User login
• Arrange for urgent transport to the hospital when a patient presents with stroke-like symptoms of acute onset, especially within the 3- to 6-hour therapeutic window. B
• Use a validated prehospital stroke identification algorithm such as the Face Arms Speech Time (FAST) test to identify possible acute stroke patients requiring urgent transport to the hospital. B
• Obtain a CBC and basic metabolic panel for all patients with signs and symptoms suggestive of stroke—and a blood alcohol, hepatic function, and toxicology screen, in select patients—to help rule out stroke mimics. C
• Ensure that patients undergo brain imaging to rule out stroke mimics before treatment for acute ischemic stroke is initiated. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Stroke is the third leading cause of death (claiming the life of 1 person every 3 to 4 minutes) and the No. 1 cause of adult disability in the United States.1 Advances in thrombolysis and clot removal can improve outcomes, but are dependent on swift and certain diagnosis. Amid the rush to ensure that treatment is initiated within the therapeutic window for cerebral reperfusion, “stroke mimics”—so called because of their ability to cause signs and symptoms similar to stroke—are sometimes mistaken for the real thing.
The prevalence of misdiagnosis ranges from about 4% of patients who receive tissue plasminogen activator (tPA) for reperfusion2 to 25% of patients who are rushed to the hospital because they are thought to be having a stroke.3 Seizures, migraine, sepsis, and peripheral vestibular disorders are among the many conditions that can masquerade as stroke.
Misdiagnosis can subject patients to unnecessary, and potentially harmful, invasive stroke therapies, and significantly delay the treatment they need. To prevent such outcomes, it is essential for primary care physicians, as well as emergency responders and emergency department (ED) physicians, to be aware of—and on the lookout for—the clues that can distinguish stroke from stroke mimics.
Suspect stroke? Establish a baseline
Despite a nationwide effort to increase public awareness of stroke and the importance of getting to the hospital within the therapeutic window for treatment,4 too few patients arrive within the time frame for cerebral reperfusion therapy. Primary care physicians can help by educating patients about stroke signs and symptoms.
When a patient presents with possible stroke, determine whether symptoms began gradually or abruptly. An acute ischemic stroke is heralded by the sudden onset of a focal neurologic deficit in a vascular pattern. Duration of symptoms can help distinguish stroke from a transient ischemic attack (TIA). Although TIAs were defined by the National Institutes of Health in 1975 as neurologic deficits that resolve within 24 hours of their onset, we now know that they typically last only 2 to 15 minutes, with the vast majority resolving within an hour.5
If the onset was sudden, find out when the patient was last seen at his or her neurologic baseline—information a family member, friend, or caregiver can often provide. This information is crucial because the neurologic baseline, rather than the time at which the symptoms were first noticed, is the basis for the therapeutic window for thrombolysis (3 hours for intravenous tPA and 6 hours for intra-arterial tPA). (Clot extraction with a mechanical embolus retrieval device [MERCI, Concentric Medical, Mountain View, Calif] has a 9-hour window.6,7)
Use a rapid stroke screening tool. To rapidly evaluate a patient with stroke-like signs and symptoms in a clinic or other outpatient setting, use a stroke screening tool with a high sensitivity,8 such as the Cincinnati Prehospital Stroke Scale (CPSS), the Face Arms Speech Time (FAST) test, or the Los Angeles Prehospital Stroke Screen (LAPSS) (TABLE 1). All 3 have a high positive predictive value (CPSS: 88%, FAST: 89%, LAPSS: 87%), but there is greater variation in the negative predictive value: 75%, 73%, and 55%, respectively.9
Patients with positive results typically require rapid transport to the ED—even if you notice red flags that may signal that you’re dealing with a stroke mimic.
TABLE 1
Stroke screening tools for outpatient use*30-32
| Cincinnati Prehospital Stroke Scale (CPSS) (www.strokecenter.org/trials/scales/cincinnati.html), which assesses the unilateral presence of any (or all) of the 3 key indicators—facial droop, arm drift, or slurred speech |
| Face Arms Speech Time (FAST) (www.stroke.org/site/PageServer?pagename=symp), a modification of CPSS based on the same criteria, has been validated in primary care clinics as well as emergency departments |
| Los Angeles Prehospital Stroke Screen (LAPSS) (www.strokecenter.org/trials/scales/lapss.html), a 1-page instrument that uses 5 criteria—age (>45 years), seizure history (none), onset of neurologic symptoms (within 24 hours), ambulatory status (ambulatory prior to event), and blood glucose level (60-400 mg/dL)—and 3 physical characteristics (facial smile/grimace, grip, and arm weakness) to screen for possible stroke |
| * A positive test is based on the presence of 1 or more key features for CPSS or FAST, and on a Yes (or Unknown) response to all the screening criteria in LAPSS. |
Be alert to the signs of conditions masquerading as stroke
Seizure at the onset of the episode; isolated mild neurological deficits, such as ataxia, sensory loss, or dysarthria alone; and/or minimal weakness are contraindications to thrombolytic therapy, according to the American Academy of Neurology (AAN).10
Rapidly improving neurological status is a probable indicator of a TIA or nonstroke etiology. Decreased level of consciousness with normal eye movements increases the likelihood that the patient has a condition that mimics stroke.11 Additional symptoms that strongly suggest a disorder other than stroke are convulsions (odds ratio [OR]: 0.1), loss of consciousness (OR: 0.1), confusion (OR: 0.2), headache (OR: 0.8), nausea (OR: 0.5), vomiting (OR: 0.6), and dizziness (OR: 0.3).9
Age is another consideration. The vast majority of patients with conditions that turn out to be stroke mimics are younger than 50 years of age. In patients older than 50, the prevalence of stroke misdiagnosis is just 3%.12
Watch for these stroke mimics
Seizures, either unwitnessed or unrecognized, and complex migraine are the most common stroke masqueraders. Other conditions frequently misdiagnosed as stroke include: systemic infections and early sepsis, central nervous system (CNS) tumors, and toxic-metabolic syndromes (including intoxication, hypoglycemia, hypercalcemia, and hyperosmolar nonketotic coma) (TABLE 2). Patients with cranial or peripheral neuropathy; dementia; labyrinthitis/benign positional vertigo; psychiatric disorders, in particular, conversion reaction; syncope; and transient global amnesia may also present with neurological symptoms suggestive of stroke. (For more on transient global amnesia, see this month’s Hospitalist Rounds at http://www.jfponline.com/CollectionContent.asp?CollectionID=286.) Characteristics of some of the more common mimics are detailed below.
Seizures. Neurologic deficits associated with seizures are reversible, with no structural CNS abnormalities. Postictal hemiparesis, also known as Todd’s paralysis—a focal weakness after a seizure, typically localized to 1 side of the body—occurs in approximately 13% of all seizures.13 Todd’s paralysis, which can be seen after either partial complex or generalized tonic-clonic seizures, may also affect speech and vision, producing a range of signs and symptoms easily mistaken for stroke. Duration ranges from minutes to 48 hours,14 but generally lasts only 3 to 22 minutes.13
Differentiating Todd’s paralysis from stroke is complicated by the fact that some strokes trigger focal seizures during the acute phase. However, a history of seizures or witnessed seizure activity points to Todd’s paralysis rather than stroke.
Complex migraine. Like Todd’s paralysis, complex migraine may result in hemiparesis. The presentation may also include vision loss, aphasia, or vertigo and other basilar symptoms—neurologic changes that can outlast the headache. Complex migraine is a diagnosis of exclusion, arrived at after a full neurologic assessment, including stroke work-up. Indeed, you can be certain of a diagnosis of complex migraine only after the patient has had recurrent complex migraine attacks.
Some basilar TIAs can also present with headache, but the onset is typically sudden, as opposed to the more gradual onset of migraine aura with posterior circulation symptoms.15 Age is a factor as well: Complex migraines usually develop well before the age of 40, while the mean age for ischemic stroke is 70. Although complex migraine is a risk factor for ischemic stroke, in most patients migraine is a benign condition.15,16
Systemic infections. Sepsis from almost any infectious agent can cause delirium, altered speech, weakness, and less specific stroke-like symptoms. Microbial seeding of the CNS can result in focal lesions (eg, the lesions shown in FIGURE 1B are associated with cryptococcal meningoencephalitis) or abscess formation with focal neurologic deficits.
Mass lesions. Primary CNS tumors, metastatic tumors, and cerebral abscesses are among the lesions that can cause symptoms that mimic stroke. In most cases, symptoms develop gradually as the lesion enlarges, but a small subset of patients have symptoms lasting less than 1 day. This is thought to be due to hemorrhage into the tumor or the acute development of obstructive hydrocephalus.17
Metabolic disorders. Diabetic hypoglycemia, among other metabolic disorders, is a classic stroke mimic, as well as a cause of seizures, so early evaluation of blood glucose is a crucial step in evaluating a patient with neurologic signs and symptoms. Patients with diabetic hypoglycemia may present with hemiplegia and aphasia; similar symptoms may occur in patients with hypoglycemia secondary to alcoholism, among other causes. Those with hyperglycemic nonketotic hyperosmolar states, severe hyponatremia, and hepatic encephalopathy may also present with focal stroke-like symptoms. Neurologic changes associated with metabolic disorders generally resolve rapidly with the administration of IV glucose, but on rare occasions may take several hours to resolve.14
Psychiatric illness. Patients with certain psychiatric disorders—including conversion reaction, a psychological condition that presents as an alteration in, or loss of, physical function—may present with dramatic focal problems and apparent deficits that mimic neurologic disease. Subtle disparities in the physical exam, such as Hoover’s sign, give-away weakness,18 and “la belle indifference,” as well as negative neuroimaging, will establish this difficult-to-treat stroke mimic.19 Grand mal pseudo-seizures can be differentiated from actual grand mal seizures by the failure of a prolactin level (drawn 10 to 20 minutes post-event) to rise at least 2-fold.20
Transient global amnesia. The rare, sudden development of dense anterograde amnesia occurs without alteration in level of consciousness, focal neurologic deficits, or seizure activity. It is self-limiting and mainly affects those older than 50. Transient global amnesia has an uncertain etiology, although atypical migraine, seizure discharge, and venous congestion with hippocampal ischemia are viewed as possible causes. Reported triggers include severe physical or emotional stress, strenuous physical activity, and orgasmic sexual intercourse.21
TABLE 2
Common stroke mimics9,11,12,14,22
| Condition | Misdiagnosed as stroke (%) |
|---|---|
| Brain tumor | 7-15 |
| Labyrinthitis | 5-6 |
| Metabolic disorder | 3-13 |
| Migraine | 11-47 |
| Psychiatric disorder | 1-40 |
| Seizures | 11-40 |
| Sepsis | 14-17 |
| Syncope | 5-22 |
| Transient global amnesia | 3-10 |
| Other | 11-37 |
In the ED: Evaluation is guided by a timeline
Current guidelines from the American Heart Association and American Stroke Association recommend that a possible stroke patient be evaluated by the physician in the ED within 10 minutes of his or her arrival—and that a decision on how to proceed be reached within 60 minutes of arrival. The guidelines call for the initial computed tomography (CT) to be completed within 25 minutes of the patient’s arrival and interpreted by a physician with expertise in reading CT studies within 45 minutes of arrival.6,24
In the ED, the National Institutes of Health Stroke Scale (NIHSS)25 (TABLE 3) is an ideal way to focus and record the neurological exam.6 The scale assesses 6 separate neurologic functions (level of consciousness, vision, motor function, sensory function, language, and cerebellar function) and can be performed within 5 to 8 minutes. It yields a score from 0 to 42, with the higher numbers indicating worse neurologic function.26 Although a score ≤10 is generally considered to be predictive of a stroke mimic, a recent study found that 19% of patients with an NIHSS score >10 also had conditions masquerading as stroke.27
Imaging leads to accurate diagnosis. The rate at which stroke mimics are mistaken for actual strokes varies with the population studied and the diagnostic tests performed. While stroke is largely a clinical diagnosis and a history and physical exam focused on onset, duration, and symptoms are key elements in differentiating stroke from a stroke mimic, studies have found that the incidence of misdiagnosis (19% with history, physical, and lab work alone) drops to 5% when noncontrast CT is added. When diffusion-weighted magnetic resonance imaging (MRI) is used instead, misdiagnosis drops to just 2%.11,12,14,22,23
Basic lab tests—a complete blood count and basic metabolic panel, with blood alcohol, hepatic function tests, and toxicology screens in select cases—help rule out stroke mimics. Radiographic imaging of the brain provides further clarification (FIGURE 1A AND 1B), serving 2 main purposes: to (1) evaluate diagnoses other than stroke and (2) identify the presence of any acute intracranial bleeding. Noncontrast CT scans detect acute hemorrhage with a sensitivity of 89% and specificity of 100%.27 CT angiography (which can identify the location of a clot) and CT perfusion (which allows an assessment of any existing penumbra) can also be obtained in a timely fashion with newer multislice scanners.
Some institutions, however, evaluate acute stroke patients with MRI. Depending on the sequences used, MRI has the advantage of being able to detect early ischemic changes, diffusion and perfusion mismatches, and abnormalities of the posterior fossa.29 In acute ischemic stroke, diffusion-weighted MRI has a sensitivity of 83% and specificity of 96%, compared with a sensitivity of 16% and specificity of 98% for noncontrast CT.28
TABLE 3
National Institutes of Health Stroke Scale25
| Item | Response score* |
|---|---|
| 1a. Level of consciousness | 0 = alert 1 = not alert 2 = obtunded 3 = unresponsive |
| 1b. Level of consciousness Questions | 0 = answers both correctly 1 = answers one correctly 2 = answers neither correctly |
| 1c. Level of consciousness Commands | 0 = performs both tasks correctly 1 = performs one task correctly 2 = performs neither task correctly |
| 2. Gaze | 0 = normal 1 = partial gaze palsy 2 = total gaze palsy |
| 3. Visual fields | 0 = no visual loss 1 = partial hemianopsia 2 = complete hemianopsia 3 = bilateral hemianopsia |
| 4. Facial palsy | 0 = normal 1 = minor paralysis 2 = partial paralysis 3 = complete paralysis |
| 5. Motor arm a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 6. Motor leg a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 7. Ataxia | 0 = absent 1 = 1 limb 2 = 2 limbs |
| 8. Sensory | 0 = normal 1 = mild loss 2 = severe loss |
| 9. Language | 0 = normal 1 = mild aphasia 2 = severe aphasia 3 = mute or global aphasia |
| 10. Dysarthria | 0 = normal 1 = mild 2 = severe |
| 11. Extinction/inattention | 0 = normal 1 = mild 2 = severe |
| * Yields a score from 0 to 42 (higher numbers indicate worse neurologic function). | |
FIGURE 1
2 patients with common symptoms, vastly different diagnoses
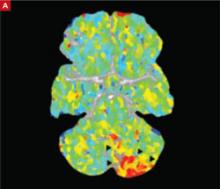
The markedly abnormal perfusion (arrows) this CT image reveals corresponds to an acute occlusion of the left vertebral artery and a subsequent infarct.
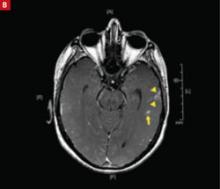
An axial postcontrast MRI reveals multiple lesions in the left temporal lobe (arrows) in a patient with rapid-onset mental changes. The diagnosis: cryptococcal meningoencephalitis.
CORRESPONDENCE
Konrad C. Nau, MD, West Virginia University Department of Family Medicine-Eastern Division, 171 Taylor Street, Harpers Ferry, WV 25425; [email protected]
1. Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480-486.
2. Scott PA, Silbergeit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med. 2003;42:611-618.
3. Morgenstern LB, Lisabeth LD, Mecozzi AC, et al. A population-based study of acute stroke and TIA diagnosis. Neurology. 2004;62:895-900.
4. Nicol MB, Thrift AG. Knowledge of risk factors and warning signs of stroke. Vasc Health Risk Manag. 2005;1:137-147.
5. Albers GW. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347:1713-1716.
6. Adams HP, del Zoppo G, Alberts MJ, et al. Guidelines for early management of adults with ischemic stroke. Circulation. 2007;115:e478-e534.
7. Rosamond WD, Reeves MJ, Johnson A, et al. Paul Coverdell National Acute Stroke Registry Prototype Investigators. Documentation of stroke onset time: challenges and recommendations. Am J Prev Med. 2006;6(suppl 2):S230-S234.
8. Crocco TJ. Streamlining stroke care: from symptom onset to emergency department. J Emerg Med. 2007;33:255-260.
9. Nor AM, Davis J, Sen B, et al. The recognition of stroke in the emergency room scale: development and validation of a stroke recognition scale. Lancet Neurol. 2005;4:727-734.
10. Practice Advisory: Thrombolytic therapy for acute ischemic stroke-summary statement. Report of the Quality standards subcommittee of the American Academy of Neurology. Neurology. 1996;47:835-839.
11. Libman RB, Wirkowski E, Alvir J, et al. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119-1122.
12. Vroomen P, Buddingh MK, Kuijckx G, et al. The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis. 2008;17:418-422.
13. Gallmetzer P, Leutmezer F, Serles W, et al. Postictal paresis in focal epilepsies: incidence, duration, and causes. Neurology. 2004;12:2160-2164.
14. Huff JS. Stroke mimics and chameleons. Emerg Med Clin N Am. 2002;20:583-595.
15. Bousser MG, Welch KM. Relation between migraine and stroke. Lancet Neurol. 2005;4:533-542.
16. Bigal ME, Kurth T, Hu H, et al. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72:1864-1871.
17. Snyder H, Robinson K, Shah D, et al. Signs and symptoms of patients with brain tumors presenting to the emergency department. J Emerg Med. 1993;11:253-258.
18. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatr. 2002;73:241-245.
19. Phoebe SC, Tobiano PS, Wang HE, et al. Case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286.
20. Chen DK, So YT, Fischer RS. Use of serum prolactin in diagnosing epileptic seizures. Report of the therapeutics and technology subcommittee of the American Academy of Neurology. Neurology. 2005;65:668-675.
21. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129:1640-1658.
22. Kothari RU, Brott T, Broderick JP, et al. Emergency physicians: accuracy in diagnosis of stroke. Stroke. 1995;26:2238-2241.
23. Ay H, Buonanno FS, Rordorf G, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999;52:1784-1792.
24. Bock BF. Response system for patients presenting with acute stroke. In: Marler JR, Jones PM, Emr M, ed. Proceeding of a National Symposium on Rapid Identification and Treatment of Acute Stroke: 1997. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health; 1997.
25. National Institutes of Health. Know stroke. Available at: http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale_Booklet.pdf. Accessed December 10, 2009.
26. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603-612.
27. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: the Brain Attack Study. Stroke. 2006;36:769-775.
28. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computerized tomography in emergency assessment of patients with suspected acute stroke—a prospective comparison. Lancet. 2007;369:293-298.
29. Kohrmann M, Jüttler E, Huttner HB, et al. Acute stroke imaging for thrombolytic therapy—an update. Cerebrovasc Dis. 2007;24:161-169.
30. Kothari RU, Panciolo A, Liu T, et al. Cincinnati prehospital stroke scale: reproducibility and validity. Ann Emerg Med. 1999;33:373-378.
31. Harbison J, Hossain O, Jenkinson D, et al. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34:71-76.
32. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field: prospective validation of the Los Angeles prehospital stroke screen (LAPPS). Stroke. 2000;31:71-76.
• Arrange for urgent transport to the hospital when a patient presents with stroke-like symptoms of acute onset, especially within the 3- to 6-hour therapeutic window. B
• Use a validated prehospital stroke identification algorithm such as the Face Arms Speech Time (FAST) test to identify possible acute stroke patients requiring urgent transport to the hospital. B
• Obtain a CBC and basic metabolic panel for all patients with signs and symptoms suggestive of stroke—and a blood alcohol, hepatic function, and toxicology screen, in select patients—to help rule out stroke mimics. C
• Ensure that patients undergo brain imaging to rule out stroke mimics before treatment for acute ischemic stroke is initiated. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Stroke is the third leading cause of death (claiming the life of 1 person every 3 to 4 minutes) and the No. 1 cause of adult disability in the United States.1 Advances in thrombolysis and clot removal can improve outcomes, but are dependent on swift and certain diagnosis. Amid the rush to ensure that treatment is initiated within the therapeutic window for cerebral reperfusion, “stroke mimics”—so called because of their ability to cause signs and symptoms similar to stroke—are sometimes mistaken for the real thing.
The prevalence of misdiagnosis ranges from about 4% of patients who receive tissue plasminogen activator (tPA) for reperfusion2 to 25% of patients who are rushed to the hospital because they are thought to be having a stroke.3 Seizures, migraine, sepsis, and peripheral vestibular disorders are among the many conditions that can masquerade as stroke.
Misdiagnosis can subject patients to unnecessary, and potentially harmful, invasive stroke therapies, and significantly delay the treatment they need. To prevent such outcomes, it is essential for primary care physicians, as well as emergency responders and emergency department (ED) physicians, to be aware of—and on the lookout for—the clues that can distinguish stroke from stroke mimics.
Suspect stroke? Establish a baseline
Despite a nationwide effort to increase public awareness of stroke and the importance of getting to the hospital within the therapeutic window for treatment,4 too few patients arrive within the time frame for cerebral reperfusion therapy. Primary care physicians can help by educating patients about stroke signs and symptoms.
When a patient presents with possible stroke, determine whether symptoms began gradually or abruptly. An acute ischemic stroke is heralded by the sudden onset of a focal neurologic deficit in a vascular pattern. Duration of symptoms can help distinguish stroke from a transient ischemic attack (TIA). Although TIAs were defined by the National Institutes of Health in 1975 as neurologic deficits that resolve within 24 hours of their onset, we now know that they typically last only 2 to 15 minutes, with the vast majority resolving within an hour.5
If the onset was sudden, find out when the patient was last seen at his or her neurologic baseline—information a family member, friend, or caregiver can often provide. This information is crucial because the neurologic baseline, rather than the time at which the symptoms were first noticed, is the basis for the therapeutic window for thrombolysis (3 hours for intravenous tPA and 6 hours for intra-arterial tPA). (Clot extraction with a mechanical embolus retrieval device [MERCI, Concentric Medical, Mountain View, Calif] has a 9-hour window.6,7)
Use a rapid stroke screening tool. To rapidly evaluate a patient with stroke-like signs and symptoms in a clinic or other outpatient setting, use a stroke screening tool with a high sensitivity,8 such as the Cincinnati Prehospital Stroke Scale (CPSS), the Face Arms Speech Time (FAST) test, or the Los Angeles Prehospital Stroke Screen (LAPSS) (TABLE 1). All 3 have a high positive predictive value (CPSS: 88%, FAST: 89%, LAPSS: 87%), but there is greater variation in the negative predictive value: 75%, 73%, and 55%, respectively.9
Patients with positive results typically require rapid transport to the ED—even if you notice red flags that may signal that you’re dealing with a stroke mimic.
TABLE 1
Stroke screening tools for outpatient use*30-32
| Cincinnati Prehospital Stroke Scale (CPSS) (www.strokecenter.org/trials/scales/cincinnati.html), which assesses the unilateral presence of any (or all) of the 3 key indicators—facial droop, arm drift, or slurred speech |
| Face Arms Speech Time (FAST) (www.stroke.org/site/PageServer?pagename=symp), a modification of CPSS based on the same criteria, has been validated in primary care clinics as well as emergency departments |
| Los Angeles Prehospital Stroke Screen (LAPSS) (www.strokecenter.org/trials/scales/lapss.html), a 1-page instrument that uses 5 criteria—age (>45 years), seizure history (none), onset of neurologic symptoms (within 24 hours), ambulatory status (ambulatory prior to event), and blood glucose level (60-400 mg/dL)—and 3 physical characteristics (facial smile/grimace, grip, and arm weakness) to screen for possible stroke |
| * A positive test is based on the presence of 1 or more key features for CPSS or FAST, and on a Yes (or Unknown) response to all the screening criteria in LAPSS. |
Be alert to the signs of conditions masquerading as stroke
Seizure at the onset of the episode; isolated mild neurological deficits, such as ataxia, sensory loss, or dysarthria alone; and/or minimal weakness are contraindications to thrombolytic therapy, according to the American Academy of Neurology (AAN).10
Rapidly improving neurological status is a probable indicator of a TIA or nonstroke etiology. Decreased level of consciousness with normal eye movements increases the likelihood that the patient has a condition that mimics stroke.11 Additional symptoms that strongly suggest a disorder other than stroke are convulsions (odds ratio [OR]: 0.1), loss of consciousness (OR: 0.1), confusion (OR: 0.2), headache (OR: 0.8), nausea (OR: 0.5), vomiting (OR: 0.6), and dizziness (OR: 0.3).9
Age is another consideration. The vast majority of patients with conditions that turn out to be stroke mimics are younger than 50 years of age. In patients older than 50, the prevalence of stroke misdiagnosis is just 3%.12
Watch for these stroke mimics
Seizures, either unwitnessed or unrecognized, and complex migraine are the most common stroke masqueraders. Other conditions frequently misdiagnosed as stroke include: systemic infections and early sepsis, central nervous system (CNS) tumors, and toxic-metabolic syndromes (including intoxication, hypoglycemia, hypercalcemia, and hyperosmolar nonketotic coma) (TABLE 2). Patients with cranial or peripheral neuropathy; dementia; labyrinthitis/benign positional vertigo; psychiatric disorders, in particular, conversion reaction; syncope; and transient global amnesia may also present with neurological symptoms suggestive of stroke. (For more on transient global amnesia, see this month’s Hospitalist Rounds at http://www.jfponline.com/CollectionContent.asp?CollectionID=286.) Characteristics of some of the more common mimics are detailed below.
Seizures. Neurologic deficits associated with seizures are reversible, with no structural CNS abnormalities. Postictal hemiparesis, also known as Todd’s paralysis—a focal weakness after a seizure, typically localized to 1 side of the body—occurs in approximately 13% of all seizures.13 Todd’s paralysis, which can be seen after either partial complex or generalized tonic-clonic seizures, may also affect speech and vision, producing a range of signs and symptoms easily mistaken for stroke. Duration ranges from minutes to 48 hours,14 but generally lasts only 3 to 22 minutes.13
Differentiating Todd’s paralysis from stroke is complicated by the fact that some strokes trigger focal seizures during the acute phase. However, a history of seizures or witnessed seizure activity points to Todd’s paralysis rather than stroke.
Complex migraine. Like Todd’s paralysis, complex migraine may result in hemiparesis. The presentation may also include vision loss, aphasia, or vertigo and other basilar symptoms—neurologic changes that can outlast the headache. Complex migraine is a diagnosis of exclusion, arrived at after a full neurologic assessment, including stroke work-up. Indeed, you can be certain of a diagnosis of complex migraine only after the patient has had recurrent complex migraine attacks.
Some basilar TIAs can also present with headache, but the onset is typically sudden, as opposed to the more gradual onset of migraine aura with posterior circulation symptoms.15 Age is a factor as well: Complex migraines usually develop well before the age of 40, while the mean age for ischemic stroke is 70. Although complex migraine is a risk factor for ischemic stroke, in most patients migraine is a benign condition.15,16
Systemic infections. Sepsis from almost any infectious agent can cause delirium, altered speech, weakness, and less specific stroke-like symptoms. Microbial seeding of the CNS can result in focal lesions (eg, the lesions shown in FIGURE 1B are associated with cryptococcal meningoencephalitis) or abscess formation with focal neurologic deficits.
Mass lesions. Primary CNS tumors, metastatic tumors, and cerebral abscesses are among the lesions that can cause symptoms that mimic stroke. In most cases, symptoms develop gradually as the lesion enlarges, but a small subset of patients have symptoms lasting less than 1 day. This is thought to be due to hemorrhage into the tumor or the acute development of obstructive hydrocephalus.17
Metabolic disorders. Diabetic hypoglycemia, among other metabolic disorders, is a classic stroke mimic, as well as a cause of seizures, so early evaluation of blood glucose is a crucial step in evaluating a patient with neurologic signs and symptoms. Patients with diabetic hypoglycemia may present with hemiplegia and aphasia; similar symptoms may occur in patients with hypoglycemia secondary to alcoholism, among other causes. Those with hyperglycemic nonketotic hyperosmolar states, severe hyponatremia, and hepatic encephalopathy may also present with focal stroke-like symptoms. Neurologic changes associated with metabolic disorders generally resolve rapidly with the administration of IV glucose, but on rare occasions may take several hours to resolve.14
Psychiatric illness. Patients with certain psychiatric disorders—including conversion reaction, a psychological condition that presents as an alteration in, or loss of, physical function—may present with dramatic focal problems and apparent deficits that mimic neurologic disease. Subtle disparities in the physical exam, such as Hoover’s sign, give-away weakness,18 and “la belle indifference,” as well as negative neuroimaging, will establish this difficult-to-treat stroke mimic.19 Grand mal pseudo-seizures can be differentiated from actual grand mal seizures by the failure of a prolactin level (drawn 10 to 20 minutes post-event) to rise at least 2-fold.20
Transient global amnesia. The rare, sudden development of dense anterograde amnesia occurs without alteration in level of consciousness, focal neurologic deficits, or seizure activity. It is self-limiting and mainly affects those older than 50. Transient global amnesia has an uncertain etiology, although atypical migraine, seizure discharge, and venous congestion with hippocampal ischemia are viewed as possible causes. Reported triggers include severe physical or emotional stress, strenuous physical activity, and orgasmic sexual intercourse.21
TABLE 2
Common stroke mimics9,11,12,14,22
| Condition | Misdiagnosed as stroke (%) |
|---|---|
| Brain tumor | 7-15 |
| Labyrinthitis | 5-6 |
| Metabolic disorder | 3-13 |
| Migraine | 11-47 |
| Psychiatric disorder | 1-40 |
| Seizures | 11-40 |
| Sepsis | 14-17 |
| Syncope | 5-22 |
| Transient global amnesia | 3-10 |
| Other | 11-37 |
In the ED: Evaluation is guided by a timeline
Current guidelines from the American Heart Association and American Stroke Association recommend that a possible stroke patient be evaluated by the physician in the ED within 10 minutes of his or her arrival—and that a decision on how to proceed be reached within 60 minutes of arrival. The guidelines call for the initial computed tomography (CT) to be completed within 25 minutes of the patient’s arrival and interpreted by a physician with expertise in reading CT studies within 45 minutes of arrival.6,24
In the ED, the National Institutes of Health Stroke Scale (NIHSS)25 (TABLE 3) is an ideal way to focus and record the neurological exam.6 The scale assesses 6 separate neurologic functions (level of consciousness, vision, motor function, sensory function, language, and cerebellar function) and can be performed within 5 to 8 minutes. It yields a score from 0 to 42, with the higher numbers indicating worse neurologic function.26 Although a score ≤10 is generally considered to be predictive of a stroke mimic, a recent study found that 19% of patients with an NIHSS score >10 also had conditions masquerading as stroke.27
Imaging leads to accurate diagnosis. The rate at which stroke mimics are mistaken for actual strokes varies with the population studied and the diagnostic tests performed. While stroke is largely a clinical diagnosis and a history and physical exam focused on onset, duration, and symptoms are key elements in differentiating stroke from a stroke mimic, studies have found that the incidence of misdiagnosis (19% with history, physical, and lab work alone) drops to 5% when noncontrast CT is added. When diffusion-weighted magnetic resonance imaging (MRI) is used instead, misdiagnosis drops to just 2%.11,12,14,22,23
Basic lab tests—a complete blood count and basic metabolic panel, with blood alcohol, hepatic function tests, and toxicology screens in select cases—help rule out stroke mimics. Radiographic imaging of the brain provides further clarification (FIGURE 1A AND 1B), serving 2 main purposes: to (1) evaluate diagnoses other than stroke and (2) identify the presence of any acute intracranial bleeding. Noncontrast CT scans detect acute hemorrhage with a sensitivity of 89% and specificity of 100%.27 CT angiography (which can identify the location of a clot) and CT perfusion (which allows an assessment of any existing penumbra) can also be obtained in a timely fashion with newer multislice scanners.
Some institutions, however, evaluate acute stroke patients with MRI. Depending on the sequences used, MRI has the advantage of being able to detect early ischemic changes, diffusion and perfusion mismatches, and abnormalities of the posterior fossa.29 In acute ischemic stroke, diffusion-weighted MRI has a sensitivity of 83% and specificity of 96%, compared with a sensitivity of 16% and specificity of 98% for noncontrast CT.28
TABLE 3
National Institutes of Health Stroke Scale25
| Item | Response score* |
|---|---|
| 1a. Level of consciousness | 0 = alert 1 = not alert 2 = obtunded 3 = unresponsive |
| 1b. Level of consciousness Questions | 0 = answers both correctly 1 = answers one correctly 2 = answers neither correctly |
| 1c. Level of consciousness Commands | 0 = performs both tasks correctly 1 = performs one task correctly 2 = performs neither task correctly |
| 2. Gaze | 0 = normal 1 = partial gaze palsy 2 = total gaze palsy |
| 3. Visual fields | 0 = no visual loss 1 = partial hemianopsia 2 = complete hemianopsia 3 = bilateral hemianopsia |
| 4. Facial palsy | 0 = normal 1 = minor paralysis 2 = partial paralysis 3 = complete paralysis |
| 5. Motor arm a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 6. Motor leg a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 7. Ataxia | 0 = absent 1 = 1 limb 2 = 2 limbs |
| 8. Sensory | 0 = normal 1 = mild loss 2 = severe loss |
| 9. Language | 0 = normal 1 = mild aphasia 2 = severe aphasia 3 = mute or global aphasia |
| 10. Dysarthria | 0 = normal 1 = mild 2 = severe |
| 11. Extinction/inattention | 0 = normal 1 = mild 2 = severe |
| * Yields a score from 0 to 42 (higher numbers indicate worse neurologic function). | |
FIGURE 1
2 patients with common symptoms, vastly different diagnoses
The markedly abnormal perfusion (arrows) this CT image reveals corresponds to an acute occlusion of the left vertebral artery and a subsequent infarct.
An axial postcontrast MRI reveals multiple lesions in the left temporal lobe (arrows) in a patient with rapid-onset mental changes. The diagnosis: cryptococcal meningoencephalitis.
CORRESPONDENCE
Konrad C. Nau, MD, West Virginia University Department of Family Medicine-Eastern Division, 171 Taylor Street, Harpers Ferry, WV 25425; [email protected]
• Arrange for urgent transport to the hospital when a patient presents with stroke-like symptoms of acute onset, especially within the 3- to 6-hour therapeutic window. B
• Use a validated prehospital stroke identification algorithm such as the Face Arms Speech Time (FAST) test to identify possible acute stroke patients requiring urgent transport to the hospital. B
• Obtain a CBC and basic metabolic panel for all patients with signs and symptoms suggestive of stroke—and a blood alcohol, hepatic function, and toxicology screen, in select patients—to help rule out stroke mimics. C
• Ensure that patients undergo brain imaging to rule out stroke mimics before treatment for acute ischemic stroke is initiated. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Stroke is the third leading cause of death (claiming the life of 1 person every 3 to 4 minutes) and the No. 1 cause of adult disability in the United States.1 Advances in thrombolysis and clot removal can improve outcomes, but are dependent on swift and certain diagnosis. Amid the rush to ensure that treatment is initiated within the therapeutic window for cerebral reperfusion, “stroke mimics”—so called because of their ability to cause signs and symptoms similar to stroke—are sometimes mistaken for the real thing.
The prevalence of misdiagnosis ranges from about 4% of patients who receive tissue plasminogen activator (tPA) for reperfusion2 to 25% of patients who are rushed to the hospital because they are thought to be having a stroke.3 Seizures, migraine, sepsis, and peripheral vestibular disorders are among the many conditions that can masquerade as stroke.
Misdiagnosis can subject patients to unnecessary, and potentially harmful, invasive stroke therapies, and significantly delay the treatment they need. To prevent such outcomes, it is essential for primary care physicians, as well as emergency responders and emergency department (ED) physicians, to be aware of—and on the lookout for—the clues that can distinguish stroke from stroke mimics.
Suspect stroke? Establish a baseline
Despite a nationwide effort to increase public awareness of stroke and the importance of getting to the hospital within the therapeutic window for treatment,4 too few patients arrive within the time frame for cerebral reperfusion therapy. Primary care physicians can help by educating patients about stroke signs and symptoms.
When a patient presents with possible stroke, determine whether symptoms began gradually or abruptly. An acute ischemic stroke is heralded by the sudden onset of a focal neurologic deficit in a vascular pattern. Duration of symptoms can help distinguish stroke from a transient ischemic attack (TIA). Although TIAs were defined by the National Institutes of Health in 1975 as neurologic deficits that resolve within 24 hours of their onset, we now know that they typically last only 2 to 15 minutes, with the vast majority resolving within an hour.5
If the onset was sudden, find out when the patient was last seen at his or her neurologic baseline—information a family member, friend, or caregiver can often provide. This information is crucial because the neurologic baseline, rather than the time at which the symptoms were first noticed, is the basis for the therapeutic window for thrombolysis (3 hours for intravenous tPA and 6 hours for intra-arterial tPA). (Clot extraction with a mechanical embolus retrieval device [MERCI, Concentric Medical, Mountain View, Calif] has a 9-hour window.6,7)
Use a rapid stroke screening tool. To rapidly evaluate a patient with stroke-like signs and symptoms in a clinic or other outpatient setting, use a stroke screening tool with a high sensitivity,8 such as the Cincinnati Prehospital Stroke Scale (CPSS), the Face Arms Speech Time (FAST) test, or the Los Angeles Prehospital Stroke Screen (LAPSS) (TABLE 1). All 3 have a high positive predictive value (CPSS: 88%, FAST: 89%, LAPSS: 87%), but there is greater variation in the negative predictive value: 75%, 73%, and 55%, respectively.9
Patients with positive results typically require rapid transport to the ED—even if you notice red flags that may signal that you’re dealing with a stroke mimic.
TABLE 1
Stroke screening tools for outpatient use*30-32
| Cincinnati Prehospital Stroke Scale (CPSS) (www.strokecenter.org/trials/scales/cincinnati.html), which assesses the unilateral presence of any (or all) of the 3 key indicators—facial droop, arm drift, or slurred speech |
| Face Arms Speech Time (FAST) (www.stroke.org/site/PageServer?pagename=symp), a modification of CPSS based on the same criteria, has been validated in primary care clinics as well as emergency departments |
| Los Angeles Prehospital Stroke Screen (LAPSS) (www.strokecenter.org/trials/scales/lapss.html), a 1-page instrument that uses 5 criteria—age (>45 years), seizure history (none), onset of neurologic symptoms (within 24 hours), ambulatory status (ambulatory prior to event), and blood glucose level (60-400 mg/dL)—and 3 physical characteristics (facial smile/grimace, grip, and arm weakness) to screen for possible stroke |
| * A positive test is based on the presence of 1 or more key features for CPSS or FAST, and on a Yes (or Unknown) response to all the screening criteria in LAPSS. |
Be alert to the signs of conditions masquerading as stroke
Seizure at the onset of the episode; isolated mild neurological deficits, such as ataxia, sensory loss, or dysarthria alone; and/or minimal weakness are contraindications to thrombolytic therapy, according to the American Academy of Neurology (AAN).10
Rapidly improving neurological status is a probable indicator of a TIA or nonstroke etiology. Decreased level of consciousness with normal eye movements increases the likelihood that the patient has a condition that mimics stroke.11 Additional symptoms that strongly suggest a disorder other than stroke are convulsions (odds ratio [OR]: 0.1), loss of consciousness (OR: 0.1), confusion (OR: 0.2), headache (OR: 0.8), nausea (OR: 0.5), vomiting (OR: 0.6), and dizziness (OR: 0.3).9
Age is another consideration. The vast majority of patients with conditions that turn out to be stroke mimics are younger than 50 years of age. In patients older than 50, the prevalence of stroke misdiagnosis is just 3%.12
Watch for these stroke mimics
Seizures, either unwitnessed or unrecognized, and complex migraine are the most common stroke masqueraders. Other conditions frequently misdiagnosed as stroke include: systemic infections and early sepsis, central nervous system (CNS) tumors, and toxic-metabolic syndromes (including intoxication, hypoglycemia, hypercalcemia, and hyperosmolar nonketotic coma) (TABLE 2). Patients with cranial or peripheral neuropathy; dementia; labyrinthitis/benign positional vertigo; psychiatric disorders, in particular, conversion reaction; syncope; and transient global amnesia may also present with neurological symptoms suggestive of stroke. (For more on transient global amnesia, see this month’s Hospitalist Rounds at http://www.jfponline.com/CollectionContent.asp?CollectionID=286.) Characteristics of some of the more common mimics are detailed below.
Seizures. Neurologic deficits associated with seizures are reversible, with no structural CNS abnormalities. Postictal hemiparesis, also known as Todd’s paralysis—a focal weakness after a seizure, typically localized to 1 side of the body—occurs in approximately 13% of all seizures.13 Todd’s paralysis, which can be seen after either partial complex or generalized tonic-clonic seizures, may also affect speech and vision, producing a range of signs and symptoms easily mistaken for stroke. Duration ranges from minutes to 48 hours,14 but generally lasts only 3 to 22 minutes.13
Differentiating Todd’s paralysis from stroke is complicated by the fact that some strokes trigger focal seizures during the acute phase. However, a history of seizures or witnessed seizure activity points to Todd’s paralysis rather than stroke.
Complex migraine. Like Todd’s paralysis, complex migraine may result in hemiparesis. The presentation may also include vision loss, aphasia, or vertigo and other basilar symptoms—neurologic changes that can outlast the headache. Complex migraine is a diagnosis of exclusion, arrived at after a full neurologic assessment, including stroke work-up. Indeed, you can be certain of a diagnosis of complex migraine only after the patient has had recurrent complex migraine attacks.
Some basilar TIAs can also present with headache, but the onset is typically sudden, as opposed to the more gradual onset of migraine aura with posterior circulation symptoms.15 Age is a factor as well: Complex migraines usually develop well before the age of 40, while the mean age for ischemic stroke is 70. Although complex migraine is a risk factor for ischemic stroke, in most patients migraine is a benign condition.15,16
Systemic infections. Sepsis from almost any infectious agent can cause delirium, altered speech, weakness, and less specific stroke-like symptoms. Microbial seeding of the CNS can result in focal lesions (eg, the lesions shown in FIGURE 1B are associated with cryptococcal meningoencephalitis) or abscess formation with focal neurologic deficits.
Mass lesions. Primary CNS tumors, metastatic tumors, and cerebral abscesses are among the lesions that can cause symptoms that mimic stroke. In most cases, symptoms develop gradually as the lesion enlarges, but a small subset of patients have symptoms lasting less than 1 day. This is thought to be due to hemorrhage into the tumor or the acute development of obstructive hydrocephalus.17
Metabolic disorders. Diabetic hypoglycemia, among other metabolic disorders, is a classic stroke mimic, as well as a cause of seizures, so early evaluation of blood glucose is a crucial step in evaluating a patient with neurologic signs and symptoms. Patients with diabetic hypoglycemia may present with hemiplegia and aphasia; similar symptoms may occur in patients with hypoglycemia secondary to alcoholism, among other causes. Those with hyperglycemic nonketotic hyperosmolar states, severe hyponatremia, and hepatic encephalopathy may also present with focal stroke-like symptoms. Neurologic changes associated with metabolic disorders generally resolve rapidly with the administration of IV glucose, but on rare occasions may take several hours to resolve.14
Psychiatric illness. Patients with certain psychiatric disorders—including conversion reaction, a psychological condition that presents as an alteration in, or loss of, physical function—may present with dramatic focal problems and apparent deficits that mimic neurologic disease. Subtle disparities in the physical exam, such as Hoover’s sign, give-away weakness,18 and “la belle indifference,” as well as negative neuroimaging, will establish this difficult-to-treat stroke mimic.19 Grand mal pseudo-seizures can be differentiated from actual grand mal seizures by the failure of a prolactin level (drawn 10 to 20 minutes post-event) to rise at least 2-fold.20
Transient global amnesia. The rare, sudden development of dense anterograde amnesia occurs without alteration in level of consciousness, focal neurologic deficits, or seizure activity. It is self-limiting and mainly affects those older than 50. Transient global amnesia has an uncertain etiology, although atypical migraine, seizure discharge, and venous congestion with hippocampal ischemia are viewed as possible causes. Reported triggers include severe physical or emotional stress, strenuous physical activity, and orgasmic sexual intercourse.21
TABLE 2
Common stroke mimics9,11,12,14,22
| Condition | Misdiagnosed as stroke (%) |
|---|---|
| Brain tumor | 7-15 |
| Labyrinthitis | 5-6 |
| Metabolic disorder | 3-13 |
| Migraine | 11-47 |
| Psychiatric disorder | 1-40 |
| Seizures | 11-40 |
| Sepsis | 14-17 |
| Syncope | 5-22 |
| Transient global amnesia | 3-10 |
| Other | 11-37 |
In the ED: Evaluation is guided by a timeline
Current guidelines from the American Heart Association and American Stroke Association recommend that a possible stroke patient be evaluated by the physician in the ED within 10 minutes of his or her arrival—and that a decision on how to proceed be reached within 60 minutes of arrival. The guidelines call for the initial computed tomography (CT) to be completed within 25 minutes of the patient’s arrival and interpreted by a physician with expertise in reading CT studies within 45 minutes of arrival.6,24
In the ED, the National Institutes of Health Stroke Scale (NIHSS)25 (TABLE 3) is an ideal way to focus and record the neurological exam.6 The scale assesses 6 separate neurologic functions (level of consciousness, vision, motor function, sensory function, language, and cerebellar function) and can be performed within 5 to 8 minutes. It yields a score from 0 to 42, with the higher numbers indicating worse neurologic function.26 Although a score ≤10 is generally considered to be predictive of a stroke mimic, a recent study found that 19% of patients with an NIHSS score >10 also had conditions masquerading as stroke.27
Imaging leads to accurate diagnosis. The rate at which stroke mimics are mistaken for actual strokes varies with the population studied and the diagnostic tests performed. While stroke is largely a clinical diagnosis and a history and physical exam focused on onset, duration, and symptoms are key elements in differentiating stroke from a stroke mimic, studies have found that the incidence of misdiagnosis (19% with history, physical, and lab work alone) drops to 5% when noncontrast CT is added. When diffusion-weighted magnetic resonance imaging (MRI) is used instead, misdiagnosis drops to just 2%.11,12,14,22,23
Basic lab tests—a complete blood count and basic metabolic panel, with blood alcohol, hepatic function tests, and toxicology screens in select cases—help rule out stroke mimics. Radiographic imaging of the brain provides further clarification (FIGURE 1A AND 1B), serving 2 main purposes: to (1) evaluate diagnoses other than stroke and (2) identify the presence of any acute intracranial bleeding. Noncontrast CT scans detect acute hemorrhage with a sensitivity of 89% and specificity of 100%.27 CT angiography (which can identify the location of a clot) and CT perfusion (which allows an assessment of any existing penumbra) can also be obtained in a timely fashion with newer multislice scanners.
Some institutions, however, evaluate acute stroke patients with MRI. Depending on the sequences used, MRI has the advantage of being able to detect early ischemic changes, diffusion and perfusion mismatches, and abnormalities of the posterior fossa.29 In acute ischemic stroke, diffusion-weighted MRI has a sensitivity of 83% and specificity of 96%, compared with a sensitivity of 16% and specificity of 98% for noncontrast CT.28
TABLE 3
National Institutes of Health Stroke Scale25
| Item | Response score* |
|---|---|
| 1a. Level of consciousness | 0 = alert 1 = not alert 2 = obtunded 3 = unresponsive |
| 1b. Level of consciousness Questions | 0 = answers both correctly 1 = answers one correctly 2 = answers neither correctly |
| 1c. Level of consciousness Commands | 0 = performs both tasks correctly 1 = performs one task correctly 2 = performs neither task correctly |
| 2. Gaze | 0 = normal 1 = partial gaze palsy 2 = total gaze palsy |
| 3. Visual fields | 0 = no visual loss 1 = partial hemianopsia 2 = complete hemianopsia 3 = bilateral hemianopsia |
| 4. Facial palsy | 0 = normal 1 = minor paralysis 2 = partial paralysis 3 = complete paralysis |
| 5. Motor arm a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 6. Motor leg a. Left b. Right | 0 = no drift 1 = drifts before 5 sec 2 = falls before 10 sec 3 = no effort against gravity 4 = no movement |
| 7. Ataxia | 0 = absent 1 = 1 limb 2 = 2 limbs |
| 8. Sensory | 0 = normal 1 = mild loss 2 = severe loss |
| 9. Language | 0 = normal 1 = mild aphasia 2 = severe aphasia 3 = mute or global aphasia |
| 10. Dysarthria | 0 = normal 1 = mild 2 = severe |
| 11. Extinction/inattention | 0 = normal 1 = mild 2 = severe |
| * Yields a score from 0 to 42 (higher numbers indicate worse neurologic function). | |
FIGURE 1
2 patients with common symptoms, vastly different diagnoses
The markedly abnormal perfusion (arrows) this CT image reveals corresponds to an acute occlusion of the left vertebral artery and a subsequent infarct.
An axial postcontrast MRI reveals multiple lesions in the left temporal lobe (arrows) in a patient with rapid-onset mental changes. The diagnosis: cryptococcal meningoencephalitis.
CORRESPONDENCE
Konrad C. Nau, MD, West Virginia University Department of Family Medicine-Eastern Division, 171 Taylor Street, Harpers Ferry, WV 25425; [email protected]
1. Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480-486.
2. Scott PA, Silbergeit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med. 2003;42:611-618.
3. Morgenstern LB, Lisabeth LD, Mecozzi AC, et al. A population-based study of acute stroke and TIA diagnosis. Neurology. 2004;62:895-900.
4. Nicol MB, Thrift AG. Knowledge of risk factors and warning signs of stroke. Vasc Health Risk Manag. 2005;1:137-147.
5. Albers GW. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347:1713-1716.
6. Adams HP, del Zoppo G, Alberts MJ, et al. Guidelines for early management of adults with ischemic stroke. Circulation. 2007;115:e478-e534.
7. Rosamond WD, Reeves MJ, Johnson A, et al. Paul Coverdell National Acute Stroke Registry Prototype Investigators. Documentation of stroke onset time: challenges and recommendations. Am J Prev Med. 2006;6(suppl 2):S230-S234.
8. Crocco TJ. Streamlining stroke care: from symptom onset to emergency department. J Emerg Med. 2007;33:255-260.
9. Nor AM, Davis J, Sen B, et al. The recognition of stroke in the emergency room scale: development and validation of a stroke recognition scale. Lancet Neurol. 2005;4:727-734.
10. Practice Advisory: Thrombolytic therapy for acute ischemic stroke-summary statement. Report of the Quality standards subcommittee of the American Academy of Neurology. Neurology. 1996;47:835-839.
11. Libman RB, Wirkowski E, Alvir J, et al. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119-1122.
12. Vroomen P, Buddingh MK, Kuijckx G, et al. The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis. 2008;17:418-422.
13. Gallmetzer P, Leutmezer F, Serles W, et al. Postictal paresis in focal epilepsies: incidence, duration, and causes. Neurology. 2004;12:2160-2164.
14. Huff JS. Stroke mimics and chameleons. Emerg Med Clin N Am. 2002;20:583-595.
15. Bousser MG, Welch KM. Relation between migraine and stroke. Lancet Neurol. 2005;4:533-542.
16. Bigal ME, Kurth T, Hu H, et al. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72:1864-1871.
17. Snyder H, Robinson K, Shah D, et al. Signs and symptoms of patients with brain tumors presenting to the emergency department. J Emerg Med. 1993;11:253-258.
18. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatr. 2002;73:241-245.
19. Phoebe SC, Tobiano PS, Wang HE, et al. Case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286.
20. Chen DK, So YT, Fischer RS. Use of serum prolactin in diagnosing epileptic seizures. Report of the therapeutics and technology subcommittee of the American Academy of Neurology. Neurology. 2005;65:668-675.
21. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129:1640-1658.
22. Kothari RU, Brott T, Broderick JP, et al. Emergency physicians: accuracy in diagnosis of stroke. Stroke. 1995;26:2238-2241.
23. Ay H, Buonanno FS, Rordorf G, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999;52:1784-1792.
24. Bock BF. Response system for patients presenting with acute stroke. In: Marler JR, Jones PM, Emr M, ed. Proceeding of a National Symposium on Rapid Identification and Treatment of Acute Stroke: 1997. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health; 1997.
25. National Institutes of Health. Know stroke. Available at: http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale_Booklet.pdf. Accessed December 10, 2009.
26. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603-612.
27. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: the Brain Attack Study. Stroke. 2006;36:769-775.
28. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computerized tomography in emergency assessment of patients with suspected acute stroke—a prospective comparison. Lancet. 2007;369:293-298.
29. Kohrmann M, Jüttler E, Huttner HB, et al. Acute stroke imaging for thrombolytic therapy—an update. Cerebrovasc Dis. 2007;24:161-169.
30. Kothari RU, Panciolo A, Liu T, et al. Cincinnati prehospital stroke scale: reproducibility and validity. Ann Emerg Med. 1999;33:373-378.
31. Harbison J, Hossain O, Jenkinson D, et al. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34:71-76.
32. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field: prospective validation of the Los Angeles prehospital stroke screen (LAPPS). Stroke. 2000;31:71-76.
1. Lloyd-Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480-486.
2. Scott PA, Silbergeit R. Misdiagnosis of stroke in tissue plasminogen activator-treated patients: characteristics and outcomes. Ann Emerg Med. 2003;42:611-618.
3. Morgenstern LB, Lisabeth LD, Mecozzi AC, et al. A population-based study of acute stroke and TIA diagnosis. Neurology. 2004;62:895-900.
4. Nicol MB, Thrift AG. Knowledge of risk factors and warning signs of stroke. Vasc Health Risk Manag. 2005;1:137-147.
5. Albers GW. Transient ischemic attack—proposal for a new definition. N Engl J Med. 2002;347:1713-1716.
6. Adams HP, del Zoppo G, Alberts MJ, et al. Guidelines for early management of adults with ischemic stroke. Circulation. 2007;115:e478-e534.
7. Rosamond WD, Reeves MJ, Johnson A, et al. Paul Coverdell National Acute Stroke Registry Prototype Investigators. Documentation of stroke onset time: challenges and recommendations. Am J Prev Med. 2006;6(suppl 2):S230-S234.
8. Crocco TJ. Streamlining stroke care: from symptom onset to emergency department. J Emerg Med. 2007;33:255-260.
9. Nor AM, Davis J, Sen B, et al. The recognition of stroke in the emergency room scale: development and validation of a stroke recognition scale. Lancet Neurol. 2005;4:727-734.
10. Practice Advisory: Thrombolytic therapy for acute ischemic stroke-summary statement. Report of the Quality standards subcommittee of the American Academy of Neurology. Neurology. 1996;47:835-839.
11. Libman RB, Wirkowski E, Alvir J, et al. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol. 1995;52:1119-1122.
12. Vroomen P, Buddingh MK, Kuijckx G, et al. The incidence of stroke mimics among stroke department admissions in relation to age group. J Stroke Cerebrovasc Dis. 2008;17:418-422.
13. Gallmetzer P, Leutmezer F, Serles W, et al. Postictal paresis in focal epilepsies: incidence, duration, and causes. Neurology. 2004;12:2160-2164.
14. Huff JS. Stroke mimics and chameleons. Emerg Med Clin N Am. 2002;20:583-595.
15. Bousser MG, Welch KM. Relation between migraine and stroke. Lancet Neurol. 2005;4:533-542.
16. Bigal ME, Kurth T, Hu H, et al. Migraine and cardiovascular disease: possible mechanisms of interaction. Neurology. 2009;72:1864-1871.
17. Snyder H, Robinson K, Shah D, et al. Signs and symptoms of patients with brain tumors presenting to the emergency department. J Emerg Med. 1993;11:253-258.
18. Stone J, Zeman A, Sharpe M. Functional weakness and sensory disturbance. J Neurol Neurosurg Psychiatr. 2002;73:241-245.
19. Phoebe SC, Tobiano PS, Wang HE, et al. Case of conversion disorder presenting as a severe acute stroke. J Emerg Med. 2006;30:283-286.
20. Chen DK, So YT, Fischer RS. Use of serum prolactin in diagnosing epileptic seizures. Report of the therapeutics and technology subcommittee of the American Academy of Neurology. Neurology. 2005;65:668-675.
21. Quinette P, Guillery-Girard B, Dayan J, et al. What does transient global amnesia really mean? Review of the literature and thorough study of 142 cases. Brain. 2006;129:1640-1658.
22. Kothari RU, Brott T, Broderick JP, et al. Emergency physicians: accuracy in diagnosis of stroke. Stroke. 1995;26:2238-2241.
23. Ay H, Buonanno FS, Rordorf G, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology. 1999;52:1784-1792.
24. Bock BF. Response system for patients presenting with acute stroke. In: Marler JR, Jones PM, Emr M, ed. Proceeding of a National Symposium on Rapid Identification and Treatment of Acute Stroke: 1997. Bethesda, MD: National Institute of Neurological Disorders and Stroke, National Institutes of Health; 1997.
25. National Institutes of Health. Know stroke. Available at: http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale_Booklet.pdf. Accessed December 10, 2009.
26. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603-612.
27. Hand PJ, Kwan J, Lindley RI, et al. Distinguishing between stroke and mimic at the bedside: the Brain Attack Study. Stroke. 2006;36:769-775.
28. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computerized tomography in emergency assessment of patients with suspected acute stroke—a prospective comparison. Lancet. 2007;369:293-298.
29. Kohrmann M, Jüttler E, Huttner HB, et al. Acute stroke imaging for thrombolytic therapy—an update. Cerebrovasc Dis. 2007;24:161-169.
30. Kothari RU, Panciolo A, Liu T, et al. Cincinnati prehospital stroke scale: reproducibility and validity. Ann Emerg Med. 1999;33:373-378.
31. Harbison J, Hossain O, Jenkinson D, et al. Diagnostic accuracy of stroke referrals from primary care, emergency room physicians, and ambulance staff using the face arm speech test. Stroke. 2003;34:71-76.
32. Kidwell CS, Starkman S, Eckstein M, et al. Identifying stroke in the field: prospective validation of the Los Angeles prehospital stroke screen (LAPPS). Stroke. 2000;31:71-76.