User login
For many women, the postmenopausal loss of estrogen is associated with uncomfortable genitourinary symptoms, collectively referred to as the genitourinary syndrome of menopause (GSM). But despite the prevalence of GSM and the availability of treatments, most women do not seek relief.
This article reviews the syndrome and offers advice on how to talk about it with patients and what treatment options to consider.
A SYNDROME RECENTLY DEFINED
The term GSM and its definition were approved by the North American Menopause Society and the International Society for the Study of Women’s Sexual Health in 2014.1 It replaces older terms such as vulvovaginal atrophy, urogenital atrophy, and atrophic vaginitis.
GSM refers collectively to the symptoms associated with estrogen loss after menopause that adversely affect the vulvovaginal area and lower urinary tract. The most common symptoms are vulvovaginal dryness, burning, or irritation; sexual pain from inadequate lubrication; and urinary urgency, dysuria, or recurrent urinary tract infection.1,2
The definition notes that symptoms are self-reported as bothersome and are not the result of another disorder. Symptoms may be chronic and progressive, are not likely to resolve without treatment (pharmacologic or nonpharmacologic), and can have a significant negative impact on a woman’s quality of life and sexual health.1,2
COMMON BUT UNDERTREATED
From 40% to 60% of postmenopausal women experience GSM, but few seek treatment.3 Nevertheless, most postmenopausal women remain sexually active. In a 2008 survey of 94,000 postmenopausal women ages 50 to 79, 52% reported that they had been sexually active with a partner in the past year.4 However, 45% of postmenopausal women experienced unpleasant vaginal symptoms, according to a 2012 international survey of 3,520 postmenopausal women ages 55 to 65.5 In this survey, most respondents (75%) felt that vaginal symptoms had a negative impact on their life, but only 4% connected their symptoms to the vulvovaginal atrophy that resulted from loss of estrogen after menopause. Moreover, almost half were unaware of management options.5
These findings were supported by a 2013 survey of more than 3,000 US women who reported unpleasant vulvar and vaginal symptoms.6 From 60% to 85% noted negative sexual consequences from vulvovaginal symptoms, 47% felt their relationship suffered, and 27% felt it had a negative impact on their general enjoyment of life. In this study, 24% attributed their symptoms to menopause and 12% to hormonal changes. Although 56% had discussed GSM symptoms with a healthcare provider, only 40% were using GSM-specific topical treatments, mostly over-the-counter preparations.
Male partners of symptomatic women also note adverse emotional and physical effects.7 In an online survey of 4,100 men and 4,100 women ages 55 to 65, 52% to 78% of men and 58% to 64% of women expressed the negative effects of vulvovaginal symptoms on intimacy, libido, and sexual pain.
GSM is a progressive disorder. Women may note symptoms many years before menopause or have no symptoms until several years after menopause. One study found the prevalence of GSM to be 4% during perimenopause, rising after menopause to 25% after 1 year and to 47% after 3 years.8
Although distressing symptoms occur mostly after menopause, they may be seen in women of any age who experience a hypoestrogenic state, even if it is transient. Causes of this include premature ovarian failure, hypothalamic amenorrhea, and hyperprolactinemia. In addition, some treatments such as gonadotropin-releasing hormone agonists and aromatase inhibitors may cause vulvovaginal and lower urinary tract symptoms. Chemotherapy, radiation, and surgical removal of ovaries may also precipitate symptoms. The abrupt onset of menopause that may occur with these treatments is often associated with significantly greater sexual dysfunction and negative impact on quality of life. Cigarette smoking also leads to lower estrogen levels, which may contribute to GSM.
WHAT CAUSES GSM?
The genitourinary system develops from common embryologic tissue, the basis for the functional and clinical connection. Estrogen maintains the epithelium of the vagina, vulva, urethra, and bladder trigone via estrogen receptors present throughout these tissues.9
Premenopausal changes
Histologically, the estrogen-exposed vagina of a premenopausal woman is lined by glycogen-rich, stratified squamous epithelium, with underlying supportive fibromuscular layers. The epithelium is composed of superficial, intermediate, and parabasal cellular layers. In the presence of estrogen, the superficial and intermediate cellular levels predominate, with few parabasal cells.
Glycogen acts as a substrate for lactobacilli, producing organic acids, primarily lactate, that help maintain an acidic pH of 2.8 to 4.0. The low pH helps protect against pathologic shifts in the microbiome. Estrogen also maintains the collagen content of the epithelium, maintains acid mucopolysaccharides and hyaluronic acid, and optimizes vaginal blood flow. These effects result in optimal epithelial thickness and elasticity, moisture, vaginal secretions, and lubrication.10
Postmenopausal changes
Low levels of estrogen after menopause result in adverse anatomic, physiologic, and clinical changes in vaginal tissue. Effects of hypoestrogenism include the loss of collagen and adipose, leading to decreased elasticity and vaginal mucosal thinning. Vascular flow is decreased. The epithelial cytology transitions to a predominance of parabasal cells and a decrease in superficial and intermediate cells. Eccrine and apocrine glands become attenuated. These changes result in decreased vaginal secretions, diminished or delayed lubrication with sexual stimulation, friability of the vaginal vault, and vaginal dryness.11
Additionally, without estrogen, glycogen content is diminished, leading to decreased lactic acid production and a rise in vaginal pH to greater than 5. As the pH rises, Lactobacillus colonization decreases, leading to a further decrease in glycogen metabolism and to propagation of an elevated vaginal pH. The loss of vaginal acidity makes the vagina more susceptible to pathologic bacteria, including those found in the bowel and skin, sexually transmitted infections, and bacterial vaginosis.12
Other affected tissues. Anatomic effects of estrogen loss are not limited to the vagina. The epithelium, connective tissue, and smooth muscle of the vulva, vagina, urethra, and bladder trigone are also affected. The labia minora become thinner and regress, the introitus retracts, and narrowing and stricture of the vaginal canal and introitus may result. In some women, the urethral meatus becomes prominent relative to the introitus and more vulnerable to physical irritation, infection, and trauma.
Clinically, estrogen-related changes are usually responsible for vaginal dryness, irritation, burning, and superficial or deep dyspareunia. Urinary frequency, urgency, and incontinence also may develop.
THE DIAGNOSIS IS CLINICAL
The diagnosis of GSM is based on the history and physical examination. Standardized diagnostic tools for GSM are lacking, but some tools are available.
In 2006, the US Food and Drug Administration (FDA) published guidelines for industry to better define patient-reported outcome measures in clinical trials.13 The most significant addition was having the patient define the symptoms and rate how “bothersome” the symptoms are. Although this measure does not help diagnose GSM, it can be used effectively to follow response to treatment.
The Vaginal Symptom Questionnaire14 can be useful for assessing symptoms. It is a validated 21-item questionnaire that measures the quality-of-life impact of genital, but not urinary, symptoms of menopause.
Ask patients about symptoms
Healthcare providers should ask about GSM symptoms (Table 1) during routine clinical visits with women who are peri- or postmenopausal or who have hypoestrogenism from other causes, as many women are reluctant to initiate this discussion. Conversely, in women who present with sexual problems, such as difficulty with arousal or dyspareunia, GSM should be considered as a possible cause.
Specifically, ask women if they have any of the following symptoms:
- Vaginal itching, burning, discomfort, or irritation
- Malodorous or irritating vaginal discharge
- Urinary frequency, urgency, dysuria, urethral discomfort, or recurrent urinary tract infections
- Sexual symptoms of entry dyspareunia, vaginal pain, or irritation with sexual activity, which may be complicated by postcoital bleeding, spotting, or fissuring.
Vulvovaginal pain or irritation may be constant or may be present in the absence of sexual activity, such as with exercise, wearing tight clothing, or sitting for long periods.
Physical examination
Characteristic physical findings of GSM include scarce pubic hair, thinning of the labia from loss of labial fat, resorption of the labia minora, or fusion of the labia minora and majora (Table 2).15,16 The vulvar skin is pale and thin. The clitoral hood may retract, exposing the glans (which may lead to increased pain with sexual stimulation), or clitoral hood fusion may occur. The vagina is pale, dry, smooth, and shiny with loss of rugae; shortening or stricturing may be present. Vaginal elasticity decreases. Inflammation and petechiae (pinpoint, nonraised, round purple-red spots) may be present. The cervix may be flush with the vaginal fornices.
Prolonged atrophy may result in introital narrowing and friability, which may cause tearing with sexual activity or insertion of a speculum during pelvic examination. In addition, the epithelium of the lower urinary tract thins, and the muscular and fibrous layers atrophy—changes that may not be obvious during examination. A urethral caruncle may form, presenting as proliferative red tissue at the entrance of the urethra. Prolapse may become more prominent.
In women with severe genitourinary atrophy, pelvic examination may cause significant discomfort. Reassuring the patient that she can ask the clinician to stop at any time due to extreme discomfort is the first step in a successful pelvic examination.
In some situations, initial examination of the pelvic area may not include insertion of a speculum. Use of a hand-held mirror so the patient can observe the examination may help her relax during the examination.
Vaginal pH and cultures, if indicated, may be obtained by gently inserting a cotton-tipped swab into the vagina without a speculum and before applying lubricant. Lubricant should be used generously; in some instances, topical lidocaine gel (diluted, as it may burn) may be placed against the perineum on a gauze pad for 3 to 5 minutes before insertion of the speculum.
When an internal pelvic examination is necessary in a timely manner, such as with postmenopausal bleeding or a history of an abnormal Papanicolaou smear, but is too painful for the patient, the examination should be done under anesthesia.
Additional considerations
The history should review current medical conditions, medication use, nongenital skin disorders (eg, eczema), and systemic menopausal symptoms, such as hot flashes.
Also, consider other potential causes of GSM during the evaluation (Table 3).17,18 Review the use of detergents, soaps, douches, or over-the-counter topical products that could cause genitourinary symptoms secondary to contact irritation or allergy.
Any isolated, ulcerated, or nonhealing lesion should be biopsied. Reevaluate patients who have not responded to previous topical therapy or consider referral to a specialist.
Assess the personal, interpersonal, social, and sexual impact of the symptoms: if they do not cause distress, GSM does not require treatment. Nevertheless, potential treatment options should be discussed as symptoms may progress, making intervention necessary.
Laboratory tests: Helpful, not essential
Laboratory tests are unnecessary for the diagnosis of GSM. However, office-based objective evaluations such as vaginal pH testing and the maturation index can support the diagnosis.
The pH of the estrogenized vagina ranges from 3.8 to 4.2, whereas in women with GSM, the pH may reach 5.5 or higher. The pH can be obtained by placing a pH-sensitive paper against the lateral vaginal wall, avoiding any discharge or cervical mucus. A vaginal pH of 5 or greater in the absence of blood, semen, or infection suggests vulvovaginal atrophy.19
The vaginal maturation index is determined by a vaginal smear using Rakoff staining, in which 100 cells are counted and the number of parabasal, intermediate, and superficial cells is determined. In general, a well-estrogenized vagina has mostly superficial and intermediate cells, which shifts to a predominance of parabasal cells as estrogen levels decline.20
A recent review of vaginal atrophy suggests that after a diagnosis of GSM, healthcare providers can consider the most bothersome symptom along with the vaginal pH to assess the response to treatment.21 In general, schedule a follow-up appointment at 8 to 12 weeks to review treatment response. If treatment has not resulted in adequate symptom relief, consider a pelvic examination and further testing.
SELECTING A TREATMENT
Symptomatic women with GSM who desire intervention should be offered over-the-counter nonhormonal products as the first line of therapy.
If nonhormonal products are ineffective and there are no contraindications, locally applied estrogen in cream, tablet, or a ring delivery system may be offered. Local dehydroepiandrosterone (DHEA) inserts or ospemifene, an oral selective estrogen-receptor modulator, are FDA-approved for moderate to severe dyspareunia secondary to GSM.
Oral estrogen therapy is not indicated for vulvovaginal symptoms, but some women taking systemic estrogen for vasomotor symptoms may need additional local estrogen application to relieve vaginal symptoms.
Nonhormonal treatments
Nonhormonal over-the-counter therapies provide sufficient relief for most women with mild symptoms. There is a plethora of products, so practitioners need to offer guidance to help women with their individual choices.
Vaginal lubricants are intended for use with sexual or penetrative activity (including pelvic examination). They provide short-term relief of symptoms, but there is no evidence of any impact on histologic changes of atrophy. They are meant to relieve friction. Lubricants may be water-based, oil-based, silicone-based, or a combination. Individual products have different effects on condom integrity. Perfumed, warming, or stimulating products may be irritating to some women and should be tried initially in small amounts.
Vaginal moisturizers are intended to treat GSM. They are applied regularly, not just with vaginal activity, usually once or twice a week. Some vaginal lubricants can maintain an acidic pH in the vagina and may reverse the histologic changes of atrophy. Symptomatic improvement over placebo or estrogen has been shown in clinical trials.22–24
Women should be advised that trial and error in choosing products may be necessary to establish a successful regimen. Products should be tried in succession, not simultaneously, with a “wash-out” period between, to be able to evaluate response.
Vaginal dilators and pelvic floor physical therapy
Sexual activity, either by self-stimulation or with a partner, helps maintain vaginal health by contributing to increased vascularity and elasticity of tissue. Women who resume sexual activity after a long period of inactivity may benefit from the use of vaginal dilators, which aid both in mechanical distention and progressive relaxation of the vaginal musculature.
In some women, long-term dyspareunia may result in vaginismus, an involuntary contraction of the vaginal musculature. For these women, dilators may be effective. Additional options focus on pelvic floor physical therapy, which can isolate trigger points, using biofeedback to teach relaxation and home exercises such as vaginal massage.
HORMONAL THERAPIES
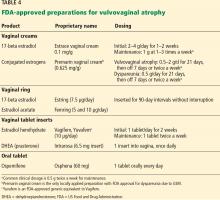
If nonhormonal lubricants and moisturizers do not achieve satisfactory symptomatic relief, FDA-approved hormonal therapies (Table 4) include estrogen-containing vaginal creams, rings, and a tablet; a vaginal tablet containing DHEA; and an oral tablet containing ospemifene.
Estrogen products
For patients whose symptoms do not respond to nonhormonal therapies, low-dose, locally applied estrogen therapy is the first treatment recommended.2 Locally applied estrogens can reverse the atrophic changes of estrogen deprivation, resulting in an increase in blood flow, elasticity, and vaginal wall thickness. This therapy also can normalize pH levels with subsequent restoration of a healthy lactobacilli-based flora. Locally applied estrogens also have been shown to decrease the frequency of recurrent urinary tract infection.25
Estrogen-containing vaginal creams, rings, and a tablet are available, and each has been shown to be effective for GSM. Locally applied estrogens at recommended dosages tend to have fewer adverse events and risks than systemic estrogens.26 Estradiol levels generally do not exceed levels found in the untreated menopausal population, although a dose- and duration-dependent increase in systemic levels may occur.27
Dosing considerations
The vaginal ring and the vaginal tablet provide the lowest prefixed daily dose of estradiol (7.5 and 10 µg daily, respectively). Estrogen creams (estradiol, conjugated equine estrogens) are more readily absorbed, and dosing should be tapered to the lowest, most effective dose for symptom relief.
The FDA-approved doses for vaginal creams containing 17-beta estradiol are higher than the dose found to be effective in clinical practice (0.5 g twice a week). Most practitioners start with the lower dose, reserving the FDA-approved higher doses for patients who do not obtain adequate relief over 6 to 8 weeks of treatment. The conjugated-estrogen vaginal cream Premarin is the only locally applied estrogen approved by the FDA to treat dyspareunia. It is dosed at 0.5 g intravaginally for 21 days and is then either withdrawn for 7 days or, more commonly, administered at 0.5 g twice a week.
Initial treatment with vaginal cream may require more frequent vulvovaginal application, such as daily for 1 to 2 weeks. Women with vaginal fissures or tearing will benefit from externally applied creams in addition to internal applications. Response to therapy is usually seen within 4 to 6 weeks from onset of treatment. Once symptom relief is obtained, treatment should continue indefinitely. Although long-term safety studies are lacking, risks are believed to be minimal.
Endometrial impact. Women with contraindications to systemic estrogen should be counseled about possible small increases in serum levels of estradiol associated with locally applied estrogens and the potential risks and benefits those increases incur. Endometrial surveillance with either transvaginal ultrasonography or endometrial sampling is not required, even with long-term use, but it should be considered with higher doses or more frequent applications.
Similarly, progesterone replacement for endometrial protection is not recommended but can be considered in women with an intact uterus at high risk of endometrial cancer, such as obese patients. If a systemic progestational agent is considered, the risks and benefits should be weighed carefully. Even in women at high risk, endometrial surveillance may be the most appropriate option.28 Uterine bleeding that occurs should be considered abnormal and should be investigated.
DHEA (prasterone)
In 2016, the FDA approved intravaginal prasterone, a DHEA-containing product for the treatment of dyspareunia secondary to moderate to severe vulvovaginal atrophy caused by menopause. DHEA is an endogenous steroid that is converted by aromatase activity into testosterone and estradiol.
Clinical trials have found that 12 weeks of vaginal DHEA supplementation (0.25%, 0.5%, and 1% DHEA ovules) was more effective than placebo in improving vaginal dryness and dyspareunia in women with GSM.29–31 In these studies, locally applied DHEA decreased parabasal cells, decreased vaginal pH, increased vaginal secretions, and improved epithelial surface thickness and integrity without any significant impact on serum levels of DHEA, DHEA-sulfate, estradiol, testosterone, or their metabolites. Importantly, transvaginal DHEA had negligible endometrial effect.
The breast cancer risk associated with vaginal DHEA has not been fully evaluated. However, labeling lists breast cancer as a warning, not a contraindication.
Selective estrogen-receptor modulator
In 2013, the FDA approved ospemifene for the treatment of dyspareunia caused by GSM. Ospemifene, an estrogen agonist in the vagina, is taken daily as a 60-mg oral dose. Long-term safety studies suggested no adverse effects on the endometrium or breast for at least 52 weeks.32
These studies also noted that ospemifene improved the vaginal maturation index (decreased parabasal cells and increased superficial cells) and decreased vaginal pH. It has further been shown to decrease severity of the self-identified most bothersome symptom—dyspareunia or vaginal dryness—compared with placebo.33
Potential increases in hot flashes, which may occur in up to 7% of patients, and the risk of blood clots should be considered. Additionally, the safety of ospemifene in women with a history of breast cancer has not been established. Although early studies suggest it either has no effect or possibly a protective effect on breast tissue, the FDA does not recommend its use in women at risk for breast cancer. Long-term effects on bone are unknown.
The labeling for ospemifene includes a boxed warning about the risk of stroke, blood clots, and cancer of the lining of the uterus. Patients should be counseled about worrisome signs or symptoms that require medical attention.
ALTERNATIVE THERAPIES
Treatments for GSM not approved by the FDA include laser and radiofrequency therapies, testosterone, isoflavones, and bioidentical hormones.
Laser and radiofrequency therapies
Both of these therapies aim to promote tissue remodeling with increased collagen and elastin production and increased vascularity. This, in turn, increases muscle support and tone.
Laser therapies act by ablating and coagulating vaginal tissues; radiofrequency therapies directly heat the tissue. Both treatments are office-based, require up to 3 initial treatments, and are followed by retreatment at approximately 1-year intervals.
Studies have reported high patient satisfaction rates (91% to 100%), improved sexual functioning, and decreased GSM symptoms of vaginal dryness, burning, itching, and dyspareunia.34–36 Data, however, are from observational studies, not placebo-controlled trials.
Although laser and radiofrequency therapies are FDA-approved for several indications, laser treatment for symptoms of vulvovaginal atrophy is not currently an approved indication. Patients should be advised of this.
Testosterone
Locally applied testosterone was shown in a small study to improve dyspareunia and vaginal dryness associated with aromatase inhibitor use in breast cancer patients.37 However, due to the lack of safety and efficacy data from larger, controlled trials, testosterone therapy is not currently recommended.
Isoflavones
Isoflavones are phytoestrogens found in soy. In a 12-week, double-blind placebo-controlled study of vaginally applied 4% soy isoflavone gel, improvements in vaginal atrophy symptoms, maturation values, and vaginal pH were found in 60 postmenopausal women.38 Additional data on efficacy and safety are needed before isoflavones should be considered as a treatment for GSM.
Bioidentical hormones
Bioidentical hormones are plant-derived hormones that are chemically similar or identical to those produced by the body. Although there are FDA-approved bioidentical hormones (eg, micronized progesterone, estradiol, DHEA), the term bioidentical usually refers to non-FDA-approved, commercially available hormones produced and compounded by specialty pharmacies.
Patients often view these substances as being better, safer, and more acceptable for use, and healthcare practitioners need to be prepared to address these beliefs. The FDA and the American College of Obstetricians and Gynecologists consider bioidentical hormones to be a marketing term and not an alternative treatment based on scientific evidence.39 Patients should be informed that bioidentical hormones have the same risks as any similar hormone preparation along with additional risks related to potential lack of purity and potency. Further, they have not been adequately studied in controlled clinical trials.
FOLLOW-UP CARE
Healthcare providers caring for women should assume a proactive role in diagnosing and treating the symptoms of GSM. And once diagnosis of GSM is established and treatment is under way, practitioners can use symptom questionnaires and vaginal pH testing as easy and reliable means of measuring clinical response to therapy.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014; 21(10):1063–1068. doi:10.1097/GME.0000000000000329
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–904. doi:10.1097/GME.0b013e3182a122c2
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health 2013; 5:437–447. doi:10.2147/IJWH.S44579
- McCall-Hosenfeld JS, Jaramillo SA, Legault C, et al; Members of Women’s Health Initiative-Observational Study. Correlates of sexual satisfaction among sexually active postmenopausal women in the Women’s Health Initiative-Observational Study. J Gen Intern Med 2008; 23(12):2000–2009. doi:10.1007/s11606-008-0820-9
- Nappi RE, Kokot-Kierepa M. Vaginal Health: Insights, Views & Attitudes (VIVA): results from an international survey. Climacteric 2012; 15(1):36–44. doi:10.3109/13697137.2011.647840
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal Changes) survey. J Sex Med 2013; 10(7):1790–1799. doi:10.1111/jsm.12190
- Nappi RE, Kingsberg S, Maamari R, Simon J. The CLOSER (Clarifying Vaginal Atrophy’s Impact On Sex and Relationships) survey: implications of vaginal discomfort in postmenopausal women and in male partners. J Sex Med 2013; 10(9):2232–2241. doi:10.1111/jsm.12235
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol 2000; 96(3):351–358. pmid:10960625
- Stika CS. Atrophic vaginitis. Dermatol Ther 2010; 23(5):514–522. doi:10.1111/j.1529-8019.2010.01354.x
- Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Juliá MD. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas 2005; 52(suppl 1):S46–S52. doi:10.1016/j.maturitas.2005.06.014
- Forsberg JG. A morphologist’s approach to the vagina—age-related changes and estrogen sensitivity. Maturitas 1995; 22(suppl):S7–S15.
- Martin DH. The microbiota of the vagina and its influence on women’s health and disease. Am J Med Sci 2012; 343(1):2–9. doi:10.1097/MAJ.0b013e31823ea228
- US Department of Health and Human Services; Food and Drug Administration (FDA). Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims, 2006. doi:10.1186/1477-7525-4-79
- Erekson EA, Yip SO, Wedderburn TS, et al. The Vulvovaginal Symptoms Questionnaire: a questionnaire for measuring vulvovaginal symptoms in postmenopausal women. Menopause 2013; 20(9):973–979. doi:10.1097/GME.0b013e318282600b
- Johnston SL, Farrell SA, Bouchard C, et al; SOGC Joint Committee-Clinical Practice Gynaecology and Urogynaecology. The detection and management of vaginal atrophy. J Obstet Gynaecol Can 2004; 26(5):503–515. doi:10.1016/S1701-2163(16)30662-4
- Oriba HA, Maibach HI. Vulvar transepidermal water loss (TEWL) decay curves. Effect of occlusion, delipidation, and age. Acta Derm Venereol 1989; 69(6):461–465. pmid:2575316
- Bachmann G, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician 2000; 61(10):3090–3096. pmid:10839558
- MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010; 85(1):87–94. doi:10.4065/mcp.2009.0413
- Nilsson K, Risberg B, Heimer G. The vaginal epithelium in the post menopause--cytology, histology and pH as methods of assessment. Maturitas 1995; 21(1):51–56. pmid:7731384
- McEndree B. Clinical application of the vaginal maturation index. Nurse Pract 1999; 24(9):48–56. pmid:10507070
- Weber MA, Limpens J, Roovers JP. Assessment of vaginal atrophy: a review. Int Urogynecol J 2015; 26(1):15–28. doi:10.1007/s00192-014-2464-0
- Lee YK, Chung HH, Kim JW, Park NH, Song YS, Kang SB. Vaginal pH-balanced gel for the control of atrophic vaginitis among breast cancer survivors: a randomized controlled trial. Obstet Gynecol 2011; 117(4):922–927. doi:10.1097/AOG.0b013e3182118790
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas 1996; 23(3):259–263. pmid:8794418
- Nachtigall LE. Comparative study: replens versus local estrogen in menopausal women. Fertil Steril 1994; 61(1):178–180. pmid:8293835
- Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis 2000; 30(1):152–156. doi:10.1086/313596
- Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2006; 4:CD001500. doi:10.1002/14651858.CD001500
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–126. doi:10.3109/13697137.2014.947254
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause 2010; 17(2):242–255. doi:10.1097/gme.0b013e3181d0f6b9
- Labrie F, Archer D, Bouchard C, et al. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause 2009; 16(5):923–931. doi:10.1097/gme.0b013e31819e85c6
- Labrie F, Archer D, Bouchard C, et al. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause 2009; 16(5):907–922. doi:10.1097/gme.0b013e31819e8e2d
- Archer DF. Dehydroepiandrosterone intra vaginal administration for the management of postmenopausal vulvovaginal atrophy. J Steroid Biochem Mol Biol 2015; 145:139–143. doi:10.1016/j.jsbmb.2014.09.003
- Wurz GT, Kao CJ, DeGregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging 2014; 9:1939–1950. doi:10.2147/CIA.S73753
- Constantine G, Graham S, Portman DJ, Rosen RC, Kingsberg SA. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric 2015; 18(2):226–232. doi:10.3109/13697137.2014.954996
- Arroyo C. Fractional CO2 laser treatment for vulvovaginal atrophy symptoms and vaginal rejuvenation in perimenopausal women. Int J Womens Health 2017; 9:591–595. doi:10.2147/IJWH.S136857
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas 2015; 80(3):296–301. doi:10.1016/j.maturitas.2014.12.006
- Salvatore S, Nappi R, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric 2014; 17(4):363–369. doi:10.3109/13697137.2014.899347
- Witherby S, Johnson J, Demers L, et al. Topical testosterone for breast cancer patients with vaginal atrophy related to aromatase inhibitors: a phase I/II study. Oncologist 2011; 16(4):424–431. doi:10.1634/theoncologist.2010-0435
- Lima SM, Bernardo BF, Yamada SS, Reis BF, da Silva GM, Galvão MA. Effects of Glycine max (L.) Merr. soy isoflavone vaginal gel on epithelium morphology and estrogen receptor expression in postmenopausal women: a 12-week, randomized, double-blind, placebo-controlled trial. Maturitas 2014; 78(3):205–211. doi:10.1016/j.maturitas.2014.04.007
- Committee on Gynecologic Practice and the American Society for Reproductive Medicine Practice Committee. Committee opinion No 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol 2012; 120(2 pt 1):411–415. doi:10.1097/AOG.0b013e318268049e
For many women, the postmenopausal loss of estrogen is associated with uncomfortable genitourinary symptoms, collectively referred to as the genitourinary syndrome of menopause (GSM). But despite the prevalence of GSM and the availability of treatments, most women do not seek relief.
This article reviews the syndrome and offers advice on how to talk about it with patients and what treatment options to consider.
A SYNDROME RECENTLY DEFINED
The term GSM and its definition were approved by the North American Menopause Society and the International Society for the Study of Women’s Sexual Health in 2014.1 It replaces older terms such as vulvovaginal atrophy, urogenital atrophy, and atrophic vaginitis.
GSM refers collectively to the symptoms associated with estrogen loss after menopause that adversely affect the vulvovaginal area and lower urinary tract. The most common symptoms are vulvovaginal dryness, burning, or irritation; sexual pain from inadequate lubrication; and urinary urgency, dysuria, or recurrent urinary tract infection.1,2
The definition notes that symptoms are self-reported as bothersome and are not the result of another disorder. Symptoms may be chronic and progressive, are not likely to resolve without treatment (pharmacologic or nonpharmacologic), and can have a significant negative impact on a woman’s quality of life and sexual health.1,2
COMMON BUT UNDERTREATED
From 40% to 60% of postmenopausal women experience GSM, but few seek treatment.3 Nevertheless, most postmenopausal women remain sexually active. In a 2008 survey of 94,000 postmenopausal women ages 50 to 79, 52% reported that they had been sexually active with a partner in the past year.4 However, 45% of postmenopausal women experienced unpleasant vaginal symptoms, according to a 2012 international survey of 3,520 postmenopausal women ages 55 to 65.5 In this survey, most respondents (75%) felt that vaginal symptoms had a negative impact on their life, but only 4% connected their symptoms to the vulvovaginal atrophy that resulted from loss of estrogen after menopause. Moreover, almost half were unaware of management options.5
These findings were supported by a 2013 survey of more than 3,000 US women who reported unpleasant vulvar and vaginal symptoms.6 From 60% to 85% noted negative sexual consequences from vulvovaginal symptoms, 47% felt their relationship suffered, and 27% felt it had a negative impact on their general enjoyment of life. In this study, 24% attributed their symptoms to menopause and 12% to hormonal changes. Although 56% had discussed GSM symptoms with a healthcare provider, only 40% were using GSM-specific topical treatments, mostly over-the-counter preparations.
Male partners of symptomatic women also note adverse emotional and physical effects.7 In an online survey of 4,100 men and 4,100 women ages 55 to 65, 52% to 78% of men and 58% to 64% of women expressed the negative effects of vulvovaginal symptoms on intimacy, libido, and sexual pain.
GSM is a progressive disorder. Women may note symptoms many years before menopause or have no symptoms until several years after menopause. One study found the prevalence of GSM to be 4% during perimenopause, rising after menopause to 25% after 1 year and to 47% after 3 years.8
Although distressing symptoms occur mostly after menopause, they may be seen in women of any age who experience a hypoestrogenic state, even if it is transient. Causes of this include premature ovarian failure, hypothalamic amenorrhea, and hyperprolactinemia. In addition, some treatments such as gonadotropin-releasing hormone agonists and aromatase inhibitors may cause vulvovaginal and lower urinary tract symptoms. Chemotherapy, radiation, and surgical removal of ovaries may also precipitate symptoms. The abrupt onset of menopause that may occur with these treatments is often associated with significantly greater sexual dysfunction and negative impact on quality of life. Cigarette smoking also leads to lower estrogen levels, which may contribute to GSM.
WHAT CAUSES GSM?
The genitourinary system develops from common embryologic tissue, the basis for the functional and clinical connection. Estrogen maintains the epithelium of the vagina, vulva, urethra, and bladder trigone via estrogen receptors present throughout these tissues.9
Premenopausal changes
Histologically, the estrogen-exposed vagina of a premenopausal woman is lined by glycogen-rich, stratified squamous epithelium, with underlying supportive fibromuscular layers. The epithelium is composed of superficial, intermediate, and parabasal cellular layers. In the presence of estrogen, the superficial and intermediate cellular levels predominate, with few parabasal cells.
Glycogen acts as a substrate for lactobacilli, producing organic acids, primarily lactate, that help maintain an acidic pH of 2.8 to 4.0. The low pH helps protect against pathologic shifts in the microbiome. Estrogen also maintains the collagen content of the epithelium, maintains acid mucopolysaccharides and hyaluronic acid, and optimizes vaginal blood flow. These effects result in optimal epithelial thickness and elasticity, moisture, vaginal secretions, and lubrication.10
Postmenopausal changes
Low levels of estrogen after menopause result in adverse anatomic, physiologic, and clinical changes in vaginal tissue. Effects of hypoestrogenism include the loss of collagen and adipose, leading to decreased elasticity and vaginal mucosal thinning. Vascular flow is decreased. The epithelial cytology transitions to a predominance of parabasal cells and a decrease in superficial and intermediate cells. Eccrine and apocrine glands become attenuated. These changes result in decreased vaginal secretions, diminished or delayed lubrication with sexual stimulation, friability of the vaginal vault, and vaginal dryness.11
Additionally, without estrogen, glycogen content is diminished, leading to decreased lactic acid production and a rise in vaginal pH to greater than 5. As the pH rises, Lactobacillus colonization decreases, leading to a further decrease in glycogen metabolism and to propagation of an elevated vaginal pH. The loss of vaginal acidity makes the vagina more susceptible to pathologic bacteria, including those found in the bowel and skin, sexually transmitted infections, and bacterial vaginosis.12
Other affected tissues. Anatomic effects of estrogen loss are not limited to the vagina. The epithelium, connective tissue, and smooth muscle of the vulva, vagina, urethra, and bladder trigone are also affected. The labia minora become thinner and regress, the introitus retracts, and narrowing and stricture of the vaginal canal and introitus may result. In some women, the urethral meatus becomes prominent relative to the introitus and more vulnerable to physical irritation, infection, and trauma.
Clinically, estrogen-related changes are usually responsible for vaginal dryness, irritation, burning, and superficial or deep dyspareunia. Urinary frequency, urgency, and incontinence also may develop.
THE DIAGNOSIS IS CLINICAL
The diagnosis of GSM is based on the history and physical examination. Standardized diagnostic tools for GSM are lacking, but some tools are available.
In 2006, the US Food and Drug Administration (FDA) published guidelines for industry to better define patient-reported outcome measures in clinical trials.13 The most significant addition was having the patient define the symptoms and rate how “bothersome” the symptoms are. Although this measure does not help diagnose GSM, it can be used effectively to follow response to treatment.
The Vaginal Symptom Questionnaire14 can be useful for assessing symptoms. It is a validated 21-item questionnaire that measures the quality-of-life impact of genital, but not urinary, symptoms of menopause.
Ask patients about symptoms
Healthcare providers should ask about GSM symptoms (Table 1) during routine clinical visits with women who are peri- or postmenopausal or who have hypoestrogenism from other causes, as many women are reluctant to initiate this discussion. Conversely, in women who present with sexual problems, such as difficulty with arousal or dyspareunia, GSM should be considered as a possible cause.
Specifically, ask women if they have any of the following symptoms:
- Vaginal itching, burning, discomfort, or irritation
- Malodorous or irritating vaginal discharge
- Urinary frequency, urgency, dysuria, urethral discomfort, or recurrent urinary tract infections
- Sexual symptoms of entry dyspareunia, vaginal pain, or irritation with sexual activity, which may be complicated by postcoital bleeding, spotting, or fissuring.
Vulvovaginal pain or irritation may be constant or may be present in the absence of sexual activity, such as with exercise, wearing tight clothing, or sitting for long periods.
Physical examination
Characteristic physical findings of GSM include scarce pubic hair, thinning of the labia from loss of labial fat, resorption of the labia minora, or fusion of the labia minora and majora (Table 2).15,16 The vulvar skin is pale and thin. The clitoral hood may retract, exposing the glans (which may lead to increased pain with sexual stimulation), or clitoral hood fusion may occur. The vagina is pale, dry, smooth, and shiny with loss of rugae; shortening or stricturing may be present. Vaginal elasticity decreases. Inflammation and petechiae (pinpoint, nonraised, round purple-red spots) may be present. The cervix may be flush with the vaginal fornices.
Prolonged atrophy may result in introital narrowing and friability, which may cause tearing with sexual activity or insertion of a speculum during pelvic examination. In addition, the epithelium of the lower urinary tract thins, and the muscular and fibrous layers atrophy—changes that may not be obvious during examination. A urethral caruncle may form, presenting as proliferative red tissue at the entrance of the urethra. Prolapse may become more prominent.
In women with severe genitourinary atrophy, pelvic examination may cause significant discomfort. Reassuring the patient that she can ask the clinician to stop at any time due to extreme discomfort is the first step in a successful pelvic examination.
In some situations, initial examination of the pelvic area may not include insertion of a speculum. Use of a hand-held mirror so the patient can observe the examination may help her relax during the examination.
Vaginal pH and cultures, if indicated, may be obtained by gently inserting a cotton-tipped swab into the vagina without a speculum and before applying lubricant. Lubricant should be used generously; in some instances, topical lidocaine gel (diluted, as it may burn) may be placed against the perineum on a gauze pad for 3 to 5 minutes before insertion of the speculum.
When an internal pelvic examination is necessary in a timely manner, such as with postmenopausal bleeding or a history of an abnormal Papanicolaou smear, but is too painful for the patient, the examination should be done under anesthesia.
Additional considerations
The history should review current medical conditions, medication use, nongenital skin disorders (eg, eczema), and systemic menopausal symptoms, such as hot flashes.
Also, consider other potential causes of GSM during the evaluation (Table 3).17,18 Review the use of detergents, soaps, douches, or over-the-counter topical products that could cause genitourinary symptoms secondary to contact irritation or allergy.
Any isolated, ulcerated, or nonhealing lesion should be biopsied. Reevaluate patients who have not responded to previous topical therapy or consider referral to a specialist.
Assess the personal, interpersonal, social, and sexual impact of the symptoms: if they do not cause distress, GSM does not require treatment. Nevertheless, potential treatment options should be discussed as symptoms may progress, making intervention necessary.
Laboratory tests: Helpful, not essential
Laboratory tests are unnecessary for the diagnosis of GSM. However, office-based objective evaluations such as vaginal pH testing and the maturation index can support the diagnosis.
The pH of the estrogenized vagina ranges from 3.8 to 4.2, whereas in women with GSM, the pH may reach 5.5 or higher. The pH can be obtained by placing a pH-sensitive paper against the lateral vaginal wall, avoiding any discharge or cervical mucus. A vaginal pH of 5 or greater in the absence of blood, semen, or infection suggests vulvovaginal atrophy.19
The vaginal maturation index is determined by a vaginal smear using Rakoff staining, in which 100 cells are counted and the number of parabasal, intermediate, and superficial cells is determined. In general, a well-estrogenized vagina has mostly superficial and intermediate cells, which shifts to a predominance of parabasal cells as estrogen levels decline.20
A recent review of vaginal atrophy suggests that after a diagnosis of GSM, healthcare providers can consider the most bothersome symptom along with the vaginal pH to assess the response to treatment.21 In general, schedule a follow-up appointment at 8 to 12 weeks to review treatment response. If treatment has not resulted in adequate symptom relief, consider a pelvic examination and further testing.
SELECTING A TREATMENT
Symptomatic women with GSM who desire intervention should be offered over-the-counter nonhormonal products as the first line of therapy.
If nonhormonal products are ineffective and there are no contraindications, locally applied estrogen in cream, tablet, or a ring delivery system may be offered. Local dehydroepiandrosterone (DHEA) inserts or ospemifene, an oral selective estrogen-receptor modulator, are FDA-approved for moderate to severe dyspareunia secondary to GSM.
Oral estrogen therapy is not indicated for vulvovaginal symptoms, but some women taking systemic estrogen for vasomotor symptoms may need additional local estrogen application to relieve vaginal symptoms.
Nonhormonal treatments
Nonhormonal over-the-counter therapies provide sufficient relief for most women with mild symptoms. There is a plethora of products, so practitioners need to offer guidance to help women with their individual choices.
Vaginal lubricants are intended for use with sexual or penetrative activity (including pelvic examination). They provide short-term relief of symptoms, but there is no evidence of any impact on histologic changes of atrophy. They are meant to relieve friction. Lubricants may be water-based, oil-based, silicone-based, or a combination. Individual products have different effects on condom integrity. Perfumed, warming, or stimulating products may be irritating to some women and should be tried initially in small amounts.
Vaginal moisturizers are intended to treat GSM. They are applied regularly, not just with vaginal activity, usually once or twice a week. Some vaginal lubricants can maintain an acidic pH in the vagina and may reverse the histologic changes of atrophy. Symptomatic improvement over placebo or estrogen has been shown in clinical trials.22–24
Women should be advised that trial and error in choosing products may be necessary to establish a successful regimen. Products should be tried in succession, not simultaneously, with a “wash-out” period between, to be able to evaluate response.
Vaginal dilators and pelvic floor physical therapy
Sexual activity, either by self-stimulation or with a partner, helps maintain vaginal health by contributing to increased vascularity and elasticity of tissue. Women who resume sexual activity after a long period of inactivity may benefit from the use of vaginal dilators, which aid both in mechanical distention and progressive relaxation of the vaginal musculature.
In some women, long-term dyspareunia may result in vaginismus, an involuntary contraction of the vaginal musculature. For these women, dilators may be effective. Additional options focus on pelvic floor physical therapy, which can isolate trigger points, using biofeedback to teach relaxation and home exercises such as vaginal massage.
HORMONAL THERAPIES
If nonhormonal lubricants and moisturizers do not achieve satisfactory symptomatic relief, FDA-approved hormonal therapies (Table 4) include estrogen-containing vaginal creams, rings, and a tablet; a vaginal tablet containing DHEA; and an oral tablet containing ospemifene.
Estrogen products
For patients whose symptoms do not respond to nonhormonal therapies, low-dose, locally applied estrogen therapy is the first treatment recommended.2 Locally applied estrogens can reverse the atrophic changes of estrogen deprivation, resulting in an increase in blood flow, elasticity, and vaginal wall thickness. This therapy also can normalize pH levels with subsequent restoration of a healthy lactobacilli-based flora. Locally applied estrogens also have been shown to decrease the frequency of recurrent urinary tract infection.25
Estrogen-containing vaginal creams, rings, and a tablet are available, and each has been shown to be effective for GSM. Locally applied estrogens at recommended dosages tend to have fewer adverse events and risks than systemic estrogens.26 Estradiol levels generally do not exceed levels found in the untreated menopausal population, although a dose- and duration-dependent increase in systemic levels may occur.27
Dosing considerations
The vaginal ring and the vaginal tablet provide the lowest prefixed daily dose of estradiol (7.5 and 10 µg daily, respectively). Estrogen creams (estradiol, conjugated equine estrogens) are more readily absorbed, and dosing should be tapered to the lowest, most effective dose for symptom relief.
The FDA-approved doses for vaginal creams containing 17-beta estradiol are higher than the dose found to be effective in clinical practice (0.5 g twice a week). Most practitioners start with the lower dose, reserving the FDA-approved higher doses for patients who do not obtain adequate relief over 6 to 8 weeks of treatment. The conjugated-estrogen vaginal cream Premarin is the only locally applied estrogen approved by the FDA to treat dyspareunia. It is dosed at 0.5 g intravaginally for 21 days and is then either withdrawn for 7 days or, more commonly, administered at 0.5 g twice a week.
Initial treatment with vaginal cream may require more frequent vulvovaginal application, such as daily for 1 to 2 weeks. Women with vaginal fissures or tearing will benefit from externally applied creams in addition to internal applications. Response to therapy is usually seen within 4 to 6 weeks from onset of treatment. Once symptom relief is obtained, treatment should continue indefinitely. Although long-term safety studies are lacking, risks are believed to be minimal.
Endometrial impact. Women with contraindications to systemic estrogen should be counseled about possible small increases in serum levels of estradiol associated with locally applied estrogens and the potential risks and benefits those increases incur. Endometrial surveillance with either transvaginal ultrasonography or endometrial sampling is not required, even with long-term use, but it should be considered with higher doses or more frequent applications.
Similarly, progesterone replacement for endometrial protection is not recommended but can be considered in women with an intact uterus at high risk of endometrial cancer, such as obese patients. If a systemic progestational agent is considered, the risks and benefits should be weighed carefully. Even in women at high risk, endometrial surveillance may be the most appropriate option.28 Uterine bleeding that occurs should be considered abnormal and should be investigated.
DHEA (prasterone)
In 2016, the FDA approved intravaginal prasterone, a DHEA-containing product for the treatment of dyspareunia secondary to moderate to severe vulvovaginal atrophy caused by menopause. DHEA is an endogenous steroid that is converted by aromatase activity into testosterone and estradiol.
Clinical trials have found that 12 weeks of vaginal DHEA supplementation (0.25%, 0.5%, and 1% DHEA ovules) was more effective than placebo in improving vaginal dryness and dyspareunia in women with GSM.29–31 In these studies, locally applied DHEA decreased parabasal cells, decreased vaginal pH, increased vaginal secretions, and improved epithelial surface thickness and integrity without any significant impact on serum levels of DHEA, DHEA-sulfate, estradiol, testosterone, or their metabolites. Importantly, transvaginal DHEA had negligible endometrial effect.
The breast cancer risk associated with vaginal DHEA has not been fully evaluated. However, labeling lists breast cancer as a warning, not a contraindication.
Selective estrogen-receptor modulator
In 2013, the FDA approved ospemifene for the treatment of dyspareunia caused by GSM. Ospemifene, an estrogen agonist in the vagina, is taken daily as a 60-mg oral dose. Long-term safety studies suggested no adverse effects on the endometrium or breast for at least 52 weeks.32
These studies also noted that ospemifene improved the vaginal maturation index (decreased parabasal cells and increased superficial cells) and decreased vaginal pH. It has further been shown to decrease severity of the self-identified most bothersome symptom—dyspareunia or vaginal dryness—compared with placebo.33
Potential increases in hot flashes, which may occur in up to 7% of patients, and the risk of blood clots should be considered. Additionally, the safety of ospemifene in women with a history of breast cancer has not been established. Although early studies suggest it either has no effect or possibly a protective effect on breast tissue, the FDA does not recommend its use in women at risk for breast cancer. Long-term effects on bone are unknown.
The labeling for ospemifene includes a boxed warning about the risk of stroke, blood clots, and cancer of the lining of the uterus. Patients should be counseled about worrisome signs or symptoms that require medical attention.
ALTERNATIVE THERAPIES
Treatments for GSM not approved by the FDA include laser and radiofrequency therapies, testosterone, isoflavones, and bioidentical hormones.
Laser and radiofrequency therapies
Both of these therapies aim to promote tissue remodeling with increased collagen and elastin production and increased vascularity. This, in turn, increases muscle support and tone.
Laser therapies act by ablating and coagulating vaginal tissues; radiofrequency therapies directly heat the tissue. Both treatments are office-based, require up to 3 initial treatments, and are followed by retreatment at approximately 1-year intervals.
Studies have reported high patient satisfaction rates (91% to 100%), improved sexual functioning, and decreased GSM symptoms of vaginal dryness, burning, itching, and dyspareunia.34–36 Data, however, are from observational studies, not placebo-controlled trials.
Although laser and radiofrequency therapies are FDA-approved for several indications, laser treatment for symptoms of vulvovaginal atrophy is not currently an approved indication. Patients should be advised of this.
Testosterone
Locally applied testosterone was shown in a small study to improve dyspareunia and vaginal dryness associated with aromatase inhibitor use in breast cancer patients.37 However, due to the lack of safety and efficacy data from larger, controlled trials, testosterone therapy is not currently recommended.
Isoflavones
Isoflavones are phytoestrogens found in soy. In a 12-week, double-blind placebo-controlled study of vaginally applied 4% soy isoflavone gel, improvements in vaginal atrophy symptoms, maturation values, and vaginal pH were found in 60 postmenopausal women.38 Additional data on efficacy and safety are needed before isoflavones should be considered as a treatment for GSM.
Bioidentical hormones
Bioidentical hormones are plant-derived hormones that are chemically similar or identical to those produced by the body. Although there are FDA-approved bioidentical hormones (eg, micronized progesterone, estradiol, DHEA), the term bioidentical usually refers to non-FDA-approved, commercially available hormones produced and compounded by specialty pharmacies.
Patients often view these substances as being better, safer, and more acceptable for use, and healthcare practitioners need to be prepared to address these beliefs. The FDA and the American College of Obstetricians and Gynecologists consider bioidentical hormones to be a marketing term and not an alternative treatment based on scientific evidence.39 Patients should be informed that bioidentical hormones have the same risks as any similar hormone preparation along with additional risks related to potential lack of purity and potency. Further, they have not been adequately studied in controlled clinical trials.
FOLLOW-UP CARE
Healthcare providers caring for women should assume a proactive role in diagnosing and treating the symptoms of GSM. And once diagnosis of GSM is established and treatment is under way, practitioners can use symptom questionnaires and vaginal pH testing as easy and reliable means of measuring clinical response to therapy.
For many women, the postmenopausal loss of estrogen is associated with uncomfortable genitourinary symptoms, collectively referred to as the genitourinary syndrome of menopause (GSM). But despite the prevalence of GSM and the availability of treatments, most women do not seek relief.
This article reviews the syndrome and offers advice on how to talk about it with patients and what treatment options to consider.
A SYNDROME RECENTLY DEFINED
The term GSM and its definition were approved by the North American Menopause Society and the International Society for the Study of Women’s Sexual Health in 2014.1 It replaces older terms such as vulvovaginal atrophy, urogenital atrophy, and atrophic vaginitis.
GSM refers collectively to the symptoms associated with estrogen loss after menopause that adversely affect the vulvovaginal area and lower urinary tract. The most common symptoms are vulvovaginal dryness, burning, or irritation; sexual pain from inadequate lubrication; and urinary urgency, dysuria, or recurrent urinary tract infection.1,2
The definition notes that symptoms are self-reported as bothersome and are not the result of another disorder. Symptoms may be chronic and progressive, are not likely to resolve without treatment (pharmacologic or nonpharmacologic), and can have a significant negative impact on a woman’s quality of life and sexual health.1,2
COMMON BUT UNDERTREATED
From 40% to 60% of postmenopausal women experience GSM, but few seek treatment.3 Nevertheless, most postmenopausal women remain sexually active. In a 2008 survey of 94,000 postmenopausal women ages 50 to 79, 52% reported that they had been sexually active with a partner in the past year.4 However, 45% of postmenopausal women experienced unpleasant vaginal symptoms, according to a 2012 international survey of 3,520 postmenopausal women ages 55 to 65.5 In this survey, most respondents (75%) felt that vaginal symptoms had a negative impact on their life, but only 4% connected their symptoms to the vulvovaginal atrophy that resulted from loss of estrogen after menopause. Moreover, almost half were unaware of management options.5
These findings were supported by a 2013 survey of more than 3,000 US women who reported unpleasant vulvar and vaginal symptoms.6 From 60% to 85% noted negative sexual consequences from vulvovaginal symptoms, 47% felt their relationship suffered, and 27% felt it had a negative impact on their general enjoyment of life. In this study, 24% attributed their symptoms to menopause and 12% to hormonal changes. Although 56% had discussed GSM symptoms with a healthcare provider, only 40% were using GSM-specific topical treatments, mostly over-the-counter preparations.
Male partners of symptomatic women also note adverse emotional and physical effects.7 In an online survey of 4,100 men and 4,100 women ages 55 to 65, 52% to 78% of men and 58% to 64% of women expressed the negative effects of vulvovaginal symptoms on intimacy, libido, and sexual pain.
GSM is a progressive disorder. Women may note symptoms many years before menopause or have no symptoms until several years after menopause. One study found the prevalence of GSM to be 4% during perimenopause, rising after menopause to 25% after 1 year and to 47% after 3 years.8
Although distressing symptoms occur mostly after menopause, they may be seen in women of any age who experience a hypoestrogenic state, even if it is transient. Causes of this include premature ovarian failure, hypothalamic amenorrhea, and hyperprolactinemia. In addition, some treatments such as gonadotropin-releasing hormone agonists and aromatase inhibitors may cause vulvovaginal and lower urinary tract symptoms. Chemotherapy, radiation, and surgical removal of ovaries may also precipitate symptoms. The abrupt onset of menopause that may occur with these treatments is often associated with significantly greater sexual dysfunction and negative impact on quality of life. Cigarette smoking also leads to lower estrogen levels, which may contribute to GSM.
WHAT CAUSES GSM?
The genitourinary system develops from common embryologic tissue, the basis for the functional and clinical connection. Estrogen maintains the epithelium of the vagina, vulva, urethra, and bladder trigone via estrogen receptors present throughout these tissues.9
Premenopausal changes
Histologically, the estrogen-exposed vagina of a premenopausal woman is lined by glycogen-rich, stratified squamous epithelium, with underlying supportive fibromuscular layers. The epithelium is composed of superficial, intermediate, and parabasal cellular layers. In the presence of estrogen, the superficial and intermediate cellular levels predominate, with few parabasal cells.
Glycogen acts as a substrate for lactobacilli, producing organic acids, primarily lactate, that help maintain an acidic pH of 2.8 to 4.0. The low pH helps protect against pathologic shifts in the microbiome. Estrogen also maintains the collagen content of the epithelium, maintains acid mucopolysaccharides and hyaluronic acid, and optimizes vaginal blood flow. These effects result in optimal epithelial thickness and elasticity, moisture, vaginal secretions, and lubrication.10
Postmenopausal changes
Low levels of estrogen after menopause result in adverse anatomic, physiologic, and clinical changes in vaginal tissue. Effects of hypoestrogenism include the loss of collagen and adipose, leading to decreased elasticity and vaginal mucosal thinning. Vascular flow is decreased. The epithelial cytology transitions to a predominance of parabasal cells and a decrease in superficial and intermediate cells. Eccrine and apocrine glands become attenuated. These changes result in decreased vaginal secretions, diminished or delayed lubrication with sexual stimulation, friability of the vaginal vault, and vaginal dryness.11
Additionally, without estrogen, glycogen content is diminished, leading to decreased lactic acid production and a rise in vaginal pH to greater than 5. As the pH rises, Lactobacillus colonization decreases, leading to a further decrease in glycogen metabolism and to propagation of an elevated vaginal pH. The loss of vaginal acidity makes the vagina more susceptible to pathologic bacteria, including those found in the bowel and skin, sexually transmitted infections, and bacterial vaginosis.12
Other affected tissues. Anatomic effects of estrogen loss are not limited to the vagina. The epithelium, connective tissue, and smooth muscle of the vulva, vagina, urethra, and bladder trigone are also affected. The labia minora become thinner and regress, the introitus retracts, and narrowing and stricture of the vaginal canal and introitus may result. In some women, the urethral meatus becomes prominent relative to the introitus and more vulnerable to physical irritation, infection, and trauma.
Clinically, estrogen-related changes are usually responsible for vaginal dryness, irritation, burning, and superficial or deep dyspareunia. Urinary frequency, urgency, and incontinence also may develop.
THE DIAGNOSIS IS CLINICAL
The diagnosis of GSM is based on the history and physical examination. Standardized diagnostic tools for GSM are lacking, but some tools are available.
In 2006, the US Food and Drug Administration (FDA) published guidelines for industry to better define patient-reported outcome measures in clinical trials.13 The most significant addition was having the patient define the symptoms and rate how “bothersome” the symptoms are. Although this measure does not help diagnose GSM, it can be used effectively to follow response to treatment.
The Vaginal Symptom Questionnaire14 can be useful for assessing symptoms. It is a validated 21-item questionnaire that measures the quality-of-life impact of genital, but not urinary, symptoms of menopause.
Ask patients about symptoms
Healthcare providers should ask about GSM symptoms (Table 1) during routine clinical visits with women who are peri- or postmenopausal or who have hypoestrogenism from other causes, as many women are reluctant to initiate this discussion. Conversely, in women who present with sexual problems, such as difficulty with arousal or dyspareunia, GSM should be considered as a possible cause.
Specifically, ask women if they have any of the following symptoms:
- Vaginal itching, burning, discomfort, or irritation
- Malodorous or irritating vaginal discharge
- Urinary frequency, urgency, dysuria, urethral discomfort, or recurrent urinary tract infections
- Sexual symptoms of entry dyspareunia, vaginal pain, or irritation with sexual activity, which may be complicated by postcoital bleeding, spotting, or fissuring.
Vulvovaginal pain or irritation may be constant or may be present in the absence of sexual activity, such as with exercise, wearing tight clothing, or sitting for long periods.
Physical examination
Characteristic physical findings of GSM include scarce pubic hair, thinning of the labia from loss of labial fat, resorption of the labia minora, or fusion of the labia minora and majora (Table 2).15,16 The vulvar skin is pale and thin. The clitoral hood may retract, exposing the glans (which may lead to increased pain with sexual stimulation), or clitoral hood fusion may occur. The vagina is pale, dry, smooth, and shiny with loss of rugae; shortening or stricturing may be present. Vaginal elasticity decreases. Inflammation and petechiae (pinpoint, nonraised, round purple-red spots) may be present. The cervix may be flush with the vaginal fornices.
Prolonged atrophy may result in introital narrowing and friability, which may cause tearing with sexual activity or insertion of a speculum during pelvic examination. In addition, the epithelium of the lower urinary tract thins, and the muscular and fibrous layers atrophy—changes that may not be obvious during examination. A urethral caruncle may form, presenting as proliferative red tissue at the entrance of the urethra. Prolapse may become more prominent.
In women with severe genitourinary atrophy, pelvic examination may cause significant discomfort. Reassuring the patient that she can ask the clinician to stop at any time due to extreme discomfort is the first step in a successful pelvic examination.
In some situations, initial examination of the pelvic area may not include insertion of a speculum. Use of a hand-held mirror so the patient can observe the examination may help her relax during the examination.
Vaginal pH and cultures, if indicated, may be obtained by gently inserting a cotton-tipped swab into the vagina without a speculum and before applying lubricant. Lubricant should be used generously; in some instances, topical lidocaine gel (diluted, as it may burn) may be placed against the perineum on a gauze pad for 3 to 5 minutes before insertion of the speculum.
When an internal pelvic examination is necessary in a timely manner, such as with postmenopausal bleeding or a history of an abnormal Papanicolaou smear, but is too painful for the patient, the examination should be done under anesthesia.
Additional considerations
The history should review current medical conditions, medication use, nongenital skin disorders (eg, eczema), and systemic menopausal symptoms, such as hot flashes.
Also, consider other potential causes of GSM during the evaluation (Table 3).17,18 Review the use of detergents, soaps, douches, or over-the-counter topical products that could cause genitourinary symptoms secondary to contact irritation or allergy.
Any isolated, ulcerated, or nonhealing lesion should be biopsied. Reevaluate patients who have not responded to previous topical therapy or consider referral to a specialist.
Assess the personal, interpersonal, social, and sexual impact of the symptoms: if they do not cause distress, GSM does not require treatment. Nevertheless, potential treatment options should be discussed as symptoms may progress, making intervention necessary.
Laboratory tests: Helpful, not essential
Laboratory tests are unnecessary for the diagnosis of GSM. However, office-based objective evaluations such as vaginal pH testing and the maturation index can support the diagnosis.
The pH of the estrogenized vagina ranges from 3.8 to 4.2, whereas in women with GSM, the pH may reach 5.5 or higher. The pH can be obtained by placing a pH-sensitive paper against the lateral vaginal wall, avoiding any discharge or cervical mucus. A vaginal pH of 5 or greater in the absence of blood, semen, or infection suggests vulvovaginal atrophy.19
The vaginal maturation index is determined by a vaginal smear using Rakoff staining, in which 100 cells are counted and the number of parabasal, intermediate, and superficial cells is determined. In general, a well-estrogenized vagina has mostly superficial and intermediate cells, which shifts to a predominance of parabasal cells as estrogen levels decline.20
A recent review of vaginal atrophy suggests that after a diagnosis of GSM, healthcare providers can consider the most bothersome symptom along with the vaginal pH to assess the response to treatment.21 In general, schedule a follow-up appointment at 8 to 12 weeks to review treatment response. If treatment has not resulted in adequate symptom relief, consider a pelvic examination and further testing.
SELECTING A TREATMENT
Symptomatic women with GSM who desire intervention should be offered over-the-counter nonhormonal products as the first line of therapy.
If nonhormonal products are ineffective and there are no contraindications, locally applied estrogen in cream, tablet, or a ring delivery system may be offered. Local dehydroepiandrosterone (DHEA) inserts or ospemifene, an oral selective estrogen-receptor modulator, are FDA-approved for moderate to severe dyspareunia secondary to GSM.
Oral estrogen therapy is not indicated for vulvovaginal symptoms, but some women taking systemic estrogen for vasomotor symptoms may need additional local estrogen application to relieve vaginal symptoms.
Nonhormonal treatments
Nonhormonal over-the-counter therapies provide sufficient relief for most women with mild symptoms. There is a plethora of products, so practitioners need to offer guidance to help women with their individual choices.
Vaginal lubricants are intended for use with sexual or penetrative activity (including pelvic examination). They provide short-term relief of symptoms, but there is no evidence of any impact on histologic changes of atrophy. They are meant to relieve friction. Lubricants may be water-based, oil-based, silicone-based, or a combination. Individual products have different effects on condom integrity. Perfumed, warming, or stimulating products may be irritating to some women and should be tried initially in small amounts.
Vaginal moisturizers are intended to treat GSM. They are applied regularly, not just with vaginal activity, usually once or twice a week. Some vaginal lubricants can maintain an acidic pH in the vagina and may reverse the histologic changes of atrophy. Symptomatic improvement over placebo or estrogen has been shown in clinical trials.22–24
Women should be advised that trial and error in choosing products may be necessary to establish a successful regimen. Products should be tried in succession, not simultaneously, with a “wash-out” period between, to be able to evaluate response.
Vaginal dilators and pelvic floor physical therapy
Sexual activity, either by self-stimulation or with a partner, helps maintain vaginal health by contributing to increased vascularity and elasticity of tissue. Women who resume sexual activity after a long period of inactivity may benefit from the use of vaginal dilators, which aid both in mechanical distention and progressive relaxation of the vaginal musculature.
In some women, long-term dyspareunia may result in vaginismus, an involuntary contraction of the vaginal musculature. For these women, dilators may be effective. Additional options focus on pelvic floor physical therapy, which can isolate trigger points, using biofeedback to teach relaxation and home exercises such as vaginal massage.
HORMONAL THERAPIES
If nonhormonal lubricants and moisturizers do not achieve satisfactory symptomatic relief, FDA-approved hormonal therapies (Table 4) include estrogen-containing vaginal creams, rings, and a tablet; a vaginal tablet containing DHEA; and an oral tablet containing ospemifene.
Estrogen products
For patients whose symptoms do not respond to nonhormonal therapies, low-dose, locally applied estrogen therapy is the first treatment recommended.2 Locally applied estrogens can reverse the atrophic changes of estrogen deprivation, resulting in an increase in blood flow, elasticity, and vaginal wall thickness. This therapy also can normalize pH levels with subsequent restoration of a healthy lactobacilli-based flora. Locally applied estrogens also have been shown to decrease the frequency of recurrent urinary tract infection.25
Estrogen-containing vaginal creams, rings, and a tablet are available, and each has been shown to be effective for GSM. Locally applied estrogens at recommended dosages tend to have fewer adverse events and risks than systemic estrogens.26 Estradiol levels generally do not exceed levels found in the untreated menopausal population, although a dose- and duration-dependent increase in systemic levels may occur.27
Dosing considerations
The vaginal ring and the vaginal tablet provide the lowest prefixed daily dose of estradiol (7.5 and 10 µg daily, respectively). Estrogen creams (estradiol, conjugated equine estrogens) are more readily absorbed, and dosing should be tapered to the lowest, most effective dose for symptom relief.
The FDA-approved doses for vaginal creams containing 17-beta estradiol are higher than the dose found to be effective in clinical practice (0.5 g twice a week). Most practitioners start with the lower dose, reserving the FDA-approved higher doses for patients who do not obtain adequate relief over 6 to 8 weeks of treatment. The conjugated-estrogen vaginal cream Premarin is the only locally applied estrogen approved by the FDA to treat dyspareunia. It is dosed at 0.5 g intravaginally for 21 days and is then either withdrawn for 7 days or, more commonly, administered at 0.5 g twice a week.
Initial treatment with vaginal cream may require more frequent vulvovaginal application, such as daily for 1 to 2 weeks. Women with vaginal fissures or tearing will benefit from externally applied creams in addition to internal applications. Response to therapy is usually seen within 4 to 6 weeks from onset of treatment. Once symptom relief is obtained, treatment should continue indefinitely. Although long-term safety studies are lacking, risks are believed to be minimal.
Endometrial impact. Women with contraindications to systemic estrogen should be counseled about possible small increases in serum levels of estradiol associated with locally applied estrogens and the potential risks and benefits those increases incur. Endometrial surveillance with either transvaginal ultrasonography or endometrial sampling is not required, even with long-term use, but it should be considered with higher doses or more frequent applications.
Similarly, progesterone replacement for endometrial protection is not recommended but can be considered in women with an intact uterus at high risk of endometrial cancer, such as obese patients. If a systemic progestational agent is considered, the risks and benefits should be weighed carefully. Even in women at high risk, endometrial surveillance may be the most appropriate option.28 Uterine bleeding that occurs should be considered abnormal and should be investigated.
DHEA (prasterone)
In 2016, the FDA approved intravaginal prasterone, a DHEA-containing product for the treatment of dyspareunia secondary to moderate to severe vulvovaginal atrophy caused by menopause. DHEA is an endogenous steroid that is converted by aromatase activity into testosterone and estradiol.
Clinical trials have found that 12 weeks of vaginal DHEA supplementation (0.25%, 0.5%, and 1% DHEA ovules) was more effective than placebo in improving vaginal dryness and dyspareunia in women with GSM.29–31 In these studies, locally applied DHEA decreased parabasal cells, decreased vaginal pH, increased vaginal secretions, and improved epithelial surface thickness and integrity without any significant impact on serum levels of DHEA, DHEA-sulfate, estradiol, testosterone, or their metabolites. Importantly, transvaginal DHEA had negligible endometrial effect.
The breast cancer risk associated with vaginal DHEA has not been fully evaluated. However, labeling lists breast cancer as a warning, not a contraindication.
Selective estrogen-receptor modulator
In 2013, the FDA approved ospemifene for the treatment of dyspareunia caused by GSM. Ospemifene, an estrogen agonist in the vagina, is taken daily as a 60-mg oral dose. Long-term safety studies suggested no adverse effects on the endometrium or breast for at least 52 weeks.32
These studies also noted that ospemifene improved the vaginal maturation index (decreased parabasal cells and increased superficial cells) and decreased vaginal pH. It has further been shown to decrease severity of the self-identified most bothersome symptom—dyspareunia or vaginal dryness—compared with placebo.33
Potential increases in hot flashes, which may occur in up to 7% of patients, and the risk of blood clots should be considered. Additionally, the safety of ospemifene in women with a history of breast cancer has not been established. Although early studies suggest it either has no effect or possibly a protective effect on breast tissue, the FDA does not recommend its use in women at risk for breast cancer. Long-term effects on bone are unknown.
The labeling for ospemifene includes a boxed warning about the risk of stroke, blood clots, and cancer of the lining of the uterus. Patients should be counseled about worrisome signs or symptoms that require medical attention.
ALTERNATIVE THERAPIES
Treatments for GSM not approved by the FDA include laser and radiofrequency therapies, testosterone, isoflavones, and bioidentical hormones.
Laser and radiofrequency therapies
Both of these therapies aim to promote tissue remodeling with increased collagen and elastin production and increased vascularity. This, in turn, increases muscle support and tone.
Laser therapies act by ablating and coagulating vaginal tissues; radiofrequency therapies directly heat the tissue. Both treatments are office-based, require up to 3 initial treatments, and are followed by retreatment at approximately 1-year intervals.
Studies have reported high patient satisfaction rates (91% to 100%), improved sexual functioning, and decreased GSM symptoms of vaginal dryness, burning, itching, and dyspareunia.34–36 Data, however, are from observational studies, not placebo-controlled trials.
Although laser and radiofrequency therapies are FDA-approved for several indications, laser treatment for symptoms of vulvovaginal atrophy is not currently an approved indication. Patients should be advised of this.
Testosterone
Locally applied testosterone was shown in a small study to improve dyspareunia and vaginal dryness associated with aromatase inhibitor use in breast cancer patients.37 However, due to the lack of safety and efficacy data from larger, controlled trials, testosterone therapy is not currently recommended.
Isoflavones
Isoflavones are phytoestrogens found in soy. In a 12-week, double-blind placebo-controlled study of vaginally applied 4% soy isoflavone gel, improvements in vaginal atrophy symptoms, maturation values, and vaginal pH were found in 60 postmenopausal women.38 Additional data on efficacy and safety are needed before isoflavones should be considered as a treatment for GSM.
Bioidentical hormones
Bioidentical hormones are plant-derived hormones that are chemically similar or identical to those produced by the body. Although there are FDA-approved bioidentical hormones (eg, micronized progesterone, estradiol, DHEA), the term bioidentical usually refers to non-FDA-approved, commercially available hormones produced and compounded by specialty pharmacies.
Patients often view these substances as being better, safer, and more acceptable for use, and healthcare practitioners need to be prepared to address these beliefs. The FDA and the American College of Obstetricians and Gynecologists consider bioidentical hormones to be a marketing term and not an alternative treatment based on scientific evidence.39 Patients should be informed that bioidentical hormones have the same risks as any similar hormone preparation along with additional risks related to potential lack of purity and potency. Further, they have not been adequately studied in controlled clinical trials.
FOLLOW-UP CARE
Healthcare providers caring for women should assume a proactive role in diagnosing and treating the symptoms of GSM. And once diagnosis of GSM is established and treatment is under way, practitioners can use symptom questionnaires and vaginal pH testing as easy and reliable means of measuring clinical response to therapy.
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014; 21(10):1063–1068. doi:10.1097/GME.0000000000000329
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–904. doi:10.1097/GME.0b013e3182a122c2
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health 2013; 5:437–447. doi:10.2147/IJWH.S44579
- McCall-Hosenfeld JS, Jaramillo SA, Legault C, et al; Members of Women’s Health Initiative-Observational Study. Correlates of sexual satisfaction among sexually active postmenopausal women in the Women’s Health Initiative-Observational Study. J Gen Intern Med 2008; 23(12):2000–2009. doi:10.1007/s11606-008-0820-9
- Nappi RE, Kokot-Kierepa M. Vaginal Health: Insights, Views & Attitudes (VIVA): results from an international survey. Climacteric 2012; 15(1):36–44. doi:10.3109/13697137.2011.647840
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal Changes) survey. J Sex Med 2013; 10(7):1790–1799. doi:10.1111/jsm.12190
- Nappi RE, Kingsberg S, Maamari R, Simon J. The CLOSER (Clarifying Vaginal Atrophy’s Impact On Sex and Relationships) survey: implications of vaginal discomfort in postmenopausal women and in male partners. J Sex Med 2013; 10(9):2232–2241. doi:10.1111/jsm.12235
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol 2000; 96(3):351–358. pmid:10960625
- Stika CS. Atrophic vaginitis. Dermatol Ther 2010; 23(5):514–522. doi:10.1111/j.1529-8019.2010.01354.x
- Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Juliá MD. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas 2005; 52(suppl 1):S46–S52. doi:10.1016/j.maturitas.2005.06.014
- Forsberg JG. A morphologist’s approach to the vagina—age-related changes and estrogen sensitivity. Maturitas 1995; 22(suppl):S7–S15.
- Martin DH. The microbiota of the vagina and its influence on women’s health and disease. Am J Med Sci 2012; 343(1):2–9. doi:10.1097/MAJ.0b013e31823ea228
- US Department of Health and Human Services; Food and Drug Administration (FDA). Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims, 2006. doi:10.1186/1477-7525-4-79
- Erekson EA, Yip SO, Wedderburn TS, et al. The Vulvovaginal Symptoms Questionnaire: a questionnaire for measuring vulvovaginal symptoms in postmenopausal women. Menopause 2013; 20(9):973–979. doi:10.1097/GME.0b013e318282600b
- Johnston SL, Farrell SA, Bouchard C, et al; SOGC Joint Committee-Clinical Practice Gynaecology and Urogynaecology. The detection and management of vaginal atrophy. J Obstet Gynaecol Can 2004; 26(5):503–515. doi:10.1016/S1701-2163(16)30662-4
- Oriba HA, Maibach HI. Vulvar transepidermal water loss (TEWL) decay curves. Effect of occlusion, delipidation, and age. Acta Derm Venereol 1989; 69(6):461–465. pmid:2575316
- Bachmann G, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician 2000; 61(10):3090–3096. pmid:10839558
- MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010; 85(1):87–94. doi:10.4065/mcp.2009.0413
- Nilsson K, Risberg B, Heimer G. The vaginal epithelium in the post menopause--cytology, histology and pH as methods of assessment. Maturitas 1995; 21(1):51–56. pmid:7731384
- McEndree B. Clinical application of the vaginal maturation index. Nurse Pract 1999; 24(9):48–56. pmid:10507070
- Weber MA, Limpens J, Roovers JP. Assessment of vaginal atrophy: a review. Int Urogynecol J 2015; 26(1):15–28. doi:10.1007/s00192-014-2464-0
- Lee YK, Chung HH, Kim JW, Park NH, Song YS, Kang SB. Vaginal pH-balanced gel for the control of atrophic vaginitis among breast cancer survivors: a randomized controlled trial. Obstet Gynecol 2011; 117(4):922–927. doi:10.1097/AOG.0b013e3182118790
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas 1996; 23(3):259–263. pmid:8794418
- Nachtigall LE. Comparative study: replens versus local estrogen in menopausal women. Fertil Steril 1994; 61(1):178–180. pmid:8293835
- Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis 2000; 30(1):152–156. doi:10.1086/313596
- Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2006; 4:CD001500. doi:10.1002/14651858.CD001500
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–126. doi:10.3109/13697137.2014.947254
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause 2010; 17(2):242–255. doi:10.1097/gme.0b013e3181d0f6b9
- Labrie F, Archer D, Bouchard C, et al. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause 2009; 16(5):923–931. doi:10.1097/gme.0b013e31819e85c6
- Labrie F, Archer D, Bouchard C, et al. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause 2009; 16(5):907–922. doi:10.1097/gme.0b013e31819e8e2d
- Archer DF. Dehydroepiandrosterone intra vaginal administration for the management of postmenopausal vulvovaginal atrophy. J Steroid Biochem Mol Biol 2015; 145:139–143. doi:10.1016/j.jsbmb.2014.09.003
- Wurz GT, Kao CJ, DeGregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging 2014; 9:1939–1950. doi:10.2147/CIA.S73753
- Constantine G, Graham S, Portman DJ, Rosen RC, Kingsberg SA. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric 2015; 18(2):226–232. doi:10.3109/13697137.2014.954996
- Arroyo C. Fractional CO2 laser treatment for vulvovaginal atrophy symptoms and vaginal rejuvenation in perimenopausal women. Int J Womens Health 2017; 9:591–595. doi:10.2147/IJWH.S136857
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas 2015; 80(3):296–301. doi:10.1016/j.maturitas.2014.12.006
- Salvatore S, Nappi R, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric 2014; 17(4):363–369. doi:10.3109/13697137.2014.899347
- Witherby S, Johnson J, Demers L, et al. Topical testosterone for breast cancer patients with vaginal atrophy related to aromatase inhibitors: a phase I/II study. Oncologist 2011; 16(4):424–431. doi:10.1634/theoncologist.2010-0435
- Lima SM, Bernardo BF, Yamada SS, Reis BF, da Silva GM, Galvão MA. Effects of Glycine max (L.) Merr. soy isoflavone vaginal gel on epithelium morphology and estrogen receptor expression in postmenopausal women: a 12-week, randomized, double-blind, placebo-controlled trial. Maturitas 2014; 78(3):205–211. doi:10.1016/j.maturitas.2014.04.007
- Committee on Gynecologic Practice and the American Society for Reproductive Medicine Practice Committee. Committee opinion No 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol 2012; 120(2 pt 1):411–415. doi:10.1097/AOG.0b013e318268049e
- Portman DJ, Gass ML; Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 2014; 21(10):1063–1068. doi:10.1097/GME.0000000000000329
- Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–904. doi:10.1097/GME.0b013e3182a122c2
- Parish SJ, Nappi RE, Krychman ML, et al. Impact of vulvovaginal health on postmenopausal women: a review of surveys on symptoms of vulvovaginal atrophy. Int J Womens Health 2013; 5:437–447. doi:10.2147/IJWH.S44579
- McCall-Hosenfeld JS, Jaramillo SA, Legault C, et al; Members of Women’s Health Initiative-Observational Study. Correlates of sexual satisfaction among sexually active postmenopausal women in the Women’s Health Initiative-Observational Study. J Gen Intern Med 2008; 23(12):2000–2009. doi:10.1007/s11606-008-0820-9
- Nappi RE, Kokot-Kierepa M. Vaginal Health: Insights, Views & Attitudes (VIVA): results from an international survey. Climacteric 2012; 15(1):36–44. doi:10.3109/13697137.2011.647840
- Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal Changes) survey. J Sex Med 2013; 10(7):1790–1799. doi:10.1111/jsm.12190
- Nappi RE, Kingsberg S, Maamari R, Simon J. The CLOSER (Clarifying Vaginal Atrophy’s Impact On Sex and Relationships) survey: implications of vaginal discomfort in postmenopausal women and in male partners. J Sex Med 2013; 10(9):2232–2241. doi:10.1111/jsm.12235
- Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol 2000; 96(3):351–358. pmid:10960625
- Stika CS. Atrophic vaginitis. Dermatol Ther 2010; 23(5):514–522. doi:10.1111/j.1529-8019.2010.01354.x
- Castelo-Branco C, Cancelo MJ, Villero J, Nohales F, Juliá MD. Management of postmenopausal vaginal atrophy and atrophic vaginitis. Maturitas 2005; 52(suppl 1):S46–S52. doi:10.1016/j.maturitas.2005.06.014
- Forsberg JG. A morphologist’s approach to the vagina—age-related changes and estrogen sensitivity. Maturitas 1995; 22(suppl):S7–S15.
- Martin DH. The microbiota of the vagina and its influence on women’s health and disease. Am J Med Sci 2012; 343(1):2–9. doi:10.1097/MAJ.0b013e31823ea228
- US Department of Health and Human Services; Food and Drug Administration (FDA). Guidance for industry. Patient-reported outcome measures: use in medical product development to support labeling claims, 2006. doi:10.1186/1477-7525-4-79
- Erekson EA, Yip SO, Wedderburn TS, et al. The Vulvovaginal Symptoms Questionnaire: a questionnaire for measuring vulvovaginal symptoms in postmenopausal women. Menopause 2013; 20(9):973–979. doi:10.1097/GME.0b013e318282600b
- Johnston SL, Farrell SA, Bouchard C, et al; SOGC Joint Committee-Clinical Practice Gynaecology and Urogynaecology. The detection and management of vaginal atrophy. J Obstet Gynaecol Can 2004; 26(5):503–515. doi:10.1016/S1701-2163(16)30662-4
- Oriba HA, Maibach HI. Vulvar transepidermal water loss (TEWL) decay curves. Effect of occlusion, delipidation, and age. Acta Derm Venereol 1989; 69(6):461–465. pmid:2575316
- Bachmann G, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. Am Fam Physician 2000; 61(10):3090–3096. pmid:10839558
- MacBride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010; 85(1):87–94. doi:10.4065/mcp.2009.0413
- Nilsson K, Risberg B, Heimer G. The vaginal epithelium in the post menopause--cytology, histology and pH as methods of assessment. Maturitas 1995; 21(1):51–56. pmid:7731384
- McEndree B. Clinical application of the vaginal maturation index. Nurse Pract 1999; 24(9):48–56. pmid:10507070
- Weber MA, Limpens J, Roovers JP. Assessment of vaginal atrophy: a review. Int Urogynecol J 2015; 26(1):15–28. doi:10.1007/s00192-014-2464-0
- Lee YK, Chung HH, Kim JW, Park NH, Song YS, Kang SB. Vaginal pH-balanced gel for the control of atrophic vaginitis among breast cancer survivors: a randomized controlled trial. Obstet Gynecol 2011; 117(4):922–927. doi:10.1097/AOG.0b013e3182118790
- Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas 1996; 23(3):259–263. pmid:8794418
- Nachtigall LE. Comparative study: replens versus local estrogen in menopausal women. Fertil Steril 1994; 61(1):178–180. pmid:8293835
- Raz R, Gennesin Y, Wasser J, et al. Recurrent urinary tract infections in postmenopausal women. Clin Infect Dis 2000; 30(1):152–156. doi:10.1086/313596
- Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2006; 4:CD001500. doi:10.1002/14651858.CD001500
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–126. doi:10.3109/13697137.2014.947254
- North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the North American Menopause Society. Menopause 2010; 17(2):242–255. doi:10.1097/gme.0b013e3181d0f6b9
- Labrie F, Archer D, Bouchard C, et al. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause 2009; 16(5):923–931. doi:10.1097/gme.0b013e31819e85c6
- Labrie F, Archer D, Bouchard C, et al. Intravaginal dehydroepiandrosterone (Prasterone), a physiological and highly efficient treatment of vaginal atrophy. Menopause 2009; 16(5):907–922. doi:10.1097/gme.0b013e31819e8e2d
- Archer DF. Dehydroepiandrosterone intra vaginal administration for the management of postmenopausal vulvovaginal atrophy. J Steroid Biochem Mol Biol 2015; 145:139–143. doi:10.1016/j.jsbmb.2014.09.003
- Wurz GT, Kao CJ, DeGregorio MW. Safety and efficacy of ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy due to menopause. Clin Interv Aging 2014; 9:1939–1950. doi:10.2147/CIA.S73753
- Constantine G, Graham S, Portman DJ, Rosen RC, Kingsberg SA. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric 2015; 18(2):226–232. doi:10.3109/13697137.2014.954996
- Arroyo C. Fractional CO2 laser treatment for vulvovaginal atrophy symptoms and vaginal rejuvenation in perimenopausal women. Int J Womens Health 2017; 9:591–595. doi:10.2147/IJWH.S136857
- Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas 2015; 80(3):296–301. doi:10.1016/j.maturitas.2014.12.006
- Salvatore S, Nappi R, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric 2014; 17(4):363–369. doi:10.3109/13697137.2014.899347
- Witherby S, Johnson J, Demers L, et al. Topical testosterone for breast cancer patients with vaginal atrophy related to aromatase inhibitors: a phase I/II study. Oncologist 2011; 16(4):424–431. doi:10.1634/theoncologist.2010-0435
- Lima SM, Bernardo BF, Yamada SS, Reis BF, da Silva GM, Galvão MA. Effects of Glycine max (L.) Merr. soy isoflavone vaginal gel on epithelium morphology and estrogen receptor expression in postmenopausal women: a 12-week, randomized, double-blind, placebo-controlled trial. Maturitas 2014; 78(3):205–211. doi:10.1016/j.maturitas.2014.04.007
- Committee on Gynecologic Practice and the American Society for Reproductive Medicine Practice Committee. Committee opinion No 532: compounded bioidentical menopausal hormone therapy. Obstet Gynecol 2012; 120(2 pt 1):411–415. doi:10.1097/AOG.0b013e318268049e
KEY POINTS
- Practitioners can use the patient’s most bothersome symptom and her vaginal pH level to assess clinical responses to therapy.
- The diagnosis is based on clinical signs and symptoms from the medical history and physical examination.
- If the symptoms are not bothersome to the patient, the syndrome does not require treatment.