User login
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.
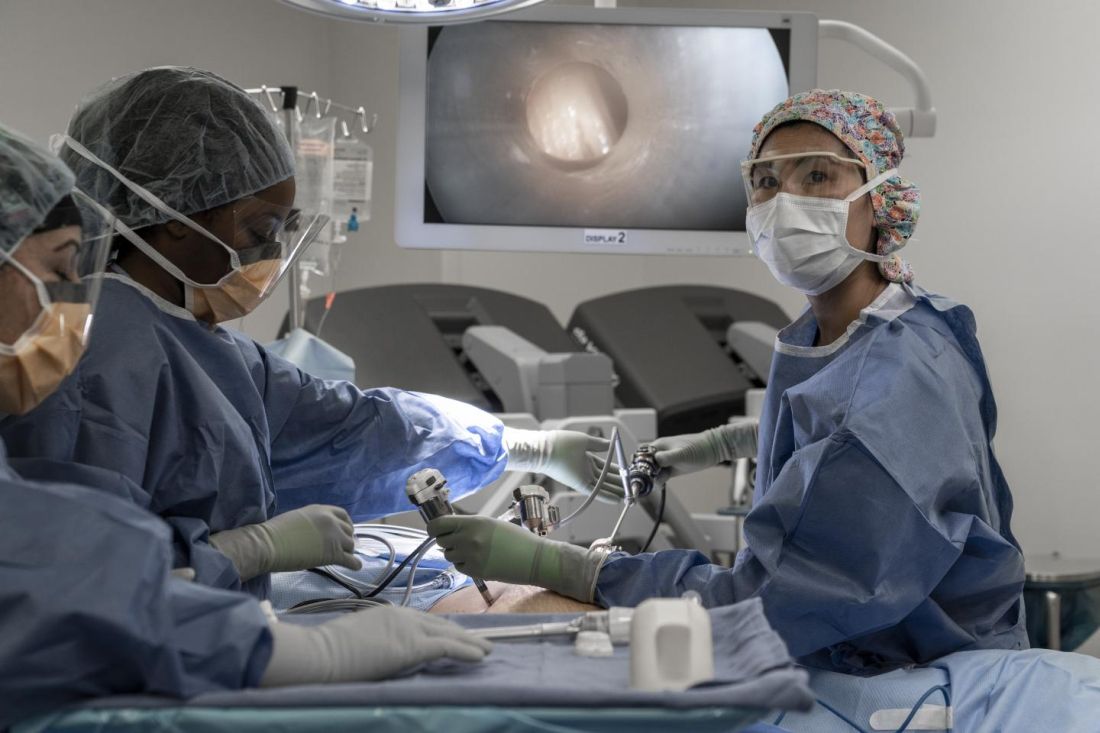
The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.

The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
A team of U.S. researchers is investigating whether pressurized intraperitoneal aerosolized chemotherapy (PIPAC) can benefit patients with advanced cancer and peritoneal carcinomatosis.

The team’s phase 1 trial is the first in the United States to test PIPAC, and it will enroll patients with ovarian, uterine, colorectal, or gastric cancer who have peritoneal carcinomatosis.
Data from studies outside the United States suggest PIPAC can induce regression of peritoneal carcinomatosis, even in end-stage, therapy-resistant gastric, ovarian, and colorectal cancers (Lancet Oncol. 2019 Jul;20[7]:e368-e377).
The current study (NCT04329494) formally introduces PIPAC to the United States and serves as a launching pad for further investigation into how the treatment should be administered and which types of chemotherapies can be used.
About PIPAC
“PIPAC is a novel therapeutic approach that is minimally invasive, does not require cytoreduction, and can be repeated frequently,” said Thanh Dellinger, MD, a gynecologic oncology surgeon at City of Hope in Duarte, Calif., and co–principal investigator of the phase 1 trial.
“[PIPAC] entails accessing the abdominal cavity using standard laparoscopic techniques and relies on the increased intra-abdominal pressure (15 mm Hg) achieved with laparoscopic surgery, which generates a convective flux that forces aerosolized chemotherapy drugs from the peritoneal cavity into the subperitoneal tissue and overcomes the tumor’s interstitial pressure,” Dr. Dellinger explained in an interview.
“The surgical procedure to deliver PIPAC does not typically cause adhesive disease and allows for repeated delivery of intraperitoneal chemotherapy, objective tumor staging, and response assessment,” she noted.
Dr. Dellinger said the advantages of PIPAC include a minimally invasive approach; no debulking surgery required; deeper uptake of drugs in tumor tissues; wider, more effective drug distribution; fewer toxicities caused by lower drug dosage; repeatable administration; and palliation of peritoneal carcinomatosis symptoms, including abdominal bloating and ascites.
PIPAC achieves a deeper peritoneal nodule penetration of several millimeters with cisplatin, compared to less than 1 mm with heated intraoperative peritoneal chemotherapy (HIPEC) and other intraperitoneal methods, according to Amit Merchea, MD, an assistant professor of surgery at the Mayo Clinic in Jacksonville, Fla.
Dr. Merchea performed the first PIPAC procedure in the United States in December 2019.
Innovative therapies needed
Peritoneal carcinomatosis is often a late-stage manifestation of abdominal cancers and is usually lethal, Dr. Dellinger said. She noted that systemic chemotherapy in the palliative setting is relatively ineffective in patients with peritoneal carcinomatosis because of pharmacokinetic limitations, poor peritoneal drug uptake, and impaired local drug distribution.
“Innovative, effective therapies are urgently needed for people who have ovarian, uterine, gastric, or colorectal cancer with peritoneal carcinomatosis,” Dr. Dellinger said.
“PIPAC is a novel treatment option that has had very favorable and exciting results,” Dr. Merchea said. “It is a potential option for patients when no other treatment options exist, and it is an avenue to provide hope to patients when often they have none.”
Potential candidates for PIPAC include patients who have peritoneal carcinomatosis, have failed other standard therapies, have more than 6 months’ life expectancy, and are not candidates for cytoreduction with HIPEC. There remains very limited data on the use of PIPAC as a neoadjuvant approach to convert patients who were previously unresectable to resectable disease, Dr. Merchea noted.
“To deliver chemotherapy directly to the tumor under pressure allows PIPAC to better penetrate the peritoneal surface and tumor nodules than traditional approaches, such as HIPEC,” Dr. Merchea said. “And the drug distribution at the tissue level is better than what is often achieved by systemic chemotherapy, but without the systemic effects of chemotherapy, such as hair loss. The treatment gives essentially a real-time, quantitative assessment of response by being able to directly assess the tumor via laparoscopic visualization and repeat biopsy.”
“Importantly, patients who undergo PIPAC don’t notice a decrease in their quality of life, and some patients note improvement, particularly with respect to nausea, vomiting, appetite, fatigue, and constipation,” Dr. Merchea said.
Trial details
The phase 1 trial of PIPAC will include a maximum of 24 patients. They will receive treatment every 6 weeks for up to three cycles and be followed for up to 3 years.
Patients with ovarian, uterine, or gastric cancer will undergo PIPAC with cisplatin, followed by doxorubicin. Patients with colorectal cancer will undergo PIPAC with oxaliplatin preceded by leucovorin and fluorouracil for cycles 2 and 3.
The researchers also plan to profile patients’ tumors.
“Tumor samples will be chronologically evaluated with genomics, spatial transcriptomics, pharmacodynamics, and single-cell sequencing throughout a patient’s treatment course, thus elucidating the treatment effects and natural history of peritoneal cancers,” Dr. Dellinger said.
The trial sites include City of Hope, Mayo Clinic in Florida, Northwell Health in New York, and the National Cancer Institute in Maryland.
The trial is sponsored by City of Hope in collaboration with the National Cancer Institute. Dr. Merchea and Dr. Dellinger reported having no conflicts of interest.
