable cardioverter defibrillator (ICD). He recently spent 2 days in the hospital after being admitted for chest pain.
As an inpatient, Joe underwent stress echocardiography, which showed no inducible ischemia and an EF of 50%. Should the normalization of Joe’s EF prompt a change in his therapy?
Heart failure (HF), which affects an estimated 5 million Americans, is the leading cause of hospitalization in people older than 65 years.1 The condition—characterized by signs and symptoms of congestion and objective evidence of structural or functional heart disease—has historically been divided into 2 categories: Patients with HF and a reduced ejection fraction (EF) were said to have systolic dysfunction, while the term “diastolic dysfunction” was applied to those with HF and a preserved EF.
The distinction between systolic and diastolic dysfunction is not so simple, though, and the definition of diastolic dysfunction, in particular, is not so clearcut.
Diastolic dysfunction is sometimes described on the basis of echocardiographic criteria, such as the ratio of early-to-late diastolic filling, short deceleration times, and isovolumic relaxation times.2,3 But demographic and physiologic variables make interpretation of these parameters difficult, and the parameters themselves are not uniformly applied. What’s more, echocardiographic evidence of diastolic dysfunction is not specific to HF with a preserved EF.4 Some patients may be shown to have diastolic dysfunction and reduced EF.
As understanding of these variations grows, momentum about the need to change the clinical terminology has begun to develop. The suggested revision is simply to distinguish between HF with reduced EF and HF with preserved EF.5
To provide the best possible care for HF patients—including those who, like Joe, have gone from a reduced to a normal EF after receiving aggressive treatment—you need to be familiar with these changing parameters, recent research findings, and implications for treatment.
Managing both types of HF: What the evidence shows
Evidence-based management of HF with reduced EF is distinctly different from that of HF with preserved EF (TABLE).6-8 In fact, the vast majority of the evidence involves patients with reduced EF, as most randomized clinical trials (RCTs)—and the only trials demonstrating a reduction in mortality—have excluded patients with preserved EF. Thus, in diagnosing and treating HF patients, it is crucial to assess for, and to distinguish between, the 2 EF states. Documentation of this assessment is a core quality measure for HF management, according to the Joint Commission.9
Treating HF with reduced EF. Barring any contraindications, ACE inhibitors and beta-blockers are core treatments for patients with reduced EF.8 Aldosterone antagonists are also indicated for patients with reduced EF who have, or recently had, rest dyspnea. They are also indicated for patients with reduced EF who are 3 to 14 days post-MI and have diabetes or symptomatic HF.8 Nitrates are indicated for African American patients who have persistent symptoms despite treatment with ACE inhibitors, beta-blockers, and diuretics, as needed.8 Consider an ICD as well, as these devices have been found to significantly reduce the risk of death for patients who have an EF <35% with either ischemic cardiomyopathy or symptomatic HF.
Treating HF with preserved EF. Because of the dearth of trials involving patients with HF and a preserved EF, there is limited evidence-based treatment. Nonetheless, it is reasonable to control signs and symptoms of congestion with diuretics.6 In addition, the CHARM-Preserved trial demonstrated the efficacy of candesartan—an angiotensin receptor blocker—in decreasing rates of hospital admissions among symptomatic patients with a preserved EF.10
TABLE
Heart failure: Ejection fraction status dictates treatment6-8
| Intervention | Indications in patients with reduced EF (evidence supporting its use) | Indications in patients with preserved EF (evidence supporting its use) |
|---|---|---|
| ACEIs | •All patients (reduced mortality) | No evidence |
| Beta-blockers | • All symptomatic patients (reduced mortality) | No evidence |
| Aldosterone antagonists | • Rest dyspnea • Post-MI with diabetes or symptomatic HF (reduced mortality) | No evidence |
| Nitrates | • African American patients with persistent symptoms despite treatment with ACEIs, beta-blockers, and diuretics • Intolerance to ACEI/ARBs due to renal impairment (reduced mortality) | No evidence |
| Diuretics | As needed for fluid overload | As needed for fluid overload |
| ARBs | • Intolerance to ACEIs due to cough • Consider for patients with ACEI intolerance due to angioedema* (reduced mortality) | Symptomatic patients (reduced hospitalization rates) |
| Digoxin | • Persistent symptoms despite background therapy • HF and atrial fibrillation (reduced hospitalization rates) | No evidence |
| ICDs | • EF <35% and ischemic cardiomyopathy or symptomatic HF (reduced mortality) | No evidence |
| *There is a possibility of cross-reactivity. | ||
| ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; EF, ejection fraction; HF, heart failure; ICDs, implantable cardioverter defibrillators; MI, myocardial infarction. | ||
How to treat the patient with normalized EF
These 2 options, however, do not clearly address the question of what to do with patients like Joe, whose case is described in our opener. Should patients whose EF has normalized after months, or years, of aggressive treatment remain on the medication regimen they followed when they had reduced EF? Should they be treated as HF patients with preserved EF? Is there another option? How often should patients who initially had a reduced EF be reassessed? To answer these questions, let’s take a closer look at the evidence.
Measurement of EF. No large clinical trials have investigated the impact of serial measurement of EF. Two small studies showed prognostic significance with serial measurements that demonstrated improvements in EF,11,12 but their reproducibility and clinical significance are unclear. The American College of Cardiology/American Heart Association and the European Society of Cardiology recommend repeat measurement of EF only when it is clinically indicated.6,13
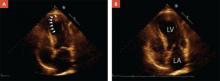
Accuracy of the results. Echocardiogram is among the most widely used cardiac imaging modalities because it is fast, portable, and noninvasive. However, physiologic limitations and the challenges of calculating a 3-dimensional parameter with 2-dimensional images (FIGURE) limit the usefulness of echocardiography for the measurement of EF. Among other things, echocardiography lacks the ability to reliably identify small changes or improvements. Newer techniques—such as contrast or radionuclide ventriculography—have shown improved reliability in early studies.14 Compared with echocardiography, however, ventriculography is more costly and more invasive.
McGowan et al15 conducted a systematic review of studies comparing echocardiography with reference standards of contrast or radionuclide ventriculography. They concluded that no method in general use to calculate EF from echocardiographic images could provide a 95% confidence interval (CI) of <±10% in the measurement of EF. The American Society of Echocardiography has published standards to improve measurement technique and minimize variability in EF measurement.16
FIGURE
Echocardiographic evidence of heart failure? Systolic image tells the story
In a patient with reduced ejection fraction, the systolic image (A) reveals septal apical akinesis (arrows) and hypokinesis in the remainder of the left ventricle. The diastolic image (B) is unremarkable.
Normalization of EF is not a cure
In treating patients with HF, it is crucial to distinguish between what is reversible and what is not. Diuretics, oxygen, and other supportive therapy may reverse symptoms of congestion. Reversible causes of HF may include alcohol toxicity, thyroid disease, tachycardia, anemia, valvular heart disease, and CAD, among others. However, reversing the symptoms or the cause does not necessarily reverse HF itself. Further, normalization of EF does not necessarily imply that HF has been cured.
In a prospective study of 42 HF patients whose EF had normalized, the aggregate initial EF was 26%. It increased to ≥40%, with an absolute increase in EF ≥10%. During 41 months of follow-up, 19% of the patients had a recurrence of reduced EF. The likelihood of recurrence was greater among those who had discontinued their HF medications, the researchers found.17
In another prospective study, researchers followed 110 patients with reduced EF who were managed medically according to guideline-prescribed therapy.18 During a 17-month follow-up period, 18% had a normal EF at some point—but in more than half the cases (55%), the improvement was transient. Factors that were predictive of normalization included the presence of arterial hypertension (odds ratio [OR]=8.5; P=.01), nonischemic etiology (OR=4.9; P=.02), the absence of diabetes (OR=9.5; P=.01), beta-blocker therapy with carvedilol (OR=3.9, P=.02), and a higher beta-blocker dosage (OR=1.1; P=.04). Normalization occurred, on average, at 13 months (±6 months). The only difference between those who had a sustained improvement and those for whom the normalization was transient was the rate of chronic obstructive pulmonary disease (COPD). None of the patients who had a sustained improvement had COPD; 36% of those with transient improvement did (P=.04).
Finally, researchers used an Italian registry to follow prospectively 581 patients with dilated cardiomyopathy who were enrolled over a 25-year period.19 The team found that “healing” (reverse remodeling) occurred in 16% of the patients in response to treatment with ACE inhibitors and beta-blockers. Thus, in the vast majority of patients, the underlying disorder that caused the cardiomyopathy was maintained.
Keep patients on the same meds
There are no prospective RCTs investigating the continuation of ACE inhibitors, beta-blockers, or other therapy among HF patients whose EF normalized in response to treatment. Given the dramatic benefit of these medications for patients with a reduced EF, no such trial is likely to be performed. The trials noted above are instructive, however, and show that maintenance of HF medications17 (and the absence of COPD18) are predictors of sustained improvement.
Other factors to consider: Diastolic dysfunction is an ill-defined condition, and normalization of EF does not necessarily restore a patient to the same status as that of someone who never had a reduced EF. In addition, many patients with a reduced EF have concomitant CAD. In such cases, beta-blockers and ACE inhibitors are indicated as part of secondary prevention—another reason for continuation of the medication regimen, despite EF normalization.
CASE That was true for Joe, who suffered from a host of comorbidities, including CAD, as well as HF, and had been hospitalized for chest pain. His negative stress echocardiogram and improved EF suggested—although neither was definitive evidence—that his chest pain may have had a noncardiac cause. At his postdischarge follow-up visit, he was not experiencing any additional pain.
Despite Joe’s improved EF, however, his medical regimen remains unchanged. He comes in every 1 to 2 months for surveillance.
CORRESPONDENCE
William E. Chavey, MD, MS, University of Michigan, 1500 East Medical Center Drive, L2003 Women’s Hospital, Ann Arbor, Mich 48109-5239; [email protected]
1. Schocken DD, Benjamin EJ, Fonarow GC, et al. American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; Functional Genomics and Translational Biology Interdisciplinary Working Group Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544-2565.
2. Quinones MA. Assessment of diastolic dysfunction. Prog Cardiovasc Dis. 2005;47:340-355
3. Smiseth OA, Thompson CR. Atrioventricular filling dynamics, diastolic dysfunction and dysfunction. Heart Fail Rev. 2000;5:291-299.
4. Pinamonti B, Zecchin M, DiLenarda A, et al. Persistence of restrictive left ventricular filling pattern in dilated cardiomyopathy: an ominous prognostic sign. J Am Coll Cardiol. 1997;29:604-612.
5. Yamamoto K, Sakata Y, Ohtani T, et al. Heart failure with preserved ejection fraction—what is known and unknown. Circ J. 2009;73:404-410.
6. Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of European Society of Cardiology, Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29:2388-2442.
7. Jessup M, Abraham WT, Casey DE, et al. Focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;199:1977-2016
8. Chavey WE, Bleske BE, Van Harrison R, et al. Pharmacologic management of heart failure caused by systolic dysfunction. Am Fam Physician. 2008;77:957-968.
9. Joint Commission Heart failure core measure set. Oak-brook Park, IL. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Heart+Failure+
Core+Measure+Set.htm. Accessed February 5, 2010.
10. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777-781.
11. Moreo A de Chiara B, Cataldo G, et al. Prognostic value of serial measurements of left ventricular function and exercise performance in chronic heart failure [in Spanish]. Rev Esp Cardiol. 2006;59:905-910.
12. Metra M, Nodari S, Parrinello G, et al. Marked improvement in left ventricular ejection fraction during long-term beta blockade in patients with chronic heart failure: clinical correlates and prognostic significance. Am Heart J. 2003;145:292-299.
13. Swedberg K, Cleland J, Dargie H, et al. For the Task Force for the Diagnosis and Treatment of Chronic Heart Failurwe of the European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005). Eur Heart J. 2005;26:1115-1140.
14. Malm S, Frigstad S, Sagberg E, et al. Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography. J Am Coll Cardiol. 2004;44:1030-1035.
15. McGowan JH, Cleland J. Reliability of reporting left ventricular systolic function by echocardiography: a systematic review of three methods. Am Heart J. 2003;146:388-397.
16. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography. J Am Soc Echocardiogr. 2005;18:1440-1463.
17. Moon J, Ko YG, Chung N, et al. Recovery and recurrence of left ventricular systolic dysfunction in patients with idiopathic dilated cardiomyopathy. Can J Cardiol. 2009;25:e147-e150.
18. Cioffi G, Stefenelli C, Tarantini L, et al. Chronic left ventricular failure in the community: prevalence, prognosis, and predictors of the complete clinical recovery with return of cardiac size and function to normal in patients undergoing optimal therapy. J Card Fail. 2004;10:250-257.
19. Di Lenarda A, Sabbadini G, Perkan A, et al. Apparent healing in dilated cardiomyopathy: incidence, long-term persistence and predictive factors. The heart muscle disease registry of Trieste [abstract]. Ital Heart J. 2001;2(suppl 2):S97.-
• Assess the ejection fraction (EF) of all heart failure patients, and treat those with reduced EF according to established guidelines. A
• Reassess EF only when the clinical situation demands it; there is no need for routine EF surveillance. B
• Continue to treat patients with heart failure medications even after their EF has normalized. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Joe H is a 64-year-old African American man with a history of heart failure, hypertension, coronary artery disease (CAD), type 2 diabetes, chronic kidney disease, atrial fibrillation, and gout. His ejection fraction (EF), measured several years ago by echocardiography, was 20%, and he has New York Heart Association class II–III symptoms. Joe is taking an angiotensin-converting enzyme (ACE) inhibitor, a beta-blocker, an aldosterone antagonist, a loop diuretic, a nitrate, and digoxin, and he has an implantable cardioverter defibrillator (ICD). He recently spent 2 days in the hospital after being admitted for chest pain.
As an inpatient, Joe underwent stress echocardiography, which showed no inducible ischemia and an EF of 50%. Should the normalization of Joe’s EF prompt a change in his therapy?
Heart failure (HF), which affects an estimated 5 million Americans, is the leading cause of hospitalization in people older than 65 years.1 The condition—characterized by signs and symptoms of congestion and objective evidence of structural or functional heart disease—has historically been divided into 2 categories: Patients with HF and a reduced ejection fraction (EF) were said to have systolic dysfunction, while the term “diastolic dysfunction” was applied to those with HF and a preserved EF.
The distinction between systolic and diastolic dysfunction is not so simple, though, and the definition of diastolic dysfunction, in particular, is not so clearcut.
Diastolic dysfunction is sometimes described on the basis of echocardiographic criteria, such as the ratio of early-to-late diastolic filling, short deceleration times, and isovolumic relaxation times.2,3 But demographic and physiologic variables make interpretation of these parameters difficult, and the parameters themselves are not uniformly applied. What’s more, echocardiographic evidence of diastolic dysfunction is not specific to HF with a preserved EF.4 Some patients may be shown to have diastolic dysfunction and reduced EF.
As understanding of these variations grows, momentum about the need to change the clinical terminology has begun to develop. The suggested revision is simply to distinguish between HF with reduced EF and HF with preserved EF.5
To provide the best possible care for HF patients—including those who, like Joe, have gone from a reduced to a normal EF after receiving aggressive treatment—you need to be familiar with these changing parameters, recent research findings, and implications for treatment.
Managing both types of HF: What the evidence shows
Evidence-based management of HF with reduced EF is distinctly different from that of HF with preserved EF (TABLE).6-8 In fact, the vast majority of the evidence involves patients with reduced EF, as most randomized clinical trials (RCTs)—and the only trials demonstrating a reduction in mortality—have excluded patients with preserved EF. Thus, in diagnosing and treating HF patients, it is crucial to assess for, and to distinguish between, the 2 EF states. Documentation of this assessment is a core quality measure for HF management, according to the Joint Commission.9
Treating HF with reduced EF. Barring any contraindications, ACE inhibitors and beta-blockers are core treatments for patients with reduced EF.8 Aldosterone antagonists are also indicated for patients with reduced EF who have, or recently had, rest dyspnea. They are also indicated for patients with reduced EF who are 3 to 14 days post-MI and have diabetes or symptomatic HF.8 Nitrates are indicated for African American patients who have persistent symptoms despite treatment with ACE inhibitors, beta-blockers, and diuretics, as needed.8 Consider an ICD as well, as these devices have been found to significantly reduce the risk of death for patients who have an EF <35% with either ischemic cardiomyopathy or symptomatic HF.
Treating HF with preserved EF. Because of the dearth of trials involving patients with HF and a preserved EF, there is limited evidence-based treatment. Nonetheless, it is reasonable to control signs and symptoms of congestion with diuretics.6 In addition, the CHARM-Preserved trial demonstrated the efficacy of candesartan—an angiotensin receptor blocker—in decreasing rates of hospital admissions among symptomatic patients with a preserved EF.10
TABLE
Heart failure: Ejection fraction status dictates treatment6-8
| Intervention | Indications in patients with reduced EF (evidence supporting its use) | Indications in patients with preserved EF (evidence supporting its use) |
|---|---|---|
| ACEIs | •All patients (reduced mortality) | No evidence |
| Beta-blockers | • All symptomatic patients (reduced mortality) | No evidence |
| Aldosterone antagonists | • Rest dyspnea • Post-MI with diabetes or symptomatic HF (reduced mortality) | No evidence |
| Nitrates | • African American patients with persistent symptoms despite treatment with ACEIs, beta-blockers, and diuretics • Intolerance to ACEI/ARBs due to renal impairment (reduced mortality) | No evidence |
| Diuretics | As needed for fluid overload | As needed for fluid overload |
| ARBs | • Intolerance to ACEIs due to cough • Consider for patients with ACEI intolerance due to angioedema* (reduced mortality) | Symptomatic patients (reduced hospitalization rates) |
| Digoxin | • Persistent symptoms despite background therapy • HF and atrial fibrillation (reduced hospitalization rates) | No evidence |
| ICDs | • EF <35% and ischemic cardiomyopathy or symptomatic HF (reduced mortality) | No evidence |
| *There is a possibility of cross-reactivity. | ||
| ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; EF, ejection fraction; HF, heart failure; ICDs, implantable cardioverter defibrillators; MI, myocardial infarction. | ||
How to treat the patient with normalized EF
These 2 options, however, do not clearly address the question of what to do with patients like Joe, whose case is described in our opener. Should patients whose EF has normalized after months, or years, of aggressive treatment remain on the medication regimen they followed when they had reduced EF? Should they be treated as HF patients with preserved EF? Is there another option? How often should patients who initially had a reduced EF be reassessed? To answer these questions, let’s take a closer look at the evidence.
Measurement of EF. No large clinical trials have investigated the impact of serial measurement of EF. Two small studies showed prognostic significance with serial measurements that demonstrated improvements in EF,11,12 but their reproducibility and clinical significance are unclear. The American College of Cardiology/American Heart Association and the European Society of Cardiology recommend repeat measurement of EF only when it is clinically indicated.6,13
Accuracy of the results. Echocardiogram is among the most widely used cardiac imaging modalities because it is fast, portable, and noninvasive. However, physiologic limitations and the challenges of calculating a 3-dimensional parameter with 2-dimensional images (FIGURE) limit the usefulness of echocardiography for the measurement of EF. Among other things, echocardiography lacks the ability to reliably identify small changes or improvements. Newer techniques—such as contrast or radionuclide ventriculography—have shown improved reliability in early studies.14 Compared with echocardiography, however, ventriculography is more costly and more invasive.
McGowan et al15 conducted a systematic review of studies comparing echocardiography with reference standards of contrast or radionuclide ventriculography. They concluded that no method in general use to calculate EF from echocardiographic images could provide a 95% confidence interval (CI) of <±10% in the measurement of EF. The American Society of Echocardiography has published standards to improve measurement technique and minimize variability in EF measurement.16
FIGURE
Echocardiographic evidence of heart failure? Systolic image tells the story
In a patient with reduced ejection fraction, the systolic image (A) reveals septal apical akinesis (arrows) and hypokinesis in the remainder of the left ventricle. The diastolic image (B) is unremarkable.
Normalization of EF is not a cure
In treating patients with HF, it is crucial to distinguish between what is reversible and what is not. Diuretics, oxygen, and other supportive therapy may reverse symptoms of congestion. Reversible causes of HF may include alcohol toxicity, thyroid disease, tachycardia, anemia, valvular heart disease, and CAD, among others. However, reversing the symptoms or the cause does not necessarily reverse HF itself. Further, normalization of EF does not necessarily imply that HF has been cured.
In a prospective study of 42 HF patients whose EF had normalized, the aggregate initial EF was 26%. It increased to ≥40%, with an absolute increase in EF ≥10%. During 41 months of follow-up, 19% of the patients had a recurrence of reduced EF. The likelihood of recurrence was greater among those who had discontinued their HF medications, the researchers found.17
In another prospective study, researchers followed 110 patients with reduced EF who were managed medically according to guideline-prescribed therapy.18 During a 17-month follow-up period, 18% had a normal EF at some point—but in more than half the cases (55%), the improvement was transient. Factors that were predictive of normalization included the presence of arterial hypertension (odds ratio [OR]=8.5; P=.01), nonischemic etiology (OR=4.9; P=.02), the absence of diabetes (OR=9.5; P=.01), beta-blocker therapy with carvedilol (OR=3.9, P=.02), and a higher beta-blocker dosage (OR=1.1; P=.04). Normalization occurred, on average, at 13 months (±6 months). The only difference between those who had a sustained improvement and those for whom the normalization was transient was the rate of chronic obstructive pulmonary disease (COPD). None of the patients who had a sustained improvement had COPD; 36% of those with transient improvement did (P=.04).
Finally, researchers used an Italian registry to follow prospectively 581 patients with dilated cardiomyopathy who were enrolled over a 25-year period.19 The team found that “healing” (reverse remodeling) occurred in 16% of the patients in response to treatment with ACE inhibitors and beta-blockers. Thus, in the vast majority of patients, the underlying disorder that caused the cardiomyopathy was maintained.
Keep patients on the same meds
There are no prospective RCTs investigating the continuation of ACE inhibitors, beta-blockers, or other therapy among HF patients whose EF normalized in response to treatment. Given the dramatic benefit of these medications for patients with a reduced EF, no such trial is likely to be performed. The trials noted above are instructive, however, and show that maintenance of HF medications17 (and the absence of COPD18) are predictors of sustained improvement.
Other factors to consider: Diastolic dysfunction is an ill-defined condition, and normalization of EF does not necessarily restore a patient to the same status as that of someone who never had a reduced EF. In addition, many patients with a reduced EF have concomitant CAD. In such cases, beta-blockers and ACE inhibitors are indicated as part of secondary prevention—another reason for continuation of the medication regimen, despite EF normalization.
CASE That was true for Joe, who suffered from a host of comorbidities, including CAD, as well as HF, and had been hospitalized for chest pain. His negative stress echocardiogram and improved EF suggested—although neither was definitive evidence—that his chest pain may have had a noncardiac cause. At his postdischarge follow-up visit, he was not experiencing any additional pain.
Despite Joe’s improved EF, however, his medical regimen remains unchanged. He comes in every 1 to 2 months for surveillance.
CORRESPONDENCE
William E. Chavey, MD, MS, University of Michigan, 1500 East Medical Center Drive, L2003 Women’s Hospital, Ann Arbor, Mich 48109-5239; [email protected]
• Assess the ejection fraction (EF) of all heart failure patients, and treat those with reduced EF according to established guidelines. A
• Reassess EF only when the clinical situation demands it; there is no need for routine EF surveillance. B
• Continue to treat patients with heart failure medications even after their EF has normalized. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Joe H is a 64-year-old African American man with a history of heart failure, hypertension, coronary artery disease (CAD), type 2 diabetes, chronic kidney disease, atrial fibrillation, and gout. His ejection fraction (EF), measured several years ago by echocardiography, was 20%, and he has New York Heart Association class II–III symptoms. Joe is taking an angiotensin-converting enzyme (ACE) inhibitor, a beta-blocker, an aldosterone antagonist, a loop diuretic, a nitrate, and digoxin, and he has an implantable cardioverter defibrillator (ICD). He recently spent 2 days in the hospital after being admitted for chest pain.
As an inpatient, Joe underwent stress echocardiography, which showed no inducible ischemia and an EF of 50%. Should the normalization of Joe’s EF prompt a change in his therapy?
Heart failure (HF), which affects an estimated 5 million Americans, is the leading cause of hospitalization in people older than 65 years.1 The condition—characterized by signs and symptoms of congestion and objective evidence of structural or functional heart disease—has historically been divided into 2 categories: Patients with HF and a reduced ejection fraction (EF) were said to have systolic dysfunction, while the term “diastolic dysfunction” was applied to those with HF and a preserved EF.
The distinction between systolic and diastolic dysfunction is not so simple, though, and the definition of diastolic dysfunction, in particular, is not so clearcut.
Diastolic dysfunction is sometimes described on the basis of echocardiographic criteria, such as the ratio of early-to-late diastolic filling, short deceleration times, and isovolumic relaxation times.2,3 But demographic and physiologic variables make interpretation of these parameters difficult, and the parameters themselves are not uniformly applied. What’s more, echocardiographic evidence of diastolic dysfunction is not specific to HF with a preserved EF.4 Some patients may be shown to have diastolic dysfunction and reduced EF.
As understanding of these variations grows, momentum about the need to change the clinical terminology has begun to develop. The suggested revision is simply to distinguish between HF with reduced EF and HF with preserved EF.5
To provide the best possible care for HF patients—including those who, like Joe, have gone from a reduced to a normal EF after receiving aggressive treatment—you need to be familiar with these changing parameters, recent research findings, and implications for treatment.
Managing both types of HF: What the evidence shows
Evidence-based management of HF with reduced EF is distinctly different from that of HF with preserved EF (TABLE).6-8 In fact, the vast majority of the evidence involves patients with reduced EF, as most randomized clinical trials (RCTs)—and the only trials demonstrating a reduction in mortality—have excluded patients with preserved EF. Thus, in diagnosing and treating HF patients, it is crucial to assess for, and to distinguish between, the 2 EF states. Documentation of this assessment is a core quality measure for HF management, according to the Joint Commission.9
Treating HF with reduced EF. Barring any contraindications, ACE inhibitors and beta-blockers are core treatments for patients with reduced EF.8 Aldosterone antagonists are also indicated for patients with reduced EF who have, or recently had, rest dyspnea. They are also indicated for patients with reduced EF who are 3 to 14 days post-MI and have diabetes or symptomatic HF.8 Nitrates are indicated for African American patients who have persistent symptoms despite treatment with ACE inhibitors, beta-blockers, and diuretics, as needed.8 Consider an ICD as well, as these devices have been found to significantly reduce the risk of death for patients who have an EF <35% with either ischemic cardiomyopathy or symptomatic HF.
Treating HF with preserved EF. Because of the dearth of trials involving patients with HF and a preserved EF, there is limited evidence-based treatment. Nonetheless, it is reasonable to control signs and symptoms of congestion with diuretics.6 In addition, the CHARM-Preserved trial demonstrated the efficacy of candesartan—an angiotensin receptor blocker—in decreasing rates of hospital admissions among symptomatic patients with a preserved EF.10
TABLE
Heart failure: Ejection fraction status dictates treatment6-8
| Intervention | Indications in patients with reduced EF (evidence supporting its use) | Indications in patients with preserved EF (evidence supporting its use) |
|---|---|---|
| ACEIs | •All patients (reduced mortality) | No evidence |
| Beta-blockers | • All symptomatic patients (reduced mortality) | No evidence |
| Aldosterone antagonists | • Rest dyspnea • Post-MI with diabetes or symptomatic HF (reduced mortality) | No evidence |
| Nitrates | • African American patients with persistent symptoms despite treatment with ACEIs, beta-blockers, and diuretics • Intolerance to ACEI/ARBs due to renal impairment (reduced mortality) | No evidence |
| Diuretics | As needed for fluid overload | As needed for fluid overload |
| ARBs | • Intolerance to ACEIs due to cough • Consider for patients with ACEI intolerance due to angioedema* (reduced mortality) | Symptomatic patients (reduced hospitalization rates) |
| Digoxin | • Persistent symptoms despite background therapy • HF and atrial fibrillation (reduced hospitalization rates) | No evidence |
| ICDs | • EF <35% and ischemic cardiomyopathy or symptomatic HF (reduced mortality) | No evidence |
| *There is a possibility of cross-reactivity. | ||
| ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; EF, ejection fraction; HF, heart failure; ICDs, implantable cardioverter defibrillators; MI, myocardial infarction. | ||
How to treat the patient with normalized EF
These 2 options, however, do not clearly address the question of what to do with patients like Joe, whose case is described in our opener. Should patients whose EF has normalized after months, or years, of aggressive treatment remain on the medication regimen they followed when they had reduced EF? Should they be treated as HF patients with preserved EF? Is there another option? How often should patients who initially had a reduced EF be reassessed? To answer these questions, let’s take a closer look at the evidence.
Measurement of EF. No large clinical trials have investigated the impact of serial measurement of EF. Two small studies showed prognostic significance with serial measurements that demonstrated improvements in EF,11,12 but their reproducibility and clinical significance are unclear. The American College of Cardiology/American Heart Association and the European Society of Cardiology recommend repeat measurement of EF only when it is clinically indicated.6,13
Accuracy of the results. Echocardiogram is among the most widely used cardiac imaging modalities because it is fast, portable, and noninvasive. However, physiologic limitations and the challenges of calculating a 3-dimensional parameter with 2-dimensional images (FIGURE) limit the usefulness of echocardiography for the measurement of EF. Among other things, echocardiography lacks the ability to reliably identify small changes or improvements. Newer techniques—such as contrast or radionuclide ventriculography—have shown improved reliability in early studies.14 Compared with echocardiography, however, ventriculography is more costly and more invasive.
McGowan et al15 conducted a systematic review of studies comparing echocardiography with reference standards of contrast or radionuclide ventriculography. They concluded that no method in general use to calculate EF from echocardiographic images could provide a 95% confidence interval (CI) of <±10% in the measurement of EF. The American Society of Echocardiography has published standards to improve measurement technique and minimize variability in EF measurement.16
FIGURE
Echocardiographic evidence of heart failure? Systolic image tells the story
In a patient with reduced ejection fraction, the systolic image (A) reveals septal apical akinesis (arrows) and hypokinesis in the remainder of the left ventricle. The diastolic image (B) is unremarkable.
Normalization of EF is not a cure
In treating patients with HF, it is crucial to distinguish between what is reversible and what is not. Diuretics, oxygen, and other supportive therapy may reverse symptoms of congestion. Reversible causes of HF may include alcohol toxicity, thyroid disease, tachycardia, anemia, valvular heart disease, and CAD, among others. However, reversing the symptoms or the cause does not necessarily reverse HF itself. Further, normalization of EF does not necessarily imply that HF has been cured.
In a prospective study of 42 HF patients whose EF had normalized, the aggregate initial EF was 26%. It increased to ≥40%, with an absolute increase in EF ≥10%. During 41 months of follow-up, 19% of the patients had a recurrence of reduced EF. The likelihood of recurrence was greater among those who had discontinued their HF medications, the researchers found.17
In another prospective study, researchers followed 110 patients with reduced EF who were managed medically according to guideline-prescribed therapy.18 During a 17-month follow-up period, 18% had a normal EF at some point—but in more than half the cases (55%), the improvement was transient. Factors that were predictive of normalization included the presence of arterial hypertension (odds ratio [OR]=8.5; P=.01), nonischemic etiology (OR=4.9; P=.02), the absence of diabetes (OR=9.5; P=.01), beta-blocker therapy with carvedilol (OR=3.9, P=.02), and a higher beta-blocker dosage (OR=1.1; P=.04). Normalization occurred, on average, at 13 months (±6 months). The only difference between those who had a sustained improvement and those for whom the normalization was transient was the rate of chronic obstructive pulmonary disease (COPD). None of the patients who had a sustained improvement had COPD; 36% of those with transient improvement did (P=.04).
Finally, researchers used an Italian registry to follow prospectively 581 patients with dilated cardiomyopathy who were enrolled over a 25-year period.19 The team found that “healing” (reverse remodeling) occurred in 16% of the patients in response to treatment with ACE inhibitors and beta-blockers. Thus, in the vast majority of patients, the underlying disorder that caused the cardiomyopathy was maintained.
Keep patients on the same meds
There are no prospective RCTs investigating the continuation of ACE inhibitors, beta-blockers, or other therapy among HF patients whose EF normalized in response to treatment. Given the dramatic benefit of these medications for patients with a reduced EF, no such trial is likely to be performed. The trials noted above are instructive, however, and show that maintenance of HF medications17 (and the absence of COPD18) are predictors of sustained improvement.
Other factors to consider: Diastolic dysfunction is an ill-defined condition, and normalization of EF does not necessarily restore a patient to the same status as that of someone who never had a reduced EF. In addition, many patients with a reduced EF have concomitant CAD. In such cases, beta-blockers and ACE inhibitors are indicated as part of secondary prevention—another reason for continuation of the medication regimen, despite EF normalization.
CASE That was true for Joe, who suffered from a host of comorbidities, including CAD, as well as HF, and had been hospitalized for chest pain. His negative stress echocardiogram and improved EF suggested—although neither was definitive evidence—that his chest pain may have had a noncardiac cause. At his postdischarge follow-up visit, he was not experiencing any additional pain.
Despite Joe’s improved EF, however, his medical regimen remains unchanged. He comes in every 1 to 2 months for surveillance.
CORRESPONDENCE
William E. Chavey, MD, MS, University of Michigan, 1500 East Medical Center Drive, L2003 Women’s Hospital, Ann Arbor, Mich 48109-5239; [email protected]
1. Schocken DD, Benjamin EJ, Fonarow GC, et al. American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; Functional Genomics and Translational Biology Interdisciplinary Working Group Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544-2565.
2. Quinones MA. Assessment of diastolic dysfunction. Prog Cardiovasc Dis. 2005;47:340-355
3. Smiseth OA, Thompson CR. Atrioventricular filling dynamics, diastolic dysfunction and dysfunction. Heart Fail Rev. 2000;5:291-299.
4. Pinamonti B, Zecchin M, DiLenarda A, et al. Persistence of restrictive left ventricular filling pattern in dilated cardiomyopathy: an ominous prognostic sign. J Am Coll Cardiol. 1997;29:604-612.
5. Yamamoto K, Sakata Y, Ohtani T, et al. Heart failure with preserved ejection fraction—what is known and unknown. Circ J. 2009;73:404-410.
6. Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of European Society of Cardiology, Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29:2388-2442.
7. Jessup M, Abraham WT, Casey DE, et al. Focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;199:1977-2016
8. Chavey WE, Bleske BE, Van Harrison R, et al. Pharmacologic management of heart failure caused by systolic dysfunction. Am Fam Physician. 2008;77:957-968.
9. Joint Commission Heart failure core measure set. Oak-brook Park, IL. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Heart+Failure+
Core+Measure+Set.htm. Accessed February 5, 2010.
10. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777-781.
11. Moreo A de Chiara B, Cataldo G, et al. Prognostic value of serial measurements of left ventricular function and exercise performance in chronic heart failure [in Spanish]. Rev Esp Cardiol. 2006;59:905-910.
12. Metra M, Nodari S, Parrinello G, et al. Marked improvement in left ventricular ejection fraction during long-term beta blockade in patients with chronic heart failure: clinical correlates and prognostic significance. Am Heart J. 2003;145:292-299.
13. Swedberg K, Cleland J, Dargie H, et al. For the Task Force for the Diagnosis and Treatment of Chronic Heart Failurwe of the European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005). Eur Heart J. 2005;26:1115-1140.
14. Malm S, Frigstad S, Sagberg E, et al. Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography. J Am Coll Cardiol. 2004;44:1030-1035.
15. McGowan JH, Cleland J. Reliability of reporting left ventricular systolic function by echocardiography: a systematic review of three methods. Am Heart J. 2003;146:388-397.
16. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography. J Am Soc Echocardiogr. 2005;18:1440-1463.
17. Moon J, Ko YG, Chung N, et al. Recovery and recurrence of left ventricular systolic dysfunction in patients with idiopathic dilated cardiomyopathy. Can J Cardiol. 2009;25:e147-e150.
18. Cioffi G, Stefenelli C, Tarantini L, et al. Chronic left ventricular failure in the community: prevalence, prognosis, and predictors of the complete clinical recovery with return of cardiac size and function to normal in patients undergoing optimal therapy. J Card Fail. 2004;10:250-257.
19. Di Lenarda A, Sabbadini G, Perkan A, et al. Apparent healing in dilated cardiomyopathy: incidence, long-term persistence and predictive factors. The heart muscle disease registry of Trieste [abstract]. Ital Heart J. 2001;2(suppl 2):S97.-
1. Schocken DD, Benjamin EJ, Fonarow GC, et al. American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; Functional Genomics and Translational Biology Interdisciplinary Working Group Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544-2565.
2. Quinones MA. Assessment of diastolic dysfunction. Prog Cardiovasc Dis. 2005;47:340-355
3. Smiseth OA, Thompson CR. Atrioventricular filling dynamics, diastolic dysfunction and dysfunction. Heart Fail Rev. 2000;5:291-299.
4. Pinamonti B, Zecchin M, DiLenarda A, et al. Persistence of restrictive left ventricular filling pattern in dilated cardiomyopathy: an ominous prognostic sign. J Am Coll Cardiol. 1997;29:604-612.
5. Yamamoto K, Sakata Y, Ohtani T, et al. Heart failure with preserved ejection fraction—what is known and unknown. Circ J. 2009;73:404-410.
6. Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of European Society of Cardiology, Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29:2388-2442.
7. Jessup M, Abraham WT, Casey DE, et al. Focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;199:1977-2016
8. Chavey WE, Bleske BE, Van Harrison R, et al. Pharmacologic management of heart failure caused by systolic dysfunction. Am Fam Physician. 2008;77:957-968.
9. Joint Commission Heart failure core measure set. Oak-brook Park, IL. Available at: http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Heart+Failure+
Core+Measure+Set.htm. Accessed February 5, 2010.
10. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777-781.
11. Moreo A de Chiara B, Cataldo G, et al. Prognostic value of serial measurements of left ventricular function and exercise performance in chronic heart failure [in Spanish]. Rev Esp Cardiol. 2006;59:905-910.
12. Metra M, Nodari S, Parrinello G, et al. Marked improvement in left ventricular ejection fraction during long-term beta blockade in patients with chronic heart failure: clinical correlates and prognostic significance. Am Heart J. 2003;145:292-299.
13. Swedberg K, Cleland J, Dargie H, et al. For the Task Force for the Diagnosis and Treatment of Chronic Heart Failurwe of the European Society of Cardiology. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005). Eur Heart J. 2005;26:1115-1140.
14. Malm S, Frigstad S, Sagberg E, et al. Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography. J Am Coll Cardiol. 2004;44:1030-1035.
15. McGowan JH, Cleland J. Reliability of reporting left ventricular systolic function by echocardiography: a systematic review of three methods. Am Heart J. 2003;146:388-397.
16. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography. J Am Soc Echocardiogr. 2005;18:1440-1463.
17. Moon J, Ko YG, Chung N, et al. Recovery and recurrence of left ventricular systolic dysfunction in patients with idiopathic dilated cardiomyopathy. Can J Cardiol. 2009;25:e147-e150.
18. Cioffi G, Stefenelli C, Tarantini L, et al. Chronic left ventricular failure in the community: prevalence, prognosis, and predictors of the complete clinical recovery with return of cardiac size and function to normal in patients undergoing optimal therapy. J Card Fail. 2004;10:250-257.
19. Di Lenarda A, Sabbadini G, Perkan A, et al. Apparent healing in dilated cardiomyopathy: incidence, long-term persistence and predictive factors. The heart muscle disease registry of Trieste [abstract]. Ital Heart J. 2001;2(suppl 2):S97.-