User login
Today’s cosmetic patient wants to look more youthful every day without spending a lot of money, feeling any pain, or having any postprocedure downtime. With continued technological improvements, dermatologists have been able to provide our patients with the more youthful appearance they desire; however, many of these procedures still are costly, painful, and may require some downtime. New cosmeceutical therapies can be used as adjuncts to these procedures, making antiaging regimens less painful for patients and requiring less postprocedure healing time. In this article, the use of cosmeceuticals in conjunction with chemical peels, lasers, and injectables will be discussed.
Chemical Peels
Chemical peels are used to create an injury of specific skin depth with a goal of stimulating new skin growth and improving surface texture and appearance. They generally are classified as superficial, medium, or deep according to the depth of action. Currently available agents for superficial chemical peels include α-hydroxy acids (AHAs)(eg, glycolic acid [GA]) and β-hydroxy acids (BHAs)(eg, salicylic acid). β-Lipohydroxy acid (up to 10%), a derivative of salicylic acid, is widely used in Europe. Trichloroacetic acid (TCA) can be used for superficial peels (10%–20%) and for medium-depth peels (35%). Combination peels such as Monheit combination (Jessner solution plus TCA), Brody combination (solid CO2 plus TCA), Coleman combination (GA 70% plus TCA), and Jessner solution with GA can be used as medium-depth peels. Deep peels typically are performed with phenol-based solutions, including the Baker-Gordon phenol peel and the Hetter peel (phenol or croton oil peel).
Specific agents for chemical peels should be selected based on the disorder being treated and should be administered using an appropriate peel depth determined by the histologic level or severity of skin pathology to maximize treatment success.1 However, other considerations, such as skin characteristics, area of skin to be treated, safety concerns, healing time, and patient adherence also should be taken into account to achieve the best overall results. Although many of the deeper peels recently have been replaced by laser-based ablative treatments, superficial to medium-depth peels still are commonly used in the treatment of fine lines, uneven texture, and dyspigmentation.2
Superficial peels are reasonably safe and well tolerated, usually with only mild discomfort (eg, transient burning, irritation, erythema). Scarring, postinflammatory hyperpigmentation (PIH), and infection are rare with superficial peels.1 Postinflammatory hyperpigmentation can be exacerbated by sun exposure, making it important for patients to be educated about sun protection and closely monitored during the recovery phase. In medium and deep peels, lines of demarcation related to the administration technique can occur. Feathering the chemical peel solution at junctions with nonpeeled skin can help to avoid this effect.1 Side effects associated with deeper chemical peels can include pigmentary changes, infections, allergic reactions, improper healing, hypersensitivity, and underlying disease exacerbation. The best way to prevent complications is to identify patients who are at risk and maintain an appropriate peel depth that balances efficacy with known adverse events.1
Many adjunctive agents (eg, AHAs, BHAs, retinoids, skin-bleaching preparations) can be used to enhance chemical peels and decrease the incidence of PIH. α-Hydroxy acids and BHAs can be beneficial when applied prior to chemical peels. Moisturizers containing AHAs and BHAs can be used for 2 to 3 weeks before superficial or medium-depth chemical peels.2 These agents cause thinning of the stratum corneum, thereby creating a more uniform cutaneous surface and allowing for deeper penetration of the chemical peeling agent. Retinoids also are superior prepeeling agents; however, retinoids also can increase the likelihood of irritation, which can be minimized by discontinuing retinoids for 1 week following chemical peels.2 A combination of chemical peels and topical bleaching agents has been shown to be effective in treating hyperpigmentation. The chemical peel causes superficial exfoliation, which allows the lightening agent to penetrate more deeply.2
Hydroquinone (HQ) is the gold standard for improvement of existing pigmentation.3 It is one of the most effective inhibitors of melanogenesis both in vitro and in vivo and is widely used for the treatment of melanosis and other hyperpigmentary disorders. It is widely accepted that the depigmentation activity of HQ may partly be related to its ability to act as an alternate substrate of tyrosinase, thereby competing for tyrosine oxidation in active melanocytes.3 Using HQ at a 4% concentration and combining it with retinoids is quite efficacious.2 Other commonly used depigmenting agents include kojic acid, ascorbic acid (vitamin C), and niacinamide, which often can be used as adjuncts with or maintenance therapy after HQ treatment.2,3
The risk for PIH is imminent for chemical peels and cosmetic laser treatments; therefore, it is crucial to educate patients about the importance of daily and aggressive sun protection. There are several methods of reducing or eliminating postprocedure melanin formation, such as inhibiting tyrosinase synthesis, using complex copper to inhibit tyrosinase function, eliminating oxidation reactions that lead to polymer formation, slowing down the transfer of melanosomes to keratinocytes, or acting upstream on the hormone that stimulates melanogenesis.3 Most of the depigmenting agents presently on the market act by inhibiting tyrosinase via one of these mechanisms.
Skin-lightening agents are primarily formulated as emulsions that have a higher aesthetic appeal. Many of the ingredients get better dispersions with emulsions, which is an added feature of these products. Recently, gel-based formulations also are being considered for their suitability in certain skin types. Efficacy studies for skin-lightening formulations are being carried out through clinical trials that utilize devices that measure skin color in addition to the dermatologist’s assessment.4 Other skin parameters (eg, moisturization, texture, barrier integrity, pH) also are being evaluated to give physicians a picture of skin health after the use of skin-lightening agents. With advances in technology and measurement techniques, it is becoming easier to identify the efficacy of these formulations in different skin types.4
Lasers
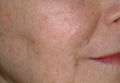
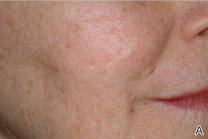
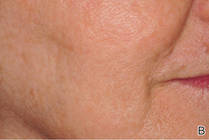
The ultimate goal of laser therapy often is to improve the canvas and color of the skin. Ablative laser resurfacing is reliably the most effective procedure for sun-damaged skin.2 This technique causes thermally induced full-thickness epidermal and dermal denudation, which in turn facilitates cytokine-led dermal collagen formation and reepithelialization. Various nonablative modalities also are used for treating photodamaged skin. The epidermis remains unaffected by these nonablative methods, thus decreasing the need for extensive wound care and downtime that is required with ablative treatments. Combining nonablative laser treatments with topical cosmeceuticals has been proven more effective than using either method alone.2 The use of topical retinoids prior to ablative laser resurfacing often results in remarkably faster postprocedure healing and reepithelialization (Figure). Retinoids are best applied nightly for at least 2 weeks and optimally for 3 months before ablative laser treatment. Application should be discontinued for 1 week immediately prior to the procedure.
|
|
| Before (A) and after (B) treatment with a fractional laser in combination with a pre- and postprocedure skin care regimen consisting of retinoids and sunscreen. |
Topical retinoids also are effective in reducing erythema and increasing dermal thickness after nonablative treatments. When used prior to laser treatments, retinoids have been shown to decrease the risk for postoperative milia and hyperpigmentation as well as to allow for better penetration of the laser beam secondary to a thinner stratum corneum.2 Following ablative resurfacing, retinoid use should be discontinued for several weeks to allow for reepithelialization and adequate healing.
Postprocedure Wound Healing
Most of the recommended products that help decrease postprocedural inflammation are cosmeceuticals containing both antioxidants and anti-inflammatories to help decrease redness and inflammation, including various barrier repair moisturizers. Restoring barrier integrity improves the overall appearance of the skin. The ingredients normally recommended in barrier repair moisturizers are epidermal lipids such as ceramides; hyaluronic acid (HA), which is a humectant; and occlusives for patients with very dry skin. Some of the ingredients in over-the-counter cosmeceuticals that can help decrease redness and inflammation include vitamin C, vitamin E, and vitamin B or niacinamide, which will help plump the barrier and also have anti-inflammatory properties. Additionally, polyphenolic flavonoids such as soy and green tea can help decrease inflammation, along with a number of other organic ingredients, such as caffeine, feverfew, and licorice.5 If topical vitamin C is being considered for postprocedure use, the non–ascorbic acid variant should be administered. The magnesium ascorbyl phosphate and ascorbyl palmitate forms of vitamin C have a neutral pH and tend to be better tolerated by patients.
In addition to current prescription and over-the-counter cosmeceuticals used for postprocedure irritation and inflammation, copper peptides and other well-tolerated and effective naturally occurring compounds are being investigated and tried. Copper is a biocide that regulates keratinocyte integrins for epithelization and extracellular matrix remodeling. The extracellular matrix consists of the structural fibrillar collagens and is remodeled or degraded by matrix metalloproteinases (MMPs) that facilitate epithelization. The predominant classes of MMPs include collagenases (ie, MMP-1) and gelatinases (ie, MMP-2, MMP-9) that degrade interstitial collagen and basement membrane proteins.6 The MMPs are endogenously inhibited by tissue inhibitors of metalloproteinases (TIMPs). Copper is a cofactor to lysyl oxidase, which cross-links collagen and stimulates expression of MMP-2 and collagen in a complex with a matrix-derived tripeptide (glycyl-histidyl-lysine or Gly-His-Lys [GHK]) in fibroblasts.6 Much attention has been focused on the tripeptides, such as GHK and Gly-Gly-His, and their copper complexes, which have high activity and good skin tolerance. These complexes have been shown to play a physiological role in the process of wound healing, tissue repair, and skin inflammation. Gly-Gly-His, GHK, copper chloride, and their copper complexes decrease tumor necrosis factor α–dependent IL-6 secretion in fibroblasts.7 IL-6 is crucial for normal wound healing, skin inflammation, and UVB-induced erythema. Because of their anti-inflammatory properties, these copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs, which have more side effects.
Botulinum Neurotoxin and Other Injectable Fillers
Acetyl Hexapeptide-3: A Topical Complement to Botulinum Neurotoxin
Acetyl hexapeptide-3 (Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2) was discovered when looking for a less toxic variation of botulinum neurotoxin (BoNT) to treat aging skin.8 It is patterned from the N-terminal end of the synaptosome-associated protein of molecular weight 25 kDa (SNAP-25), which is essential for docking and fusion of synaptic vesicles to the presynaptic membrane for acetylcholine release.9 It prevents formation and stability of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex, inhibiting vesicle docking and calcium-dependent catecholamine exocytosis.8 It also has been found to substantially inhibit the repetitive muscular contraction of facial expression similar to BoNT type A but with somewhat lower efficacy. Acetyl hexapeptide-3 was shown to inhibit 30% of total catecholamine exocytosis and had a remarkable capacity to permeate the skin.10 Thus this topical form of BoNT is a useful complement to intramuscular BoNT.
Studies showing the efficacy and safety of acetyl hexapeptide-3 have demonstrated reductions in wrinkle intensity, mainly in the lateral periorbital areas. In one early study, 10 women applied an emulsion containing 10% of the hexapeptide to one lateral periorbital region and the same emulsion without the hexapeptide to the contralateral side, both twice daily for 30 days.10 A 30% decrease in the depth of skin wrinkles was seen on the hexapeptide side compared with a 10% decrease in the depth of wrinkles on the side treated without hexapeptide. No irritation or toxicity was noted.10 In another trial, 10 women applied an acetyl hexapeptide-3 cream 5% twice daily to lateral periorbital rhytides, with a 27% improvement in wrinkle depth after a 30-day treatment period.9 A double-blind, placebo-controlled study of 60 women assessing the safety and efficacy of topical hexapeptide showed a total antiwrinkle efficacy of 48.9% on the side treated with an emulsion containing 10% of the hexapeptide compared with 0% efficacy on the placebo side.8 Similar to Blanes-Mira et al,10 no adverse events such as skin irritation or toxicity were seen.8 In all of these studies, wrinkle depth was measured by silicone replica analysis.
Topical acetyl hexapeptide-3 is effective in decreasing wrinkles, and its best use will likely be as an adjunct to intramuscular BoNT, as the intramuscular form likely has higher efficacy with the toxin injected directly into the target muscle; however, patients who want the effects of BoNT without the pain of injections may choose to use topical acetyl hexapeptide-3 alone. Patients who do use acetyl hexapeptide-3 as a complement to their intramuscular BoNT regimen may not need as many units of BoNT with each treatment or may not need certain areas injected as often, leading to fewer injections and less pain with each visit. Skin irritation was not seen as a side effect in these trials. Additionally, the topical form has insignificant acute toxicity (≥2000 mg/kg) compared to BoNT type A (20 ng/kg), and genotoxicity was not seen with testing, making it a safe complementary option to an injectable regimen.8
Topical Hyaluronic Acid: A Complement to Injectable Fillers
Hyaluronic acid (HA) is a glycosaminoglycan found in the extracellular matrix of the skin that greatly contributes to tissue hydration. Additionally, it plays a crucial role in the synthesis of extracellular matrix molecules and epidermal cell interaction with the environment.11 The water-binding capacity of HA approximates 1000 times its volume or 6 L of water per gram of HA; however, once an individual reaches adulthood, the amount of HA decreases to 5% of baseline levels, thus contributing to xerosis, loss of skin elasticity, and atrophy.11,12 Although photoaged skin can have increased glycosaminoglycans due to an increase in chondroitin sulfate proteoglycans, they are abnormally deposited on elastotic material in the superficial dermis rather than diffusely scattered, as seen in youthful skin.12
Many topical antiaging products contain HA, though evidence for efficacy in reducing wrinkles has been lacking, along with concerns that HA cannot penetrate the skin. This concern stems from the fact that the original molecule is 3000 nm in diameter and the intercellular space is only 15 to 50 nm. This space is only 6 to 10 nm at the hyaline membrane. Recently, scientists in Japan found a way to reduce the size of HA molecules to 5 nm (nano-HA) without changing its structure. A study of 33 women who applied the topical nano-HA twice daily for 8 weeks to one periorbital area while the contralateral side was left untreated showed improved hydration of the treated side that continued to increase when measured at 2, 4, and 8 weeks using corneometry.11 Roughness decreased and elasticity increased after week 2, which were maintained throughout the study. Additionally, erythema was measured using a chroma meter, which was found to have decreased at day 57 versus day 1.11 An earlier study by Pavicic et al12 evaluated the efficacy of topical hyalu-ronan 0.1% formulations of different molecular weights—50, 130, 300, 800, or 2000 kDa—in the periocular area. A randomized group of 76 women were treated twice daily for 2 months with HA cream on one side of the periocular area and placebo cream on the other. With regard to antiwrinkle properties, only the 50- and 130-kDa HA formulations showed marked effects compared with placebo after 2 months.12
Topical HA would be an effective addition to an antiwrinkle regimen, especially in patients who are averse to needles or are just starting to get wrinkles and are looking for a noninvasive therapy. Additionally, it would be beneficial for patients who have an injectable filler and BoNT regimen, as these patients will be able to target wrinkles simultaneously with both topical cosmeceuticals and injectables and likely will need fewer units of BoNT and/or filler and possibly fewer injections over time, which translates to decreased pain and adverse outcomes for patients.
Conclusion
The myriad of options dermatologists have to offer patients for cosmetic enhancement provides alternatives for patients who have contraindications to certain treatments, are needle averse, or have lifestyles that do not afford them a great deal of postprocedural healing time. Being knowledgeable about these options and how to combine them for improved outcomes is essential to any cosmetic practice.
1. Rendon MI, Berson DS, Cohen JL, et al. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3:32-43.
2. Lupo MP, Jacob LG. Cosmeceuticals for enhancing cosmetic procedures. In: Farris PK, ed. Cosmeceuticals and Cosmetic Practice. Oxford, United Kingdom: Wiley-Blackwell; 2014:268-276.
3. Gruber JV, Holtz R. Examining the impact of skin lighteners in vitro [published online ahead of print April 28, 2013]. Oxid Med Cell Longev. 2013;2013:702120.
4. Antonio JR, Antonio CR, Cardeal ILS, et al. Nanotechnology in dermatology. An Bras Dermatol. 2014;89:126-136.
5. Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308-319.
6. Gruchlik A, Jurzak M, Chodurek, E, et al. Effect of GLY-GLY-HIS, GLY-HIS-LYS and their copper complexes on TNF-α-dependant IL-6 secretion in normal human dermal fibroblasts. Acta Pol Pharm. 2012;69:1303-1306.
7. Philips N, Hwang H, Chauhan S, et al. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res. 2010;51:224-229.
8. Wang Y, Wang M, Xiao S, et al. The anti-wrinkle efficacy of Argireline, a synthetic hexapeptide, in Chinese subjects. Am J Clin Dermatol. 2013;14:147-153.
9. Lupo MP, Cole A. Cosmeceutical peptides. Dermatol Ther. 2007;20:343-349.
10. Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24:303-310.
11. Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J Clin Aesthet Dermatol. 2014;7:27-29.
12. Pavicic T, Gauglitz G, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990-1000.
Today’s cosmetic patient wants to look more youthful every day without spending a lot of money, feeling any pain, or having any postprocedure downtime. With continued technological improvements, dermatologists have been able to provide our patients with the more youthful appearance they desire; however, many of these procedures still are costly, painful, and may require some downtime. New cosmeceutical therapies can be used as adjuncts to these procedures, making antiaging regimens less painful for patients and requiring less postprocedure healing time. In this article, the use of cosmeceuticals in conjunction with chemical peels, lasers, and injectables will be discussed.
Chemical Peels
Chemical peels are used to create an injury of specific skin depth with a goal of stimulating new skin growth and improving surface texture and appearance. They generally are classified as superficial, medium, or deep according to the depth of action. Currently available agents for superficial chemical peels include α-hydroxy acids (AHAs)(eg, glycolic acid [GA]) and β-hydroxy acids (BHAs)(eg, salicylic acid). β-Lipohydroxy acid (up to 10%), a derivative of salicylic acid, is widely used in Europe. Trichloroacetic acid (TCA) can be used for superficial peels (10%–20%) and for medium-depth peels (35%). Combination peels such as Monheit combination (Jessner solution plus TCA), Brody combination (solid CO2 plus TCA), Coleman combination (GA 70% plus TCA), and Jessner solution with GA can be used as medium-depth peels. Deep peels typically are performed with phenol-based solutions, including the Baker-Gordon phenol peel and the Hetter peel (phenol or croton oil peel).
Specific agents for chemical peels should be selected based on the disorder being treated and should be administered using an appropriate peel depth determined by the histologic level or severity of skin pathology to maximize treatment success.1 However, other considerations, such as skin characteristics, area of skin to be treated, safety concerns, healing time, and patient adherence also should be taken into account to achieve the best overall results. Although many of the deeper peels recently have been replaced by laser-based ablative treatments, superficial to medium-depth peels still are commonly used in the treatment of fine lines, uneven texture, and dyspigmentation.2
Superficial peels are reasonably safe and well tolerated, usually with only mild discomfort (eg, transient burning, irritation, erythema). Scarring, postinflammatory hyperpigmentation (PIH), and infection are rare with superficial peels.1 Postinflammatory hyperpigmentation can be exacerbated by sun exposure, making it important for patients to be educated about sun protection and closely monitored during the recovery phase. In medium and deep peels, lines of demarcation related to the administration technique can occur. Feathering the chemical peel solution at junctions with nonpeeled skin can help to avoid this effect.1 Side effects associated with deeper chemical peels can include pigmentary changes, infections, allergic reactions, improper healing, hypersensitivity, and underlying disease exacerbation. The best way to prevent complications is to identify patients who are at risk and maintain an appropriate peel depth that balances efficacy with known adverse events.1
Many adjunctive agents (eg, AHAs, BHAs, retinoids, skin-bleaching preparations) can be used to enhance chemical peels and decrease the incidence of PIH. α-Hydroxy acids and BHAs can be beneficial when applied prior to chemical peels. Moisturizers containing AHAs and BHAs can be used for 2 to 3 weeks before superficial or medium-depth chemical peels.2 These agents cause thinning of the stratum corneum, thereby creating a more uniform cutaneous surface and allowing for deeper penetration of the chemical peeling agent. Retinoids also are superior prepeeling agents; however, retinoids also can increase the likelihood of irritation, which can be minimized by discontinuing retinoids for 1 week following chemical peels.2 A combination of chemical peels and topical bleaching agents has been shown to be effective in treating hyperpigmentation. The chemical peel causes superficial exfoliation, which allows the lightening agent to penetrate more deeply.2
Hydroquinone (HQ) is the gold standard for improvement of existing pigmentation.3 It is one of the most effective inhibitors of melanogenesis both in vitro and in vivo and is widely used for the treatment of melanosis and other hyperpigmentary disorders. It is widely accepted that the depigmentation activity of HQ may partly be related to its ability to act as an alternate substrate of tyrosinase, thereby competing for tyrosine oxidation in active melanocytes.3 Using HQ at a 4% concentration and combining it with retinoids is quite efficacious.2 Other commonly used depigmenting agents include kojic acid, ascorbic acid (vitamin C), and niacinamide, which often can be used as adjuncts with or maintenance therapy after HQ treatment.2,3
The risk for PIH is imminent for chemical peels and cosmetic laser treatments; therefore, it is crucial to educate patients about the importance of daily and aggressive sun protection. There are several methods of reducing or eliminating postprocedure melanin formation, such as inhibiting tyrosinase synthesis, using complex copper to inhibit tyrosinase function, eliminating oxidation reactions that lead to polymer formation, slowing down the transfer of melanosomes to keratinocytes, or acting upstream on the hormone that stimulates melanogenesis.3 Most of the depigmenting agents presently on the market act by inhibiting tyrosinase via one of these mechanisms.
Skin-lightening agents are primarily formulated as emulsions that have a higher aesthetic appeal. Many of the ingredients get better dispersions with emulsions, which is an added feature of these products. Recently, gel-based formulations also are being considered for their suitability in certain skin types. Efficacy studies for skin-lightening formulations are being carried out through clinical trials that utilize devices that measure skin color in addition to the dermatologist’s assessment.4 Other skin parameters (eg, moisturization, texture, barrier integrity, pH) also are being evaluated to give physicians a picture of skin health after the use of skin-lightening agents. With advances in technology and measurement techniques, it is becoming easier to identify the efficacy of these formulations in different skin types.4
Lasers
The ultimate goal of laser therapy often is to improve the canvas and color of the skin. Ablative laser resurfacing is reliably the most effective procedure for sun-damaged skin.2 This technique causes thermally induced full-thickness epidermal and dermal denudation, which in turn facilitates cytokine-led dermal collagen formation and reepithelialization. Various nonablative modalities also are used for treating photodamaged skin. The epidermis remains unaffected by these nonablative methods, thus decreasing the need for extensive wound care and downtime that is required with ablative treatments. Combining nonablative laser treatments with topical cosmeceuticals has been proven more effective than using either method alone.2 The use of topical retinoids prior to ablative laser resurfacing often results in remarkably faster postprocedure healing and reepithelialization (Figure). Retinoids are best applied nightly for at least 2 weeks and optimally for 3 months before ablative laser treatment. Application should be discontinued for 1 week immediately prior to the procedure.

|

|
| Before (A) and after (B) treatment with a fractional laser in combination with a pre- and postprocedure skin care regimen consisting of retinoids and sunscreen. |
Topical retinoids also are effective in reducing erythema and increasing dermal thickness after nonablative treatments. When used prior to laser treatments, retinoids have been shown to decrease the risk for postoperative milia and hyperpigmentation as well as to allow for better penetration of the laser beam secondary to a thinner stratum corneum.2 Following ablative resurfacing, retinoid use should be discontinued for several weeks to allow for reepithelialization and adequate healing.
Postprocedure Wound Healing
Most of the recommended products that help decrease postprocedural inflammation are cosmeceuticals containing both antioxidants and anti-inflammatories to help decrease redness and inflammation, including various barrier repair moisturizers. Restoring barrier integrity improves the overall appearance of the skin. The ingredients normally recommended in barrier repair moisturizers are epidermal lipids such as ceramides; hyaluronic acid (HA), which is a humectant; and occlusives for patients with very dry skin. Some of the ingredients in over-the-counter cosmeceuticals that can help decrease redness and inflammation include vitamin C, vitamin E, and vitamin B or niacinamide, which will help plump the barrier and also have anti-inflammatory properties. Additionally, polyphenolic flavonoids such as soy and green tea can help decrease inflammation, along with a number of other organic ingredients, such as caffeine, feverfew, and licorice.5 If topical vitamin C is being considered for postprocedure use, the non–ascorbic acid variant should be administered. The magnesium ascorbyl phosphate and ascorbyl palmitate forms of vitamin C have a neutral pH and tend to be better tolerated by patients.
In addition to current prescription and over-the-counter cosmeceuticals used for postprocedure irritation and inflammation, copper peptides and other well-tolerated and effective naturally occurring compounds are being investigated and tried. Copper is a biocide that regulates keratinocyte integrins for epithelization and extracellular matrix remodeling. The extracellular matrix consists of the structural fibrillar collagens and is remodeled or degraded by matrix metalloproteinases (MMPs) that facilitate epithelization. The predominant classes of MMPs include collagenases (ie, MMP-1) and gelatinases (ie, MMP-2, MMP-9) that degrade interstitial collagen and basement membrane proteins.6 The MMPs are endogenously inhibited by tissue inhibitors of metalloproteinases (TIMPs). Copper is a cofactor to lysyl oxidase, which cross-links collagen and stimulates expression of MMP-2 and collagen in a complex with a matrix-derived tripeptide (glycyl-histidyl-lysine or Gly-His-Lys [GHK]) in fibroblasts.6 Much attention has been focused on the tripeptides, such as GHK and Gly-Gly-His, and their copper complexes, which have high activity and good skin tolerance. These complexes have been shown to play a physiological role in the process of wound healing, tissue repair, and skin inflammation. Gly-Gly-His, GHK, copper chloride, and their copper complexes decrease tumor necrosis factor α–dependent IL-6 secretion in fibroblasts.7 IL-6 is crucial for normal wound healing, skin inflammation, and UVB-induced erythema. Because of their anti-inflammatory properties, these copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs, which have more side effects.
Botulinum Neurotoxin and Other Injectable Fillers
Acetyl Hexapeptide-3: A Topical Complement to Botulinum Neurotoxin
Acetyl hexapeptide-3 (Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2) was discovered when looking for a less toxic variation of botulinum neurotoxin (BoNT) to treat aging skin.8 It is patterned from the N-terminal end of the synaptosome-associated protein of molecular weight 25 kDa (SNAP-25), which is essential for docking and fusion of synaptic vesicles to the presynaptic membrane for acetylcholine release.9 It prevents formation and stability of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex, inhibiting vesicle docking and calcium-dependent catecholamine exocytosis.8 It also has been found to substantially inhibit the repetitive muscular contraction of facial expression similar to BoNT type A but with somewhat lower efficacy. Acetyl hexapeptide-3 was shown to inhibit 30% of total catecholamine exocytosis and had a remarkable capacity to permeate the skin.10 Thus this topical form of BoNT is a useful complement to intramuscular BoNT.
Studies showing the efficacy and safety of acetyl hexapeptide-3 have demonstrated reductions in wrinkle intensity, mainly in the lateral periorbital areas. In one early study, 10 women applied an emulsion containing 10% of the hexapeptide to one lateral periorbital region and the same emulsion without the hexapeptide to the contralateral side, both twice daily for 30 days.10 A 30% decrease in the depth of skin wrinkles was seen on the hexapeptide side compared with a 10% decrease in the depth of wrinkles on the side treated without hexapeptide. No irritation or toxicity was noted.10 In another trial, 10 women applied an acetyl hexapeptide-3 cream 5% twice daily to lateral periorbital rhytides, with a 27% improvement in wrinkle depth after a 30-day treatment period.9 A double-blind, placebo-controlled study of 60 women assessing the safety and efficacy of topical hexapeptide showed a total antiwrinkle efficacy of 48.9% on the side treated with an emulsion containing 10% of the hexapeptide compared with 0% efficacy on the placebo side.8 Similar to Blanes-Mira et al,10 no adverse events such as skin irritation or toxicity were seen.8 In all of these studies, wrinkle depth was measured by silicone replica analysis.
Topical acetyl hexapeptide-3 is effective in decreasing wrinkles, and its best use will likely be as an adjunct to intramuscular BoNT, as the intramuscular form likely has higher efficacy with the toxin injected directly into the target muscle; however, patients who want the effects of BoNT without the pain of injections may choose to use topical acetyl hexapeptide-3 alone. Patients who do use acetyl hexapeptide-3 as a complement to their intramuscular BoNT regimen may not need as many units of BoNT with each treatment or may not need certain areas injected as often, leading to fewer injections and less pain with each visit. Skin irritation was not seen as a side effect in these trials. Additionally, the topical form has insignificant acute toxicity (≥2000 mg/kg) compared to BoNT type A (20 ng/kg), and genotoxicity was not seen with testing, making it a safe complementary option to an injectable regimen.8
Topical Hyaluronic Acid: A Complement to Injectable Fillers
Hyaluronic acid (HA) is a glycosaminoglycan found in the extracellular matrix of the skin that greatly contributes to tissue hydration. Additionally, it plays a crucial role in the synthesis of extracellular matrix molecules and epidermal cell interaction with the environment.11 The water-binding capacity of HA approximates 1000 times its volume or 6 L of water per gram of HA; however, once an individual reaches adulthood, the amount of HA decreases to 5% of baseline levels, thus contributing to xerosis, loss of skin elasticity, and atrophy.11,12 Although photoaged skin can have increased glycosaminoglycans due to an increase in chondroitin sulfate proteoglycans, they are abnormally deposited on elastotic material in the superficial dermis rather than diffusely scattered, as seen in youthful skin.12
Many topical antiaging products contain HA, though evidence for efficacy in reducing wrinkles has been lacking, along with concerns that HA cannot penetrate the skin. This concern stems from the fact that the original molecule is 3000 nm in diameter and the intercellular space is only 15 to 50 nm. This space is only 6 to 10 nm at the hyaline membrane. Recently, scientists in Japan found a way to reduce the size of HA molecules to 5 nm (nano-HA) without changing its structure. A study of 33 women who applied the topical nano-HA twice daily for 8 weeks to one periorbital area while the contralateral side was left untreated showed improved hydration of the treated side that continued to increase when measured at 2, 4, and 8 weeks using corneometry.11 Roughness decreased and elasticity increased after week 2, which were maintained throughout the study. Additionally, erythema was measured using a chroma meter, which was found to have decreased at day 57 versus day 1.11 An earlier study by Pavicic et al12 evaluated the efficacy of topical hyalu-ronan 0.1% formulations of different molecular weights—50, 130, 300, 800, or 2000 kDa—in the periocular area. A randomized group of 76 women were treated twice daily for 2 months with HA cream on one side of the periocular area and placebo cream on the other. With regard to antiwrinkle properties, only the 50- and 130-kDa HA formulations showed marked effects compared with placebo after 2 months.12
Topical HA would be an effective addition to an antiwrinkle regimen, especially in patients who are averse to needles or are just starting to get wrinkles and are looking for a noninvasive therapy. Additionally, it would be beneficial for patients who have an injectable filler and BoNT regimen, as these patients will be able to target wrinkles simultaneously with both topical cosmeceuticals and injectables and likely will need fewer units of BoNT and/or filler and possibly fewer injections over time, which translates to decreased pain and adverse outcomes for patients.
Conclusion
The myriad of options dermatologists have to offer patients for cosmetic enhancement provides alternatives for patients who have contraindications to certain treatments, are needle averse, or have lifestyles that do not afford them a great deal of postprocedural healing time. Being knowledgeable about these options and how to combine them for improved outcomes is essential to any cosmetic practice.
Today’s cosmetic patient wants to look more youthful every day without spending a lot of money, feeling any pain, or having any postprocedure downtime. With continued technological improvements, dermatologists have been able to provide our patients with the more youthful appearance they desire; however, many of these procedures still are costly, painful, and may require some downtime. New cosmeceutical therapies can be used as adjuncts to these procedures, making antiaging regimens less painful for patients and requiring less postprocedure healing time. In this article, the use of cosmeceuticals in conjunction with chemical peels, lasers, and injectables will be discussed.
Chemical Peels
Chemical peels are used to create an injury of specific skin depth with a goal of stimulating new skin growth and improving surface texture and appearance. They generally are classified as superficial, medium, or deep according to the depth of action. Currently available agents for superficial chemical peels include α-hydroxy acids (AHAs)(eg, glycolic acid [GA]) and β-hydroxy acids (BHAs)(eg, salicylic acid). β-Lipohydroxy acid (up to 10%), a derivative of salicylic acid, is widely used in Europe. Trichloroacetic acid (TCA) can be used for superficial peels (10%–20%) and for medium-depth peels (35%). Combination peels such as Monheit combination (Jessner solution plus TCA), Brody combination (solid CO2 plus TCA), Coleman combination (GA 70% plus TCA), and Jessner solution with GA can be used as medium-depth peels. Deep peels typically are performed with phenol-based solutions, including the Baker-Gordon phenol peel and the Hetter peel (phenol or croton oil peel).
Specific agents for chemical peels should be selected based on the disorder being treated and should be administered using an appropriate peel depth determined by the histologic level or severity of skin pathology to maximize treatment success.1 However, other considerations, such as skin characteristics, area of skin to be treated, safety concerns, healing time, and patient adherence also should be taken into account to achieve the best overall results. Although many of the deeper peels recently have been replaced by laser-based ablative treatments, superficial to medium-depth peels still are commonly used in the treatment of fine lines, uneven texture, and dyspigmentation.2
Superficial peels are reasonably safe and well tolerated, usually with only mild discomfort (eg, transient burning, irritation, erythema). Scarring, postinflammatory hyperpigmentation (PIH), and infection are rare with superficial peels.1 Postinflammatory hyperpigmentation can be exacerbated by sun exposure, making it important for patients to be educated about sun protection and closely monitored during the recovery phase. In medium and deep peels, lines of demarcation related to the administration technique can occur. Feathering the chemical peel solution at junctions with nonpeeled skin can help to avoid this effect.1 Side effects associated with deeper chemical peels can include pigmentary changes, infections, allergic reactions, improper healing, hypersensitivity, and underlying disease exacerbation. The best way to prevent complications is to identify patients who are at risk and maintain an appropriate peel depth that balances efficacy with known adverse events.1
Many adjunctive agents (eg, AHAs, BHAs, retinoids, skin-bleaching preparations) can be used to enhance chemical peels and decrease the incidence of PIH. α-Hydroxy acids and BHAs can be beneficial when applied prior to chemical peels. Moisturizers containing AHAs and BHAs can be used for 2 to 3 weeks before superficial or medium-depth chemical peels.2 These agents cause thinning of the stratum corneum, thereby creating a more uniform cutaneous surface and allowing for deeper penetration of the chemical peeling agent. Retinoids also are superior prepeeling agents; however, retinoids also can increase the likelihood of irritation, which can be minimized by discontinuing retinoids for 1 week following chemical peels.2 A combination of chemical peels and topical bleaching agents has been shown to be effective in treating hyperpigmentation. The chemical peel causes superficial exfoliation, which allows the lightening agent to penetrate more deeply.2
Hydroquinone (HQ) is the gold standard for improvement of existing pigmentation.3 It is one of the most effective inhibitors of melanogenesis both in vitro and in vivo and is widely used for the treatment of melanosis and other hyperpigmentary disorders. It is widely accepted that the depigmentation activity of HQ may partly be related to its ability to act as an alternate substrate of tyrosinase, thereby competing for tyrosine oxidation in active melanocytes.3 Using HQ at a 4% concentration and combining it with retinoids is quite efficacious.2 Other commonly used depigmenting agents include kojic acid, ascorbic acid (vitamin C), and niacinamide, which often can be used as adjuncts with or maintenance therapy after HQ treatment.2,3
The risk for PIH is imminent for chemical peels and cosmetic laser treatments; therefore, it is crucial to educate patients about the importance of daily and aggressive sun protection. There are several methods of reducing or eliminating postprocedure melanin formation, such as inhibiting tyrosinase synthesis, using complex copper to inhibit tyrosinase function, eliminating oxidation reactions that lead to polymer formation, slowing down the transfer of melanosomes to keratinocytes, or acting upstream on the hormone that stimulates melanogenesis.3 Most of the depigmenting agents presently on the market act by inhibiting tyrosinase via one of these mechanisms.
Skin-lightening agents are primarily formulated as emulsions that have a higher aesthetic appeal. Many of the ingredients get better dispersions with emulsions, which is an added feature of these products. Recently, gel-based formulations also are being considered for their suitability in certain skin types. Efficacy studies for skin-lightening formulations are being carried out through clinical trials that utilize devices that measure skin color in addition to the dermatologist’s assessment.4 Other skin parameters (eg, moisturization, texture, barrier integrity, pH) also are being evaluated to give physicians a picture of skin health after the use of skin-lightening agents. With advances in technology and measurement techniques, it is becoming easier to identify the efficacy of these formulations in different skin types.4
Lasers
The ultimate goal of laser therapy often is to improve the canvas and color of the skin. Ablative laser resurfacing is reliably the most effective procedure for sun-damaged skin.2 This technique causes thermally induced full-thickness epidermal and dermal denudation, which in turn facilitates cytokine-led dermal collagen formation and reepithelialization. Various nonablative modalities also are used for treating photodamaged skin. The epidermis remains unaffected by these nonablative methods, thus decreasing the need for extensive wound care and downtime that is required with ablative treatments. Combining nonablative laser treatments with topical cosmeceuticals has been proven more effective than using either method alone.2 The use of topical retinoids prior to ablative laser resurfacing often results in remarkably faster postprocedure healing and reepithelialization (Figure). Retinoids are best applied nightly for at least 2 weeks and optimally for 3 months before ablative laser treatment. Application should be discontinued for 1 week immediately prior to the procedure.

|

|
| Before (A) and after (B) treatment with a fractional laser in combination with a pre- and postprocedure skin care regimen consisting of retinoids and sunscreen. |
Topical retinoids also are effective in reducing erythema and increasing dermal thickness after nonablative treatments. When used prior to laser treatments, retinoids have been shown to decrease the risk for postoperative milia and hyperpigmentation as well as to allow for better penetration of the laser beam secondary to a thinner stratum corneum.2 Following ablative resurfacing, retinoid use should be discontinued for several weeks to allow for reepithelialization and adequate healing.
Postprocedure Wound Healing
Most of the recommended products that help decrease postprocedural inflammation are cosmeceuticals containing both antioxidants and anti-inflammatories to help decrease redness and inflammation, including various barrier repair moisturizers. Restoring barrier integrity improves the overall appearance of the skin. The ingredients normally recommended in barrier repair moisturizers are epidermal lipids such as ceramides; hyaluronic acid (HA), which is a humectant; and occlusives for patients with very dry skin. Some of the ingredients in over-the-counter cosmeceuticals that can help decrease redness and inflammation include vitamin C, vitamin E, and vitamin B or niacinamide, which will help plump the barrier and also have anti-inflammatory properties. Additionally, polyphenolic flavonoids such as soy and green tea can help decrease inflammation, along with a number of other organic ingredients, such as caffeine, feverfew, and licorice.5 If topical vitamin C is being considered for postprocedure use, the non–ascorbic acid variant should be administered. The magnesium ascorbyl phosphate and ascorbyl palmitate forms of vitamin C have a neutral pH and tend to be better tolerated by patients.
In addition to current prescription and over-the-counter cosmeceuticals used for postprocedure irritation and inflammation, copper peptides and other well-tolerated and effective naturally occurring compounds are being investigated and tried. Copper is a biocide that regulates keratinocyte integrins for epithelization and extracellular matrix remodeling. The extracellular matrix consists of the structural fibrillar collagens and is remodeled or degraded by matrix metalloproteinases (MMPs) that facilitate epithelization. The predominant classes of MMPs include collagenases (ie, MMP-1) and gelatinases (ie, MMP-2, MMP-9) that degrade interstitial collagen and basement membrane proteins.6 The MMPs are endogenously inhibited by tissue inhibitors of metalloproteinases (TIMPs). Copper is a cofactor to lysyl oxidase, which cross-links collagen and stimulates expression of MMP-2 and collagen in a complex with a matrix-derived tripeptide (glycyl-histidyl-lysine or Gly-His-Lys [GHK]) in fibroblasts.6 Much attention has been focused on the tripeptides, such as GHK and Gly-Gly-His, and their copper complexes, which have high activity and good skin tolerance. These complexes have been shown to play a physiological role in the process of wound healing, tissue repair, and skin inflammation. Gly-Gly-His, GHK, copper chloride, and their copper complexes decrease tumor necrosis factor α–dependent IL-6 secretion in fibroblasts.7 IL-6 is crucial for normal wound healing, skin inflammation, and UVB-induced erythema. Because of their anti-inflammatory properties, these copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs, which have more side effects.
Botulinum Neurotoxin and Other Injectable Fillers
Acetyl Hexapeptide-3: A Topical Complement to Botulinum Neurotoxin
Acetyl hexapeptide-3 (Ac-Glu-Glu-Met-Gln-Arg-Arg-NH2) was discovered when looking for a less toxic variation of botulinum neurotoxin (BoNT) to treat aging skin.8 It is patterned from the N-terminal end of the synaptosome-associated protein of molecular weight 25 kDa (SNAP-25), which is essential for docking and fusion of synaptic vesicles to the presynaptic membrane for acetylcholine release.9 It prevents formation and stability of the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complex, inhibiting vesicle docking and calcium-dependent catecholamine exocytosis.8 It also has been found to substantially inhibit the repetitive muscular contraction of facial expression similar to BoNT type A but with somewhat lower efficacy. Acetyl hexapeptide-3 was shown to inhibit 30% of total catecholamine exocytosis and had a remarkable capacity to permeate the skin.10 Thus this topical form of BoNT is a useful complement to intramuscular BoNT.
Studies showing the efficacy and safety of acetyl hexapeptide-3 have demonstrated reductions in wrinkle intensity, mainly in the lateral periorbital areas. In one early study, 10 women applied an emulsion containing 10% of the hexapeptide to one lateral periorbital region and the same emulsion without the hexapeptide to the contralateral side, both twice daily for 30 days.10 A 30% decrease in the depth of skin wrinkles was seen on the hexapeptide side compared with a 10% decrease in the depth of wrinkles on the side treated without hexapeptide. No irritation or toxicity was noted.10 In another trial, 10 women applied an acetyl hexapeptide-3 cream 5% twice daily to lateral periorbital rhytides, with a 27% improvement in wrinkle depth after a 30-day treatment period.9 A double-blind, placebo-controlled study of 60 women assessing the safety and efficacy of topical hexapeptide showed a total antiwrinkle efficacy of 48.9% on the side treated with an emulsion containing 10% of the hexapeptide compared with 0% efficacy on the placebo side.8 Similar to Blanes-Mira et al,10 no adverse events such as skin irritation or toxicity were seen.8 In all of these studies, wrinkle depth was measured by silicone replica analysis.
Topical acetyl hexapeptide-3 is effective in decreasing wrinkles, and its best use will likely be as an adjunct to intramuscular BoNT, as the intramuscular form likely has higher efficacy with the toxin injected directly into the target muscle; however, patients who want the effects of BoNT without the pain of injections may choose to use topical acetyl hexapeptide-3 alone. Patients who do use acetyl hexapeptide-3 as a complement to their intramuscular BoNT regimen may not need as many units of BoNT with each treatment or may not need certain areas injected as often, leading to fewer injections and less pain with each visit. Skin irritation was not seen as a side effect in these trials. Additionally, the topical form has insignificant acute toxicity (≥2000 mg/kg) compared to BoNT type A (20 ng/kg), and genotoxicity was not seen with testing, making it a safe complementary option to an injectable regimen.8
Topical Hyaluronic Acid: A Complement to Injectable Fillers
Hyaluronic acid (HA) is a glycosaminoglycan found in the extracellular matrix of the skin that greatly contributes to tissue hydration. Additionally, it plays a crucial role in the synthesis of extracellular matrix molecules and epidermal cell interaction with the environment.11 The water-binding capacity of HA approximates 1000 times its volume or 6 L of water per gram of HA; however, once an individual reaches adulthood, the amount of HA decreases to 5% of baseline levels, thus contributing to xerosis, loss of skin elasticity, and atrophy.11,12 Although photoaged skin can have increased glycosaminoglycans due to an increase in chondroitin sulfate proteoglycans, they are abnormally deposited on elastotic material in the superficial dermis rather than diffusely scattered, as seen in youthful skin.12
Many topical antiaging products contain HA, though evidence for efficacy in reducing wrinkles has been lacking, along with concerns that HA cannot penetrate the skin. This concern stems from the fact that the original molecule is 3000 nm in diameter and the intercellular space is only 15 to 50 nm. This space is only 6 to 10 nm at the hyaline membrane. Recently, scientists in Japan found a way to reduce the size of HA molecules to 5 nm (nano-HA) without changing its structure. A study of 33 women who applied the topical nano-HA twice daily for 8 weeks to one periorbital area while the contralateral side was left untreated showed improved hydration of the treated side that continued to increase when measured at 2, 4, and 8 weeks using corneometry.11 Roughness decreased and elasticity increased after week 2, which were maintained throughout the study. Additionally, erythema was measured using a chroma meter, which was found to have decreased at day 57 versus day 1.11 An earlier study by Pavicic et al12 evaluated the efficacy of topical hyalu-ronan 0.1% formulations of different molecular weights—50, 130, 300, 800, or 2000 kDa—in the periocular area. A randomized group of 76 women were treated twice daily for 2 months with HA cream on one side of the periocular area and placebo cream on the other. With regard to antiwrinkle properties, only the 50- and 130-kDa HA formulations showed marked effects compared with placebo after 2 months.12
Topical HA would be an effective addition to an antiwrinkle regimen, especially in patients who are averse to needles or are just starting to get wrinkles and are looking for a noninvasive therapy. Additionally, it would be beneficial for patients who have an injectable filler and BoNT regimen, as these patients will be able to target wrinkles simultaneously with both topical cosmeceuticals and injectables and likely will need fewer units of BoNT and/or filler and possibly fewer injections over time, which translates to decreased pain and adverse outcomes for patients.
Conclusion
The myriad of options dermatologists have to offer patients for cosmetic enhancement provides alternatives for patients who have contraindications to certain treatments, are needle averse, or have lifestyles that do not afford them a great deal of postprocedural healing time. Being knowledgeable about these options and how to combine them for improved outcomes is essential to any cosmetic practice.
1. Rendon MI, Berson DS, Cohen JL, et al. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3:32-43.
2. Lupo MP, Jacob LG. Cosmeceuticals for enhancing cosmetic procedures. In: Farris PK, ed. Cosmeceuticals and Cosmetic Practice. Oxford, United Kingdom: Wiley-Blackwell; 2014:268-276.
3. Gruber JV, Holtz R. Examining the impact of skin lighteners in vitro [published online ahead of print April 28, 2013]. Oxid Med Cell Longev. 2013;2013:702120.
4. Antonio JR, Antonio CR, Cardeal ILS, et al. Nanotechnology in dermatology. An Bras Dermatol. 2014;89:126-136.
5. Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308-319.
6. Gruchlik A, Jurzak M, Chodurek, E, et al. Effect of GLY-GLY-HIS, GLY-HIS-LYS and their copper complexes on TNF-α-dependant IL-6 secretion in normal human dermal fibroblasts. Acta Pol Pharm. 2012;69:1303-1306.
7. Philips N, Hwang H, Chauhan S, et al. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res. 2010;51:224-229.
8. Wang Y, Wang M, Xiao S, et al. The anti-wrinkle efficacy of Argireline, a synthetic hexapeptide, in Chinese subjects. Am J Clin Dermatol. 2013;14:147-153.
9. Lupo MP, Cole A. Cosmeceutical peptides. Dermatol Ther. 2007;20:343-349.
10. Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24:303-310.
11. Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J Clin Aesthet Dermatol. 2014;7:27-29.
12. Pavicic T, Gauglitz G, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990-1000.
1. Rendon MI, Berson DS, Cohen JL, et al. Evidence and considerations in the application of chemical peels in skin disorders and aesthetic resurfacing. J Clin Aesthet Dermatol. 2010;3:32-43.
2. Lupo MP, Jacob LG. Cosmeceuticals for enhancing cosmetic procedures. In: Farris PK, ed. Cosmeceuticals and Cosmetic Practice. Oxford, United Kingdom: Wiley-Blackwell; 2014:268-276.
3. Gruber JV, Holtz R. Examining the impact of skin lighteners in vitro [published online ahead of print April 28, 2013]. Oxid Med Cell Longev. 2013;2013:702120.
4. Antonio JR, Antonio CR, Cardeal ILS, et al. Nanotechnology in dermatology. An Bras Dermatol. 2014;89:126-136.
5. Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinol. 2012;4:308-319.
6. Gruchlik A, Jurzak M, Chodurek, E, et al. Effect of GLY-GLY-HIS, GLY-HIS-LYS and their copper complexes on TNF-α-dependant IL-6 secretion in normal human dermal fibroblasts. Acta Pol Pharm. 2012;69:1303-1306.
7. Philips N, Hwang H, Chauhan S, et al. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect Tissue Res. 2010;51:224-229.
8. Wang Y, Wang M, Xiao S, et al. The anti-wrinkle efficacy of Argireline, a synthetic hexapeptide, in Chinese subjects. Am J Clin Dermatol. 2013;14:147-153.
9. Lupo MP, Cole A. Cosmeceutical peptides. Dermatol Ther. 2007;20:343-349.
10. Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24:303-310.
11. Jegasothy SM, Zabolotniaia V, Bielfeldt S. Efficacy of a new topical nano-hyaluronic acid in humans. J Clin Aesthet Dermatol. 2014;7:27-29.
12. Pavicic T, Gauglitz G, Lersch P, et al. Efficacy of cream-based novel formulations of hyaluronic acid of different molecular weights in anti-wrinkle treatment. J Drugs Dermatol. 2011;10:990-1000.
Practice Points
- Copper peptides could potentially be used in place of corticosteroids or nonsteroidal anti-inflammatory drugs for postprocedure irritation and inflammation.
- Acetyl hexapeptide-3 is a topical variation of botulinum toxin to be used on its own or adjunctively with the injectable form.
- Topical hyaluronic acid can be used on its own or adjunctively with injectable fillers.