User login
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.
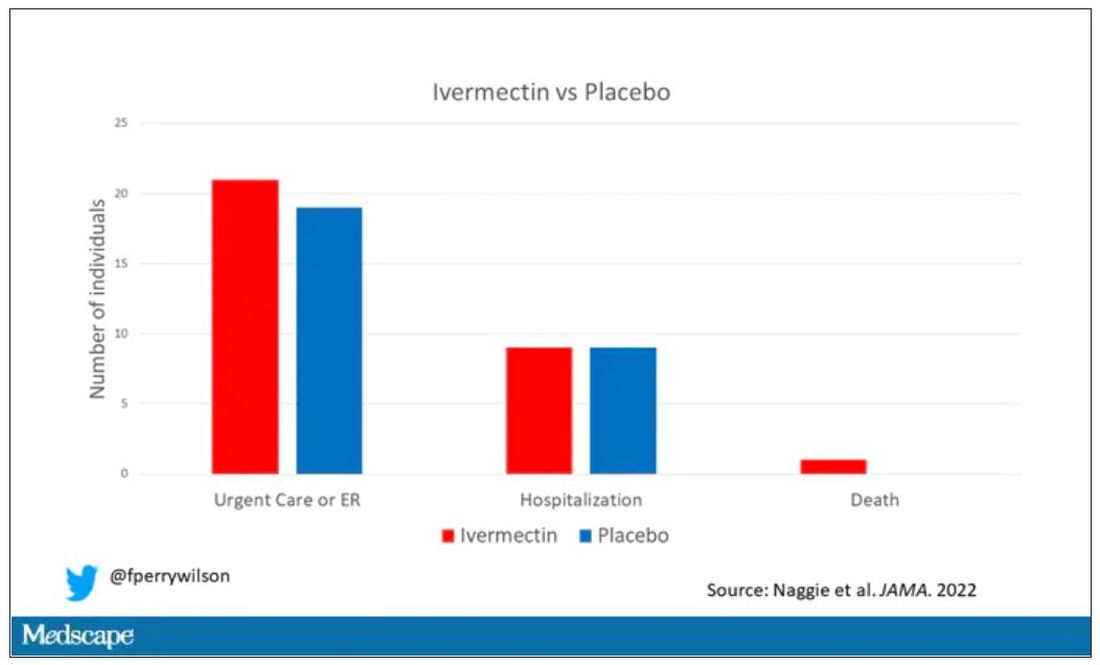
But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.

But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
It began in a petri dish.
Ivermectin, a widely available, cheap, and well-tolerated drug on the WHO’s list of essential medicines for its critical role in treating river blindness, was shown to dramatically reduce the proliferation of SARS-CoV-2 virus in cell culture.
You know the rest of the story. Despite the fact that the median inhibitory concentration in cell culture is about 100-fold higher than what one can achieve with oral dosing in humans, anecdotal reports of miraculous cures proliferated.
Cohort studies suggested that people who got ivermectin did very well in terms of COVID outcomes.
A narrative started to develop online – one that is still quite present today – that authorities were suppressing the good news about ivermectin in order to line their own pockets and those of the execs at Big Pharma. The official Twitter account of the Food and Drug Administration clapped back, reminding the populace that we are not horses or cows.
And every time a study came out that seemed like the nail in the coffin for the so-called horse paste, it rose again, vampire-like, feasting on the blood of social media outrage.
The truth is that, while excitement for ivermectin mounted online, it crashed quite quickly in scientific circles. Most randomized trials showed no effect of the drug. A couple of larger trials which seemed to show dramatic effects were subsequently shown to be fraudulent.
Then the TOGETHER trial was published. The 1,400-patient study from Brazil, which treated outpatients with COVID-19, found no significant difference in hospitalization or ER visits – the primary outcome – between those randomized to ivermectin vs. placebo or another therapy.
But still, Brazil. Different population than the United States. Different health systems. And very different rates of Strongyloides infections (this is a parasite that may be incidentally treated by ivermectin, leading to improvement independent of the drug’s effect on COVID). We all wanted a U.S. trial.
And now we have it. ACTIV-6 was published Oct. 21 in JAMA, a study randomizing outpatients with COVID-19 from 93 sites around the United States to ivermectin or placebo.
A total of 1,591 individuals – median age 47, 60% female – with confirmed symptomatic COVID-19 were randomized from June 2021 to February 2022. About half had been vaccinated.
The primary outcome was straightforward: time to clinical recovery. The time to recovery, defined as having three symptom-free days, was 12 days in the ivermectin group and 13 days in the placebo group – that’s within the margin of error.

But overall, everyone in the trial did fairly well. Serious outcomes, like death, hospitalization, urgent care, or ER visits, occurred in 32 people in the ivermectin group and 28 in the placebo group. Death itself was rare – just one occurred in the trial, in someone receiving ivermectin.OK, are we done with this drug yet? Is this nice U.S. randomized trial enough to convince people that results from a petri dish don’t always transfer to humans, regardless of the presence or absence of an evil pharmaceutical cabal?
No, of course not. At this point, I can predict the responses. The dose wasn’t high enough. It wasn’t given early enough. The patients weren’t sick enough, or they were too sick. This is motivated reasoning, plain and simple. It’s not to say that there isn’t a chance that this drug has some off-target effects on COVID that we haven’t adequately measured, but studies like ACTIV-6 effectively rule out the idea that it’s a miracle cure. And you know what? That’s OK. Miracle cures are vanishingly rare. Most things that work in medicine work OK; they make us a little better, and we learn why they do that and improve on them, and try again and again. It’s not flashy; it doesn’t have that allure of secret knowledge. But it’s what separates science from magic.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator; his science communication work can be found in the Huffington Post, on NPR, and on Medscape.
A version of this article first appeared on Medscape.com.