User login
NEW ORLEANS – When used as an add-on therapy to metformin in patients with type 2 diabetes mellitus, empagliflozin has sustained safety and efficacy in reducing in hemoglobin A1c (HbA1c) and other key metabolic measures for up to 4 years, according to results of the EMPA-REG H2H-SU trial presented at the annual scientific sessions of the American Diabetes Association.
The latest results of the double-blind, phase III trial extended out to 4 years the previously published 2-year results (Lancet Diabetes Endocrinol. 2014;2:691-700) in comparing empagliflozin, a sodium glucose cotransporter inhibitor, 25 mg daily, and the sulfonylurea glimepiride, 1-4 mg daily, as add-on therapy to metformin. “As previously reported, after 2 years there was a modest difference in HbA1c between empagliflozin and glimepiride,” said Dr. Afshin Salsali, reporting for the trial group. “This difference continued for the remainder of the study, although there was a slight rebound in each group,” according to Dr. Salsali, executive director of global clinical development at Boehringer Ingelheim, Ridgefield, Conn.
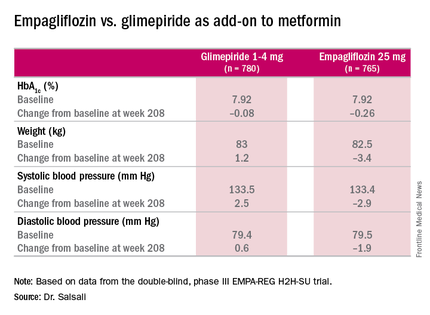
The 4-year results involved more than 73% of the 1,545 patients who participated in the 2-year study, Dr. Salsali said. The majority of patients were white, with average HbA1c levels of 7.92% and weight of 82.5 kg at the outset of the 2-year study. After 4 years, average HbA1c levels declined 0.08% in those on glimepiride vs. 0.26% for empagliflozin, Dr. Salsali said, achieving the primary study endpoint of noninferiority to glimepiride. However, he noted that rates of hypoglycemia varied dramatically between the two therapies. “This was achieved at the rate of much lower hypoglycemia on empagliflozin, compared with glimepiride, 3% vs. 25% (P less than .001),” he said.
Dr. Salsali pointed out that patients in the empagliflozin group were less likely to need rescue therapy, with an odds ratio of 0.56 (P less than.001), and a much later need for intervention. The advantage empagliflozin showed in both weight loss and blood pressure reduction in the 2-year study also held up for 4 years, Dr. Salsali said. “The pattern of blood pressure reduction achieved at initiation in the first few weeks after using empagliflozin more or less remained at the same level throughout this study,” he said.
One of most important messages from the study, Dr. Salsali said, is the impact empagliflozin had on the estimated glomerular filtration rate (eGFR). “Previously in the pivotal diabetes trial that we had, we showed a reduction in eGFR with a slight rebound, but we didn’t have enough time to see the full picture,” he said. “Now we have the luxury of looking into the full eGFR change over time up to 4 years. After the initial reduction in eGFR, there is a gradual increase and return of eGFR to the baseline level which remained stable for the duration of this study.” However, the glimepiride arm showed the “expected” average rate of eGFR reduction of 2 mL/min per year for type 2 diabetes, he said.
The reporting of adverse events was about the same between both groups, but serious adverse events were slightly higher among those on empagliflozin: 7.36/100 patient years vs. 7.06/100 patient years on glimepiride. The former had higher rates of urinary tract infections (6.96 vs. 5.82 per 100 patient-years) and volume depletion (0.82 vs. 0.63 per 100 patient years), but rates of bone fractures were almost identical (4.1% vs. 4.2%). There was no reported diabetic ketoacidosis in either group.
“Empagliflozin 25 mg after 208 weeks of treatment as an add-on to metformin led to modest numerical advantage in mean HbA1c change from baseline and clinically relevant reduction in weight, systolic blood pressure, and diastolic blood pressure,” Dr. Salsali said. “The difference in changes in HbA1c between empagliflozin and glimepiride were small, but empagliflozin was associated with a significant lower risk of hypoglycemia and significantly fewer patients required rescue therapy.”
Besides Dr. Salsali’s disclosure, coauthors disclosed relationships with Novo Nordisk, Medtronic, and the Steno Diabetes Center. Three other coauthors were employees of Boehringer Ingelheim.
NEW ORLEANS – When used as an add-on therapy to metformin in patients with type 2 diabetes mellitus, empagliflozin has sustained safety and efficacy in reducing in hemoglobin A1c (HbA1c) and other key metabolic measures for up to 4 years, according to results of the EMPA-REG H2H-SU trial presented at the annual scientific sessions of the American Diabetes Association.
The latest results of the double-blind, phase III trial extended out to 4 years the previously published 2-year results (Lancet Diabetes Endocrinol. 2014;2:691-700) in comparing empagliflozin, a sodium glucose cotransporter inhibitor, 25 mg daily, and the sulfonylurea glimepiride, 1-4 mg daily, as add-on therapy to metformin. “As previously reported, after 2 years there was a modest difference in HbA1c between empagliflozin and glimepiride,” said Dr. Afshin Salsali, reporting for the trial group. “This difference continued for the remainder of the study, although there was a slight rebound in each group,” according to Dr. Salsali, executive director of global clinical development at Boehringer Ingelheim, Ridgefield, Conn.
The 4-year results involved more than 73% of the 1,545 patients who participated in the 2-year study, Dr. Salsali said. The majority of patients were white, with average HbA1c levels of 7.92% and weight of 82.5 kg at the outset of the 2-year study. After 4 years, average HbA1c levels declined 0.08% in those on glimepiride vs. 0.26% for empagliflozin, Dr. Salsali said, achieving the primary study endpoint of noninferiority to glimepiride. However, he noted that rates of hypoglycemia varied dramatically between the two therapies. “This was achieved at the rate of much lower hypoglycemia on empagliflozin, compared with glimepiride, 3% vs. 25% (P less than .001),” he said.
Dr. Salsali pointed out that patients in the empagliflozin group were less likely to need rescue therapy, with an odds ratio of 0.56 (P less than.001), and a much later need for intervention. The advantage empagliflozin showed in both weight loss and blood pressure reduction in the 2-year study also held up for 4 years, Dr. Salsali said. “The pattern of blood pressure reduction achieved at initiation in the first few weeks after using empagliflozin more or less remained at the same level throughout this study,” he said.

One of most important messages from the study, Dr. Salsali said, is the impact empagliflozin had on the estimated glomerular filtration rate (eGFR). “Previously in the pivotal diabetes trial that we had, we showed a reduction in eGFR with a slight rebound, but we didn’t have enough time to see the full picture,” he said. “Now we have the luxury of looking into the full eGFR change over time up to 4 years. After the initial reduction in eGFR, there is a gradual increase and return of eGFR to the baseline level which remained stable for the duration of this study.” However, the glimepiride arm showed the “expected” average rate of eGFR reduction of 2 mL/min per year for type 2 diabetes, he said.
The reporting of adverse events was about the same between both groups, but serious adverse events were slightly higher among those on empagliflozin: 7.36/100 patient years vs. 7.06/100 patient years on glimepiride. The former had higher rates of urinary tract infections (6.96 vs. 5.82 per 100 patient-years) and volume depletion (0.82 vs. 0.63 per 100 patient years), but rates of bone fractures were almost identical (4.1% vs. 4.2%). There was no reported diabetic ketoacidosis in either group.
“Empagliflozin 25 mg after 208 weeks of treatment as an add-on to metformin led to modest numerical advantage in mean HbA1c change from baseline and clinically relevant reduction in weight, systolic blood pressure, and diastolic blood pressure,” Dr. Salsali said. “The difference in changes in HbA1c between empagliflozin and glimepiride were small, but empagliflozin was associated with a significant lower risk of hypoglycemia and significantly fewer patients required rescue therapy.”
Besides Dr. Salsali’s disclosure, coauthors disclosed relationships with Novo Nordisk, Medtronic, and the Steno Diabetes Center. Three other coauthors were employees of Boehringer Ingelheim.
NEW ORLEANS – When used as an add-on therapy to metformin in patients with type 2 diabetes mellitus, empagliflozin has sustained safety and efficacy in reducing in hemoglobin A1c (HbA1c) and other key metabolic measures for up to 4 years, according to results of the EMPA-REG H2H-SU trial presented at the annual scientific sessions of the American Diabetes Association.
The latest results of the double-blind, phase III trial extended out to 4 years the previously published 2-year results (Lancet Diabetes Endocrinol. 2014;2:691-700) in comparing empagliflozin, a sodium glucose cotransporter inhibitor, 25 mg daily, and the sulfonylurea glimepiride, 1-4 mg daily, as add-on therapy to metformin. “As previously reported, after 2 years there was a modest difference in HbA1c between empagliflozin and glimepiride,” said Dr. Afshin Salsali, reporting for the trial group. “This difference continued for the remainder of the study, although there was a slight rebound in each group,” according to Dr. Salsali, executive director of global clinical development at Boehringer Ingelheim, Ridgefield, Conn.
The 4-year results involved more than 73% of the 1,545 patients who participated in the 2-year study, Dr. Salsali said. The majority of patients were white, with average HbA1c levels of 7.92% and weight of 82.5 kg at the outset of the 2-year study. After 4 years, average HbA1c levels declined 0.08% in those on glimepiride vs. 0.26% for empagliflozin, Dr. Salsali said, achieving the primary study endpoint of noninferiority to glimepiride. However, he noted that rates of hypoglycemia varied dramatically between the two therapies. “This was achieved at the rate of much lower hypoglycemia on empagliflozin, compared with glimepiride, 3% vs. 25% (P less than .001),” he said.
Dr. Salsali pointed out that patients in the empagliflozin group were less likely to need rescue therapy, with an odds ratio of 0.56 (P less than.001), and a much later need for intervention. The advantage empagliflozin showed in both weight loss and blood pressure reduction in the 2-year study also held up for 4 years, Dr. Salsali said. “The pattern of blood pressure reduction achieved at initiation in the first few weeks after using empagliflozin more or less remained at the same level throughout this study,” he said.

One of most important messages from the study, Dr. Salsali said, is the impact empagliflozin had on the estimated glomerular filtration rate (eGFR). “Previously in the pivotal diabetes trial that we had, we showed a reduction in eGFR with a slight rebound, but we didn’t have enough time to see the full picture,” he said. “Now we have the luxury of looking into the full eGFR change over time up to 4 years. After the initial reduction in eGFR, there is a gradual increase and return of eGFR to the baseline level which remained stable for the duration of this study.” However, the glimepiride arm showed the “expected” average rate of eGFR reduction of 2 mL/min per year for type 2 diabetes, he said.
The reporting of adverse events was about the same between both groups, but serious adverse events were slightly higher among those on empagliflozin: 7.36/100 patient years vs. 7.06/100 patient years on glimepiride. The former had higher rates of urinary tract infections (6.96 vs. 5.82 per 100 patient-years) and volume depletion (0.82 vs. 0.63 per 100 patient years), but rates of bone fractures were almost identical (4.1% vs. 4.2%). There was no reported diabetic ketoacidosis in either group.
“Empagliflozin 25 mg after 208 weeks of treatment as an add-on to metformin led to modest numerical advantage in mean HbA1c change from baseline and clinically relevant reduction in weight, systolic blood pressure, and diastolic blood pressure,” Dr. Salsali said. “The difference in changes in HbA1c between empagliflozin and glimepiride were small, but empagliflozin was associated with a significant lower risk of hypoglycemia and significantly fewer patients required rescue therapy.”
Besides Dr. Salsali’s disclosure, coauthors disclosed relationships with Novo Nordisk, Medtronic, and the Steno Diabetes Center. Three other coauthors were employees of Boehringer Ingelheim.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Clinical trial results show the HBA1c-lowering effects of empagliflozin endure at least 4 years.
Major finding: Average HbA1c levels declined 0.26% for those on empagliflozin vs. 0.08% for people taking glimepiride as add-on therapy to metformin.
Data source: Double-blind, phase III clinical trial involving 1,545 patients.
Disclosures: Dr. Salsali is an employee of Boehringer Ingelheim, as are three other coauthors. Other coauthors disclosed relationships with Novo Nordisk, Medtronic, and the Steno Diabetes Center.

