User login
A frail 75-year-old woman with diabetes, hyperlipidemia, and a history of rheumatic fever in childhood presents to the emergency department of a community hospital with complaints of chest pressure, shortness of breath on exertion, and easy fatigability. Her shortness of breath started 6 months ago but has become much worse over the past few days.
On examination, her pulse is 110 and irregular, and she has markedly distended neck veins and evidence of pulmonary edema. She has a systolic murmur, but it is difficult to characterize due to tachycardia. Electrocardiography shows atrial fibrillation with rapid ventricular response and right axis deviation. Chest radiography shows bilateral pleural effusions.
The patient is given a diuretic, anticoagulation is started to prevent thromboembolism, and she undergoes cardioversion for the atrial fibrillation.
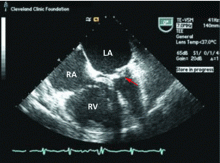
Transthoracic echocardiography is performed and reveals biatrial enlargement, anterior mitral valve leaflet thickening, mitral valve calcification, and moderate mitral regurgitation. Her ejection fraction is normal, but she has mild right ventricular systolic dysfunction with moderate tricuspid regurgitation and an estimated right ventricular systolic pressure of 90 mm Hg (normal range 15–30 mm Hg).
After an uneventful hospital course, she is discharged on a stable dose of a diuretic and oral anticoagulation. Despite adequate diuresis and maintenance of normal sinus rhythm, however, she continues to experience severe dyspnea, which limits her ability to perform simple tasks, such as dusting the furniture in her home. She is referred to Cleveland Clinic for further evaluation.
WHAT IS THE CAUSE OF HER SYMPTOMS?
1. What was the most likely cause of this patient’s initial acute presentation to the emergency department?
- An acute decrease in mitral valve area
- Rheumatic mitral valve stenosis
- Acute coronary syndrome
- Atrial fibrillation
Although an acute decrease in mitral valve area caused by an atrial myxoma, thrombus, or vegetation is a possibility, this patient’s symptoms gradually increased over a period of months. She also did not have signs of infection, nor did she have any signs of embolic phenomena to suggest myxoma, thrombus, or vegetation, all of which frequently present with emboli.
Given her history of rheumatic fever and her echocardiographic findings, rheumatic mitral valve stenosis is high on the list of differential diagnoses. Rheumatic mitral valve stenosis is a chronic process in which the valve area decreases at a rate of about 0.1 cm2/year.1,2
Acute coronary syndrome is a possibility in this elderly woman with multiple risk factors for coronary artery disease. An evaluation for coronary insufficiency should be considered, but the most striking finding on her initial electrocardiogram is atrial fibrillation with a rapid ventricular response.
The onset of atrial fibrillation in the setting of valvular heart disease is the most likely cause of her acute decompensation with signs and symptoms of congestive heart failure. In severe mitral stenosis, because the mitral valve orifice is narrowed, a higher left atrial pressure and longer ventricular filling time are required to maintain forward flow. Now add atrial fibrillation to this situation: the left atrium no longer contracts properly, so less blood is forced through the narrowed valve, and with the rapid heart rate the left ventricle has less time to fill. These two conditions result in elevated left atrial and pulmonary venous pressures, which in turn result in pulmonary edema and congestive heart failure. Thus, patients with mitral stenosis tolerate atrial fibrillation poorly.
WHAT IS THE NEXT STEP?
2. What would be the most appropriate next step for our patient?
- Transesophageal echocardiography
- Right heart catheterization
- An exercise treadmill stress test0
Transesophageal echocardiography is a reasonable option, as mitral stenosis is strongly suspected but transthoracic echocardiography did not reveal severe mitral valve disease. The use of transesophageal echocardiography for the diagnosis of mitral stenosis in this situation is a class IC recommendation (ie, the procedure is recommended, although very few trials have been done) in the American College of Cardiology and American Heart Association Valvular Disease guidelines.3
Right heart catheterization is the diagnostic test of choice in this situation, as the patient has evidence of biventricular heart failure and her right ventricular systolic pressure of 90 mm Hg is consistent with severe pulmonary hypertension. Right heart catheterization would help differentiate primary pulmonary hypertension causing dyspnea from pulmonary hypertension secondary to elevated left-sided pressures. It would also provide a direct hemodynamic estimate of her cardiac function.
Exercise treadmill testing. Evaluation for myocardial ischemia is a reasonable option, as our patient is elderly and has hypertension and diabetes, both of which are risk factors for coronary artery disease. Moreover, in a patient with diabetes, myocardial ischemia can present as dyspnea without typical anginal chest pain. Because of her age and severely limiting dyspnea, however, she would be unlikely to achieve an adequate heart rate during exercise treadmill testing, so this may not be the optimal type of stress test.
Although asymptomatic patients with moderate or severe mitral stenosis should undergo evaluation of exercise capacity and change in pulmonary artery pressures with exercise to determine the need for percutaneous balloon mitral valvuloplasty (see below),3 our patient is symptomatic and already has evidence of severe pulmonary hypertension on transthoracic echocardiography.
Other options for evaluating for myocardial ischemia include pharmacologic stress testing with imaging (eg, dobutamine echocardiography or adenosine nuclear imaging) or proceeding directly to coronary angiography.
CASE CONTINUED: RIGHT HEART CATHETERIZATION, CORONARY ANGIOGRAPHY
- Mean right atrial pressure 6 mm Hg (normal 2–7 mm Hg)
- Right ventricular pressure 102/6 mm Hg (consistent with severe pulmonary hypertension) (normal 15–30/1–7 mm Hg)
- Pulmonary artery pressure 102/40 mm Hg (normal 15–30/4–12 mm Hg)
- Mean pulmonary capillary wedge pressure 25 mm Hg (normal 4–12 mm Hg)
- Cardiac output 3.16 L/min (normal 4–8 L/min)
- Cardiac index 2.10 L/min/m2 (normal 2.5–4.2 L/min/m2)
- v waves are not prominent.
Because her symptoms raise concern for ischemia, coronary angiography is also performed and shows minimal, nonobstructive coronary artery disease. Her left ventricular end-diastolic pressure is 8 mm Hg (normal 5–12 mm Hg).
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis?
- Tricuspid stenosis
- Pulmonic stenosis
- Mitral stenosis
- Mitral regurgitation
Tricuspid stenosis would result in a higher pressure in the right atrium than in the right ventricle. In our patient, the right atrial pressure and the right ventricular diastolic pressure are both 6 mm Hg, eliminating this possibility.
Similarly, pulmonic stenosis would result in a higher pressure in the right ventricle than in the pulmonary artery. In our patient both the right ventricular systolic pressure and the pulmonary artery systolic pressures are 102 mm Hg.
Acute mitral regurgitation may result in increased wedge pressure and tall v waves (reflecting left atrial filling during ventricular systole). In chronic mitral regurgitation, however, the wedge pressure may be normal and the patient may have relatively normal-appearing v waves.4
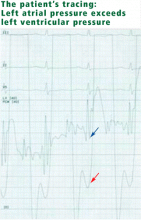
Mitral stenosis results in a marked gradient between the pulmonary capillary wedge pressure and the left ventricular diastolic pressure in the absence of pulmonary veno-occlusive disease. This gradient can be measured by simultaneous catheterization of the right heart (to measure the wedge pressure, which is an indirect measure of left atrial pressure) and the left heart (to measure the left ventricular diastolic pressure). If the patient does not have significant mitral stenosis, the wedge pressure should be approximately equal to the left ventricular diastolic pressure. In our patient, the wedge pressure (and therefore the left atrial pressure) is 25 mm Hg, and the left ventricular end-diastolic pressure is 8 mm Hg—a difference of 17 mm Hg, consistent with significant mitral stenosis.
CASE CONTINUED: TRANSESOPHAGEAL ECHOCARDIOGRAPHY
WHAT IS THE TREATMENT?
4. Which of the following is the preferred technique for correcting mitral stenosis in this patient?
- Percutaneous balloon mitral valvuloplasty
- Mitral valve surgery
- Percutaneous mitral valve replacement
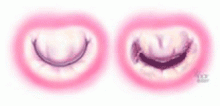
Although there are several options for mechanical treatment of mitral stenosis, percutaneous balloon mitral valvuloplasty by experienced operators is the procedure of choice for patients who have symptomatic moderate-to-severe mitral stenosis with favorable valve morphology but do not have significant mitral regurgitation or left atrial thrombus.3 The hemodynamic and symptomatic improvement that can be expected after this procedure can be predicted using several echocardiographic criteria, including valve mobility, subvalvular thickening, valve leaflet thickening, and valve leaflet calcification,5 as well as the degree of commissural calcification or commissural fusion.6 Success rates are better if the valve is relatively more mobile and has lesser degrees of valvular and subvalvular thickening, calcification, and commissural fusion.
Mitral valve surgery (repair if possible) is indicated in patients with acceptable operative risk who have symptomatic (New York Heart Association class III or IV) moderate-to-severe mitral stenosis if percutaneous balloon mitral valvuloplasty is unavailable, in cases in which an atrial thrombus or moderate-to-severe mitral regurgitation precludes balloon valvuloplasty, or when the valve morphology is not favorable for balloon valvulo-plasty.3
Although she has moderate mitral regurgitation and poor valve morphology, our patient is a poor surgical candidate because of her advanced age, severe pulmonary hypertension, and poor functional status. Patients with moderate-to-severe mitral stenosis and class III or IV symptoms who have nonpliable, calcified valves but are not candidates for open heart surgery have a class IIb indication for percutaneous balloon mitral valvuloplasty—ie, the procedure may be considered.3
In addition, the procedure also carries a class IIb recommendation in patients with moderate-to-severe mitral stenosis and new-onset atrial fibrillation (provided that they do not have a thrombus in the left atrium or moderate-to-severe mitral regurgitation), even without symptoms.3
Percutaneous mitral valve replacement is not available in clinical practice, although this is an active area of clinical research and may be available in the future.
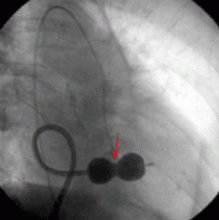
CASE CONTINUED: THE PATIENT UNDERGOES BALLOON VALVULOPLASTY
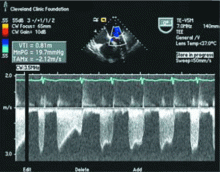
Transthoracic echocardiography performed 4 months later shows moderate mitral stenosis with a mean gradient of 9.0 mm Hg, a mitral valve area of 1.7 cm2, and moderate mitral regurgitation. Her right ventricular systolic pressure is estimated to be 74 mm Hg. The patient reports less dyspnea during her housework and now has New York Heart Association class II symptoms. Her treatment regimen includes warfarin (Coumadin) for atrial fibrillation, a beta-blocker to control her heart rate in atrial fibrillation and increase her left ventricular filling time, and a low-dose diuretic.
- Gordon SP, Douglas PS, Come PC, et al. Two-dimensional and Doppler echocardiographic determinants of the natural history of mitral valve narrowing in patients with rheumatic mitral stenosis: implications for follow-up. J Am Coll Cardiol 1992; 19:968–973.
- Sagie A, Freitas N, Padial LR, et al. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: valve area and right heart disease. J Am Coll Cardiol 1996; 28:472–479.
- Bonow ROC, Blase A, Chatterjee K, et al. ACC/AHA 2006 Practice Guidelines for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Developed in Collaboration With the Society of Cardiovascular Anesthesiologists Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:598–675.
- Braunwald E. The syndrome of severe mitral regurgitation with normal left atrial pressure. Circulation 1963; 27:29–35.
- Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 1988; 60:299–308.
- Cannan CR, Nishimura RA, Reeder GS, et al. Echocardiographic assessment of commissural calcium: a simple predictor of outcome after percutaneous mitral balloon valvotomy. J Am Coll Cardiol 1997; 29:175–180.
A frail 75-year-old woman with diabetes, hyperlipidemia, and a history of rheumatic fever in childhood presents to the emergency department of a community hospital with complaints of chest pressure, shortness of breath on exertion, and easy fatigability. Her shortness of breath started 6 months ago but has become much worse over the past few days.
On examination, her pulse is 110 and irregular, and she has markedly distended neck veins and evidence of pulmonary edema. She has a systolic murmur, but it is difficult to characterize due to tachycardia. Electrocardiography shows atrial fibrillation with rapid ventricular response and right axis deviation. Chest radiography shows bilateral pleural effusions.
The patient is given a diuretic, anticoagulation is started to prevent thromboembolism, and she undergoes cardioversion for the atrial fibrillation.
Transthoracic echocardiography is performed and reveals biatrial enlargement, anterior mitral valve leaflet thickening, mitral valve calcification, and moderate mitral regurgitation. Her ejection fraction is normal, but she has mild right ventricular systolic dysfunction with moderate tricuspid regurgitation and an estimated right ventricular systolic pressure of 90 mm Hg (normal range 15–30 mm Hg).
After an uneventful hospital course, she is discharged on a stable dose of a diuretic and oral anticoagulation. Despite adequate diuresis and maintenance of normal sinus rhythm, however, she continues to experience severe dyspnea, which limits her ability to perform simple tasks, such as dusting the furniture in her home. She is referred to Cleveland Clinic for further evaluation.
WHAT IS THE CAUSE OF HER SYMPTOMS?
1. What was the most likely cause of this patient’s initial acute presentation to the emergency department?
- An acute decrease in mitral valve area
- Rheumatic mitral valve stenosis
- Acute coronary syndrome
- Atrial fibrillation
Although an acute decrease in mitral valve area caused by an atrial myxoma, thrombus, or vegetation is a possibility, this patient’s symptoms gradually increased over a period of months. She also did not have signs of infection, nor did she have any signs of embolic phenomena to suggest myxoma, thrombus, or vegetation, all of which frequently present with emboli.
Given her history of rheumatic fever and her echocardiographic findings, rheumatic mitral valve stenosis is high on the list of differential diagnoses. Rheumatic mitral valve stenosis is a chronic process in which the valve area decreases at a rate of about 0.1 cm2/year.1,2
Acute coronary syndrome is a possibility in this elderly woman with multiple risk factors for coronary artery disease. An evaluation for coronary insufficiency should be considered, but the most striking finding on her initial electrocardiogram is atrial fibrillation with a rapid ventricular response.
The onset of atrial fibrillation in the setting of valvular heart disease is the most likely cause of her acute decompensation with signs and symptoms of congestive heart failure. In severe mitral stenosis, because the mitral valve orifice is narrowed, a higher left atrial pressure and longer ventricular filling time are required to maintain forward flow. Now add atrial fibrillation to this situation: the left atrium no longer contracts properly, so less blood is forced through the narrowed valve, and with the rapid heart rate the left ventricle has less time to fill. These two conditions result in elevated left atrial and pulmonary venous pressures, which in turn result in pulmonary edema and congestive heart failure. Thus, patients with mitral stenosis tolerate atrial fibrillation poorly.
WHAT IS THE NEXT STEP?
2. What would be the most appropriate next step for our patient?
- Transesophageal echocardiography
- Right heart catheterization
- An exercise treadmill stress test0
Transesophageal echocardiography is a reasonable option, as mitral stenosis is strongly suspected but transthoracic echocardiography did not reveal severe mitral valve disease. The use of transesophageal echocardiography for the diagnosis of mitral stenosis in this situation is a class IC recommendation (ie, the procedure is recommended, although very few trials have been done) in the American College of Cardiology and American Heart Association Valvular Disease guidelines.3
Right heart catheterization is the diagnostic test of choice in this situation, as the patient has evidence of biventricular heart failure and her right ventricular systolic pressure of 90 mm Hg is consistent with severe pulmonary hypertension. Right heart catheterization would help differentiate primary pulmonary hypertension causing dyspnea from pulmonary hypertension secondary to elevated left-sided pressures. It would also provide a direct hemodynamic estimate of her cardiac function.
Exercise treadmill testing. Evaluation for myocardial ischemia is a reasonable option, as our patient is elderly and has hypertension and diabetes, both of which are risk factors for coronary artery disease. Moreover, in a patient with diabetes, myocardial ischemia can present as dyspnea without typical anginal chest pain. Because of her age and severely limiting dyspnea, however, she would be unlikely to achieve an adequate heart rate during exercise treadmill testing, so this may not be the optimal type of stress test.
Although asymptomatic patients with moderate or severe mitral stenosis should undergo evaluation of exercise capacity and change in pulmonary artery pressures with exercise to determine the need for percutaneous balloon mitral valvuloplasty (see below),3 our patient is symptomatic and already has evidence of severe pulmonary hypertension on transthoracic echocardiography.
Other options for evaluating for myocardial ischemia include pharmacologic stress testing with imaging (eg, dobutamine echocardiography or adenosine nuclear imaging) or proceeding directly to coronary angiography.
CASE CONTINUED: RIGHT HEART CATHETERIZATION, CORONARY ANGIOGRAPHY
- Mean right atrial pressure 6 mm Hg (normal 2–7 mm Hg)
- Right ventricular pressure 102/6 mm Hg (consistent with severe pulmonary hypertension) (normal 15–30/1–7 mm Hg)
- Pulmonary artery pressure 102/40 mm Hg (normal 15–30/4–12 mm Hg)
- Mean pulmonary capillary wedge pressure 25 mm Hg (normal 4–12 mm Hg)
- Cardiac output 3.16 L/min (normal 4–8 L/min)
- Cardiac index 2.10 L/min/m2 (normal 2.5–4.2 L/min/m2)
- v waves are not prominent.
Because her symptoms raise concern for ischemia, coronary angiography is also performed and shows minimal, nonobstructive coronary artery disease. Her left ventricular end-diastolic pressure is 8 mm Hg (normal 5–12 mm Hg).
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis?
- Tricuspid stenosis
- Pulmonic stenosis
- Mitral stenosis
- Mitral regurgitation
Tricuspid stenosis would result in a higher pressure in the right atrium than in the right ventricle. In our patient, the right atrial pressure and the right ventricular diastolic pressure are both 6 mm Hg, eliminating this possibility.
Similarly, pulmonic stenosis would result in a higher pressure in the right ventricle than in the pulmonary artery. In our patient both the right ventricular systolic pressure and the pulmonary artery systolic pressures are 102 mm Hg.
Acute mitral regurgitation may result in increased wedge pressure and tall v waves (reflecting left atrial filling during ventricular systole). In chronic mitral regurgitation, however, the wedge pressure may be normal and the patient may have relatively normal-appearing v waves.4
Mitral stenosis results in a marked gradient between the pulmonary capillary wedge pressure and the left ventricular diastolic pressure in the absence of pulmonary veno-occlusive disease. This gradient can be measured by simultaneous catheterization of the right heart (to measure the wedge pressure, which is an indirect measure of left atrial pressure) and the left heart (to measure the left ventricular diastolic pressure). If the patient does not have significant mitral stenosis, the wedge pressure should be approximately equal to the left ventricular diastolic pressure. In our patient, the wedge pressure (and therefore the left atrial pressure) is 25 mm Hg, and the left ventricular end-diastolic pressure is 8 mm Hg—a difference of 17 mm Hg, consistent with significant mitral stenosis.
CASE CONTINUED: TRANSESOPHAGEAL ECHOCARDIOGRAPHY
WHAT IS THE TREATMENT?
4. Which of the following is the preferred technique for correcting mitral stenosis in this patient?
- Percutaneous balloon mitral valvuloplasty
- Mitral valve surgery
- Percutaneous mitral valve replacement
Although there are several options for mechanical treatment of mitral stenosis, percutaneous balloon mitral valvuloplasty by experienced operators is the procedure of choice for patients who have symptomatic moderate-to-severe mitral stenosis with favorable valve morphology but do not have significant mitral regurgitation or left atrial thrombus.3 The hemodynamic and symptomatic improvement that can be expected after this procedure can be predicted using several echocardiographic criteria, including valve mobility, subvalvular thickening, valve leaflet thickening, and valve leaflet calcification,5 as well as the degree of commissural calcification or commissural fusion.6 Success rates are better if the valve is relatively more mobile and has lesser degrees of valvular and subvalvular thickening, calcification, and commissural fusion.
Mitral valve surgery (repair if possible) is indicated in patients with acceptable operative risk who have symptomatic (New York Heart Association class III or IV) moderate-to-severe mitral stenosis if percutaneous balloon mitral valvuloplasty is unavailable, in cases in which an atrial thrombus or moderate-to-severe mitral regurgitation precludes balloon valvuloplasty, or when the valve morphology is not favorable for balloon valvulo-plasty.3
Although she has moderate mitral regurgitation and poor valve morphology, our patient is a poor surgical candidate because of her advanced age, severe pulmonary hypertension, and poor functional status. Patients with moderate-to-severe mitral stenosis and class III or IV symptoms who have nonpliable, calcified valves but are not candidates for open heart surgery have a class IIb indication for percutaneous balloon mitral valvuloplasty—ie, the procedure may be considered.3
In addition, the procedure also carries a class IIb recommendation in patients with moderate-to-severe mitral stenosis and new-onset atrial fibrillation (provided that they do not have a thrombus in the left atrium or moderate-to-severe mitral regurgitation), even without symptoms.3
Percutaneous mitral valve replacement is not available in clinical practice, although this is an active area of clinical research and may be available in the future.
CASE CONTINUED: THE PATIENT UNDERGOES BALLOON VALVULOPLASTY
Transthoracic echocardiography performed 4 months later shows moderate mitral stenosis with a mean gradient of 9.0 mm Hg, a mitral valve area of 1.7 cm2, and moderate mitral regurgitation. Her right ventricular systolic pressure is estimated to be 74 mm Hg. The patient reports less dyspnea during her housework and now has New York Heart Association class II symptoms. Her treatment regimen includes warfarin (Coumadin) for atrial fibrillation, a beta-blocker to control her heart rate in atrial fibrillation and increase her left ventricular filling time, and a low-dose diuretic.
A frail 75-year-old woman with diabetes, hyperlipidemia, and a history of rheumatic fever in childhood presents to the emergency department of a community hospital with complaints of chest pressure, shortness of breath on exertion, and easy fatigability. Her shortness of breath started 6 months ago but has become much worse over the past few days.
On examination, her pulse is 110 and irregular, and she has markedly distended neck veins and evidence of pulmonary edema. She has a systolic murmur, but it is difficult to characterize due to tachycardia. Electrocardiography shows atrial fibrillation with rapid ventricular response and right axis deviation. Chest radiography shows bilateral pleural effusions.
The patient is given a diuretic, anticoagulation is started to prevent thromboembolism, and she undergoes cardioversion for the atrial fibrillation.
Transthoracic echocardiography is performed and reveals biatrial enlargement, anterior mitral valve leaflet thickening, mitral valve calcification, and moderate mitral regurgitation. Her ejection fraction is normal, but she has mild right ventricular systolic dysfunction with moderate tricuspid regurgitation and an estimated right ventricular systolic pressure of 90 mm Hg (normal range 15–30 mm Hg).
After an uneventful hospital course, she is discharged on a stable dose of a diuretic and oral anticoagulation. Despite adequate diuresis and maintenance of normal sinus rhythm, however, she continues to experience severe dyspnea, which limits her ability to perform simple tasks, such as dusting the furniture in her home. She is referred to Cleveland Clinic for further evaluation.
WHAT IS THE CAUSE OF HER SYMPTOMS?
1. What was the most likely cause of this patient’s initial acute presentation to the emergency department?
- An acute decrease in mitral valve area
- Rheumatic mitral valve stenosis
- Acute coronary syndrome
- Atrial fibrillation
Although an acute decrease in mitral valve area caused by an atrial myxoma, thrombus, or vegetation is a possibility, this patient’s symptoms gradually increased over a period of months. She also did not have signs of infection, nor did she have any signs of embolic phenomena to suggest myxoma, thrombus, or vegetation, all of which frequently present with emboli.
Given her history of rheumatic fever and her echocardiographic findings, rheumatic mitral valve stenosis is high on the list of differential diagnoses. Rheumatic mitral valve stenosis is a chronic process in which the valve area decreases at a rate of about 0.1 cm2/year.1,2
Acute coronary syndrome is a possibility in this elderly woman with multiple risk factors for coronary artery disease. An evaluation for coronary insufficiency should be considered, but the most striking finding on her initial electrocardiogram is atrial fibrillation with a rapid ventricular response.
The onset of atrial fibrillation in the setting of valvular heart disease is the most likely cause of her acute decompensation with signs and symptoms of congestive heart failure. In severe mitral stenosis, because the mitral valve orifice is narrowed, a higher left atrial pressure and longer ventricular filling time are required to maintain forward flow. Now add atrial fibrillation to this situation: the left atrium no longer contracts properly, so less blood is forced through the narrowed valve, and with the rapid heart rate the left ventricle has less time to fill. These two conditions result in elevated left atrial and pulmonary venous pressures, which in turn result in pulmonary edema and congestive heart failure. Thus, patients with mitral stenosis tolerate atrial fibrillation poorly.
WHAT IS THE NEXT STEP?
2. What would be the most appropriate next step for our patient?
- Transesophageal echocardiography
- Right heart catheterization
- An exercise treadmill stress test0
Transesophageal echocardiography is a reasonable option, as mitral stenosis is strongly suspected but transthoracic echocardiography did not reveal severe mitral valve disease. The use of transesophageal echocardiography for the diagnosis of mitral stenosis in this situation is a class IC recommendation (ie, the procedure is recommended, although very few trials have been done) in the American College of Cardiology and American Heart Association Valvular Disease guidelines.3
Right heart catheterization is the diagnostic test of choice in this situation, as the patient has evidence of biventricular heart failure and her right ventricular systolic pressure of 90 mm Hg is consistent with severe pulmonary hypertension. Right heart catheterization would help differentiate primary pulmonary hypertension causing dyspnea from pulmonary hypertension secondary to elevated left-sided pressures. It would also provide a direct hemodynamic estimate of her cardiac function.
Exercise treadmill testing. Evaluation for myocardial ischemia is a reasonable option, as our patient is elderly and has hypertension and diabetes, both of which are risk factors for coronary artery disease. Moreover, in a patient with diabetes, myocardial ischemia can present as dyspnea without typical anginal chest pain. Because of her age and severely limiting dyspnea, however, she would be unlikely to achieve an adequate heart rate during exercise treadmill testing, so this may not be the optimal type of stress test.
Although asymptomatic patients with moderate or severe mitral stenosis should undergo evaluation of exercise capacity and change in pulmonary artery pressures with exercise to determine the need for percutaneous balloon mitral valvuloplasty (see below),3 our patient is symptomatic and already has evidence of severe pulmonary hypertension on transthoracic echocardiography.
Other options for evaluating for myocardial ischemia include pharmacologic stress testing with imaging (eg, dobutamine echocardiography or adenosine nuclear imaging) or proceeding directly to coronary angiography.
CASE CONTINUED: RIGHT HEART CATHETERIZATION, CORONARY ANGIOGRAPHY
- Mean right atrial pressure 6 mm Hg (normal 2–7 mm Hg)
- Right ventricular pressure 102/6 mm Hg (consistent with severe pulmonary hypertension) (normal 15–30/1–7 mm Hg)
- Pulmonary artery pressure 102/40 mm Hg (normal 15–30/4–12 mm Hg)
- Mean pulmonary capillary wedge pressure 25 mm Hg (normal 4–12 mm Hg)
- Cardiac output 3.16 L/min (normal 4–8 L/min)
- Cardiac index 2.10 L/min/m2 (normal 2.5–4.2 L/min/m2)
- v waves are not prominent.
Because her symptoms raise concern for ischemia, coronary angiography is also performed and shows minimal, nonobstructive coronary artery disease. Her left ventricular end-diastolic pressure is 8 mm Hg (normal 5–12 mm Hg).
WHAT IS THE DIAGNOSIS?
3. What is the most likely diagnosis?
- Tricuspid stenosis
- Pulmonic stenosis
- Mitral stenosis
- Mitral regurgitation
Tricuspid stenosis would result in a higher pressure in the right atrium than in the right ventricle. In our patient, the right atrial pressure and the right ventricular diastolic pressure are both 6 mm Hg, eliminating this possibility.
Similarly, pulmonic stenosis would result in a higher pressure in the right ventricle than in the pulmonary artery. In our patient both the right ventricular systolic pressure and the pulmonary artery systolic pressures are 102 mm Hg.
Acute mitral regurgitation may result in increased wedge pressure and tall v waves (reflecting left atrial filling during ventricular systole). In chronic mitral regurgitation, however, the wedge pressure may be normal and the patient may have relatively normal-appearing v waves.4
Mitral stenosis results in a marked gradient between the pulmonary capillary wedge pressure and the left ventricular diastolic pressure in the absence of pulmonary veno-occlusive disease. This gradient can be measured by simultaneous catheterization of the right heart (to measure the wedge pressure, which is an indirect measure of left atrial pressure) and the left heart (to measure the left ventricular diastolic pressure). If the patient does not have significant mitral stenosis, the wedge pressure should be approximately equal to the left ventricular diastolic pressure. In our patient, the wedge pressure (and therefore the left atrial pressure) is 25 mm Hg, and the left ventricular end-diastolic pressure is 8 mm Hg—a difference of 17 mm Hg, consistent with significant mitral stenosis.
CASE CONTINUED: TRANSESOPHAGEAL ECHOCARDIOGRAPHY
WHAT IS THE TREATMENT?
4. Which of the following is the preferred technique for correcting mitral stenosis in this patient?
- Percutaneous balloon mitral valvuloplasty
- Mitral valve surgery
- Percutaneous mitral valve replacement
Although there are several options for mechanical treatment of mitral stenosis, percutaneous balloon mitral valvuloplasty by experienced operators is the procedure of choice for patients who have symptomatic moderate-to-severe mitral stenosis with favorable valve morphology but do not have significant mitral regurgitation or left atrial thrombus.3 The hemodynamic and symptomatic improvement that can be expected after this procedure can be predicted using several echocardiographic criteria, including valve mobility, subvalvular thickening, valve leaflet thickening, and valve leaflet calcification,5 as well as the degree of commissural calcification or commissural fusion.6 Success rates are better if the valve is relatively more mobile and has lesser degrees of valvular and subvalvular thickening, calcification, and commissural fusion.
Mitral valve surgery (repair if possible) is indicated in patients with acceptable operative risk who have symptomatic (New York Heart Association class III or IV) moderate-to-severe mitral stenosis if percutaneous balloon mitral valvuloplasty is unavailable, in cases in which an atrial thrombus or moderate-to-severe mitral regurgitation precludes balloon valvuloplasty, or when the valve morphology is not favorable for balloon valvulo-plasty.3
Although she has moderate mitral regurgitation and poor valve morphology, our patient is a poor surgical candidate because of her advanced age, severe pulmonary hypertension, and poor functional status. Patients with moderate-to-severe mitral stenosis and class III or IV symptoms who have nonpliable, calcified valves but are not candidates for open heart surgery have a class IIb indication for percutaneous balloon mitral valvuloplasty—ie, the procedure may be considered.3
In addition, the procedure also carries a class IIb recommendation in patients with moderate-to-severe mitral stenosis and new-onset atrial fibrillation (provided that they do not have a thrombus in the left atrium or moderate-to-severe mitral regurgitation), even without symptoms.3
Percutaneous mitral valve replacement is not available in clinical practice, although this is an active area of clinical research and may be available in the future.
CASE CONTINUED: THE PATIENT UNDERGOES BALLOON VALVULOPLASTY
Transthoracic echocardiography performed 4 months later shows moderate mitral stenosis with a mean gradient of 9.0 mm Hg, a mitral valve area of 1.7 cm2, and moderate mitral regurgitation. Her right ventricular systolic pressure is estimated to be 74 mm Hg. The patient reports less dyspnea during her housework and now has New York Heart Association class II symptoms. Her treatment regimen includes warfarin (Coumadin) for atrial fibrillation, a beta-blocker to control her heart rate in atrial fibrillation and increase her left ventricular filling time, and a low-dose diuretic.
- Gordon SP, Douglas PS, Come PC, et al. Two-dimensional and Doppler echocardiographic determinants of the natural history of mitral valve narrowing in patients with rheumatic mitral stenosis: implications for follow-up. J Am Coll Cardiol 1992; 19:968–973.
- Sagie A, Freitas N, Padial LR, et al. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: valve area and right heart disease. J Am Coll Cardiol 1996; 28:472–479.
- Bonow ROC, Blase A, Chatterjee K, et al. ACC/AHA 2006 Practice Guidelines for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Developed in Collaboration With the Society of Cardiovascular Anesthesiologists Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:598–675.
- Braunwald E. The syndrome of severe mitral regurgitation with normal left atrial pressure. Circulation 1963; 27:29–35.
- Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 1988; 60:299–308.
- Cannan CR, Nishimura RA, Reeder GS, et al. Echocardiographic assessment of commissural calcium: a simple predictor of outcome after percutaneous mitral balloon valvotomy. J Am Coll Cardiol 1997; 29:175–180.
- Gordon SP, Douglas PS, Come PC, et al. Two-dimensional and Doppler echocardiographic determinants of the natural history of mitral valve narrowing in patients with rheumatic mitral stenosis: implications for follow-up. J Am Coll Cardiol 1992; 19:968–973.
- Sagie A, Freitas N, Padial LR, et al. Doppler echocardiographic assessment of long-term progression of mitral stenosis in 103 patients: valve area and right heart disease. J Am Coll Cardiol 1996; 28:472–479.
- Bonow ROC, Blase A, Chatterjee K, et al. ACC/AHA 2006 Practice Guidelines for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease) Developed in Collaboration With the Society of Cardiovascular Anesthesiologists Endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006; 48:598–675.
- Braunwald E. The syndrome of severe mitral regurgitation with normal left atrial pressure. Circulation 1963; 27:29–35.
- Wilkins GT, Weyman AE, Abascal VM, et al. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 1988; 60:299–308.
- Cannan CR, Nishimura RA, Reeder GS, et al. Echocardiographic assessment of commissural calcium: a simple predictor of outcome after percutaneous mitral balloon valvotomy. J Am Coll Cardiol 1997; 29:175–180.