User login
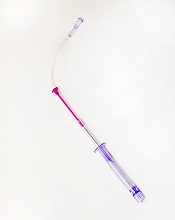
Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has cleared for marketing a device that reduces the need for venipunctures for in-hospital blood draws.
Velano Vascular’s blood-draw device resembles a common syringe.
It allows peripheral intravenous catheters to be repurposed to draw blood from patients, thereby reducing the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The single-use device will soon be used for clinical evaluation in select hospitals, including the University of Pennsylvania in Philadelphia and University Hospitals Case Medical Center of Cleveland in Ohio.
“A fundamental benefit of this technology is reducing the ‘pin cushion effect,’ in which hospitalized patients are ‘stuck’ several times daily to obtain blood tests,” said Eric M. Stone, co-founder and chief executive officer of Velano Vascular, the company developing the blood-draw device.
“Oftentimes, the draw procedure is plagued by multiple failed attempts. The FDA’s clearance of this novel technology validates the existing clinical need and will allow us to expedite our efforts to bring this innovation to patients, healthcare providers, and hospitals around the world.”
According to research conducted by Velano Vascular, 1 of every 3 hospital patients is stuck 2 or more times daily for blood draws, with a significant subset of these patients receiving 3 or more blood draws, along with numerous needle sticks.
Twenty-eight percent of adult venipunctures and 44% of pediatric venipunctures require more than one stick to successfully draw blood.
“Traditional blood draws are one of the most common and most problematic healthcare procedures,” said Karen Daley, PhD, RN, past president of the American Nurses Association and a healthcare worker safety advocate.
“It is an antiquated technology that creates pain and anxiety for many patients, a significant safety risk for healthcare professionals, and a real inefficiency in our healthcare system. Velano Vascular has developed a common-sense solution to this pervasive, long-standing problem.” ![]()

Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has cleared for marketing a device that reduces the need for venipunctures for in-hospital blood draws.
Velano Vascular’s blood-draw device resembles a common syringe.
It allows peripheral intravenous catheters to be repurposed to draw blood from patients, thereby reducing the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The single-use device will soon be used for clinical evaluation in select hospitals, including the University of Pennsylvania in Philadelphia and University Hospitals Case Medical Center of Cleveland in Ohio.
“A fundamental benefit of this technology is reducing the ‘pin cushion effect,’ in which hospitalized patients are ‘stuck’ several times daily to obtain blood tests,” said Eric M. Stone, co-founder and chief executive officer of Velano Vascular, the company developing the blood-draw device.
“Oftentimes, the draw procedure is plagued by multiple failed attempts. The FDA’s clearance of this novel technology validates the existing clinical need and will allow us to expedite our efforts to bring this innovation to patients, healthcare providers, and hospitals around the world.”
According to research conducted by Velano Vascular, 1 of every 3 hospital patients is stuck 2 or more times daily for blood draws, with a significant subset of these patients receiving 3 or more blood draws, along with numerous needle sticks.
Twenty-eight percent of adult venipunctures and 44% of pediatric venipunctures require more than one stick to successfully draw blood.
“Traditional blood draws are one of the most common and most problematic healthcare procedures,” said Karen Daley, PhD, RN, past president of the American Nurses Association and a healthcare worker safety advocate.
“It is an antiquated technology that creates pain and anxiety for many patients, a significant safety risk for healthcare professionals, and a real inefficiency in our healthcare system. Velano Vascular has developed a common-sense solution to this pervasive, long-standing problem.” ![]()

Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has cleared for marketing a device that reduces the need for venipunctures for in-hospital blood draws.
Velano Vascular’s blood-draw device resembles a common syringe.
It allows peripheral intravenous catheters to be repurposed to draw blood from patients, thereby reducing the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The single-use device will soon be used for clinical evaluation in select hospitals, including the University of Pennsylvania in Philadelphia and University Hospitals Case Medical Center of Cleveland in Ohio.
“A fundamental benefit of this technology is reducing the ‘pin cushion effect,’ in which hospitalized patients are ‘stuck’ several times daily to obtain blood tests,” said Eric M. Stone, co-founder and chief executive officer of Velano Vascular, the company developing the blood-draw device.
“Oftentimes, the draw procedure is plagued by multiple failed attempts. The FDA’s clearance of this novel technology validates the existing clinical need and will allow us to expedite our efforts to bring this innovation to patients, healthcare providers, and hospitals around the world.”
According to research conducted by Velano Vascular, 1 of every 3 hospital patients is stuck 2 or more times daily for blood draws, with a significant subset of these patients receiving 3 or more blood draws, along with numerous needle sticks.
Twenty-eight percent of adult venipunctures and 44% of pediatric venipunctures require more than one stick to successfully draw blood.
“Traditional blood draws are one of the most common and most problematic healthcare procedures,” said Karen Daley, PhD, RN, past president of the American Nurses Association and a healthcare worker safety advocate.
“It is an antiquated technology that creates pain and anxiety for many patients, a significant safety risk for healthcare professionals, and a real inefficiency in our healthcare system. Velano Vascular has developed a common-sense solution to this pervasive, long-standing problem.” ![]()