User login
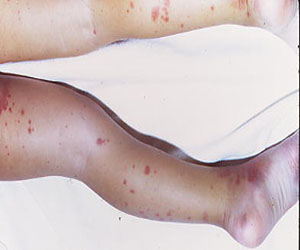
Treatment with the thrombopoietin receptor agonist eltrombopag led to a sustained platelet response in 40% of children and adolescents with chronic immune thrombocytopenia, compared with only 3% of the placebo group, according to a randomized multicenter trial published online in The Lancet.
Eltrombopag is approved in the United States for adults with chronic immune thrombocytopenia (CIT) who have not responded adequately to corticosteroids, immunoglobulins, or splenectomy, but few trials have assessed CIT therapies in children, said Dr. John Grainger of the Royal Manchester Children’s Hospital and the University of Manchester (England) and his associates.
Their multicenter, international study included 92 patients up to age 17 with CIT. During the 13-week double-blinded period of the study, patients received once-daily placebo or eltrombopag dosed at 0.89-1.2 mg/kg for patients aged 1-5 years and at 25-50 mg for patients aged 6-17 years. Dosing ranges were adjusted for ethnicity as well as body weight because east Asians have higher eltrombopag exposures and need lower starting doses, the investigators noted. After the double-blinded period, all patients entered 24 weeks of open-label treatment with eltrombopag (Lancet. 2015 Jul 29. doi: 10.1016/S0140-6736(15)61107-2.).
A total of 25 (40%) patients who received eltrombopag achieved platelet counts of at least 50 × 10⁹ per L for at least 6 of the last 8 weeks of the double-blinded period, compared with only one patient (3%) on placebo (odds ratio, 18; 95% confidence interval, 2.3-140.9; P = .0004), said the researchers. Based on the World Health Organization bleeding scale, the percentage of patients who experienced grade 1-4 bleeding events fell from 63% at the start of the open-label period to 24% at the end, and clinically significant (grade 2-4) bleeding events dropped from 20% to 6%. Seven of 87 patients were able to stop all other drugs they were taking for CIT without needing rescue therapy during open-label treatment, the researchers said.
Two patients stopped eltrombopag because of elevated liver aminotransferases. Rates of nasopharyngitis, rhinitis, upper respiratory tract infection, and cough were more common for eltrombopag than placebo, the investigators reported. However, serious adverse events were more common with placebo (14% vs. 8%), and there were no deaths, thrombotic events, or malignancies. Safety trends were similar during the double-blinded and open-label study periods, they added.
GlaxoSmithKline funded the study. Dr. Grainger reported receiving honoraria from GlaxoSmithKline, Amgen, and Baxter. Eleven coauthors reported financial conflicts of interest with GlaxoSmithKline and a number of other pharmaceutical companies.
Treatment with the thrombopoietin receptor agonist eltrombopag led to a sustained platelet response in 40% of children and adolescents with chronic immune thrombocytopenia, compared with only 3% of the placebo group, according to a randomized multicenter trial published online in The Lancet.
Eltrombopag is approved in the United States for adults with chronic immune thrombocytopenia (CIT) who have not responded adequately to corticosteroids, immunoglobulins, or splenectomy, but few trials have assessed CIT therapies in children, said Dr. John Grainger of the Royal Manchester Children’s Hospital and the University of Manchester (England) and his associates.
Their multicenter, international study included 92 patients up to age 17 with CIT. During the 13-week double-blinded period of the study, patients received once-daily placebo or eltrombopag dosed at 0.89-1.2 mg/kg for patients aged 1-5 years and at 25-50 mg for patients aged 6-17 years. Dosing ranges were adjusted for ethnicity as well as body weight because east Asians have higher eltrombopag exposures and need lower starting doses, the investigators noted. After the double-blinded period, all patients entered 24 weeks of open-label treatment with eltrombopag (Lancet. 2015 Jul 29. doi: 10.1016/S0140-6736(15)61107-2.).
A total of 25 (40%) patients who received eltrombopag achieved platelet counts of at least 50 × 10⁹ per L for at least 6 of the last 8 weeks of the double-blinded period, compared with only one patient (3%) on placebo (odds ratio, 18; 95% confidence interval, 2.3-140.9; P = .0004), said the researchers. Based on the World Health Organization bleeding scale, the percentage of patients who experienced grade 1-4 bleeding events fell from 63% at the start of the open-label period to 24% at the end, and clinically significant (grade 2-4) bleeding events dropped from 20% to 6%. Seven of 87 patients were able to stop all other drugs they were taking for CIT without needing rescue therapy during open-label treatment, the researchers said.
Two patients stopped eltrombopag because of elevated liver aminotransferases. Rates of nasopharyngitis, rhinitis, upper respiratory tract infection, and cough were more common for eltrombopag than placebo, the investigators reported. However, serious adverse events were more common with placebo (14% vs. 8%), and there were no deaths, thrombotic events, or malignancies. Safety trends were similar during the double-blinded and open-label study periods, they added.
GlaxoSmithKline funded the study. Dr. Grainger reported receiving honoraria from GlaxoSmithKline, Amgen, and Baxter. Eleven coauthors reported financial conflicts of interest with GlaxoSmithKline and a number of other pharmaceutical companies.
Treatment with the thrombopoietin receptor agonist eltrombopag led to a sustained platelet response in 40% of children and adolescents with chronic immune thrombocytopenia, compared with only 3% of the placebo group, according to a randomized multicenter trial published online in The Lancet.
Eltrombopag is approved in the United States for adults with chronic immune thrombocytopenia (CIT) who have not responded adequately to corticosteroids, immunoglobulins, or splenectomy, but few trials have assessed CIT therapies in children, said Dr. John Grainger of the Royal Manchester Children’s Hospital and the University of Manchester (England) and his associates.
Their multicenter, international study included 92 patients up to age 17 with CIT. During the 13-week double-blinded period of the study, patients received once-daily placebo or eltrombopag dosed at 0.89-1.2 mg/kg for patients aged 1-5 years and at 25-50 mg for patients aged 6-17 years. Dosing ranges were adjusted for ethnicity as well as body weight because east Asians have higher eltrombopag exposures and need lower starting doses, the investigators noted. After the double-blinded period, all patients entered 24 weeks of open-label treatment with eltrombopag (Lancet. 2015 Jul 29. doi: 10.1016/S0140-6736(15)61107-2.).
A total of 25 (40%) patients who received eltrombopag achieved platelet counts of at least 50 × 10⁹ per L for at least 6 of the last 8 weeks of the double-blinded period, compared with only one patient (3%) on placebo (odds ratio, 18; 95% confidence interval, 2.3-140.9; P = .0004), said the researchers. Based on the World Health Organization bleeding scale, the percentage of patients who experienced grade 1-4 bleeding events fell from 63% at the start of the open-label period to 24% at the end, and clinically significant (grade 2-4) bleeding events dropped from 20% to 6%. Seven of 87 patients were able to stop all other drugs they were taking for CIT without needing rescue therapy during open-label treatment, the researchers said.
Two patients stopped eltrombopag because of elevated liver aminotransferases. Rates of nasopharyngitis, rhinitis, upper respiratory tract infection, and cough were more common for eltrombopag than placebo, the investigators reported. However, serious adverse events were more common with placebo (14% vs. 8%), and there were no deaths, thrombotic events, or malignancies. Safety trends were similar during the double-blinded and open-label study periods, they added.
GlaxoSmithKline funded the study. Dr. Grainger reported receiving honoraria from GlaxoSmithKline, Amgen, and Baxter. Eleven coauthors reported financial conflicts of interest with GlaxoSmithKline and a number of other pharmaceutical companies.
FROM THE LANCET
Key clinical point: Eltrombopag markedly outperformed placebo and had no new safety signals in children and adolescents with chronic immune thrombocytopenia.
Major finding: Forty percent of patients achieved sustained platelet response on eltrombopag, compared with 3% of the placebo group (OR, 18.0; P = .0004).
Data source: Randomized, double-blinded, multicenter, international trial of 92 patients aged 1-17 years.
Disclosures: GlaxoSmithKline funded the study. Dr. Grainger reported receiving honoraria from GlaxoSmithKline, Amgen, and Baxter. Eleven coauthors reported financial conflicts of interest with GlaxoSmithKline and a number of other pharmaceutical companies.