User login
The Diagnosis: Reactive Perforating Collagenosis
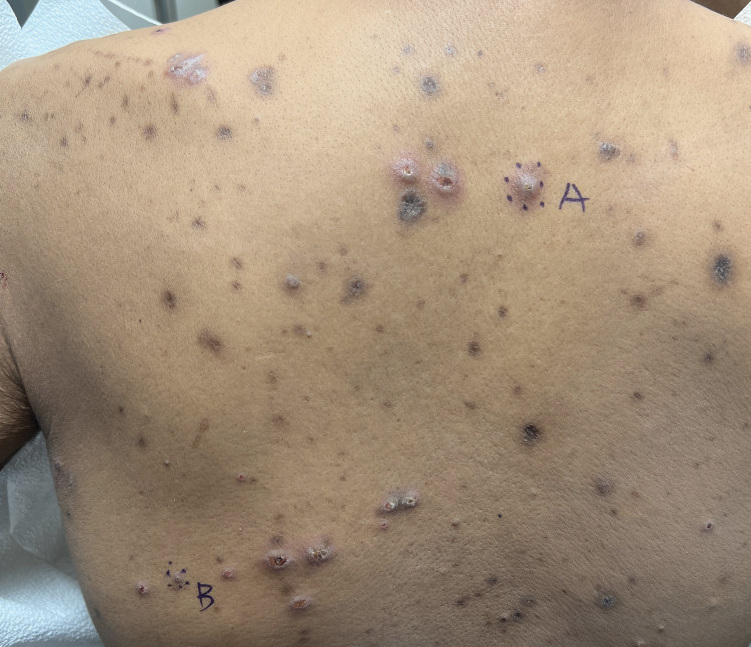
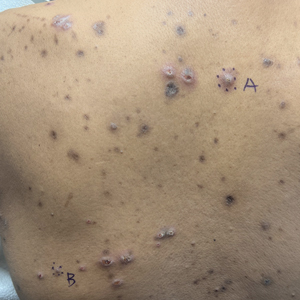
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
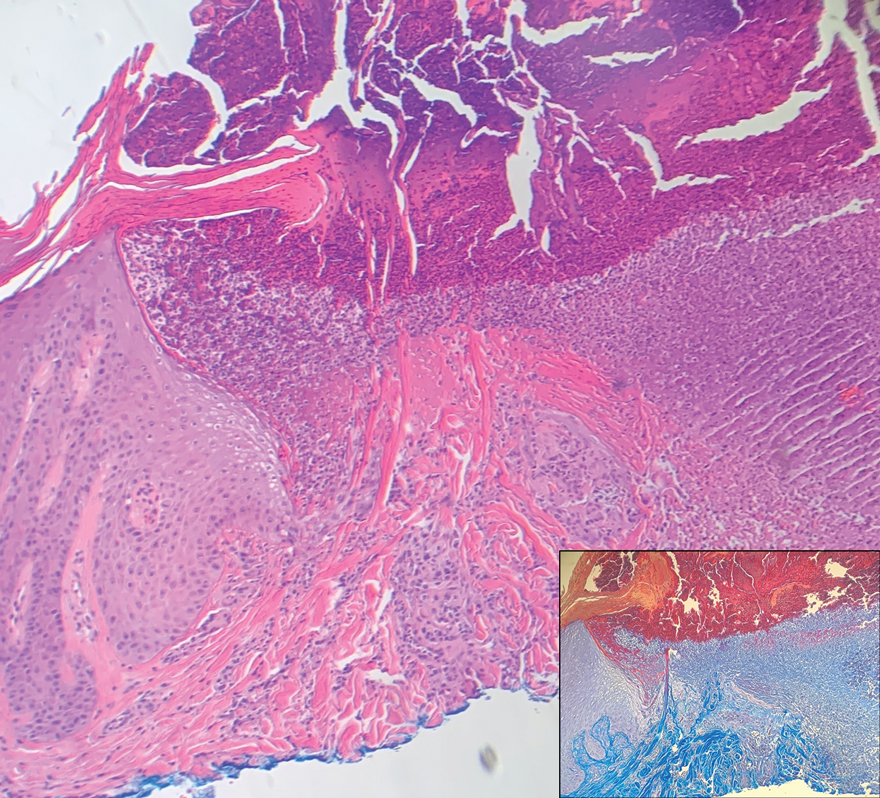
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
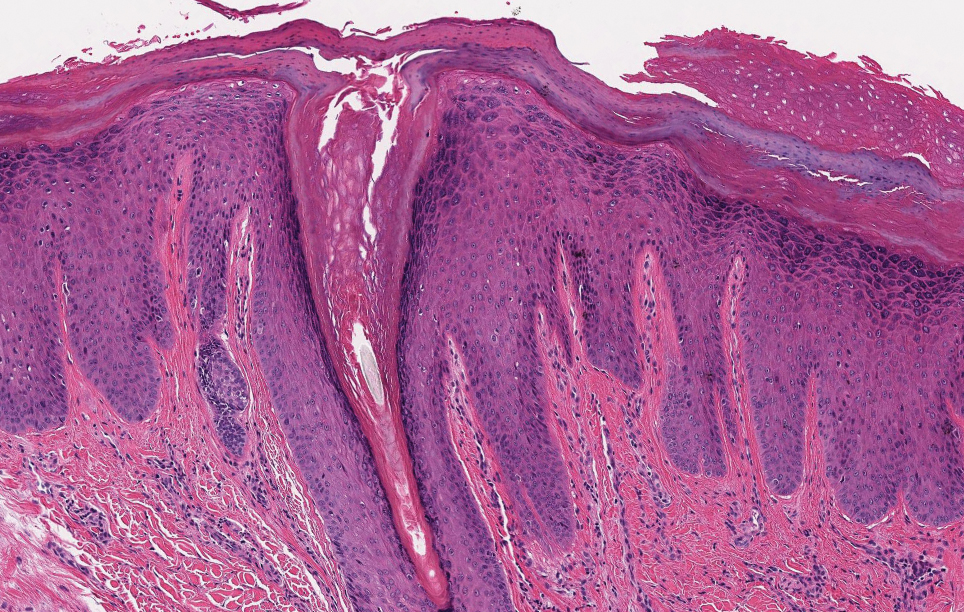
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.
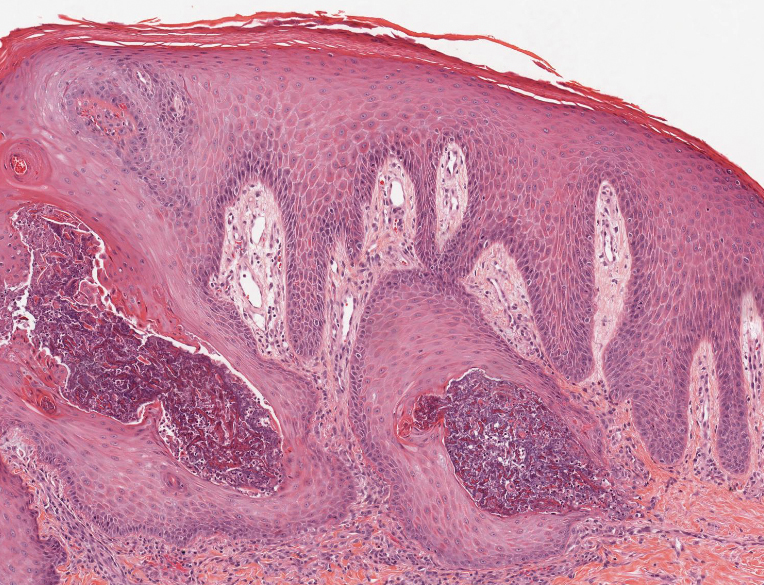
Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.
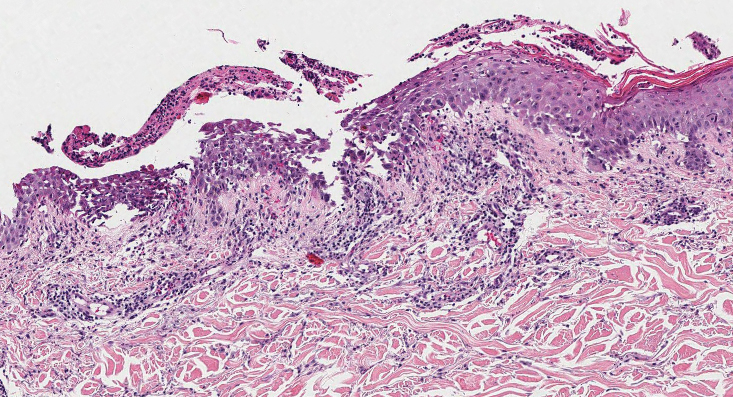
Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15
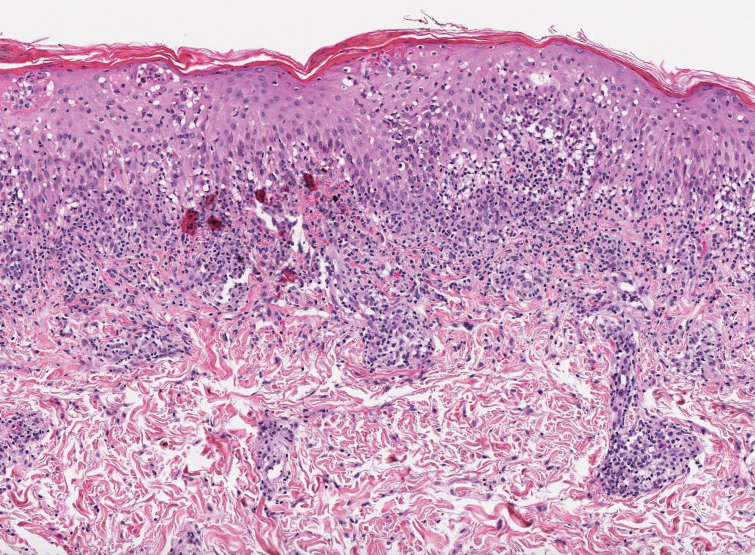
Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.

Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.

Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15

Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16

The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.

Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.

Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15

Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16

- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
A 42-year-old man with end-stage renal disease on hemodialysis presented with generalized body itching and nodules on the scalp and back of 1 year’s duration. Physical examination revealed diffuse, hyperpigmented, pruritic, keratotic nodules and macules on the scalp and back (top). A punch biopsy was performed (bottom).