User login
- Avoid cognitive screening solely on the basis of age (SOR A).
- Screen vulnerable elderly patients at their initial visit and annually thereafter (SOR A).
- Ensure that all patients who undergo cognitive screening are tested for depression (SOR A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented
Janet M, a 69-year-old woman with a history of hypertension, comes for a visit because she thinks she has Alzheimer’s disease (AD). She recently had an episode of acute confusion while shopping at the mall; when she returned to her car, she couldn’t remember how to get home. The episode cleared within minutes and hasn’t recurred.
Jack S, an 84-year-old man, seeks medical care for pain in his right shoulder. He injured the rotator cuff several years ago, but it’s been fine since he completed physical therapy—until he tripped and fell while walking outside about a week ago. His daughter is concerned about his “forgetfulness” and increasing inability to remember certain words, but the patient thinks this is a natural consequence of age.
Fran B, a 72-year-old, presents with complaints of memory problems that began about 6 months ago. She’s worried about her son and has had increasing difficulty concentrating, sleeping, and keeping track of her things.
If these were your patients, whom would you screen for dementia? Would you decide whether to screen based on your “gut,” or a defined set of criteria? Would you have several screening tools on hand, and know enough about them to determine which one might be best suited for a particular patient?
If you make decisions about screening based on your gut or aren’t sure which tools are best for which patients, you’re far from alone. Cognitive impairment, particularly in the early stages, can be difficult and time-consuming to detect, and community physicians fail to diagnose mild-to-moderate dementia more than 50% of the time.1-5
Family members and caregivers often overlook declines in cognitive function, as well. In a study of 741 caregivers of patients with AD, an average of 4 months went by between the time symptoms were first noticed and the patient was seen by a physician—and 22% of the caregivers waited more than a year.6
Cognitive decline can be slowed with early Dx
Early diagnosis of AD or any dementia is important for a number of reasons. In some cases, cognitive impairment may be related to medical conditions—head trauma, Parkinson’s disease, human immunodeficiency virus, thyroid disorder, among others—that can be modified or reversed with treatment.7 There is evidence, too, that medical, behavioral, and social interventions can delay the cognitive and functional decline associated with AD, thereby helping to prolong the time the patient can remain at home. Early diagnosis also facilitates legal and financial family planning, and makes it possible to take appropriate safety measures.8-13
AD affects approximately 5 million US residents.14 With an aging population, that number is expected to surge in the decades ahead. To help you provide optimal care for your geriatric patients, this review will detail when to screen, which tools to use, and how best to care for the 3 elderly patients in the opener.
When and whether to screen
Despite the benefits of early detection, population-based screening based on age alone is not recommended. Guidelines recommend focused screening of patients in high-risk groups and on a case-by-case basis.15
US Preventive Services Task Force (USPSTF) guidelines recommend that physicians evaluate older patients for dementia whenever there is a suggestion of cognitive impairment, based on clinical observation or concern expressed by the individuals themselves or by family members, caretakers, or friends.15
However, cognitive screening generally provides better results in populations at higher risk of dementia.16,17 With that in mind, the Assessing Care of Vulnerable Elders (ACOVE), a collaborative project to develop a set of quality indicators for care of the elderly, recently recommended cognitive and functional screening of all “vulnerable elderly.”18,19
Who are the vulnerable elderly?
ACOVE defines the vulnerable elderly as individuals who are 65 years of age and older who live in the community and are at high risk of death or functional decline over the next 2 years. You can use a screening tool to identify members of this high-risk group. Or you can simply ask a few questions and classify any noninstitutionalized older person who reports being in poor health and/or acknowledges difficulty with activities of daily living—eg, money or medication management, dressing, grooming, or preparing simple meals—as a vulnerable elderly patient.
ACOVE recommends screening all vulnerable elderly patients who are new to a primary care practice or inpatient service, and following up with an annual evaluation to detect any changes in memory and function.18,19 Based on the recommendations of ACOVE and the USPSTF, all 3 patients described earlier would be candidates for screening.
Choosing the best screening tool
There is no serum or radiographic test available for the diagnosis of AD, and a thorough clinical evaluation can be extremely time-consuming. In the primary care setting, focused evaluations are both useful and cost effective.
There are a number of valid and reliable cognitive screening tools, including the Mini-Mental State Examination (MMSE), the Mini-Cog, the Montreal Cognitive Assessment (MoCA), the AD 8 Dementia Screening Interview, and the 7-Minute Neurocognitive Screen. In evaluating these and other screening tools, consider the sensitivity and specificity of each. Consider, too, features that may make one test better suited than another for a particular patient or specific circumstance (TABLE).
TABLE
Cognitive screening tools: How they compare
| TOOL (TIME TO ADMINISTER) | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| MMSE (5-10 min) | Tracks/quantifies changes; easy to administer; widely accepted; available in >50 languages | Not specific to AD or sensitive to mild dementia; influenced by age, education, and language skills |
| Mini-Cog (3 min) | Brief, easy to administer and score; unaffected by education, language skill; high sensitivity; easily paired with FAQ | |
| MoCA (10 min) | High sensitivity for MCI and mild AD; available in >20 languages; especially useful for patients with memory complaints but normal MMSE score | More time-consuming than other screening tools |
| AD 8 (3 min) | Administered in person or by phone; identifies earliest stages of dementia | Designed for informant, who may not be available, but can be given to patient |
| 7-Minute Neurocognitive Screen | Highly sensitive to early stages of AD | |
| AD, Alzheimer’s disease; FAQ, Functional Activities Questionnaire; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment. | ||
The Mini-Mental State Exam is the gold standard
Considered the gold standard in dementia assessment, the MMSE is the most widely used cognitive screening tool in the United States.20 The MMSE samples 6 cognitive areas—orientation, registration, attention and calculation, recall, language, and constructional skill.21 It has a high degree of validity in detecting dementia, and is particularly useful in tracking and quantifying changes over time. Well-known features include the serial 7s, in which patients are asked to count backwards from 100 by 7s, and the 3-stage command: Take a paper in your right hand, fold it in half, and put it on the floor.
Besides being relatively short (testing takes 5-10 minutes), the MMSE is easy to administer. It has been translated into more than 50 languages, and can therefore be used in many cultural settings.
Interpreting the MMSE. A total score of ≤23 out of a maximum of 30 points is suggestive of dementia. However, patient performance on the MMSE is influenced by a number of factors unrelated to cognitive function, such as age, educational level, deficits in language skills, and motor or visual impairment.22 Thus, the cut point may be adjusted depending on the patient or population being screened, and the positive predictive value and sensitivity and specificity for dementia vary accordingly. In a population-based sample of >18,000 individuals with a cut point of 24, the sensitivity was 87% and the specificity was 82%.23
The usefulness of the MMSE is also limited because the test is not specific to AD and is not sensitive to mild dementia. When the test is used to screen highly intelligent, well-educated patients, it may fail to detect any decline in memory or cognitive function.
The Mini-Cog is a quick and easy alternative
The Mini-Cog screen consists of 2 simple components that evaluate executive function: a clock-drawing test and word recall.24 The patient is given paper and pencil and asked to draw a clock with hands set on a specified time—5:10, say. First, however, the screener recites 3 common but unrelated words; the patient is asked to recite the words before drawing the clock and to recall them afterwards. The entire test takes about 3 minutes.
Interpreting the Mini-Cog. Besides being quick and easy to administer, the Mini-Cog is easy to score:
- Patients who do not recall any of the words are classified as demented.
- Patients who recall all of the words are classified as nondemented
- Patients who recall 1 or 2 words are classified based on their clock drawing: They’re considered nondemented if the clock is normal and demented if it is not.
When clock-drawing is part of the Mini-Cog screen, the results are simply considered normal or abnormal. For a clock to be considered normal, all the numbers must be in the correct sequence and the hands correctly positioned to show the designated time.24 (The clock-drawing can also be used as an independent screen. For more about that, see “Clock-drawing: What to look for”.)
The Mini-Cog has been found to be highly accurate. And, unlike the MMSE, the Mini-Cog is not skewed by education level or language skill. One study based on interviews with “informants”—the family, friends, or caregivers of patients being tested for dementia—examined the Mini-Cog’s ability to differentiate between 129 demented and 120 nondemented older adults who were culturally, linguistically, and educationally heterogeneous. The Mini-Cog classified 96% of participants correctly and had a sensitivity of 99%.24
Another retrospective analysis of a random sample of more than 1100 elderly people compared the Mini-Cog to the MMSE. In this case, the Mini-Cog and MMSE (with a cut point of 25) demonstrated similar sensitivity (76% vs 79%) and specificity (89% vs 88%) for identifying individuals with dementia.25
Jack S, whose daughter worried about his “forgetfulness,” was given the Mini-Cog. He drew a clock with the big hand incorrectly placed, and recalled only 2 words. Because his daughter was with him, the physician asked her to complete a Functional Activities Questionnaire (FAQ), a test designed for family members, caregivers, and other informants. The findings indicated that Jack had dementia, and the physician gave the family a referral to a specialist in elder care.
The Mini-Cog–FAQ. A recent study paired the Mini-Cog with the FAQ, which asks the informant to rate the patient’s ability to perform a variety of activities—eg, paying bills, shopping, engaging in hobbies, and preparing a meal. When used alone, the FAQ has a high sensitivity and specificity, but patient testing is still necessary. Used together, the Mini-Cog–FAQ allowed researchers to classify patients as cognitively normal, demented, or mildly cognitively impaired with 83% accuracy.26
Montreal Cognitive Assessment detects mild impairment
The MoCA is a 10-minute screening tool designed to help physicians detect mild cognitive impairment, which is considered to be predictive of dementia.27 This tool is especially useful for individuals who present with memory complaints but achieve a normal score (26-30) on the MMSE, as the MoCA is a better measure of executive function. It tests visuospatial skills, for example, by asking patients to draw lines connecting letters and numbers in a numerical/alphabetical sequence. It also requires abstract reasoning, by asking patients to explain the similarity between, say, a banana and an orange or a train and a bicycle.
Indicators point to ischemic disease. Janet M, the patient who had an episode of acute confusion at the mall, was an ideal candidate for the MoCA. But her physician was more familiar with the MMSE and screened her with that tool first. She received a perfect score on the MMSE, but continued to worry that the episode at the mall was the beginning of dementia, so her physician followed up with the MoCA. Only after receiving a 28 of a possible 30 on the MoCA (≥26 is considered normal) was Janet reassured that she was not suffering from dementia. Based on evidence of poorly controlled blood pressure (167/89 mm Hg), the physician determined that the brief episode was more consistent with a transient ischemic attack. The patient was referred for brain imaging to be evaluated for ischemic disease.
Like the MMSE, the MoCA has been widely translated. It is available online in more than 20 languages.
Interpreting the MoCA. In a validation study of patients from a community clinic and an academic center, the test was administered to 94 patients with mild cognitive impairment, 93 patients with mild AD (MMSE score ≥17), and 90 healthy elderly adults.27 At a cut point of 26, the MMSE demonstrated a sensitivity of 18% in detecting mild impairment; among patients with mild AD, its sensitivity was 78%. In contrast, the MoCA detected 90% of cases of mild impairment and had 100% sensitivity for mild AD. Specificity was excellent for both the MMSE and the MoCA (100% and 87%, respectively). Thus, physicians can reassure patients who achieve high scores on the MoCA that there is no indication of cognitive impairment, and schedule follow-up testing in a year. Those whose MoCA scores indicate some impairment can be referred to memory clinics or consultants for a more thorough work-up.
The AD 8 Dementia Screening Interview detects early changes
This brief screening tool—a simple 8-question interview—takes about 3 minutes to administer, and accurately identifies patients in the earliest stages of AD or another dementing disorder.28 The questions examine the domains of memory, orientation, judgment, and function.
The test, which is designed primarily for an informant but is sometimes given to patients themselves, can be administered in a variety of ways: by reading the questions to the respondent, either in person or by telephone, or on a clipboard for self-administration. The AD 8 simply asks whether specific changes have been noted, without attributing causality. The respondent answers Yes, No, or Don’t Know. The final score is a sum of the number of Yes answers. A score of ≥2 is suggestive of cognitive impairment. The AD 8 has a sensitivity of 84% and specificity of 80%.28
7-Minute Neurocognitive Screen considers normal aging
This neurocognitive screen is highly sensitive and specific (mean, 92% and 96%, respectively) for the early stages of AD and can distinguish between cognitive changes due to normal aging and those due to dementia.29 The screen consists of 4 brief tests: orientation for date and time, a 5-word recall test to assess memory function, a clock-drawing test to evaluate visuospatial skills, and a semantic verbal fluency or output task. Examples of verbal fluency measures include category generation (eg, names of animals), letter (eg, number of words beginning with a particular letter), and first names.
Interpreting the 7-minute screen. This instrument was initially validated on 60 patients diagnosed as having probable AD and 60 community-dwelling volunteers of comparable age, sex distribution, and education. The neurocognitive screen has reasonable interrater and test-retest reliability and can be administered in a short time by an individual with little clinical judgment and minimal training. Unlike the MMSE, its outcome is not affected by age or education.29
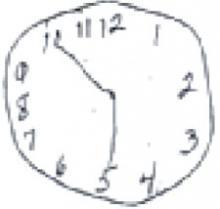
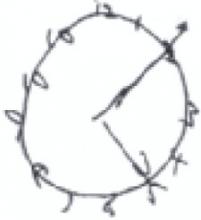
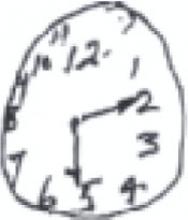
When clock drawing is used as an independent screen, it is generally graded 0 to 5, based on the shape of the circle, the distribution and inclusion of all the numbers, and the length and placement of the hands. A score of ≤3 is suggestive of dementia. All the patients here were asked to show the time as 10 minutes past 5.
The numbers on this clock are not proportionally distributed, and only 1 hand is correctly placed.
Total score: 2
The numbers are outside the circle, and there are 2 number 10s on this clock, but both hands are correctly placed.
Total score: 3
A reasonable circle with both hands correctly placed, but the numbers are disproportionately distributed.
Total score: 4
Could it be depression?
Patients with suspected dementia should also be screened for depression, because this psychiatric disorder can impersonate dementia, or exist concurrently. Depression occurs in 25% of dementia patients; it is an independent risk factor for institutionalization, and should be detected and treated.30 Subclinical depression in older women is a risk factor for cognitive decline, as well.31 Although patient presentation is not a substitute for a screening tool, the following can be helpful in distinguishing dementia and depression:
Memory. A patient suffering from depression alone is more likely to complain of memory problems than a patient with cognitive dysfunction.
Psychomotor. A depressed patient is also more likely to exhibit psychomotor retardation. Function is rarely, or only mildly, impaired in the early stages of dementia, and the movements and reactions of a patient with mild cognitive impairment often appear normal.
Concentration. Depression interferes with the ability to concentrate, and the patient may exhibit an obvious disturbance. Patients with dementia often appear to have a normal ability to concentrate, but demonstrate greater intellectual strain in attempting to answer test questions.
A 5-item geriatric screen for depression
One useful screen for depression is the 5-item version of the Geriatric Depression Scale, which has been validated in older adults.32,33 It asks the following questions:
Are you basically satisfied with your life? Do you often get bored? Do you often feel helpless? Do you prefer to stay at home rather than going out and doing new things? Do you feel pretty worthless the way you are now? This 5-item test is scored by awarding 1 point if the answer to the first question is No, and 1 additional point for each Yes answer to the remaining questions. A score of 2 or more is considered to be diagnostic for depression.
Did you suspect depression?
As you may have suspected, Fran B—the last of our 3 patients—was suffering from depression rather than dementia. Fran had a score of 4, and was referred to a psychotherapist.
Mini-Mental State Examination
http://www.nia.nih.gov/NR/rdonlyres/1C0BFA48-8280-422B-97E6-195BBFD84BA2/0/mmse.PDF
Montreal Cognitive Assessment
www.mocatest.org
AD 8 Dementia Screening Interview
http://alzheimer.wustl.edu/About_Us/PDFs/AD8form2005.pdf
7-Minute Neurocognitive Screen
http://memorydoc.org/7minutescreen
Keep these tools on hand
The assessment tools discussed in this review can help you identify mild cognitive impairment or early dementia, rule out depression, and—in many cases—reassure patients (or concerned family members) that their experiences are consistent with normal aging. All are easy to incorporate into a busy primary care practice without undue concern about the time they will take to administer.
Correspondence
Diana R. Kerwin, MD, Division of Geriatrics and Gerontology, Medical College of Wisconsin, 9200 West Wisconsin Avenue, Milwaukee, WI 53226; [email protected]
1. O’Connor DW, Pollitt PA, Hyde JB, et al. Do general practitioners miss dementia in elderly patients? BMJ. 1988;297:1107-1110.
2. Lagaay AM, van der Meij JC, Hijmans W. Validation of medical history taking as part of a population based survey in subjects aged 85 and over. BMJ. 1992;304:1091-1092.
3. Cooper B, Bickel H, Schaufele M. Early development and progression of dementing illness in the elderly: a general-practice based study. Psychol Med. 1996;26:411-419.
4. Olafsdottir M, Skoog I, Marcusson J. Detection of dementia in primary care: the Linkoping study. Dement Geriatr Cogn Disord. 2000;11:223-229.
5. Valcour VG, Masaki KH, Curb JD, et al. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964-2968.
6. Wilkinson D, Stave C, Keohane D, et al. The role of general practitioners in the diagnosis and treatment of Alzheimer’s disease: a multinational survey. J Int Med Res. 2004;32:149-159.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
8. Ritchie CW, Ames D, Clayton T, et al. Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am J Geriatr Psych. 2004;12:358-369.
9. Winblad B, Engedal K, Soinenen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489-495.
10. Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136-145.
11. Doody RS, Geldmacher DS, Gordon B, et al. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58:427-433.
12. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia. Arch Intern Med. 2006;166:2182-2188.
13. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease. A randomized controlled trial. JAMA. 1996;276:1725-1731.
14. 2008 Alzheimer’s disease facts and figures. Available at: http://www.alz.org/alzheimers_disease_publications_reports.asp. Accessed March 19, 2008.
15. US Preventive Services Task Force. Screening for dementia: recommendations and rationale. June 2003. Available at: http://www.ahcpr.gov/clinic/3rduspstf/dementia/dementrr.htm. Accessed March 18, 2008.
16. AGS Clinical Practice Committee. Guidelines abstracted from the American Academy of Neurology’s Dementia Guidelines for early detection, diagnosis, and management of dementia. J Am Geriatr Soc. 2003;51:869-873.
17. Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: Mild Cognitive Impairment(an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133-1142.
18. Wenger NS, Roth CP, Shekelle P. ACOVE Investigators. Introduction to the Assessing Care of Vulnerable Elders-3 Quality Indicator Measurement Set. J Am Geriatr Soc. 2007;55(suppl 2):s247-s251.
19. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):s293-s301.
20. Adelman AM. Initial evaluation of the patient with suspected dementia. Am Fam Physician. 2005;71:1745-1750.
21. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
22. Boustani M, Peterson B, Hanson L, et al. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138:927-937.
23. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level. JAMA. 1993;18:2386-2391.
24. Borson S, Scanlan J, Brush M, et al. The Mini-Cog: a cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021-1027.
25. Borson S, Scanlan JM, Chen P, et al. The mini-cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451-1454.
26. Steenland NK, Auman CM, Patel PM, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis. 2008;15:419-427.
27. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
28. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559-564.
29. Solomon PR, Hirschoff A, Kelly B, et al. A 7 minute neurocognitive screening battery highly sensitive to Alzheimer’s disease. Arch Neurol. 1998;55:349-355.
30. Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer’s disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction. The DIADS. Arch Gen Psychol. 2003;60:737-746.
31. Artero S, Ancelin ML, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979-984.
32. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51:694-698.
33. Sheikh JL, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173.
- Avoid cognitive screening solely on the basis of age (SOR A).
- Screen vulnerable elderly patients at their initial visit and annually thereafter (SOR A).
- Ensure that all patients who undergo cognitive screening are tested for depression (SOR A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented
Janet M, a 69-year-old woman with a history of hypertension, comes for a visit because she thinks she has Alzheimer’s disease (AD). She recently had an episode of acute confusion while shopping at the mall; when she returned to her car, she couldn’t remember how to get home. The episode cleared within minutes and hasn’t recurred.
Jack S, an 84-year-old man, seeks medical care for pain in his right shoulder. He injured the rotator cuff several years ago, but it’s been fine since he completed physical therapy—until he tripped and fell while walking outside about a week ago. His daughter is concerned about his “forgetfulness” and increasing inability to remember certain words, but the patient thinks this is a natural consequence of age.
Fran B, a 72-year-old, presents with complaints of memory problems that began about 6 months ago. She’s worried about her son and has had increasing difficulty concentrating, sleeping, and keeping track of her things.
If these were your patients, whom would you screen for dementia? Would you decide whether to screen based on your “gut,” or a defined set of criteria? Would you have several screening tools on hand, and know enough about them to determine which one might be best suited for a particular patient?
If you make decisions about screening based on your gut or aren’t sure which tools are best for which patients, you’re far from alone. Cognitive impairment, particularly in the early stages, can be difficult and time-consuming to detect, and community physicians fail to diagnose mild-to-moderate dementia more than 50% of the time.1-5
Family members and caregivers often overlook declines in cognitive function, as well. In a study of 741 caregivers of patients with AD, an average of 4 months went by between the time symptoms were first noticed and the patient was seen by a physician—and 22% of the caregivers waited more than a year.6
Cognitive decline can be slowed with early Dx
Early diagnosis of AD or any dementia is important for a number of reasons. In some cases, cognitive impairment may be related to medical conditions—head trauma, Parkinson’s disease, human immunodeficiency virus, thyroid disorder, among others—that can be modified or reversed with treatment.7 There is evidence, too, that medical, behavioral, and social interventions can delay the cognitive and functional decline associated with AD, thereby helping to prolong the time the patient can remain at home. Early diagnosis also facilitates legal and financial family planning, and makes it possible to take appropriate safety measures.8-13
AD affects approximately 5 million US residents.14 With an aging population, that number is expected to surge in the decades ahead. To help you provide optimal care for your geriatric patients, this review will detail when to screen, which tools to use, and how best to care for the 3 elderly patients in the opener.
When and whether to screen
Despite the benefits of early detection, population-based screening based on age alone is not recommended. Guidelines recommend focused screening of patients in high-risk groups and on a case-by-case basis.15
US Preventive Services Task Force (USPSTF) guidelines recommend that physicians evaluate older patients for dementia whenever there is a suggestion of cognitive impairment, based on clinical observation or concern expressed by the individuals themselves or by family members, caretakers, or friends.15
However, cognitive screening generally provides better results in populations at higher risk of dementia.16,17 With that in mind, the Assessing Care of Vulnerable Elders (ACOVE), a collaborative project to develop a set of quality indicators for care of the elderly, recently recommended cognitive and functional screening of all “vulnerable elderly.”18,19
Who are the vulnerable elderly?
ACOVE defines the vulnerable elderly as individuals who are 65 years of age and older who live in the community and are at high risk of death or functional decline over the next 2 years. You can use a screening tool to identify members of this high-risk group. Or you can simply ask a few questions and classify any noninstitutionalized older person who reports being in poor health and/or acknowledges difficulty with activities of daily living—eg, money or medication management, dressing, grooming, or preparing simple meals—as a vulnerable elderly patient.
ACOVE recommends screening all vulnerable elderly patients who are new to a primary care practice or inpatient service, and following up with an annual evaluation to detect any changes in memory and function.18,19 Based on the recommendations of ACOVE and the USPSTF, all 3 patients described earlier would be candidates for screening.
Choosing the best screening tool
There is no serum or radiographic test available for the diagnosis of AD, and a thorough clinical evaluation can be extremely time-consuming. In the primary care setting, focused evaluations are both useful and cost effective.
There are a number of valid and reliable cognitive screening tools, including the Mini-Mental State Examination (MMSE), the Mini-Cog, the Montreal Cognitive Assessment (MoCA), the AD 8 Dementia Screening Interview, and the 7-Minute Neurocognitive Screen. In evaluating these and other screening tools, consider the sensitivity and specificity of each. Consider, too, features that may make one test better suited than another for a particular patient or specific circumstance (TABLE).
TABLE
Cognitive screening tools: How they compare
| TOOL (TIME TO ADMINISTER) | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| MMSE (5-10 min) | Tracks/quantifies changes; easy to administer; widely accepted; available in >50 languages | Not specific to AD or sensitive to mild dementia; influenced by age, education, and language skills |
| Mini-Cog (3 min) | Brief, easy to administer and score; unaffected by education, language skill; high sensitivity; easily paired with FAQ | |
| MoCA (10 min) | High sensitivity for MCI and mild AD; available in >20 languages; especially useful for patients with memory complaints but normal MMSE score | More time-consuming than other screening tools |
| AD 8 (3 min) | Administered in person or by phone; identifies earliest stages of dementia | Designed for informant, who may not be available, but can be given to patient |
| 7-Minute Neurocognitive Screen | Highly sensitive to early stages of AD | |
| AD, Alzheimer’s disease; FAQ, Functional Activities Questionnaire; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment. | ||
The Mini-Mental State Exam is the gold standard
Considered the gold standard in dementia assessment, the MMSE is the most widely used cognitive screening tool in the United States.20 The MMSE samples 6 cognitive areas—orientation, registration, attention and calculation, recall, language, and constructional skill.21 It has a high degree of validity in detecting dementia, and is particularly useful in tracking and quantifying changes over time. Well-known features include the serial 7s, in which patients are asked to count backwards from 100 by 7s, and the 3-stage command: Take a paper in your right hand, fold it in half, and put it on the floor.
Besides being relatively short (testing takes 5-10 minutes), the MMSE is easy to administer. It has been translated into more than 50 languages, and can therefore be used in many cultural settings.
Interpreting the MMSE. A total score of ≤23 out of a maximum of 30 points is suggestive of dementia. However, patient performance on the MMSE is influenced by a number of factors unrelated to cognitive function, such as age, educational level, deficits in language skills, and motor or visual impairment.22 Thus, the cut point may be adjusted depending on the patient or population being screened, and the positive predictive value and sensitivity and specificity for dementia vary accordingly. In a population-based sample of >18,000 individuals with a cut point of 24, the sensitivity was 87% and the specificity was 82%.23
The usefulness of the MMSE is also limited because the test is not specific to AD and is not sensitive to mild dementia. When the test is used to screen highly intelligent, well-educated patients, it may fail to detect any decline in memory or cognitive function.
The Mini-Cog is a quick and easy alternative
The Mini-Cog screen consists of 2 simple components that evaluate executive function: a clock-drawing test and word recall.24 The patient is given paper and pencil and asked to draw a clock with hands set on a specified time—5:10, say. First, however, the screener recites 3 common but unrelated words; the patient is asked to recite the words before drawing the clock and to recall them afterwards. The entire test takes about 3 minutes.
Interpreting the Mini-Cog. Besides being quick and easy to administer, the Mini-Cog is easy to score:
- Patients who do not recall any of the words are classified as demented.
- Patients who recall all of the words are classified as nondemented
- Patients who recall 1 or 2 words are classified based on their clock drawing: They’re considered nondemented if the clock is normal and demented if it is not.
When clock-drawing is part of the Mini-Cog screen, the results are simply considered normal or abnormal. For a clock to be considered normal, all the numbers must be in the correct sequence and the hands correctly positioned to show the designated time.24 (The clock-drawing can also be used as an independent screen. For more about that, see “Clock-drawing: What to look for”.)
The Mini-Cog has been found to be highly accurate. And, unlike the MMSE, the Mini-Cog is not skewed by education level or language skill. One study based on interviews with “informants”—the family, friends, or caregivers of patients being tested for dementia—examined the Mini-Cog’s ability to differentiate between 129 demented and 120 nondemented older adults who were culturally, linguistically, and educationally heterogeneous. The Mini-Cog classified 96% of participants correctly and had a sensitivity of 99%.24
Another retrospective analysis of a random sample of more than 1100 elderly people compared the Mini-Cog to the MMSE. In this case, the Mini-Cog and MMSE (with a cut point of 25) demonstrated similar sensitivity (76% vs 79%) and specificity (89% vs 88%) for identifying individuals with dementia.25
Jack S, whose daughter worried about his “forgetfulness,” was given the Mini-Cog. He drew a clock with the big hand incorrectly placed, and recalled only 2 words. Because his daughter was with him, the physician asked her to complete a Functional Activities Questionnaire (FAQ), a test designed for family members, caregivers, and other informants. The findings indicated that Jack had dementia, and the physician gave the family a referral to a specialist in elder care.
The Mini-Cog–FAQ. A recent study paired the Mini-Cog with the FAQ, which asks the informant to rate the patient’s ability to perform a variety of activities—eg, paying bills, shopping, engaging in hobbies, and preparing a meal. When used alone, the FAQ has a high sensitivity and specificity, but patient testing is still necessary. Used together, the Mini-Cog–FAQ allowed researchers to classify patients as cognitively normal, demented, or mildly cognitively impaired with 83% accuracy.26
Montreal Cognitive Assessment detects mild impairment
The MoCA is a 10-minute screening tool designed to help physicians detect mild cognitive impairment, which is considered to be predictive of dementia.27 This tool is especially useful for individuals who present with memory complaints but achieve a normal score (26-30) on the MMSE, as the MoCA is a better measure of executive function. It tests visuospatial skills, for example, by asking patients to draw lines connecting letters and numbers in a numerical/alphabetical sequence. It also requires abstract reasoning, by asking patients to explain the similarity between, say, a banana and an orange or a train and a bicycle.
Indicators point to ischemic disease. Janet M, the patient who had an episode of acute confusion at the mall, was an ideal candidate for the MoCA. But her physician was more familiar with the MMSE and screened her with that tool first. She received a perfect score on the MMSE, but continued to worry that the episode at the mall was the beginning of dementia, so her physician followed up with the MoCA. Only after receiving a 28 of a possible 30 on the MoCA (≥26 is considered normal) was Janet reassured that she was not suffering from dementia. Based on evidence of poorly controlled blood pressure (167/89 mm Hg), the physician determined that the brief episode was more consistent with a transient ischemic attack. The patient was referred for brain imaging to be evaluated for ischemic disease.
Like the MMSE, the MoCA has been widely translated. It is available online in more than 20 languages.
Interpreting the MoCA. In a validation study of patients from a community clinic and an academic center, the test was administered to 94 patients with mild cognitive impairment, 93 patients with mild AD (MMSE score ≥17), and 90 healthy elderly adults.27 At a cut point of 26, the MMSE demonstrated a sensitivity of 18% in detecting mild impairment; among patients with mild AD, its sensitivity was 78%. In contrast, the MoCA detected 90% of cases of mild impairment and had 100% sensitivity for mild AD. Specificity was excellent for both the MMSE and the MoCA (100% and 87%, respectively). Thus, physicians can reassure patients who achieve high scores on the MoCA that there is no indication of cognitive impairment, and schedule follow-up testing in a year. Those whose MoCA scores indicate some impairment can be referred to memory clinics or consultants for a more thorough work-up.
The AD 8 Dementia Screening Interview detects early changes
This brief screening tool—a simple 8-question interview—takes about 3 minutes to administer, and accurately identifies patients in the earliest stages of AD or another dementing disorder.28 The questions examine the domains of memory, orientation, judgment, and function.
The test, which is designed primarily for an informant but is sometimes given to patients themselves, can be administered in a variety of ways: by reading the questions to the respondent, either in person or by telephone, or on a clipboard for self-administration. The AD 8 simply asks whether specific changes have been noted, without attributing causality. The respondent answers Yes, No, or Don’t Know. The final score is a sum of the number of Yes answers. A score of ≥2 is suggestive of cognitive impairment. The AD 8 has a sensitivity of 84% and specificity of 80%.28
7-Minute Neurocognitive Screen considers normal aging
This neurocognitive screen is highly sensitive and specific (mean, 92% and 96%, respectively) for the early stages of AD and can distinguish between cognitive changes due to normal aging and those due to dementia.29 The screen consists of 4 brief tests: orientation for date and time, a 5-word recall test to assess memory function, a clock-drawing test to evaluate visuospatial skills, and a semantic verbal fluency or output task. Examples of verbal fluency measures include category generation (eg, names of animals), letter (eg, number of words beginning with a particular letter), and first names.
Interpreting the 7-minute screen. This instrument was initially validated on 60 patients diagnosed as having probable AD and 60 community-dwelling volunteers of comparable age, sex distribution, and education. The neurocognitive screen has reasonable interrater and test-retest reliability and can be administered in a short time by an individual with little clinical judgment and minimal training. Unlike the MMSE, its outcome is not affected by age or education.29
When clock drawing is used as an independent screen, it is generally graded 0 to 5, based on the shape of the circle, the distribution and inclusion of all the numbers, and the length and placement of the hands. A score of ≤3 is suggestive of dementia. All the patients here were asked to show the time as 10 minutes past 5.
The numbers on this clock are not proportionally distributed, and only 1 hand is correctly placed.
Total score: 2
The numbers are outside the circle, and there are 2 number 10s on this clock, but both hands are correctly placed.
Total score: 3
A reasonable circle with both hands correctly placed, but the numbers are disproportionately distributed.
Total score: 4
Could it be depression?
Patients with suspected dementia should also be screened for depression, because this psychiatric disorder can impersonate dementia, or exist concurrently. Depression occurs in 25% of dementia patients; it is an independent risk factor for institutionalization, and should be detected and treated.30 Subclinical depression in older women is a risk factor for cognitive decline, as well.31 Although patient presentation is not a substitute for a screening tool, the following can be helpful in distinguishing dementia and depression:
Memory. A patient suffering from depression alone is more likely to complain of memory problems than a patient with cognitive dysfunction.
Psychomotor. A depressed patient is also more likely to exhibit psychomotor retardation. Function is rarely, or only mildly, impaired in the early stages of dementia, and the movements and reactions of a patient with mild cognitive impairment often appear normal.
Concentration. Depression interferes with the ability to concentrate, and the patient may exhibit an obvious disturbance. Patients with dementia often appear to have a normal ability to concentrate, but demonstrate greater intellectual strain in attempting to answer test questions.
A 5-item geriatric screen for depression
One useful screen for depression is the 5-item version of the Geriatric Depression Scale, which has been validated in older adults.32,33 It asks the following questions:
Are you basically satisfied with your life? Do you often get bored? Do you often feel helpless? Do you prefer to stay at home rather than going out and doing new things? Do you feel pretty worthless the way you are now? This 5-item test is scored by awarding 1 point if the answer to the first question is No, and 1 additional point for each Yes answer to the remaining questions. A score of 2 or more is considered to be diagnostic for depression.
Did you suspect depression?
As you may have suspected, Fran B—the last of our 3 patients—was suffering from depression rather than dementia. Fran had a score of 4, and was referred to a psychotherapist.
Mini-Mental State Examination
http://www.nia.nih.gov/NR/rdonlyres/1C0BFA48-8280-422B-97E6-195BBFD84BA2/0/mmse.PDF
Montreal Cognitive Assessment
www.mocatest.org
AD 8 Dementia Screening Interview
http://alzheimer.wustl.edu/About_Us/PDFs/AD8form2005.pdf
7-Minute Neurocognitive Screen
http://memorydoc.org/7minutescreen
Keep these tools on hand
The assessment tools discussed in this review can help you identify mild cognitive impairment or early dementia, rule out depression, and—in many cases—reassure patients (or concerned family members) that their experiences are consistent with normal aging. All are easy to incorporate into a busy primary care practice without undue concern about the time they will take to administer.
Correspondence
Diana R. Kerwin, MD, Division of Geriatrics and Gerontology, Medical College of Wisconsin, 9200 West Wisconsin Avenue, Milwaukee, WI 53226; [email protected]
- Avoid cognitive screening solely on the basis of age (SOR A).
- Screen vulnerable elderly patients at their initial visit and annually thereafter (SOR A).
- Ensure that all patients who undergo cognitive screening are tested for depression (SOR A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented
Janet M, a 69-year-old woman with a history of hypertension, comes for a visit because she thinks she has Alzheimer’s disease (AD). She recently had an episode of acute confusion while shopping at the mall; when she returned to her car, she couldn’t remember how to get home. The episode cleared within minutes and hasn’t recurred.
Jack S, an 84-year-old man, seeks medical care for pain in his right shoulder. He injured the rotator cuff several years ago, but it’s been fine since he completed physical therapy—until he tripped and fell while walking outside about a week ago. His daughter is concerned about his “forgetfulness” and increasing inability to remember certain words, but the patient thinks this is a natural consequence of age.
Fran B, a 72-year-old, presents with complaints of memory problems that began about 6 months ago. She’s worried about her son and has had increasing difficulty concentrating, sleeping, and keeping track of her things.
If these were your patients, whom would you screen for dementia? Would you decide whether to screen based on your “gut,” or a defined set of criteria? Would you have several screening tools on hand, and know enough about them to determine which one might be best suited for a particular patient?
If you make decisions about screening based on your gut or aren’t sure which tools are best for which patients, you’re far from alone. Cognitive impairment, particularly in the early stages, can be difficult and time-consuming to detect, and community physicians fail to diagnose mild-to-moderate dementia more than 50% of the time.1-5
Family members and caregivers often overlook declines in cognitive function, as well. In a study of 741 caregivers of patients with AD, an average of 4 months went by between the time symptoms were first noticed and the patient was seen by a physician—and 22% of the caregivers waited more than a year.6
Cognitive decline can be slowed with early Dx
Early diagnosis of AD or any dementia is important for a number of reasons. In some cases, cognitive impairment may be related to medical conditions—head trauma, Parkinson’s disease, human immunodeficiency virus, thyroid disorder, among others—that can be modified or reversed with treatment.7 There is evidence, too, that medical, behavioral, and social interventions can delay the cognitive and functional decline associated with AD, thereby helping to prolong the time the patient can remain at home. Early diagnosis also facilitates legal and financial family planning, and makes it possible to take appropriate safety measures.8-13
AD affects approximately 5 million US residents.14 With an aging population, that number is expected to surge in the decades ahead. To help you provide optimal care for your geriatric patients, this review will detail when to screen, which tools to use, and how best to care for the 3 elderly patients in the opener.
When and whether to screen
Despite the benefits of early detection, population-based screening based on age alone is not recommended. Guidelines recommend focused screening of patients in high-risk groups and on a case-by-case basis.15
US Preventive Services Task Force (USPSTF) guidelines recommend that physicians evaluate older patients for dementia whenever there is a suggestion of cognitive impairment, based on clinical observation or concern expressed by the individuals themselves or by family members, caretakers, or friends.15
However, cognitive screening generally provides better results in populations at higher risk of dementia.16,17 With that in mind, the Assessing Care of Vulnerable Elders (ACOVE), a collaborative project to develop a set of quality indicators for care of the elderly, recently recommended cognitive and functional screening of all “vulnerable elderly.”18,19
Who are the vulnerable elderly?
ACOVE defines the vulnerable elderly as individuals who are 65 years of age and older who live in the community and are at high risk of death or functional decline over the next 2 years. You can use a screening tool to identify members of this high-risk group. Or you can simply ask a few questions and classify any noninstitutionalized older person who reports being in poor health and/or acknowledges difficulty with activities of daily living—eg, money or medication management, dressing, grooming, or preparing simple meals—as a vulnerable elderly patient.
ACOVE recommends screening all vulnerable elderly patients who are new to a primary care practice or inpatient service, and following up with an annual evaluation to detect any changes in memory and function.18,19 Based on the recommendations of ACOVE and the USPSTF, all 3 patients described earlier would be candidates for screening.
Choosing the best screening tool
There is no serum or radiographic test available for the diagnosis of AD, and a thorough clinical evaluation can be extremely time-consuming. In the primary care setting, focused evaluations are both useful and cost effective.
There are a number of valid and reliable cognitive screening tools, including the Mini-Mental State Examination (MMSE), the Mini-Cog, the Montreal Cognitive Assessment (MoCA), the AD 8 Dementia Screening Interview, and the 7-Minute Neurocognitive Screen. In evaluating these and other screening tools, consider the sensitivity and specificity of each. Consider, too, features that may make one test better suited than another for a particular patient or specific circumstance (TABLE).
TABLE
Cognitive screening tools: How they compare
| TOOL (TIME TO ADMINISTER) | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| MMSE (5-10 min) | Tracks/quantifies changes; easy to administer; widely accepted; available in >50 languages | Not specific to AD or sensitive to mild dementia; influenced by age, education, and language skills |
| Mini-Cog (3 min) | Brief, easy to administer and score; unaffected by education, language skill; high sensitivity; easily paired with FAQ | |
| MoCA (10 min) | High sensitivity for MCI and mild AD; available in >20 languages; especially useful for patients with memory complaints but normal MMSE score | More time-consuming than other screening tools |
| AD 8 (3 min) | Administered in person or by phone; identifies earliest stages of dementia | Designed for informant, who may not be available, but can be given to patient |
| 7-Minute Neurocognitive Screen | Highly sensitive to early stages of AD | |
| AD, Alzheimer’s disease; FAQ, Functional Activities Questionnaire; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment. | ||
The Mini-Mental State Exam is the gold standard
Considered the gold standard in dementia assessment, the MMSE is the most widely used cognitive screening tool in the United States.20 The MMSE samples 6 cognitive areas—orientation, registration, attention and calculation, recall, language, and constructional skill.21 It has a high degree of validity in detecting dementia, and is particularly useful in tracking and quantifying changes over time. Well-known features include the serial 7s, in which patients are asked to count backwards from 100 by 7s, and the 3-stage command: Take a paper in your right hand, fold it in half, and put it on the floor.
Besides being relatively short (testing takes 5-10 minutes), the MMSE is easy to administer. It has been translated into more than 50 languages, and can therefore be used in many cultural settings.
Interpreting the MMSE. A total score of ≤23 out of a maximum of 30 points is suggestive of dementia. However, patient performance on the MMSE is influenced by a number of factors unrelated to cognitive function, such as age, educational level, deficits in language skills, and motor or visual impairment.22 Thus, the cut point may be adjusted depending on the patient or population being screened, and the positive predictive value and sensitivity and specificity for dementia vary accordingly. In a population-based sample of >18,000 individuals with a cut point of 24, the sensitivity was 87% and the specificity was 82%.23
The usefulness of the MMSE is also limited because the test is not specific to AD and is not sensitive to mild dementia. When the test is used to screen highly intelligent, well-educated patients, it may fail to detect any decline in memory or cognitive function.
The Mini-Cog is a quick and easy alternative
The Mini-Cog screen consists of 2 simple components that evaluate executive function: a clock-drawing test and word recall.24 The patient is given paper and pencil and asked to draw a clock with hands set on a specified time—5:10, say. First, however, the screener recites 3 common but unrelated words; the patient is asked to recite the words before drawing the clock and to recall them afterwards. The entire test takes about 3 minutes.
Interpreting the Mini-Cog. Besides being quick and easy to administer, the Mini-Cog is easy to score:
- Patients who do not recall any of the words are classified as demented.
- Patients who recall all of the words are classified as nondemented
- Patients who recall 1 or 2 words are classified based on their clock drawing: They’re considered nondemented if the clock is normal and demented if it is not.
When clock-drawing is part of the Mini-Cog screen, the results are simply considered normal or abnormal. For a clock to be considered normal, all the numbers must be in the correct sequence and the hands correctly positioned to show the designated time.24 (The clock-drawing can also be used as an independent screen. For more about that, see “Clock-drawing: What to look for”.)
The Mini-Cog has been found to be highly accurate. And, unlike the MMSE, the Mini-Cog is not skewed by education level or language skill. One study based on interviews with “informants”—the family, friends, or caregivers of patients being tested for dementia—examined the Mini-Cog’s ability to differentiate between 129 demented and 120 nondemented older adults who were culturally, linguistically, and educationally heterogeneous. The Mini-Cog classified 96% of participants correctly and had a sensitivity of 99%.24
Another retrospective analysis of a random sample of more than 1100 elderly people compared the Mini-Cog to the MMSE. In this case, the Mini-Cog and MMSE (with a cut point of 25) demonstrated similar sensitivity (76% vs 79%) and specificity (89% vs 88%) for identifying individuals with dementia.25
Jack S, whose daughter worried about his “forgetfulness,” was given the Mini-Cog. He drew a clock with the big hand incorrectly placed, and recalled only 2 words. Because his daughter was with him, the physician asked her to complete a Functional Activities Questionnaire (FAQ), a test designed for family members, caregivers, and other informants. The findings indicated that Jack had dementia, and the physician gave the family a referral to a specialist in elder care.
The Mini-Cog–FAQ. A recent study paired the Mini-Cog with the FAQ, which asks the informant to rate the patient’s ability to perform a variety of activities—eg, paying bills, shopping, engaging in hobbies, and preparing a meal. When used alone, the FAQ has a high sensitivity and specificity, but patient testing is still necessary. Used together, the Mini-Cog–FAQ allowed researchers to classify patients as cognitively normal, demented, or mildly cognitively impaired with 83% accuracy.26
Montreal Cognitive Assessment detects mild impairment
The MoCA is a 10-minute screening tool designed to help physicians detect mild cognitive impairment, which is considered to be predictive of dementia.27 This tool is especially useful for individuals who present with memory complaints but achieve a normal score (26-30) on the MMSE, as the MoCA is a better measure of executive function. It tests visuospatial skills, for example, by asking patients to draw lines connecting letters and numbers in a numerical/alphabetical sequence. It also requires abstract reasoning, by asking patients to explain the similarity between, say, a banana and an orange or a train and a bicycle.
Indicators point to ischemic disease. Janet M, the patient who had an episode of acute confusion at the mall, was an ideal candidate for the MoCA. But her physician was more familiar with the MMSE and screened her with that tool first. She received a perfect score on the MMSE, but continued to worry that the episode at the mall was the beginning of dementia, so her physician followed up with the MoCA. Only after receiving a 28 of a possible 30 on the MoCA (≥26 is considered normal) was Janet reassured that she was not suffering from dementia. Based on evidence of poorly controlled blood pressure (167/89 mm Hg), the physician determined that the brief episode was more consistent with a transient ischemic attack. The patient was referred for brain imaging to be evaluated for ischemic disease.
Like the MMSE, the MoCA has been widely translated. It is available online in more than 20 languages.
Interpreting the MoCA. In a validation study of patients from a community clinic and an academic center, the test was administered to 94 patients with mild cognitive impairment, 93 patients with mild AD (MMSE score ≥17), and 90 healthy elderly adults.27 At a cut point of 26, the MMSE demonstrated a sensitivity of 18% in detecting mild impairment; among patients with mild AD, its sensitivity was 78%. In contrast, the MoCA detected 90% of cases of mild impairment and had 100% sensitivity for mild AD. Specificity was excellent for both the MMSE and the MoCA (100% and 87%, respectively). Thus, physicians can reassure patients who achieve high scores on the MoCA that there is no indication of cognitive impairment, and schedule follow-up testing in a year. Those whose MoCA scores indicate some impairment can be referred to memory clinics or consultants for a more thorough work-up.
The AD 8 Dementia Screening Interview detects early changes
This brief screening tool—a simple 8-question interview—takes about 3 minutes to administer, and accurately identifies patients in the earliest stages of AD or another dementing disorder.28 The questions examine the domains of memory, orientation, judgment, and function.
The test, which is designed primarily for an informant but is sometimes given to patients themselves, can be administered in a variety of ways: by reading the questions to the respondent, either in person or by telephone, or on a clipboard for self-administration. The AD 8 simply asks whether specific changes have been noted, without attributing causality. The respondent answers Yes, No, or Don’t Know. The final score is a sum of the number of Yes answers. A score of ≥2 is suggestive of cognitive impairment. The AD 8 has a sensitivity of 84% and specificity of 80%.28
7-Minute Neurocognitive Screen considers normal aging
This neurocognitive screen is highly sensitive and specific (mean, 92% and 96%, respectively) for the early stages of AD and can distinguish between cognitive changes due to normal aging and those due to dementia.29 The screen consists of 4 brief tests: orientation for date and time, a 5-word recall test to assess memory function, a clock-drawing test to evaluate visuospatial skills, and a semantic verbal fluency or output task. Examples of verbal fluency measures include category generation (eg, names of animals), letter (eg, number of words beginning with a particular letter), and first names.
Interpreting the 7-minute screen. This instrument was initially validated on 60 patients diagnosed as having probable AD and 60 community-dwelling volunteers of comparable age, sex distribution, and education. The neurocognitive screen has reasonable interrater and test-retest reliability and can be administered in a short time by an individual with little clinical judgment and minimal training. Unlike the MMSE, its outcome is not affected by age or education.29
When clock drawing is used as an independent screen, it is generally graded 0 to 5, based on the shape of the circle, the distribution and inclusion of all the numbers, and the length and placement of the hands. A score of ≤3 is suggestive of dementia. All the patients here were asked to show the time as 10 minutes past 5.
The numbers on this clock are not proportionally distributed, and only 1 hand is correctly placed.
Total score: 2
The numbers are outside the circle, and there are 2 number 10s on this clock, but both hands are correctly placed.
Total score: 3
A reasonable circle with both hands correctly placed, but the numbers are disproportionately distributed.
Total score: 4
Could it be depression?
Patients with suspected dementia should also be screened for depression, because this psychiatric disorder can impersonate dementia, or exist concurrently. Depression occurs in 25% of dementia patients; it is an independent risk factor for institutionalization, and should be detected and treated.30 Subclinical depression in older women is a risk factor for cognitive decline, as well.31 Although patient presentation is not a substitute for a screening tool, the following can be helpful in distinguishing dementia and depression:
Memory. A patient suffering from depression alone is more likely to complain of memory problems than a patient with cognitive dysfunction.
Psychomotor. A depressed patient is also more likely to exhibit psychomotor retardation. Function is rarely, or only mildly, impaired in the early stages of dementia, and the movements and reactions of a patient with mild cognitive impairment often appear normal.
Concentration. Depression interferes with the ability to concentrate, and the patient may exhibit an obvious disturbance. Patients with dementia often appear to have a normal ability to concentrate, but demonstrate greater intellectual strain in attempting to answer test questions.
A 5-item geriatric screen for depression
One useful screen for depression is the 5-item version of the Geriatric Depression Scale, which has been validated in older adults.32,33 It asks the following questions:
Are you basically satisfied with your life? Do you often get bored? Do you often feel helpless? Do you prefer to stay at home rather than going out and doing new things? Do you feel pretty worthless the way you are now? This 5-item test is scored by awarding 1 point if the answer to the first question is No, and 1 additional point for each Yes answer to the remaining questions. A score of 2 or more is considered to be diagnostic for depression.
Did you suspect depression?
As you may have suspected, Fran B—the last of our 3 patients—was suffering from depression rather than dementia. Fran had a score of 4, and was referred to a psychotherapist.
Mini-Mental State Examination
http://www.nia.nih.gov/NR/rdonlyres/1C0BFA48-8280-422B-97E6-195BBFD84BA2/0/mmse.PDF
Montreal Cognitive Assessment
www.mocatest.org
AD 8 Dementia Screening Interview
http://alzheimer.wustl.edu/About_Us/PDFs/AD8form2005.pdf
7-Minute Neurocognitive Screen
http://memorydoc.org/7minutescreen
Keep these tools on hand
The assessment tools discussed in this review can help you identify mild cognitive impairment or early dementia, rule out depression, and—in many cases—reassure patients (or concerned family members) that their experiences are consistent with normal aging. All are easy to incorporate into a busy primary care practice without undue concern about the time they will take to administer.
Correspondence
Diana R. Kerwin, MD, Division of Geriatrics and Gerontology, Medical College of Wisconsin, 9200 West Wisconsin Avenue, Milwaukee, WI 53226; [email protected]
1. O’Connor DW, Pollitt PA, Hyde JB, et al. Do general practitioners miss dementia in elderly patients? BMJ. 1988;297:1107-1110.
2. Lagaay AM, van der Meij JC, Hijmans W. Validation of medical history taking as part of a population based survey in subjects aged 85 and over. BMJ. 1992;304:1091-1092.
3. Cooper B, Bickel H, Schaufele M. Early development and progression of dementing illness in the elderly: a general-practice based study. Psychol Med. 1996;26:411-419.
4. Olafsdottir M, Skoog I, Marcusson J. Detection of dementia in primary care: the Linkoping study. Dement Geriatr Cogn Disord. 2000;11:223-229.
5. Valcour VG, Masaki KH, Curb JD, et al. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964-2968.
6. Wilkinson D, Stave C, Keohane D, et al. The role of general practitioners in the diagnosis and treatment of Alzheimer’s disease: a multinational survey. J Int Med Res. 2004;32:149-159.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
8. Ritchie CW, Ames D, Clayton T, et al. Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am J Geriatr Psych. 2004;12:358-369.
9. Winblad B, Engedal K, Soinenen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489-495.
10. Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136-145.
11. Doody RS, Geldmacher DS, Gordon B, et al. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58:427-433.
12. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia. Arch Intern Med. 2006;166:2182-2188.
13. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease. A randomized controlled trial. JAMA. 1996;276:1725-1731.
14. 2008 Alzheimer’s disease facts and figures. Available at: http://www.alz.org/alzheimers_disease_publications_reports.asp. Accessed March 19, 2008.
15. US Preventive Services Task Force. Screening for dementia: recommendations and rationale. June 2003. Available at: http://www.ahcpr.gov/clinic/3rduspstf/dementia/dementrr.htm. Accessed March 18, 2008.
16. AGS Clinical Practice Committee. Guidelines abstracted from the American Academy of Neurology’s Dementia Guidelines for early detection, diagnosis, and management of dementia. J Am Geriatr Soc. 2003;51:869-873.
17. Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: Mild Cognitive Impairment(an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133-1142.
18. Wenger NS, Roth CP, Shekelle P. ACOVE Investigators. Introduction to the Assessing Care of Vulnerable Elders-3 Quality Indicator Measurement Set. J Am Geriatr Soc. 2007;55(suppl 2):s247-s251.
19. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):s293-s301.
20. Adelman AM. Initial evaluation of the patient with suspected dementia. Am Fam Physician. 2005;71:1745-1750.
21. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
22. Boustani M, Peterson B, Hanson L, et al. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138:927-937.
23. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level. JAMA. 1993;18:2386-2391.
24. Borson S, Scanlan J, Brush M, et al. The Mini-Cog: a cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021-1027.
25. Borson S, Scanlan JM, Chen P, et al. The mini-cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451-1454.
26. Steenland NK, Auman CM, Patel PM, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis. 2008;15:419-427.
27. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
28. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559-564.
29. Solomon PR, Hirschoff A, Kelly B, et al. A 7 minute neurocognitive screening battery highly sensitive to Alzheimer’s disease. Arch Neurol. 1998;55:349-355.
30. Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer’s disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction. The DIADS. Arch Gen Psychol. 2003;60:737-746.
31. Artero S, Ancelin ML, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979-984.
32. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51:694-698.
33. Sheikh JL, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173.
1. O’Connor DW, Pollitt PA, Hyde JB, et al. Do general practitioners miss dementia in elderly patients? BMJ. 1988;297:1107-1110.
2. Lagaay AM, van der Meij JC, Hijmans W. Validation of medical history taking as part of a population based survey in subjects aged 85 and over. BMJ. 1992;304:1091-1092.
3. Cooper B, Bickel H, Schaufele M. Early development and progression of dementing illness in the elderly: a general-practice based study. Psychol Med. 1996;26:411-419.
4. Olafsdottir M, Skoog I, Marcusson J. Detection of dementia in primary care: the Linkoping study. Dement Geriatr Cogn Disord. 2000;11:223-229.
5. Valcour VG, Masaki KH, Curb JD, et al. The detection of dementia in the primary care setting. Arch Intern Med. 2000;160:2964-2968.
6. Wilkinson D, Stave C, Keohane D, et al. The role of general practitioners in the diagnosis and treatment of Alzheimer’s disease: a multinational survey. J Int Med Res. 2004;32:149-159.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000.
8. Ritchie CW, Ames D, Clayton T, et al. Metaanalysis of randomized trials of the efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer disease. Am J Geriatr Psych. 2004;12:358-369.
9. Winblad B, Engedal K, Soinenen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489-495.
10. Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136-145.
11. Doody RS, Geldmacher DS, Gordon B, et al. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58:427-433.
12. Ayalon L, Gum AM, Feliciano L, et al. Effectiveness of nonpharmacological interventions for the management of neuropsychiatric symptoms in patients with dementia. Arch Intern Med. 2006;166:2182-2188.
13. Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease. A randomized controlled trial. JAMA. 1996;276:1725-1731.
14. 2008 Alzheimer’s disease facts and figures. Available at: http://www.alz.org/alzheimers_disease_publications_reports.asp. Accessed March 19, 2008.
15. US Preventive Services Task Force. Screening for dementia: recommendations and rationale. June 2003. Available at: http://www.ahcpr.gov/clinic/3rduspstf/dementia/dementrr.htm. Accessed March 18, 2008.
16. AGS Clinical Practice Committee. Guidelines abstracted from the American Academy of Neurology’s Dementia Guidelines for early detection, diagnosis, and management of dementia. J Am Geriatr Soc. 2003;51:869-873.
17. Petersen RC, Stevens JC, Ganguli M, et al. Practice parameter: Mild Cognitive Impairment(an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133-1142.
18. Wenger NS, Roth CP, Shekelle P. ACOVE Investigators. Introduction to the Assessing Care of Vulnerable Elders-3 Quality Indicator Measurement Set. J Am Geriatr Soc. 2007;55(suppl 2):s247-s251.
19. Feil DG, MacLean C, Sultzer D. Quality indicators for the care of dementia in vulnerable elders. J Am Geriatr Soc. 2007;55(suppl 2):s293-s301.
20. Adelman AM. Initial evaluation of the patient with suspected dementia. Am Fam Physician. 2005;71:1745-1750.
21. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
22. Boustani M, Peterson B, Hanson L, et al. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003;138:927-937.
23. Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the mini-mental state examination by age and educational level. JAMA. 1993;18:2386-2391.
24. Borson S, Scanlan J, Brush M, et al. The Mini-Cog: a cognitive “vital signs” measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021-1027.
25. Borson S, Scanlan JM, Chen P, et al. The mini-cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51:1451-1454.
26. Steenland NK, Auman CM, Patel PM, et al. Development of a rapid screening instrument for mild cognitive impairment and undiagnosed dementia. J Alzheimers Dis. 2008;15:419-427.
27. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699.
28. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559-564.
29. Solomon PR, Hirschoff A, Kelly B, et al. A 7 minute neurocognitive screening battery highly sensitive to Alzheimer’s disease. Arch Neurol. 1998;55:349-355.
30. Lyketsos CG, DelCampo L, Steinberg M, et al. Treating depression in Alzheimer’s disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction. The DIADS. Arch Gen Psychol. 2003;60:737-746.
31. Artero S, Ancelin ML, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979-984.
32. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51:694-698.
33. Sheikh JL, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165-173.