User login
Mobile Tender Papule on the Scalp
Mobile Tender Papule on the Scalp
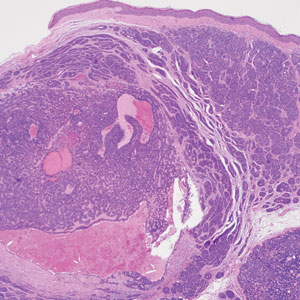
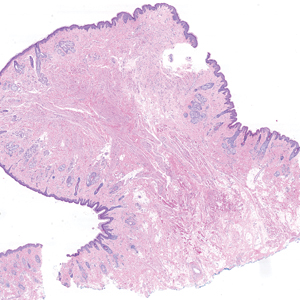
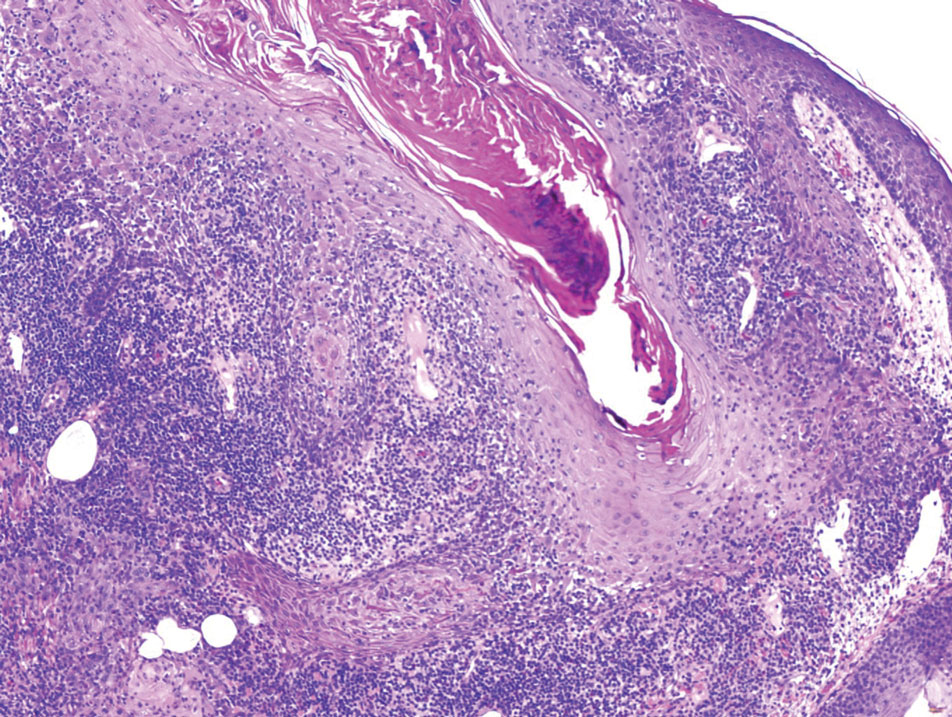
The Diagnosis: Spiradenocylindroma
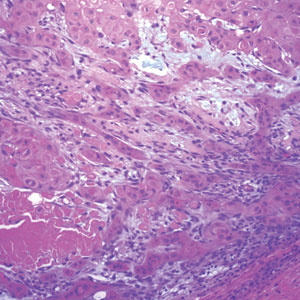
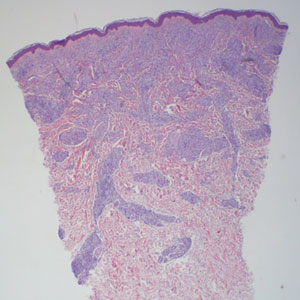
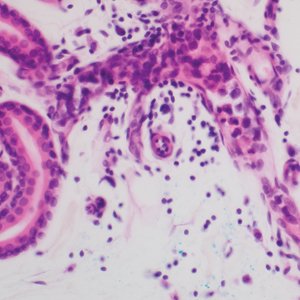
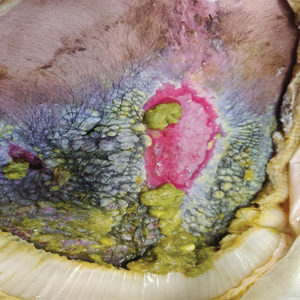
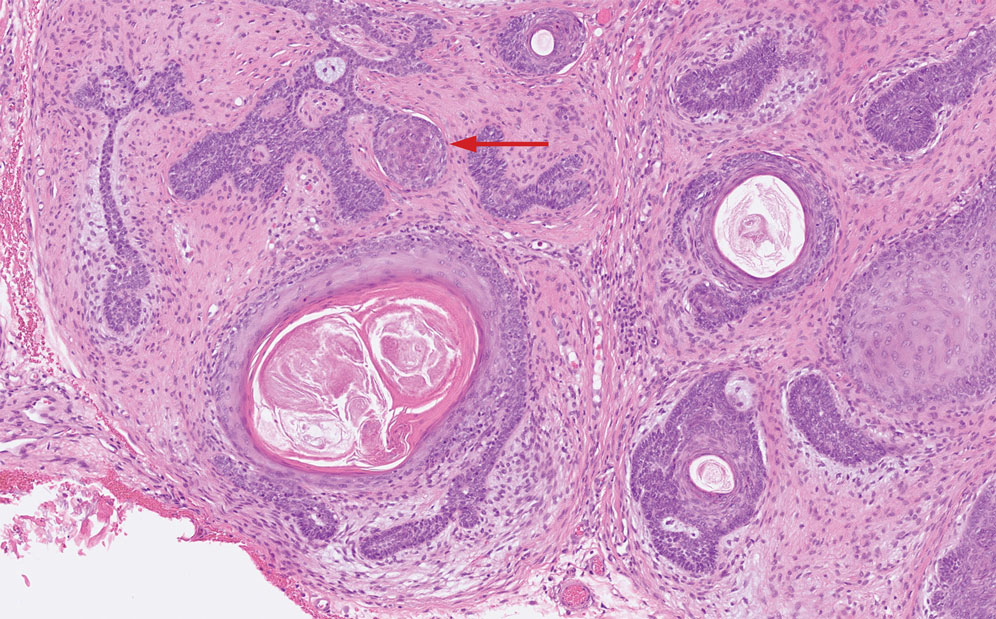
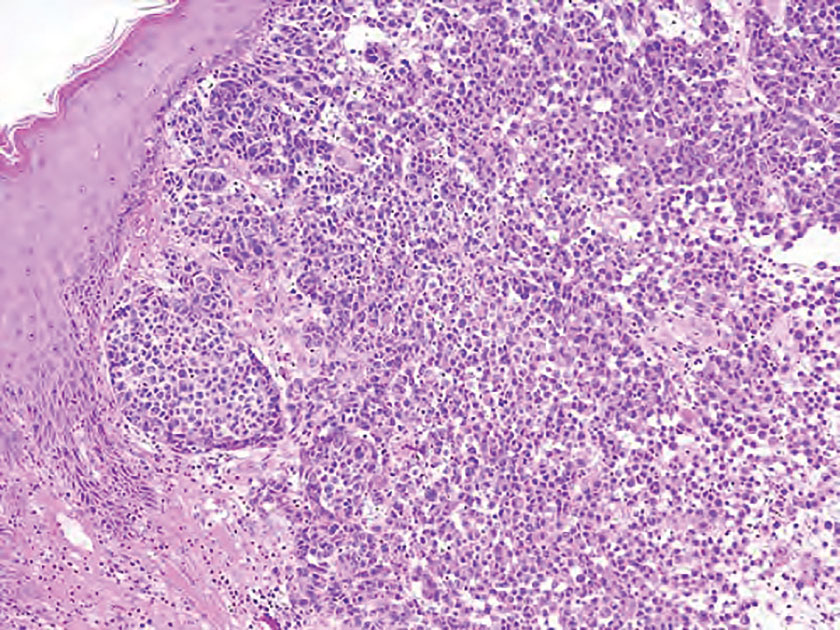
T he biopsy results confirmed the diagnosis of spiradenocylindroma with negative margins. At 6-week follow-up, the patient had no signs of recurrence. Spiradenocylindroma is a benign hybrid neoplasm consisting of histologically intermixed areas representing the spectrum of morphology between spiradenoma and cylindromas.1,2 Both spiradenoma and cylindroma comprise 2 distinct populations of dark and pale basaloid cells.2,3 The spiradenomatous areas of the spiradenocylindroma are arranged in large, well-circumscribed collections of small, darkly staining cells with interspersed lymphocytes and a thin basement membrane surrounding spiradenocylindroma component.2,3 The spiradenocylindroma regions also may contain tubular structures dilated by hemorrhage.2 In contrast, the cylindromatous regions have a jigsaw-puzzle configuration of polygonal tumor nests containing peripherally palisading dark cells and central pale cells, surrounded by a thick basement membrane (top quiz image).2,3
Clinically, sporadic spiradenocylindromas may resemble other lesions, manifesting as a papule or nodule with coloration ranging from gray-blue to salmon pink along with arborizing telangiectasias.4,5 Although spiradenocylindromas typically are found on the head, neck, and trunk, they also have been reported in the kidney, vulva, anus, and rectum.2,6,7 Not only are spiradenocylindromas clinically indistinct from other adnexal growths, but they also share some features with basal cell carcinomas (BCCs) and amelanotic melanomas.8 Features of arborizing telangiectasias on a papule may resemble BCC, requiring histopathology for a definitive diagnosis.
Spiradenocylindromas classically are associated with Brooke-Spiegler syndrome, a rare, autosomal-dominant genodermatosis caused by a germline mutation in the cylindromatosis lysine 63 deubiquitinase tumor-suppressor gene.5 Patients develop adnexal neoplasms of the folliculosebaceous-apocrine unit, including spiradenomas, cylindromas, and trichoepitheliomas.5 Rarely, malignant transformation to spiradenocylindrocarcinoma has been reported.9 Features of malignant transformation include loss of the 2-cell population, cytologic atypia, increased mitotic activity, and loss of intratumoral lymphocytes.10
Trichoepitheliomas are benign, firm, flesh-colored papules to nodules that commonly are found on the mid face but may appear on the scalp, neck, and upper trunk.5-11 Trichoepitheliomas are closely related to spiradenomas and cylindromas; the familial form, multiple familial trichoepitheliomas, exists on a spectrum with Brooke-Spiegler syndrome.3,11 Multiple familial trichoepithelioma is characterized by multiple trichoepitheliomas without accompanying spiradenomas, cylindromas, or spiradenocylindromas.3 On histopathology, trichoepitheliomas are distinguished by cribriform clusters or nests of basaloid follicular germinative cells with bulbar differentiation, known as papillary mesenchymal bodies, surrounded by an adherent stroma (eFigure 1).3,5,11 In addition to follicular bulbar differentiation, trichoepitheliomas are surrounded by an adherent cellular stroma without the retraction artifact around tumor islands seen in BCC, although artifactual clefts may occur within the stroma.11 In contrast, spiradenocylindromas do not demonstrate keratin cysts or artifactual clefts within the stroma.
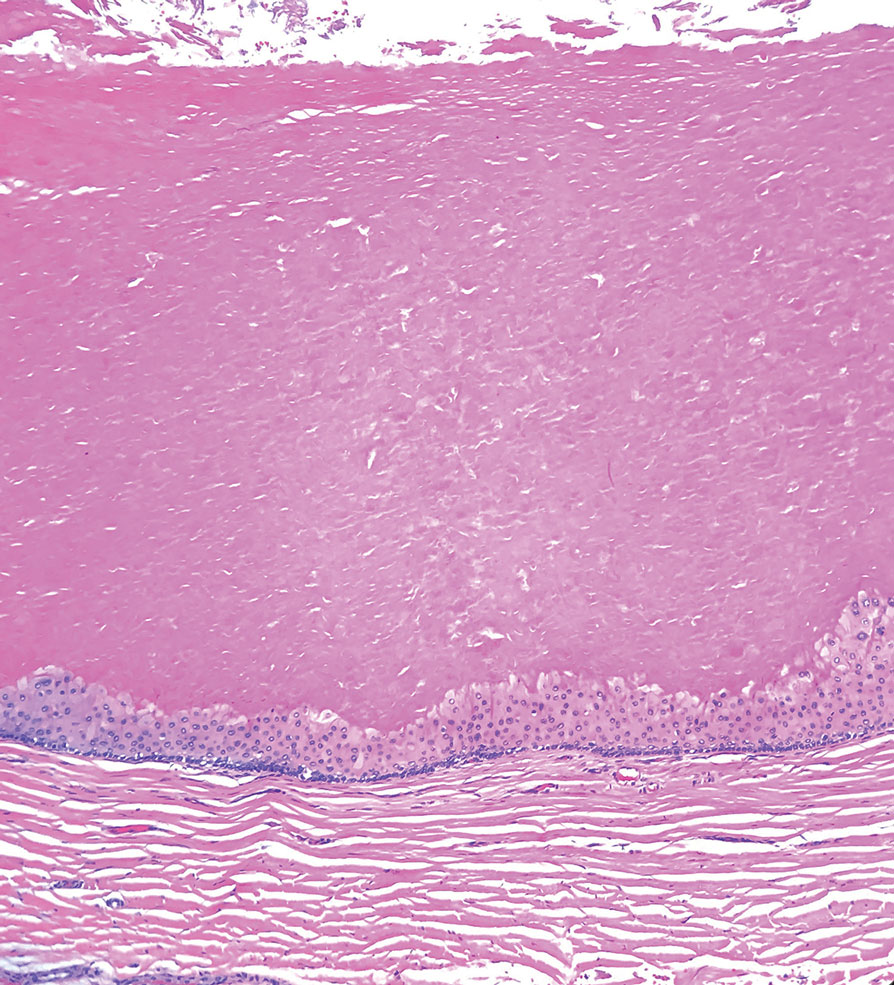
Trichilemmal cysts, or pilar cysts, are benign adnexal neoplasms derived from the outer root sheath at the isthmus.12-14 Approximately 90% of pilar cysts are found on the scalp and 2% of trichilemmal cysts may progress to a proliferating trichilemmal cyst, which is locally aggressive and contains an expanding buckled epithelium within the cyst space.12,14 Clinically, trichilemmal cysts are slow-growing, smooth, round, mobile nodules without a central punctum.12,13 On histopathology, the cyst wall contains peripherally palisading basal cells and maturing cells showing no intercellular bridging (eFigure 2). As the cells mature, they swell with pale cytoplasm and abruptly keratinize without a granular layer, a process known as trichilemmal keratinization.12-14 Additionally, cholesterol clefts are common in the keratinous lumen, and about 25% of cysts contain calcifications.13,14 The broadly basophilic spiradenocylindromas sharply contrast the abundant eosinophilic keratin of trichilemmal cysts.
Basal cell carcinoma is a slow-growing, locally destructive neoplasm that develops due to chronic sun exposure; thus, BCCs commonly arise on exposed areas of the face, head, neck, arms, and legs.15 Nodular BCC is the most common subtype and typically manifests as a shiny pearly papule or nodule with a smooth surface, rolled borders, and arborizing telangiectasias.16 On histopathology, nodular BCCs demonstrate nests or nodules of basaloid keratinocytes with peripheral palisading and retraction artifact between the tumor and stroma (eFigure 3).15,16 A lack of retraction artifact, cystic dilation of tubular structures, jigsaw molding of nests, and a distinct 2-cell population distinguish spiradenocylindroma from BCC. Of note, in rare instances BCCs also may display a thick fibrous stroma, similar to the stroma of cylindromas.15
Amelanotic melanoma is a variant of melanoma characterized by little to no pigment. Any of the 4 classic subtypes of melanoma (nodular, superficial spreading, lentigo maligna, acral lentiginous) can be amelanotic.17 Clinically, amelanotic melanomas can vary greatly, manifesting as erythematous macules, dermal plaques, or papulonodular lesions, often with scaling.18 On histopathology, findings common to all melanomas include cellular atypia, mitoses, pagetoid spread, and pleomorphism (eFigure 4).18,19 Immunohistochemistry is an important method to distinguish melanoma from other melanocytic proliferations and to aid in the assessment of Breslow depth. Markers include SOX10 (sex-determining region Y-box transcription factor 10), S100, and MART-1 (melanoma antigen recognized by T cells 1/melan-A).19,20 Expression of PRAME (preferentially expressed antigen in melanoma) often is positive but is not necessary for diagnosis.21 Histologically, the atypical pleomorphic cells of melanoma are markedly distinct from both spiradenomas and cylindromas.
- Soyer HP, Kerl H, Ott A. Spiradenocylindroma—more than a coincidence? Am J Dermatopathol. 1998;20:315-317.
- Michal M, Lamovec J, Mukenˇ snabl P, et al. Spiradenocylindromas of the skin: tumors with morphological features of spiradenoma and cylindroma in the same lesion: report of 12 cases. Pathol Int. 1999;49:419-425.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bostan E, Boynuyogun E, Gokoz O, et al. Hybrid tumor “spiradenocylindroma” with unusual dermoscopic features. An Bras Dermatol. 2023;98:382-384.
- Pinho AC, Gouveia MJ, Gameiro AR, et al. Brooke-Spiegler syndrome—an underrecognized cause of multiple familial scalp tumors: report of a new germline mutation. J Dermatol Case Rep. 2015;9:67-70.
- Ströbel P, Zettl A, Ren Z, et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am J Surg Pathol. 2002;26:119-124.
- Kacerovska D, Szepe P, Vanecek T, et al. Spiradenocylindroma-like basaloid carcinoma of the anus and rectum: case report, including HPV studies and analysis of the CYLD gene mutations. Am J Dermatopathol. 2008;30:472-476.
- Silvestri F, Maida P, Venturi F, et al. Scalp spiradenocylindroma: a challenging dermoscopic diagnosis. Dermatol Ther. 2020;33:E14307.
- Held L, Ruetten A, Saggini A, et al. Metaplastic spiradenocarcinoma: report of two cases with sarcomatous differentiation. J Cutan Pathol. 2021;48:384-389.
- Płachta I, Kleibert M, Czarnecka AM, et al. Current diagnosis and treatment options for cutaneous adnexal neoplasms with apocrine and eccrine differentiation. Int J Mol Sci. 2021;22:5077.
- Johnson H, Robles M, Kamino H, et al. Trichoepithelioma. Dermatol Online J. 2008;14:5.
- He P, Cui LG, Wang JR, et al. Trichilemmal cyst: clinical and sonographic feature. J Ultrasound Med. 2019;38:91-96.
- Liu M, Han H, Zheng Y, et al. Pilar cyst on the dorsum of hand: a case report and review of literature. Medicine (United States). 2020;99:E21519.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. In: Archives of Pathology and Laboratory Medicine. Vol 141. College of American Pathologists; 2017:1490-1502.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Kaizer-Salk KA, Herten RJ, Ragsdale BD, et al. Amelanotic melanoma: a unique case study and review of the literature. BMJ Case Rep. 2018:bcr2017222751.
- Silva TS, de Araujo LR, Faro GB de A, et al. Nodular amelanotic melanoma. An Bras Dermatol. 2019;94:497-498.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321.
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
The Diagnosis: Spiradenocylindroma
T he biopsy results confirmed the diagnosis of spiradenocylindroma with negative margins. At 6-week follow-up, the patient had no signs of recurrence. Spiradenocylindroma is a benign hybrid neoplasm consisting of histologically intermixed areas representing the spectrum of morphology between spiradenoma and cylindromas.1,2 Both spiradenoma and cylindroma comprise 2 distinct populations of dark and pale basaloid cells.2,3 The spiradenomatous areas of the spiradenocylindroma are arranged in large, well-circumscribed collections of small, darkly staining cells with interspersed lymphocytes and a thin basement membrane surrounding spiradenocylindroma component.2,3 The spiradenocylindroma regions also may contain tubular structures dilated by hemorrhage.2 In contrast, the cylindromatous regions have a jigsaw-puzzle configuration of polygonal tumor nests containing peripherally palisading dark cells and central pale cells, surrounded by a thick basement membrane (top quiz image).2,3
Clinically, sporadic spiradenocylindromas may resemble other lesions, manifesting as a papule or nodule with coloration ranging from gray-blue to salmon pink along with arborizing telangiectasias.4,5 Although spiradenocylindromas typically are found on the head, neck, and trunk, they also have been reported in the kidney, vulva, anus, and rectum.2,6,7 Not only are spiradenocylindromas clinically indistinct from other adnexal growths, but they also share some features with basal cell carcinomas (BCCs) and amelanotic melanomas.8 Features of arborizing telangiectasias on a papule may resemble BCC, requiring histopathology for a definitive diagnosis.
Spiradenocylindromas classically are associated with Brooke-Spiegler syndrome, a rare, autosomal-dominant genodermatosis caused by a germline mutation in the cylindromatosis lysine 63 deubiquitinase tumor-suppressor gene.5 Patients develop adnexal neoplasms of the folliculosebaceous-apocrine unit, including spiradenomas, cylindromas, and trichoepitheliomas.5 Rarely, malignant transformation to spiradenocylindrocarcinoma has been reported.9 Features of malignant transformation include loss of the 2-cell population, cytologic atypia, increased mitotic activity, and loss of intratumoral lymphocytes.10
Trichoepitheliomas are benign, firm, flesh-colored papules to nodules that commonly are found on the mid face but may appear on the scalp, neck, and upper trunk.5-11 Trichoepitheliomas are closely related to spiradenomas and cylindromas; the familial form, multiple familial trichoepitheliomas, exists on a spectrum with Brooke-Spiegler syndrome.3,11 Multiple familial trichoepithelioma is characterized by multiple trichoepitheliomas without accompanying spiradenomas, cylindromas, or spiradenocylindromas.3 On histopathology, trichoepitheliomas are distinguished by cribriform clusters or nests of basaloid follicular germinative cells with bulbar differentiation, known as papillary mesenchymal bodies, surrounded by an adherent stroma (eFigure 1).3,5,11 In addition to follicular bulbar differentiation, trichoepitheliomas are surrounded by an adherent cellular stroma without the retraction artifact around tumor islands seen in BCC, although artifactual clefts may occur within the stroma.11 In contrast, spiradenocylindromas do not demonstrate keratin cysts or artifactual clefts within the stroma.

Trichilemmal cysts, or pilar cysts, are benign adnexal neoplasms derived from the outer root sheath at the isthmus.12-14 Approximately 90% of pilar cysts are found on the scalp and 2% of trichilemmal cysts may progress to a proliferating trichilemmal cyst, which is locally aggressive and contains an expanding buckled epithelium within the cyst space.12,14 Clinically, trichilemmal cysts are slow-growing, smooth, round, mobile nodules without a central punctum.12,13 On histopathology, the cyst wall contains peripherally palisading basal cells and maturing cells showing no intercellular bridging (eFigure 2). As the cells mature, they swell with pale cytoplasm and abruptly keratinize without a granular layer, a process known as trichilemmal keratinization.12-14 Additionally, cholesterol clefts are common in the keratinous lumen, and about 25% of cysts contain calcifications.13,14 The broadly basophilic spiradenocylindromas sharply contrast the abundant eosinophilic keratin of trichilemmal cysts.

Basal cell carcinoma is a slow-growing, locally destructive neoplasm that develops due to chronic sun exposure; thus, BCCs commonly arise on exposed areas of the face, head, neck, arms, and legs.15 Nodular BCC is the most common subtype and typically manifests as a shiny pearly papule or nodule with a smooth surface, rolled borders, and arborizing telangiectasias.16 On histopathology, nodular BCCs demonstrate nests or nodules of basaloid keratinocytes with peripheral palisading and retraction artifact between the tumor and stroma (eFigure 3).15,16 A lack of retraction artifact, cystic dilation of tubular structures, jigsaw molding of nests, and a distinct 2-cell population distinguish spiradenocylindroma from BCC. Of note, in rare instances BCCs also may display a thick fibrous stroma, similar to the stroma of cylindromas.15

Amelanotic melanoma is a variant of melanoma characterized by little to no pigment. Any of the 4 classic subtypes of melanoma (nodular, superficial spreading, lentigo maligna, acral lentiginous) can be amelanotic.17 Clinically, amelanotic melanomas can vary greatly, manifesting as erythematous macules, dermal plaques, or papulonodular lesions, often with scaling.18 On histopathology, findings common to all melanomas include cellular atypia, mitoses, pagetoid spread, and pleomorphism (eFigure 4).18,19 Immunohistochemistry is an important method to distinguish melanoma from other melanocytic proliferations and to aid in the assessment of Breslow depth. Markers include SOX10 (sex-determining region Y-box transcription factor 10), S100, and MART-1 (melanoma antigen recognized by T cells 1/melan-A).19,20 Expression of PRAME (preferentially expressed antigen in melanoma) often is positive but is not necessary for diagnosis.21 Histologically, the atypical pleomorphic cells of melanoma are markedly distinct from both spiradenomas and cylindromas.

The Diagnosis: Spiradenocylindroma
T he biopsy results confirmed the diagnosis of spiradenocylindroma with negative margins. At 6-week follow-up, the patient had no signs of recurrence. Spiradenocylindroma is a benign hybrid neoplasm consisting of histologically intermixed areas representing the spectrum of morphology between spiradenoma and cylindromas.1,2 Both spiradenoma and cylindroma comprise 2 distinct populations of dark and pale basaloid cells.2,3 The spiradenomatous areas of the spiradenocylindroma are arranged in large, well-circumscribed collections of small, darkly staining cells with interspersed lymphocytes and a thin basement membrane surrounding spiradenocylindroma component.2,3 The spiradenocylindroma regions also may contain tubular structures dilated by hemorrhage.2 In contrast, the cylindromatous regions have a jigsaw-puzzle configuration of polygonal tumor nests containing peripherally palisading dark cells and central pale cells, surrounded by a thick basement membrane (top quiz image).2,3
Clinically, sporadic spiradenocylindromas may resemble other lesions, manifesting as a papule or nodule with coloration ranging from gray-blue to salmon pink along with arborizing telangiectasias.4,5 Although spiradenocylindromas typically are found on the head, neck, and trunk, they also have been reported in the kidney, vulva, anus, and rectum.2,6,7 Not only are spiradenocylindromas clinically indistinct from other adnexal growths, but they also share some features with basal cell carcinomas (BCCs) and amelanotic melanomas.8 Features of arborizing telangiectasias on a papule may resemble BCC, requiring histopathology for a definitive diagnosis.
Spiradenocylindromas classically are associated with Brooke-Spiegler syndrome, a rare, autosomal-dominant genodermatosis caused by a germline mutation in the cylindromatosis lysine 63 deubiquitinase tumor-suppressor gene.5 Patients develop adnexal neoplasms of the folliculosebaceous-apocrine unit, including spiradenomas, cylindromas, and trichoepitheliomas.5 Rarely, malignant transformation to spiradenocylindrocarcinoma has been reported.9 Features of malignant transformation include loss of the 2-cell population, cytologic atypia, increased mitotic activity, and loss of intratumoral lymphocytes.10
Trichoepitheliomas are benign, firm, flesh-colored papules to nodules that commonly are found on the mid face but may appear on the scalp, neck, and upper trunk.5-11 Trichoepitheliomas are closely related to spiradenomas and cylindromas; the familial form, multiple familial trichoepitheliomas, exists on a spectrum with Brooke-Spiegler syndrome.3,11 Multiple familial trichoepithelioma is characterized by multiple trichoepitheliomas without accompanying spiradenomas, cylindromas, or spiradenocylindromas.3 On histopathology, trichoepitheliomas are distinguished by cribriform clusters or nests of basaloid follicular germinative cells with bulbar differentiation, known as papillary mesenchymal bodies, surrounded by an adherent stroma (eFigure 1).3,5,11 In addition to follicular bulbar differentiation, trichoepitheliomas are surrounded by an adherent cellular stroma without the retraction artifact around tumor islands seen in BCC, although artifactual clefts may occur within the stroma.11 In contrast, spiradenocylindromas do not demonstrate keratin cysts or artifactual clefts within the stroma.

Trichilemmal cysts, or pilar cysts, are benign adnexal neoplasms derived from the outer root sheath at the isthmus.12-14 Approximately 90% of pilar cysts are found on the scalp and 2% of trichilemmal cysts may progress to a proliferating trichilemmal cyst, which is locally aggressive and contains an expanding buckled epithelium within the cyst space.12,14 Clinically, trichilemmal cysts are slow-growing, smooth, round, mobile nodules without a central punctum.12,13 On histopathology, the cyst wall contains peripherally palisading basal cells and maturing cells showing no intercellular bridging (eFigure 2). As the cells mature, they swell with pale cytoplasm and abruptly keratinize without a granular layer, a process known as trichilemmal keratinization.12-14 Additionally, cholesterol clefts are common in the keratinous lumen, and about 25% of cysts contain calcifications.13,14 The broadly basophilic spiradenocylindromas sharply contrast the abundant eosinophilic keratin of trichilemmal cysts.

Basal cell carcinoma is a slow-growing, locally destructive neoplasm that develops due to chronic sun exposure; thus, BCCs commonly arise on exposed areas of the face, head, neck, arms, and legs.15 Nodular BCC is the most common subtype and typically manifests as a shiny pearly papule or nodule with a smooth surface, rolled borders, and arborizing telangiectasias.16 On histopathology, nodular BCCs demonstrate nests or nodules of basaloid keratinocytes with peripheral palisading and retraction artifact between the tumor and stroma (eFigure 3).15,16 A lack of retraction artifact, cystic dilation of tubular structures, jigsaw molding of nests, and a distinct 2-cell population distinguish spiradenocylindroma from BCC. Of note, in rare instances BCCs also may display a thick fibrous stroma, similar to the stroma of cylindromas.15

Amelanotic melanoma is a variant of melanoma characterized by little to no pigment. Any of the 4 classic subtypes of melanoma (nodular, superficial spreading, lentigo maligna, acral lentiginous) can be amelanotic.17 Clinically, amelanotic melanomas can vary greatly, manifesting as erythematous macules, dermal plaques, or papulonodular lesions, often with scaling.18 On histopathology, findings common to all melanomas include cellular atypia, mitoses, pagetoid spread, and pleomorphism (eFigure 4).18,19 Immunohistochemistry is an important method to distinguish melanoma from other melanocytic proliferations and to aid in the assessment of Breslow depth. Markers include SOX10 (sex-determining region Y-box transcription factor 10), S100, and MART-1 (melanoma antigen recognized by T cells 1/melan-A).19,20 Expression of PRAME (preferentially expressed antigen in melanoma) often is positive but is not necessary for diagnosis.21 Histologically, the atypical pleomorphic cells of melanoma are markedly distinct from both spiradenomas and cylindromas.

- Soyer HP, Kerl H, Ott A. Spiradenocylindroma—more than a coincidence? Am J Dermatopathol. 1998;20:315-317.
- Michal M, Lamovec J, Mukenˇ snabl P, et al. Spiradenocylindromas of the skin: tumors with morphological features of spiradenoma and cylindroma in the same lesion: report of 12 cases. Pathol Int. 1999;49:419-425.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bostan E, Boynuyogun E, Gokoz O, et al. Hybrid tumor “spiradenocylindroma” with unusual dermoscopic features. An Bras Dermatol. 2023;98:382-384.
- Pinho AC, Gouveia MJ, Gameiro AR, et al. Brooke-Spiegler syndrome—an underrecognized cause of multiple familial scalp tumors: report of a new germline mutation. J Dermatol Case Rep. 2015;9:67-70.
- Ströbel P, Zettl A, Ren Z, et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am J Surg Pathol. 2002;26:119-124.
- Kacerovska D, Szepe P, Vanecek T, et al. Spiradenocylindroma-like basaloid carcinoma of the anus and rectum: case report, including HPV studies and analysis of the CYLD gene mutations. Am J Dermatopathol. 2008;30:472-476.
- Silvestri F, Maida P, Venturi F, et al. Scalp spiradenocylindroma: a challenging dermoscopic diagnosis. Dermatol Ther. 2020;33:E14307.
- Held L, Ruetten A, Saggini A, et al. Metaplastic spiradenocarcinoma: report of two cases with sarcomatous differentiation. J Cutan Pathol. 2021;48:384-389.
- Płachta I, Kleibert M, Czarnecka AM, et al. Current diagnosis and treatment options for cutaneous adnexal neoplasms with apocrine and eccrine differentiation. Int J Mol Sci. 2021;22:5077.
- Johnson H, Robles M, Kamino H, et al. Trichoepithelioma. Dermatol Online J. 2008;14:5.
- He P, Cui LG, Wang JR, et al. Trichilemmal cyst: clinical and sonographic feature. J Ultrasound Med. 2019;38:91-96.
- Liu M, Han H, Zheng Y, et al. Pilar cyst on the dorsum of hand: a case report and review of literature. Medicine (United States). 2020;99:E21519.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. In: Archives of Pathology and Laboratory Medicine. Vol 141. College of American Pathologists; 2017:1490-1502.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Kaizer-Salk KA, Herten RJ, Ragsdale BD, et al. Amelanotic melanoma: a unique case study and review of the literature. BMJ Case Rep. 2018:bcr2017222751.
- Silva TS, de Araujo LR, Faro GB de A, et al. Nodular amelanotic melanoma. An Bras Dermatol. 2019;94:497-498.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321.
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
- Soyer HP, Kerl H, Ott A. Spiradenocylindroma—more than a coincidence? Am J Dermatopathol. 1998;20:315-317.
- Michal M, Lamovec J, Mukenˇ snabl P, et al. Spiradenocylindromas of the skin: tumors with morphological features of spiradenoma and cylindroma in the same lesion: report of 12 cases. Pathol Int. 1999;49:419-425.
- Kazakov DV. Brooke-Spiegler syndrome and phenotypic variants: an update. Head Neck Pathol. 2016;10:125-130.
- Bostan E, Boynuyogun E, Gokoz O, et al. Hybrid tumor “spiradenocylindroma” with unusual dermoscopic features. An Bras Dermatol. 2023;98:382-384.
- Pinho AC, Gouveia MJ, Gameiro AR, et al. Brooke-Spiegler syndrome—an underrecognized cause of multiple familial scalp tumors: report of a new germline mutation. J Dermatol Case Rep. 2015;9:67-70.
- Ströbel P, Zettl A, Ren Z, et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am J Surg Pathol. 2002;26:119-124.
- Kacerovska D, Szepe P, Vanecek T, et al. Spiradenocylindroma-like basaloid carcinoma of the anus and rectum: case report, including HPV studies and analysis of the CYLD gene mutations. Am J Dermatopathol. 2008;30:472-476.
- Silvestri F, Maida P, Venturi F, et al. Scalp spiradenocylindroma: a challenging dermoscopic diagnosis. Dermatol Ther. 2020;33:E14307.
- Held L, Ruetten A, Saggini A, et al. Metaplastic spiradenocarcinoma: report of two cases with sarcomatous differentiation. J Cutan Pathol. 2021;48:384-389.
- Płachta I, Kleibert M, Czarnecka AM, et al. Current diagnosis and treatment options for cutaneous adnexal neoplasms with apocrine and eccrine differentiation. Int J Mol Sci. 2021;22:5077.
- Johnson H, Robles M, Kamino H, et al. Trichoepithelioma. Dermatol Online J. 2008;14:5.
- He P, Cui LG, Wang JR, et al. Trichilemmal cyst: clinical and sonographic feature. J Ultrasound Med. 2019;38:91-96.
- Liu M, Han H, Zheng Y, et al. Pilar cyst on the dorsum of hand: a case report and review of literature. Medicine (United States). 2020;99:E21519.
- Ramaswamy AS, Manjunatha HK, Sunilkumar B, et al. Morphological spectrum of pilar cysts. N Am J Med Sci. 2013;5:124-128.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. In: Archives of Pathology and Laboratory Medicine. Vol 141. College of American Pathologists; 2017:1490-1502.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Kaizer-Salk KA, Herten RJ, Ragsdale BD, et al. Amelanotic melanoma: a unique case study and review of the literature. BMJ Case Rep. 2018:bcr2017222751.
- Silva TS, de Araujo LR, Faro GB de A, et al. Nodular amelanotic melanoma. An Bras Dermatol. 2019;94:497-498.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321.
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444.
- Lezcano C, Jungbluth AA, Nehal KS, et al. PRAME expression in melanocytic tumors. Am J Surg Pathol. 2018;42:1456-1465.
Mobile Tender Papule on the Scalp
Mobile Tender Papule on the Scalp
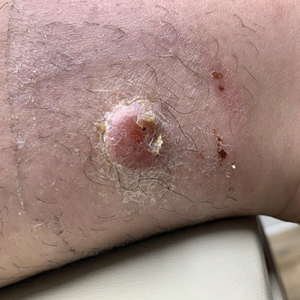
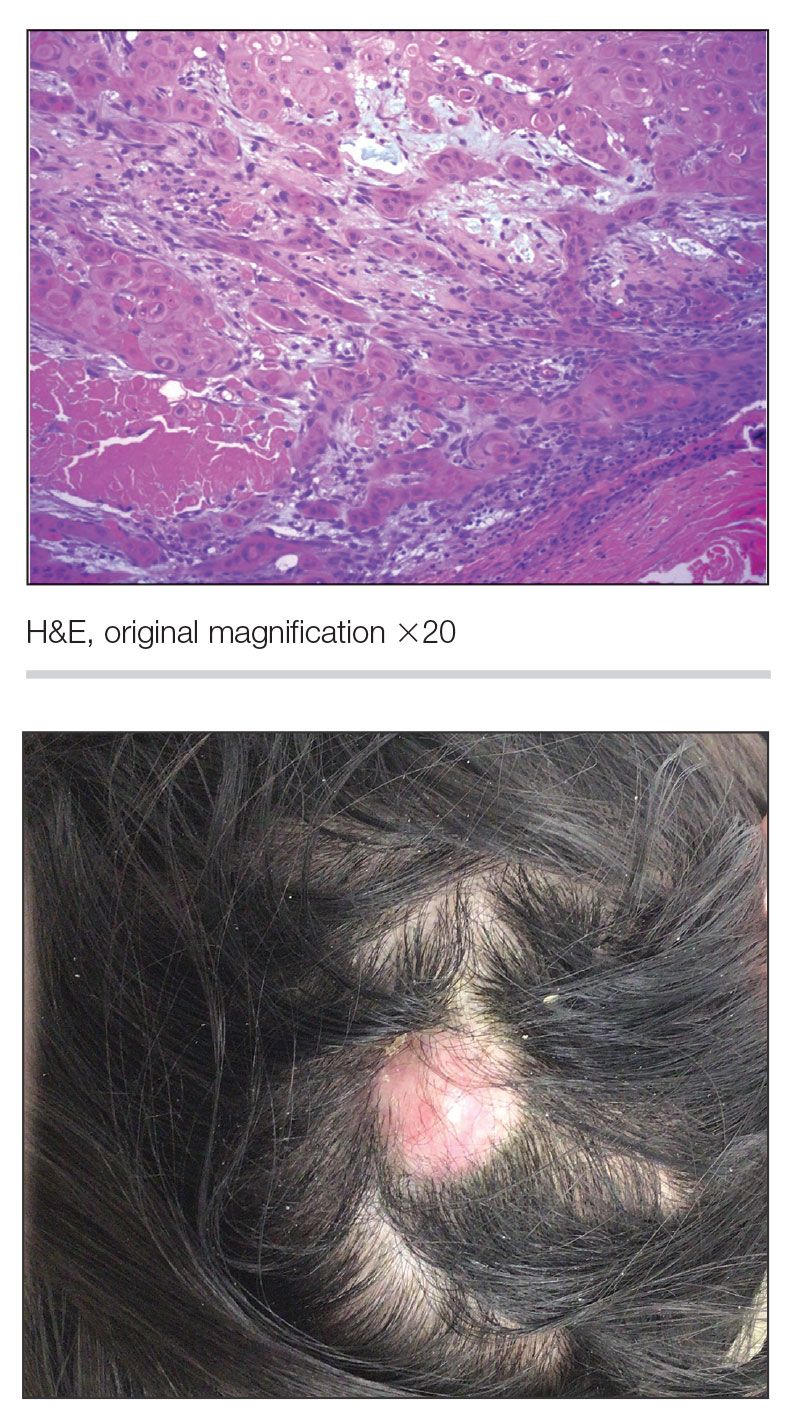
A 73-year-old man presented to the plastic surgery department with a single, progressively enlarging nodule on the scalp of 1 year’s duration. Dermatologic examination revealed a 0.8-cm, soft, mobile, gray-blue, dome-shaped papule on the left postauricular scalp that was tender to palpation. There was no central punctum, and the patient denied any history of drainage or odor. He had no personal or family history of similar lesions. An excisional biopsy of the papule was performed.
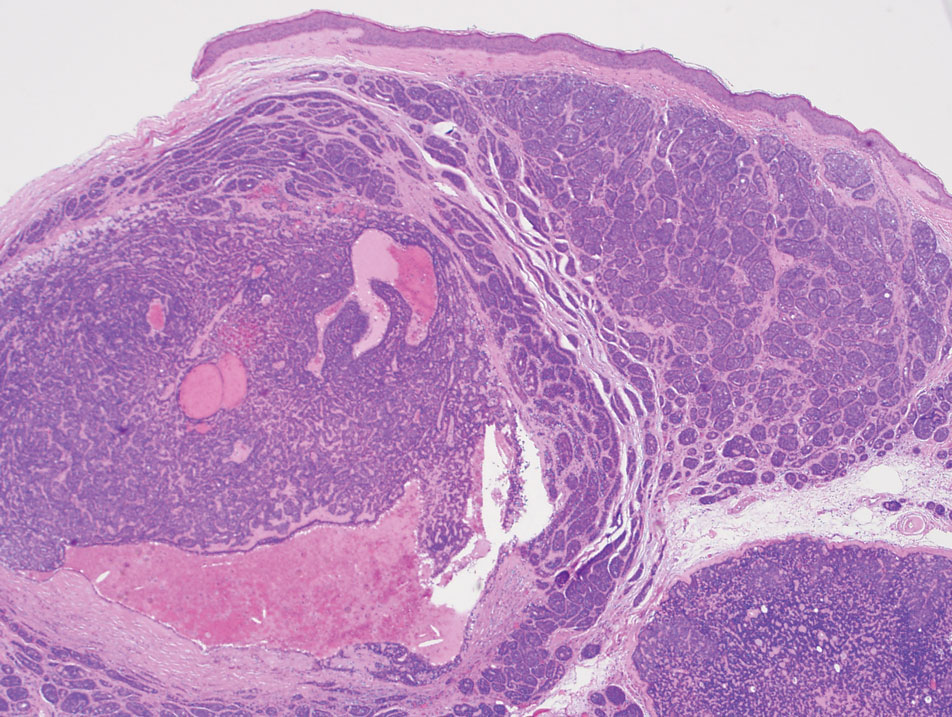
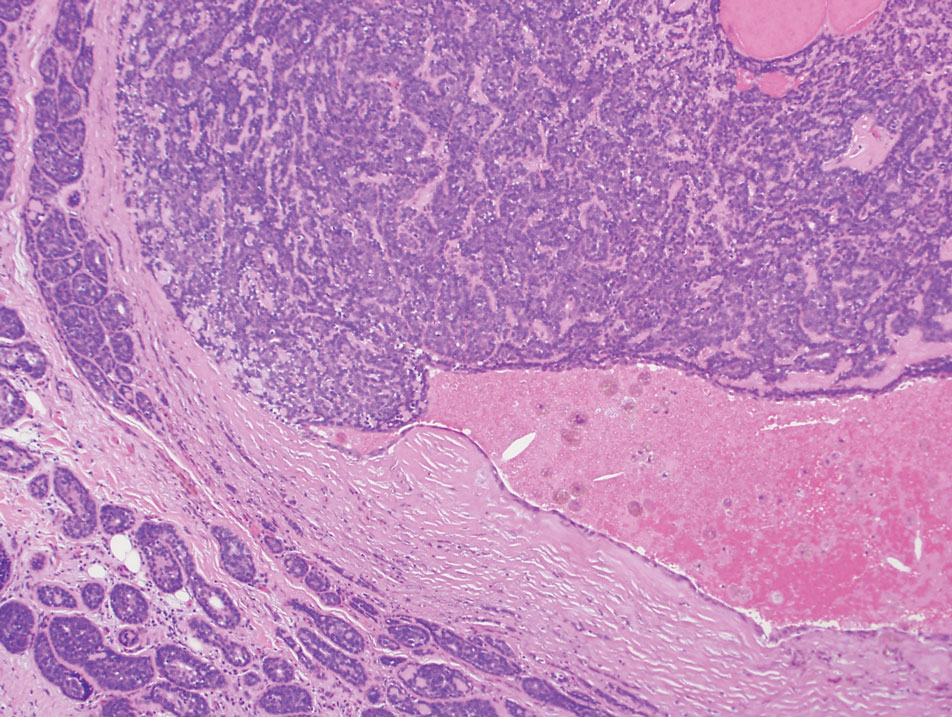
Growing Nodule on the Parietal Scalp
Growing Nodule on the Parietal Scalp
THE DIAGNOSIS: Malignant Proliferating Trichilemmal Tumor
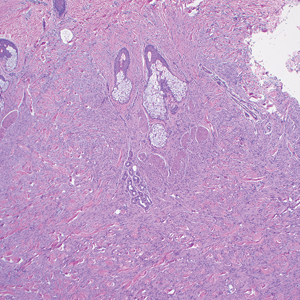
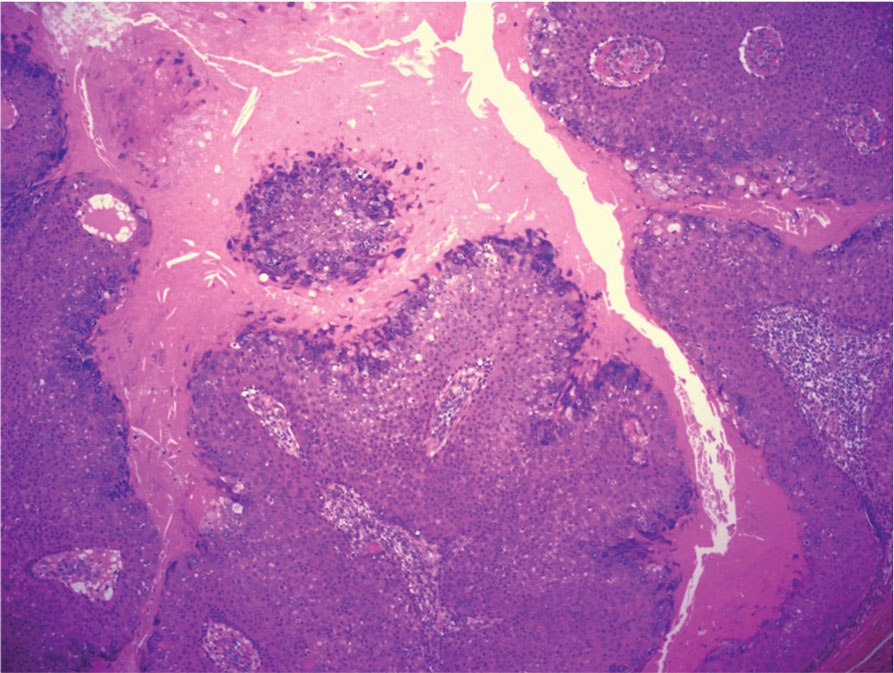
Biopsy revealed a squamous epithelium with cystic changes, trichilemmal differentiation, squamous eddy formation, keratinocyte atypia, focal necrotic changes, and a focus of atypical keratinocytes invading the dermis (Figure 1). Based on these findings, a diagnosis of malignant proliferating trichilemmal tumor (MPTT) was made.

Malignant proliferating trichilemmal tumor is a rare adnexal tumor that develops from the outer root sheath of the hair follicle. It often arises due to malignant transformation of pre-existing trichilemmal cysts, but some cases occur de novo.1 Malignant transformation is thought to start from a trichilemmal cyst in an adenomatous histologic stage, progressing to a proliferating trichilemmal cyst (PTC) in an epitheliomatous phase, ultimately becoming carcinomatous with MPTT.2-4 This transformation has been categorized into 3 morphologic groups to predict tumor behavior, including benign PTCs (curable by excision), low-grade malignant PTCs (minor risk for local recurrence), and high-grade malignant PTCs (risk for regional spread and metastasis with cytologic atypical features and potential for aggressive growth).1
More commonly observed in women in the fourth to eighth decades of life, MPTT may manifest as a fast- growing, painless, solitary nodule or as a progressively enlarging nodule at the site of a previously stable, long-standing lesion. Malignant proliferating trichilemmal tumor manifests frequently on the scalp, face, or neck, but there are reports of MPTT manifesting on the trunk and even as multiple concurrent lesions.1-4 The variability in clinical presentation and the potential to be mistaken for benign conditions makes excisional biopsy essential for diagnosis of MPTT. Histopathology classically demonstrates trichilemmal keratinization, a high mitotic index, and cellular atypia with invasion into the dermis.4 Malignant transformation frequently follows a prior history of trauma to the area or local inflammation.
Given the locally aggressive nature of MPTT, our patient was referred to a Mohs micrographic surgeon. While both wide excision with tumor-free margins and Mohs micrographic surgery are accepted surgical procedures for MPTT, there is no consensus in the literature on a standard treatment recommendation. Following surgery, close monitoring is needed for potential recurrence and metastases intracranially to the dura and muscles,5 as well as to the lungs.6 Further imaging using computed tomography or positron emission tomography can be ordered to rule out metastatic disease.4
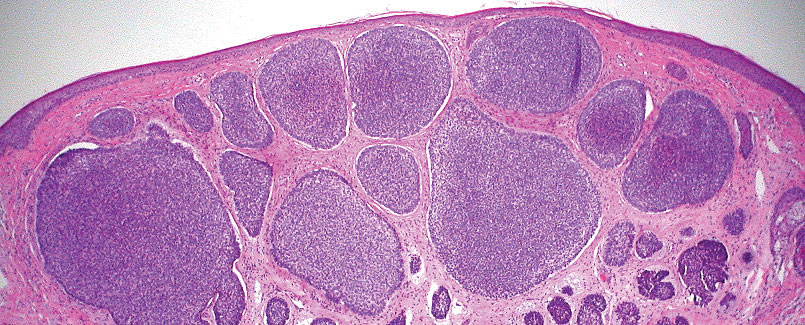
Pilomatrixomas are benign neoplasms that arise from hair matrix cells and have been associated with catenin beta-1 gene mutations, as well as genetic syndromes and trauma.7 Clinically, pilomatrixomas manifest as solitary, firm, painless, slow-growing nodules that commonly are found in the head and neck region. This tumor has a slight predominance in women and occurs frequently in adolescent years. The overlying skin may appear normal or show grey-bluish discoloration.8 Histopathology shows basaloid cells resembling primitive hair matrix cells with an abrupt transition to shadow cells composed of transformed keratinocytes without nuclei and calcification.7-8 This tumor can be differentiated by the presence of basaloid and shadow cells with calcification on histopathology, while MPTT will show atypical, mitotically active squamous cells with trichilemmal keratinization (Figure 2).

Proliferating trichilemmal cyst is a variant of trichilemmal cyst (TC) arising from the outer root sheath cells of the hair follicle. While TCs usually are slow growing and benign, the proliferating variant can be more aggressive with malignant potential. Patients often present with a solitary, well-circumscribed, rapidly growing nodule on the scalp. The lesion may be painful, and ulceration can occur, exposing the cystic contents. Histopathologically, PTCs resemble TCs with trichilemmal keratinization but also exhibit notable epithelial proliferation within the cystic space.9 While there can be considerable histopathologic overlap between PTC and MPTT—including extensive trichilemmal keratinization, variable atypia, and mitotic activity—PTC typically should not demonstrate invasion into the surrounding soft tissue or the degree of high-grade atypia, brisk mitoses, or necrosis seen in MPTT (eFigure 1).1 Immunohistochemistry may help distinguish PTC from MPTT and squamous cell carcinoma (SCC).10-11 The pattern of Ki-67 and p53 expression may be helpful with classification of PTC/MPTT into the 3 groups (benign, low-grade malignant, and high-grade malignant) proposed by Ye et al.1 Other investigators have suggested that Ki-67 expression may correlate potential for recurrence and clinical prognosis.12 Expression of CD34 (a marker that supports outer root sheath origin) might favor PTC/MPTT over SCC; however, cases of CD34- negative MPTT have been reported, particularly those with poorly differentiated histopathology.
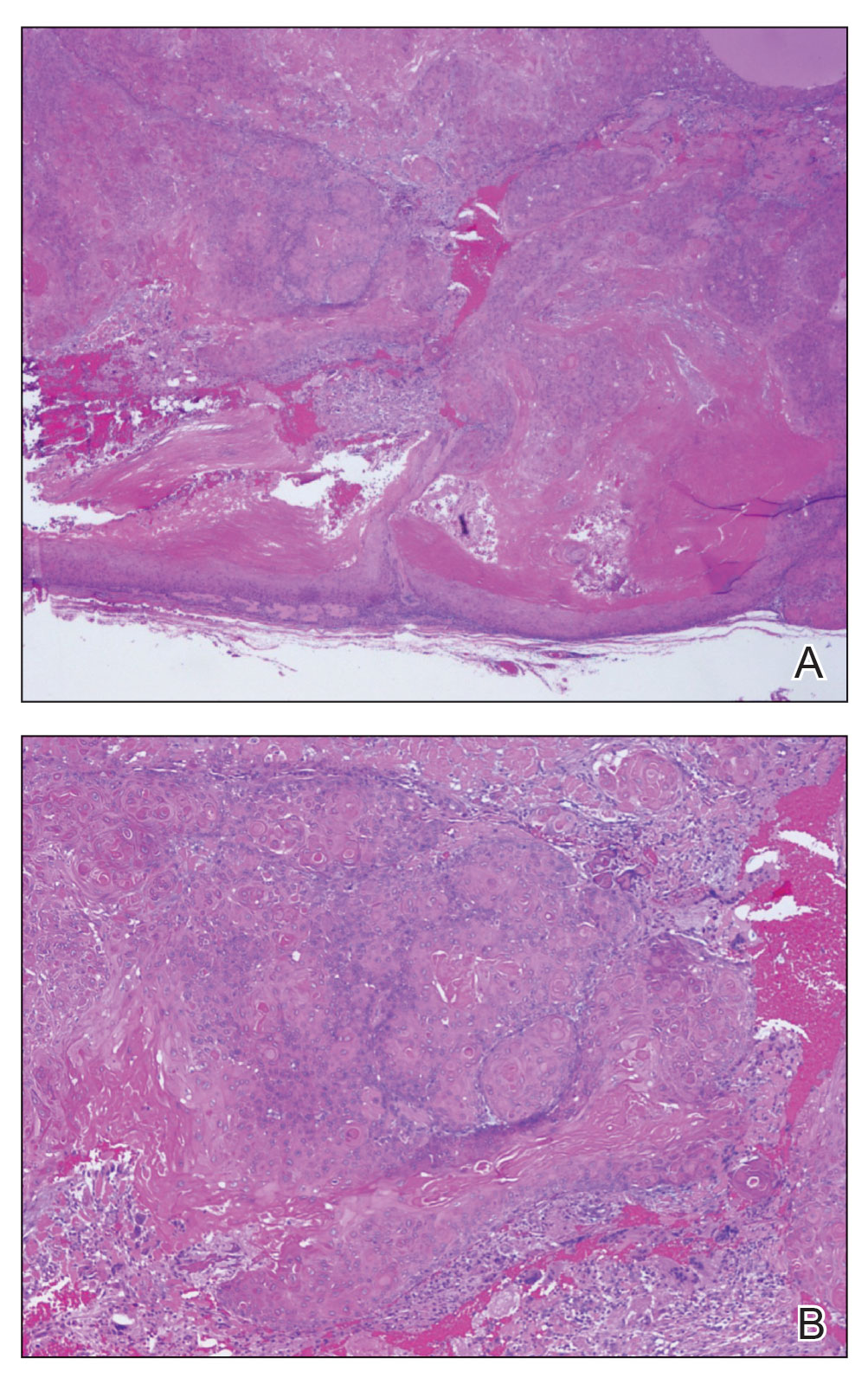
Squamous cell carcinoma with cystic features is a histologic variant of SCC characterized by cystlike spaces containing malignant squamous epithelial cells.13 Squamous cell carcinoma with cystic features can manifest as a firm nodule with ulceration similar to MPTT or PTC but also can mimic a benign cyst.14 The diagnosis of invasive SCC with cystic features typically is straightforward and characterized by cords and nests of atypical keratinocytes extending into the dermis with areas of cystic architecture (eFigure 2). While both SCC with cystic features and MPTT may show cystic histopathologic architecture, MPTT typically shows areas of PTC, whereas SCC with cystic features lacks such areas.
Verrucous cysts refer to infundibular cysts or less commonly pilar cysts or hybrid pilar-epidermoid cysts that exhibit superimposed human papillomavirus (HPV) cytopathic changes. Clinically, a verrucous cyst manifests as a single, asymptomatic, slow-growing, firm lesion most commonly manifesting on the face and back. Histopathologically, the cyst wall may show acanthosis, papillomatosis, hypergranulosis with coarse keratohyalin granules, and koilocytic changes (eFigure 3). These histopathologic features are believed to be induced by secondary HPV infection. While HPV-related change, characterized by koilocytic alteration, papillomatosis, and verruciform hyperplasia, more commonly affects epidermal cysts, occasionally trichilemmal (pilar) cysts are involved. In these cases, verrucous cysts should be distinguished from MPTT. Verrucous cysts may contain rare normal mitotic figures, but do not contain atypical mitosis, marked cellular pleomorphism, or an infiltrating pattern similar to MPTT.15
- Ye J, Nappi O, Swanson PE, et al. Proliferating pilar tumors: a clinicopathologic study of 76 cases with a proposal for definition of benign and malignant variants. Am J Clin Pathol. 2004;122:566-574. doi:10.1309/0XLEGFQ64XYJU4G6
- Saida T, Oohara K, Hori Y, et al. Development of a malignant proliferating trichilemmal cyst in a patient with multiple trichilemmal cysts. Dermatologica. 1983;166:203-208. doi:10.1159/000249868
- Rao S, Ramakrishnan R, Kamakshi D, et al. Malignant proliferating trichilemmal tumour presenting early in life: an uncommon feature. J Cutan Aesthet Surg. 2011;4:51-55. doi:10.4103/0974-2077.79196
- Kearns-Turcotte S, Thériault M, Blouin MM. Malignant proliferating trichilemmal tumors arising in patients with multiple trichilemmal cysts: a case series. JAAD Case Rep. 2022;22:42-46. doi:10.1016
- Karamese M, Akatekin A, Abaci M, et al. Unusual invasion of trichilemmal tumors: two case reports. Modern Plastic Surg. 2012; 2:54-57. doi:10.4236/MPS.2012.23014 /j.jdcr.2022.01.033
- Lobo L, Amonkar AD, Dontamsetty VV. Malignant proliferating trichilemmal tumour of the scalp with intra-cranial extension and lung metastasis-a case report. Indian J Surg. 2016;78:493-495. doi:10.1007/s12262-015-1427-0
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018;40:631-641. doi:10.1097/DAD.0000000000001118
- Sharma D, Agarwal S, Jain LS, et al. Pilomatrixoma masquerading as metastatic adenocarcinoma. A diagnostic pitfall on cytology. J Clin Diagn Res. 2014;8:FD13-FD14. doi:10.7860/JCDR/2014/9696.5064
- Valerio E, Parro FHS, Macedo MP, et al. Proliferating trichilemmal cyst with clinical, radiological, macroscopic, and microscopic orrelation. An Bras Dermatol. 2019;94:452-454. doi:10.1590 /abd1806-4841.20198199
- Joshi TP, Marchand S, Tschen J. Malignant proliferating trichilemmal tumor: a subtle presentation in an African American woman and review of immunohistochemical markers for this rare condition. Cureus. 2021;13:E17289. doi:10.7759/cureus.17289
- Gulati HK, Deshmukh SD, Anand M, et al. Low-grade malignant proliferating pilar tumor simulating a squamous-cell carcinoma in an elderly female: a case report and immunohistochemical study. Int J Trichology. 2011;3:98-101. doi:10.4103/0974-7753.90818
- Rangel-Gamboa L, Reyes-Castro M, Dominguez-Cherit J, et al. Proliferating trichilemmal cyst: the value of ki67 immunostaining. Int J Trichology. 2013;5:115-117. doi:10.4103/0974-7753.125599
- Asad U, Alkul S, Shimizu I, et al. Squamous cell carcinoma with unusual benign-appearing cystic features on histology. Cureus. 2023;15:E33610. doi:10.7759/cureus.33610
- Alkul S, Nguyen CN, Ramani NS, et al. Squamous cell carcinoma arising in an epidermal inclusion cyst. Baylor Univ Med Cent Proc. 2022;35:688-690. doi:10.1080/08998280.2022.207760
- Nanes BA, Laknezhad S, Chamseddin B, et al. Verrucous pilar cysts infected with beta human papillomavirus. J Cutan Pathol. 2020;47:381-386. doi:10.1111/cup.13599
THE DIAGNOSIS: Malignant Proliferating Trichilemmal Tumor
Biopsy revealed a squamous epithelium with cystic changes, trichilemmal differentiation, squamous eddy formation, keratinocyte atypia, focal necrotic changes, and a focus of atypical keratinocytes invading the dermis (Figure 1). Based on these findings, a diagnosis of malignant proliferating trichilemmal tumor (MPTT) was made.

Malignant proliferating trichilemmal tumor is a rare adnexal tumor that develops from the outer root sheath of the hair follicle. It often arises due to malignant transformation of pre-existing trichilemmal cysts, but some cases occur de novo.1 Malignant transformation is thought to start from a trichilemmal cyst in an adenomatous histologic stage, progressing to a proliferating trichilemmal cyst (PTC) in an epitheliomatous phase, ultimately becoming carcinomatous with MPTT.2-4 This transformation has been categorized into 3 morphologic groups to predict tumor behavior, including benign PTCs (curable by excision), low-grade malignant PTCs (minor risk for local recurrence), and high-grade malignant PTCs (risk for regional spread and metastasis with cytologic atypical features and potential for aggressive growth).1
More commonly observed in women in the fourth to eighth decades of life, MPTT may manifest as a fast- growing, painless, solitary nodule or as a progressively enlarging nodule at the site of a previously stable, long-standing lesion. Malignant proliferating trichilemmal tumor manifests frequently on the scalp, face, or neck, but there are reports of MPTT manifesting on the trunk and even as multiple concurrent lesions.1-4 The variability in clinical presentation and the potential to be mistaken for benign conditions makes excisional biopsy essential for diagnosis of MPTT. Histopathology classically demonstrates trichilemmal keratinization, a high mitotic index, and cellular atypia with invasion into the dermis.4 Malignant transformation frequently follows a prior history of trauma to the area or local inflammation.
Given the locally aggressive nature of MPTT, our patient was referred to a Mohs micrographic surgeon. While both wide excision with tumor-free margins and Mohs micrographic surgery are accepted surgical procedures for MPTT, there is no consensus in the literature on a standard treatment recommendation. Following surgery, close monitoring is needed for potential recurrence and metastases intracranially to the dura and muscles,5 as well as to the lungs.6 Further imaging using computed tomography or positron emission tomography can be ordered to rule out metastatic disease.4
Pilomatrixomas are benign neoplasms that arise from hair matrix cells and have been associated with catenin beta-1 gene mutations, as well as genetic syndromes and trauma.7 Clinically, pilomatrixomas manifest as solitary, firm, painless, slow-growing nodules that commonly are found in the head and neck region. This tumor has a slight predominance in women and occurs frequently in adolescent years. The overlying skin may appear normal or show grey-bluish discoloration.8 Histopathology shows basaloid cells resembling primitive hair matrix cells with an abrupt transition to shadow cells composed of transformed keratinocytes without nuclei and calcification.7-8 This tumor can be differentiated by the presence of basaloid and shadow cells with calcification on histopathology, while MPTT will show atypical, mitotically active squamous cells with trichilemmal keratinization (Figure 2).

Proliferating trichilemmal cyst is a variant of trichilemmal cyst (TC) arising from the outer root sheath cells of the hair follicle. While TCs usually are slow growing and benign, the proliferating variant can be more aggressive with malignant potential. Patients often present with a solitary, well-circumscribed, rapidly growing nodule on the scalp. The lesion may be painful, and ulceration can occur, exposing the cystic contents. Histopathologically, PTCs resemble TCs with trichilemmal keratinization but also exhibit notable epithelial proliferation within the cystic space.9 While there can be considerable histopathologic overlap between PTC and MPTT—including extensive trichilemmal keratinization, variable atypia, and mitotic activity—PTC typically should not demonstrate invasion into the surrounding soft tissue or the degree of high-grade atypia, brisk mitoses, or necrosis seen in MPTT (eFigure 1).1 Immunohistochemistry may help distinguish PTC from MPTT and squamous cell carcinoma (SCC).10-11 The pattern of Ki-67 and p53 expression may be helpful with classification of PTC/MPTT into the 3 groups (benign, low-grade malignant, and high-grade malignant) proposed by Ye et al.1 Other investigators have suggested that Ki-67 expression may correlate potential for recurrence and clinical prognosis.12 Expression of CD34 (a marker that supports outer root sheath origin) might favor PTC/MPTT over SCC; however, cases of CD34- negative MPTT have been reported, particularly those with poorly differentiated histopathology.

Squamous cell carcinoma with cystic features is a histologic variant of SCC characterized by cystlike spaces containing malignant squamous epithelial cells.13 Squamous cell carcinoma with cystic features can manifest as a firm nodule with ulceration similar to MPTT or PTC but also can mimic a benign cyst.14 The diagnosis of invasive SCC with cystic features typically is straightforward and characterized by cords and nests of atypical keratinocytes extending into the dermis with areas of cystic architecture (eFigure 2). While both SCC with cystic features and MPTT may show cystic histopathologic architecture, MPTT typically shows areas of PTC, whereas SCC with cystic features lacks such areas.

Verrucous cysts refer to infundibular cysts or less commonly pilar cysts or hybrid pilar-epidermoid cysts that exhibit superimposed human papillomavirus (HPV) cytopathic changes. Clinically, a verrucous cyst manifests as a single, asymptomatic, slow-growing, firm lesion most commonly manifesting on the face and back. Histopathologically, the cyst wall may show acanthosis, papillomatosis, hypergranulosis with coarse keratohyalin granules, and koilocytic changes (eFigure 3). These histopathologic features are believed to be induced by secondary HPV infection. While HPV-related change, characterized by koilocytic alteration, papillomatosis, and verruciform hyperplasia, more commonly affects epidermal cysts, occasionally trichilemmal (pilar) cysts are involved. In these cases, verrucous cysts should be distinguished from MPTT. Verrucous cysts may contain rare normal mitotic figures, but do not contain atypical mitosis, marked cellular pleomorphism, or an infiltrating pattern similar to MPTT.15

THE DIAGNOSIS: Malignant Proliferating Trichilemmal Tumor
Biopsy revealed a squamous epithelium with cystic changes, trichilemmal differentiation, squamous eddy formation, keratinocyte atypia, focal necrotic changes, and a focus of atypical keratinocytes invading the dermis (Figure 1). Based on these findings, a diagnosis of malignant proliferating trichilemmal tumor (MPTT) was made.

Malignant proliferating trichilemmal tumor is a rare adnexal tumor that develops from the outer root sheath of the hair follicle. It often arises due to malignant transformation of pre-existing trichilemmal cysts, but some cases occur de novo.1 Malignant transformation is thought to start from a trichilemmal cyst in an adenomatous histologic stage, progressing to a proliferating trichilemmal cyst (PTC) in an epitheliomatous phase, ultimately becoming carcinomatous with MPTT.2-4 This transformation has been categorized into 3 morphologic groups to predict tumor behavior, including benign PTCs (curable by excision), low-grade malignant PTCs (minor risk for local recurrence), and high-grade malignant PTCs (risk for regional spread and metastasis with cytologic atypical features and potential for aggressive growth).1
More commonly observed in women in the fourth to eighth decades of life, MPTT may manifest as a fast- growing, painless, solitary nodule or as a progressively enlarging nodule at the site of a previously stable, long-standing lesion. Malignant proliferating trichilemmal tumor manifests frequently on the scalp, face, or neck, but there are reports of MPTT manifesting on the trunk and even as multiple concurrent lesions.1-4 The variability in clinical presentation and the potential to be mistaken for benign conditions makes excisional biopsy essential for diagnosis of MPTT. Histopathology classically demonstrates trichilemmal keratinization, a high mitotic index, and cellular atypia with invasion into the dermis.4 Malignant transformation frequently follows a prior history of trauma to the area or local inflammation.
Given the locally aggressive nature of MPTT, our patient was referred to a Mohs micrographic surgeon. While both wide excision with tumor-free margins and Mohs micrographic surgery are accepted surgical procedures for MPTT, there is no consensus in the literature on a standard treatment recommendation. Following surgery, close monitoring is needed for potential recurrence and metastases intracranially to the dura and muscles,5 as well as to the lungs.6 Further imaging using computed tomography or positron emission tomography can be ordered to rule out metastatic disease.4
Pilomatrixomas are benign neoplasms that arise from hair matrix cells and have been associated with catenin beta-1 gene mutations, as well as genetic syndromes and trauma.7 Clinically, pilomatrixomas manifest as solitary, firm, painless, slow-growing nodules that commonly are found in the head and neck region. This tumor has a slight predominance in women and occurs frequently in adolescent years. The overlying skin may appear normal or show grey-bluish discoloration.8 Histopathology shows basaloid cells resembling primitive hair matrix cells with an abrupt transition to shadow cells composed of transformed keratinocytes without nuclei and calcification.7-8 This tumor can be differentiated by the presence of basaloid and shadow cells with calcification on histopathology, while MPTT will show atypical, mitotically active squamous cells with trichilemmal keratinization (Figure 2).

Proliferating trichilemmal cyst is a variant of trichilemmal cyst (TC) arising from the outer root sheath cells of the hair follicle. While TCs usually are slow growing and benign, the proliferating variant can be more aggressive with malignant potential. Patients often present with a solitary, well-circumscribed, rapidly growing nodule on the scalp. The lesion may be painful, and ulceration can occur, exposing the cystic contents. Histopathologically, PTCs resemble TCs with trichilemmal keratinization but also exhibit notable epithelial proliferation within the cystic space.9 While there can be considerable histopathologic overlap between PTC and MPTT—including extensive trichilemmal keratinization, variable atypia, and mitotic activity—PTC typically should not demonstrate invasion into the surrounding soft tissue or the degree of high-grade atypia, brisk mitoses, or necrosis seen in MPTT (eFigure 1).1 Immunohistochemistry may help distinguish PTC from MPTT and squamous cell carcinoma (SCC).10-11 The pattern of Ki-67 and p53 expression may be helpful with classification of PTC/MPTT into the 3 groups (benign, low-grade malignant, and high-grade malignant) proposed by Ye et al.1 Other investigators have suggested that Ki-67 expression may correlate potential for recurrence and clinical prognosis.12 Expression of CD34 (a marker that supports outer root sheath origin) might favor PTC/MPTT over SCC; however, cases of CD34- negative MPTT have been reported, particularly those with poorly differentiated histopathology.

Squamous cell carcinoma with cystic features is a histologic variant of SCC characterized by cystlike spaces containing malignant squamous epithelial cells.13 Squamous cell carcinoma with cystic features can manifest as a firm nodule with ulceration similar to MPTT or PTC but also can mimic a benign cyst.14 The diagnosis of invasive SCC with cystic features typically is straightforward and characterized by cords and nests of atypical keratinocytes extending into the dermis with areas of cystic architecture (eFigure 2). While both SCC with cystic features and MPTT may show cystic histopathologic architecture, MPTT typically shows areas of PTC, whereas SCC with cystic features lacks such areas.

Verrucous cysts refer to infundibular cysts or less commonly pilar cysts or hybrid pilar-epidermoid cysts that exhibit superimposed human papillomavirus (HPV) cytopathic changes. Clinically, a verrucous cyst manifests as a single, asymptomatic, slow-growing, firm lesion most commonly manifesting on the face and back. Histopathologically, the cyst wall may show acanthosis, papillomatosis, hypergranulosis with coarse keratohyalin granules, and koilocytic changes (eFigure 3). These histopathologic features are believed to be induced by secondary HPV infection. While HPV-related change, characterized by koilocytic alteration, papillomatosis, and verruciform hyperplasia, more commonly affects epidermal cysts, occasionally trichilemmal (pilar) cysts are involved. In these cases, verrucous cysts should be distinguished from MPTT. Verrucous cysts may contain rare normal mitotic figures, but do not contain atypical mitosis, marked cellular pleomorphism, or an infiltrating pattern similar to MPTT.15

- Ye J, Nappi O, Swanson PE, et al. Proliferating pilar tumors: a clinicopathologic study of 76 cases with a proposal for definition of benign and malignant variants. Am J Clin Pathol. 2004;122:566-574. doi:10.1309/0XLEGFQ64XYJU4G6
- Saida T, Oohara K, Hori Y, et al. Development of a malignant proliferating trichilemmal cyst in a patient with multiple trichilemmal cysts. Dermatologica. 1983;166:203-208. doi:10.1159/000249868
- Rao S, Ramakrishnan R, Kamakshi D, et al. Malignant proliferating trichilemmal tumour presenting early in life: an uncommon feature. J Cutan Aesthet Surg. 2011;4:51-55. doi:10.4103/0974-2077.79196
- Kearns-Turcotte S, Thériault M, Blouin MM. Malignant proliferating trichilemmal tumors arising in patients with multiple trichilemmal cysts: a case series. JAAD Case Rep. 2022;22:42-46. doi:10.1016
- Karamese M, Akatekin A, Abaci M, et al. Unusual invasion of trichilemmal tumors: two case reports. Modern Plastic Surg. 2012; 2:54-57. doi:10.4236/MPS.2012.23014 /j.jdcr.2022.01.033
- Lobo L, Amonkar AD, Dontamsetty VV. Malignant proliferating trichilemmal tumour of the scalp with intra-cranial extension and lung metastasis-a case report. Indian J Surg. 2016;78:493-495. doi:10.1007/s12262-015-1427-0
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018;40:631-641. doi:10.1097/DAD.0000000000001118
- Sharma D, Agarwal S, Jain LS, et al. Pilomatrixoma masquerading as metastatic adenocarcinoma. A diagnostic pitfall on cytology. J Clin Diagn Res. 2014;8:FD13-FD14. doi:10.7860/JCDR/2014/9696.5064
- Valerio E, Parro FHS, Macedo MP, et al. Proliferating trichilemmal cyst with clinical, radiological, macroscopic, and microscopic orrelation. An Bras Dermatol. 2019;94:452-454. doi:10.1590 /abd1806-4841.20198199
- Joshi TP, Marchand S, Tschen J. Malignant proliferating trichilemmal tumor: a subtle presentation in an African American woman and review of immunohistochemical markers for this rare condition. Cureus. 2021;13:E17289. doi:10.7759/cureus.17289
- Gulati HK, Deshmukh SD, Anand M, et al. Low-grade malignant proliferating pilar tumor simulating a squamous-cell carcinoma in an elderly female: a case report and immunohistochemical study. Int J Trichology. 2011;3:98-101. doi:10.4103/0974-7753.90818
- Rangel-Gamboa L, Reyes-Castro M, Dominguez-Cherit J, et al. Proliferating trichilemmal cyst: the value of ki67 immunostaining. Int J Trichology. 2013;5:115-117. doi:10.4103/0974-7753.125599
- Asad U, Alkul S, Shimizu I, et al. Squamous cell carcinoma with unusual benign-appearing cystic features on histology. Cureus. 2023;15:E33610. doi:10.7759/cureus.33610
- Alkul S, Nguyen CN, Ramani NS, et al. Squamous cell carcinoma arising in an epidermal inclusion cyst. Baylor Univ Med Cent Proc. 2022;35:688-690. doi:10.1080/08998280.2022.207760
- Nanes BA, Laknezhad S, Chamseddin B, et al. Verrucous pilar cysts infected with beta human papillomavirus. J Cutan Pathol. 2020;47:381-386. doi:10.1111/cup.13599
- Ye J, Nappi O, Swanson PE, et al. Proliferating pilar tumors: a clinicopathologic study of 76 cases with a proposal for definition of benign and malignant variants. Am J Clin Pathol. 2004;122:566-574. doi:10.1309/0XLEGFQ64XYJU4G6
- Saida T, Oohara K, Hori Y, et al. Development of a malignant proliferating trichilemmal cyst in a patient with multiple trichilemmal cysts. Dermatologica. 1983;166:203-208. doi:10.1159/000249868
- Rao S, Ramakrishnan R, Kamakshi D, et al. Malignant proliferating trichilemmal tumour presenting early in life: an uncommon feature. J Cutan Aesthet Surg. 2011;4:51-55. doi:10.4103/0974-2077.79196
- Kearns-Turcotte S, Thériault M, Blouin MM. Malignant proliferating trichilemmal tumors arising in patients with multiple trichilemmal cysts: a case series. JAAD Case Rep. 2022;22:42-46. doi:10.1016
- Karamese M, Akatekin A, Abaci M, et al. Unusual invasion of trichilemmal tumors: two case reports. Modern Plastic Surg. 2012; 2:54-57. doi:10.4236/MPS.2012.23014 /j.jdcr.2022.01.033
- Lobo L, Amonkar AD, Dontamsetty VV. Malignant proliferating trichilemmal tumour of the scalp with intra-cranial extension and lung metastasis-a case report. Indian J Surg. 2016;78:493-495. doi:10.1007/s12262-015-1427-0
- Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018;40:631-641. doi:10.1097/DAD.0000000000001118
- Sharma D, Agarwal S, Jain LS, et al. Pilomatrixoma masquerading as metastatic adenocarcinoma. A diagnostic pitfall on cytology. J Clin Diagn Res. 2014;8:FD13-FD14. doi:10.7860/JCDR/2014/9696.5064
- Valerio E, Parro FHS, Macedo MP, et al. Proliferating trichilemmal cyst with clinical, radiological, macroscopic, and microscopic orrelation. An Bras Dermatol. 2019;94:452-454. doi:10.1590 /abd1806-4841.20198199
- Joshi TP, Marchand S, Tschen J. Malignant proliferating trichilemmal tumor: a subtle presentation in an African American woman and review of immunohistochemical markers for this rare condition. Cureus. 2021;13:E17289. doi:10.7759/cureus.17289
- Gulati HK, Deshmukh SD, Anand M, et al. Low-grade malignant proliferating pilar tumor simulating a squamous-cell carcinoma in an elderly female: a case report and immunohistochemical study. Int J Trichology. 2011;3:98-101. doi:10.4103/0974-7753.90818
- Rangel-Gamboa L, Reyes-Castro M, Dominguez-Cherit J, et al. Proliferating trichilemmal cyst: the value of ki67 immunostaining. Int J Trichology. 2013;5:115-117. doi:10.4103/0974-7753.125599
- Asad U, Alkul S, Shimizu I, et al. Squamous cell carcinoma with unusual benign-appearing cystic features on histology. Cureus. 2023;15:E33610. doi:10.7759/cureus.33610
- Alkul S, Nguyen CN, Ramani NS, et al. Squamous cell carcinoma arising in an epidermal inclusion cyst. Baylor Univ Med Cent Proc. 2022;35:688-690. doi:10.1080/08998280.2022.207760
- Nanes BA, Laknezhad S, Chamseddin B, et al. Verrucous pilar cysts infected with beta human papillomavirus. J Cutan Pathol. 2020;47:381-386. doi:10.1111/cup.13599
Growing Nodule on the Parietal Scalp
Growing Nodule on the Parietal Scalp
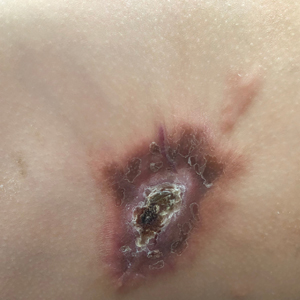
A 38-year-old woman with no notable medical history presented to the dermatology department with a firm enlarging nodule on the scalp of many years’ duration. The patient noted there was no drainage or bleeding. Physical examination revealed a mobile, 2.5-cm, subcutaneous nodule on the right parietal medial scalp. An excisional biopsy was performed.
Smooth Symmetric Plaques on the Face, Trunk, and Extremities
Smooth Symmetric Plaques on the Face, Trunk, and Extremities
THE DIAGNOSIS: Lepromatous Leprosy
Histopathology showed collections of epithelioid to sarcoidal granulomas throughout the dermis and clustered around nerve bundles with a grenz zone at the dermoepidermal junction. Fite stain was positive for acid-fast bacteria, which were confirmed to be Mycobacterium leprae by by the National Hansen’s Disease program. Based on these findings, a diagnosis of lepromatous leprosy (LL) was made. The patient was treated by the infectious disease department with multidrug therapy that included monthly rifampin, moxifloxacin, and minocycline; weekly methotrexate with daily folic acid; and an extended prednisone taper with prophylactic cholecalciferol.
Lepromatous leprosy is characterized by high antibody titers to the acid-fast, gram-positive bacillus Mycobacterium leprae as well as a high bacillary load.1 Patients typically present with muscle weakness, anesthetic skin patches, and claw hands. Patients also may present with foot drop, ulcerations of the hands and feet, autonomic dysfunction with anhidrosis or impaired sweating, and localized alopecia.2 Over months to years, LL may progress to extensive sensory loss and indurated lesions that infiltrate the skin and cause thickening, especially on the face (known as leonine facies). Furthermore, LL is characterized by extensive bilaterally symmetric cutaneous lesions with poorly defined borders and raised indurated centers.3
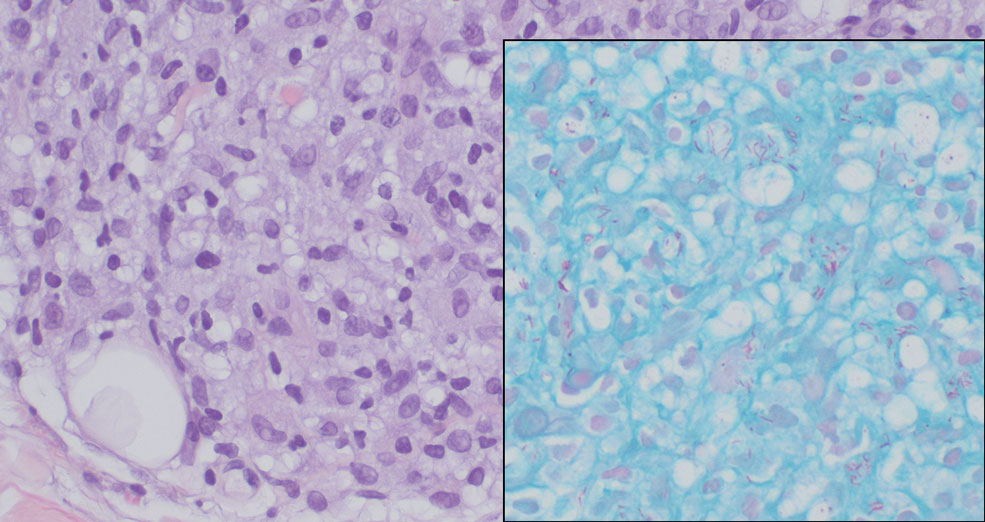
Lepromatous leprosy transmission is not fully understood but is thought to occur via airborne droplets from coughing/sneezing and nasal secretions.2 Histopathology generally shows a dense and diffuse granulomatous infiltrate that involves the dermis but is separated from the epidermis by a zone of collagen (grenz zone).3 Histology is characterized by the presence of lymphocytes and numerous foamy macrophages (lepra or Virchow cells) containing M leprae organisms. In persistent lesions, the high density of uncleared bacilli forms spherical cytoplasmic clumps known as globi within enlarged foamy histiocytes (Figure 1).4 The macrophages form granulomatous lesions in the skin and around nerve bundles, resulting in tissue damage and decreased sensation. The current standard of care for LL is a multidrug combination of dapsone, rifampin, and clofazimine. Early diagnosis and complete treatment of LL is crucial, as this approach typically leads to complete cure of the disease.
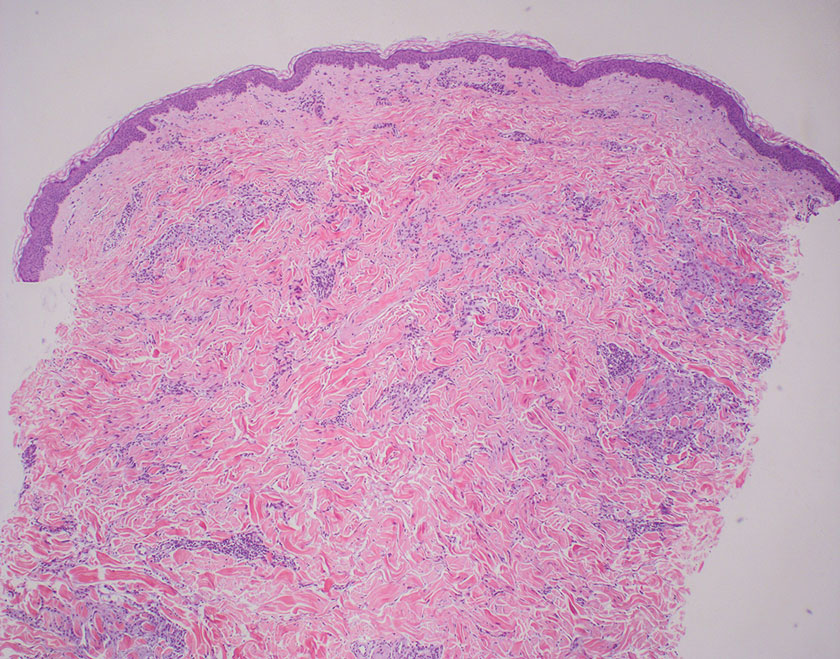
The differential diagnosis for LL includes granuloma annulare (GA), mycosis fungoides (MF), sarcoidosis, and subacute cutaneous lupus erythematosus (SCLE). Granuloma annulare is a noninfectious inflammatory granulomatous skin disease that manifests in a localized, generalized, or subcutaneous pattern. Localized GA is the most common form and manifests as self-resolving, flesh-colored or erythematous papules or plaques limited to the extremities.5,6 Generalized GA is defined by more than 10 widespread annular plaques involving the trunk and extremities and can persist for decades.6 This form can be associated with hyperlipidemia, diabetes, autoimmune disease and immunodeficiency (eg, HIV), and rarely with lymphoma or solid tumors. On histology, GA shows necrobiosis surrounded by palisading histiocytes and mucin (palisading GA) or patchy interstitial histiocytes and lymphocytes (interstitial GA)(Figure 2).6 This palisading pattern differs from the histiocytes in LL, which contain numerous acid-fast bacilli and bacterial clumps. Topical and intralesional corticosteroids are first-line therapies for GA.
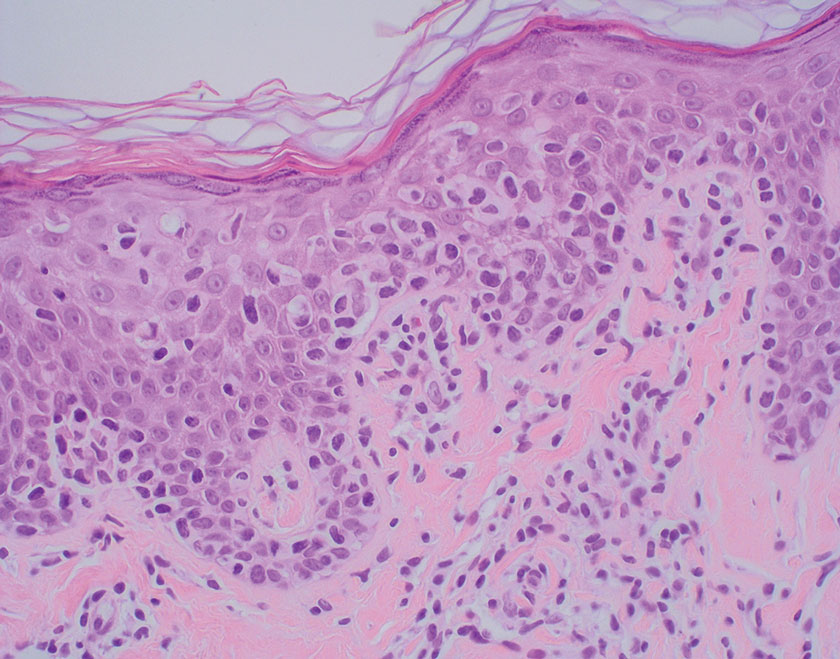
Mycosis fungoides is a cutaneous T-cell lymphoma characterized by proliferation of CD4+ T cells.7 In the early stages of MF, patients may present with multiple erythematous and scaly patches, plaques, or nodules that most commonly develop on unexposed areas of the skin, but specific variants frequently may cause lesions on the face or scalp.8 Tumors may be solitary, localized, or generalized and may be observed alongside patches and plaques or in the absence of cutaneous lesions.7 The pathologic features of MF include fibrosis of the papillary dermis, individual haloed atypical lymphocytes in the epidermis, and atypical lymphoid cells with cerebriform nuclei (Figure 3).9 Granulomatous MF is characterized by diffuse nodular and perivascular infiltrates of histiocytes with small lymphocytes without atypia, eosinophils, and plasma cells. Small lymphocytes with cerebriform nuclei and larger lymphocytes with hyperconvoluted nuclei also may be seen, in addition to multinucleated histiocytic giant cells. Although MF commonly manifests with epidermotropism, it typically is absent in granulomatous MF (GMF).10 Granulomatous MF may manifest similarly to LL. Noduloulcerative lesions and infiltration of atypical lymphocytes into the epidermis (epidermotropism) are much more common in GMF than in LL; however, although ulcerative nodules are not a common feature in patients with leprosy (except during reactional states [ie, Lucio phenomenon]) or secondary to neuropathies, they also can occur in LL.11 In GMF, the infiltrate does not follow a specific pattern, whereas LL infiltrates tend to follow a nerve distribution. Treatment for MF is determined by disease severity.12 First-line therapy includes local corticosteroids and phototherapy with UVB irradiation.
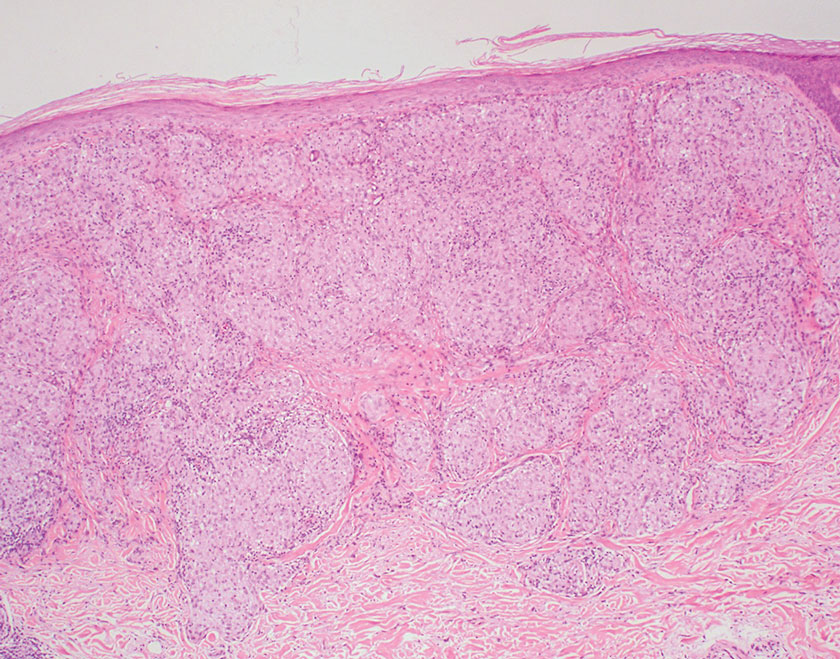
Sarcoidosis is a multisystem disease that demonstrates nonspecific clinical manifestations affecting the lungs, eyes, liver, and skin.13 Environmental exposures to silica and inorganic matter have been linked to an increased risk for sarcoidosis, with patients presenting with fatigue, fever, and arthralgia.13 Skin manifestations include subcutaneous nodules, polymorphous plaques, and erythema nodosum—nodosum—the most common cutaneous presentation of sarcoidosis. Erythema nodosum manifests as symmetrically distributed, nonulcerative, painful red nodules on the skin, especially the lower legs. The histopathology of sarcoidosis shows noncaseating granulomas with activated T-lymphocytes, epithelioid cells, and multinucleated giant cells (Figure 4). Although granulomas occur in both LL and sarcoidosis, those in sarcoidosis typically consist of epithelioid cells surrounded by a rim of lymphocytes, whereas LL granulomas contain foamy histiocytes and multinucleated giant cells. Treatment of sarcoidosis depends on disease progression and generally involves oral corticosteroids, followed by corticosteroid-sparing regimens.
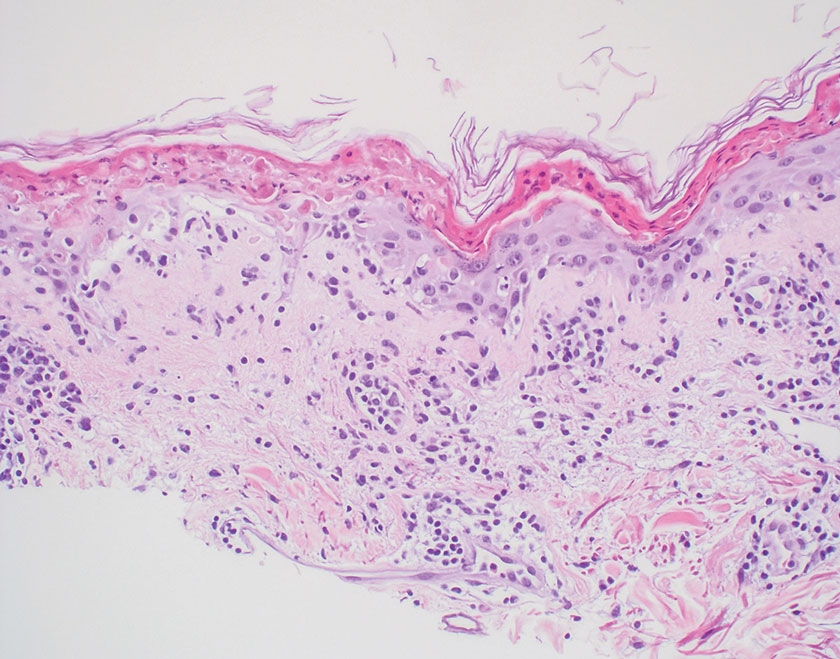
Subacute cutaneous lupus erythematosus is a chronic autoimmune disease that predominantly affects younger women. Common findings in SCLE include red scaly plaques and ring-shaped lesions on sun-exposed areas of the skin.14 Subacute cutaneous lupus erythematosus primarily is characterized by a photosensitive rash, often with arthralgia, myalgia, and/or oral ulcers; less commonly, a small percentage of patients can experience central nervous system involvement, vasculitis, or nephritis. The histologic findings of SCLE include hydropic degeneration of the basal cell layer and periadnexal infiltrates (Figure 5). The incidence of SCLE often is associated with anti-Ro (SSA) and anti-La (SSB) antibodies.15 Treatment of SCLE focuses on managing skin symptoms with corticosteroids, antimalarials, and sun protection.
- Bobosha K, Wilson L, van Meijgaarden KE, et al. T-cell regulation in lepromatous leprosy. PLoS Negl Trop Dis. 2014;8:E2773. doi:10.1371 /journal.pntd.0002773
- Fischer M. Leprosy–an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827. doi:10.1111/ddg.13301
- Jolly M, Pickard SA, Mikolaitis RA, et al. Lupus QoL-US benchmarks for US patients with systemic lupus erythematosus. J Rheumatol. 2010;37:1828-1833. doi:10.3899/jrheum.091443
- Chan MMF, Smoller BR. Overview of the histopathology and other laboratory investigations in leprosy. Curr Trop Med Rep. 2016;3:131-137. doi:10.1007/s40475-016-0086-y
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016; 75:457-465. doi:10.1016/j.jaad.2015.03.054
- Lukács J, Schliemann S, Elsner P. Treatment of generalized granuloma annulare–a systematic review. J Eur Acad Dermatol Venereol. 2015;29:1467-1480. doi:10.1111/jdv.12976
- Zinzani PL, Ferreri AJM, Cerroni L. Mycosis fungoides. Crit Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Ahn CS, ALSayyah A, Sangüeza OP. Mycosis fungoides: an updated review of clinicopathologic variants. Am J Dermatopathol. 2014;36:933- 951. doi:10.1097/DAD.0000000000000207
- Gutte R, Kharkar V, Mahajan S, et al. Granulomatous mycosis fungoides with hypohidrosis mimicking lepromatous leprosy. Indian J Dermatol Venereol Leprol. 2010;76:686. doi:10.4103/0378-6323.72470
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the cutaneous lymphoma histopathology task force group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001 /archdermatol.2008.46
- Miyashiro D, Cardona C, Valente N, et al. Ulcers in leprosy patients, an unrecognized clinical manifestation: a report of 8 cases. BMC Infect Dis. 2019;19:1013. doi:10.1186/s12879-019-4639-2
- Cerroni L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37:2-10. doi:10.12788/j.sder.2018.002
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390 /jcm9041081
- Zÿ ychowska M, Reich A. Dermoscopic features of acute, subacute, chronic and intermittent subtypes of cutaneous lupus erythematosus in Caucasians. J Clin Med. 2022;11:4088. doi:10.3390/jcm11144088
- Lazar AL. Subacute cutaneous lupus erythematosus: a facultative paraneoplastic dermatosis. Clin Dermatol. 2022;40:728-742. doi:10.1016 /j.clindermatol.2022.07.007
THE DIAGNOSIS: Lepromatous Leprosy
Histopathology showed collections of epithelioid to sarcoidal granulomas throughout the dermis and clustered around nerve bundles with a grenz zone at the dermoepidermal junction. Fite stain was positive for acid-fast bacteria, which were confirmed to be Mycobacterium leprae by by the National Hansen’s Disease program. Based on these findings, a diagnosis of lepromatous leprosy (LL) was made. The patient was treated by the infectious disease department with multidrug therapy that included monthly rifampin, moxifloxacin, and minocycline; weekly methotrexate with daily folic acid; and an extended prednisone taper with prophylactic cholecalciferol.
Lepromatous leprosy is characterized by high antibody titers to the acid-fast, gram-positive bacillus Mycobacterium leprae as well as a high bacillary load.1 Patients typically present with muscle weakness, anesthetic skin patches, and claw hands. Patients also may present with foot drop, ulcerations of the hands and feet, autonomic dysfunction with anhidrosis or impaired sweating, and localized alopecia.2 Over months to years, LL may progress to extensive sensory loss and indurated lesions that infiltrate the skin and cause thickening, especially on the face (known as leonine facies). Furthermore, LL is characterized by extensive bilaterally symmetric cutaneous lesions with poorly defined borders and raised indurated centers.3
Lepromatous leprosy transmission is not fully understood but is thought to occur via airborne droplets from coughing/sneezing and nasal secretions.2 Histopathology generally shows a dense and diffuse granulomatous infiltrate that involves the dermis but is separated from the epidermis by a zone of collagen (grenz zone).3 Histology is characterized by the presence of lymphocytes and numerous foamy macrophages (lepra or Virchow cells) containing M leprae organisms. In persistent lesions, the high density of uncleared bacilli forms spherical cytoplasmic clumps known as globi within enlarged foamy histiocytes (Figure 1).4 The macrophages form granulomatous lesions in the skin and around nerve bundles, resulting in tissue damage and decreased sensation. The current standard of care for LL is a multidrug combination of dapsone, rifampin, and clofazimine. Early diagnosis and complete treatment of LL is crucial, as this approach typically leads to complete cure of the disease.

The differential diagnosis for LL includes granuloma annulare (GA), mycosis fungoides (MF), sarcoidosis, and subacute cutaneous lupus erythematosus (SCLE). Granuloma annulare is a noninfectious inflammatory granulomatous skin disease that manifests in a localized, generalized, or subcutaneous pattern. Localized GA is the most common form and manifests as self-resolving, flesh-colored or erythematous papules or plaques limited to the extremities.5,6 Generalized GA is defined by more than 10 widespread annular plaques involving the trunk and extremities and can persist for decades.6 This form can be associated with hyperlipidemia, diabetes, autoimmune disease and immunodeficiency (eg, HIV), and rarely with lymphoma or solid tumors. On histology, GA shows necrobiosis surrounded by palisading histiocytes and mucin (palisading GA) or patchy interstitial histiocytes and lymphocytes (interstitial GA)(Figure 2).6 This palisading pattern differs from the histiocytes in LL, which contain numerous acid-fast bacilli and bacterial clumps. Topical and intralesional corticosteroids are first-line therapies for GA.

Mycosis fungoides is a cutaneous T-cell lymphoma characterized by proliferation of CD4+ T cells.7 In the early stages of MF, patients may present with multiple erythematous and scaly patches, plaques, or nodules that most commonly develop on unexposed areas of the skin, but specific variants frequently may cause lesions on the face or scalp.8 Tumors may be solitary, localized, or generalized and may be observed alongside patches and plaques or in the absence of cutaneous lesions.7 The pathologic features of MF include fibrosis of the papillary dermis, individual haloed atypical lymphocytes in the epidermis, and atypical lymphoid cells with cerebriform nuclei (Figure 3).9 Granulomatous MF is characterized by diffuse nodular and perivascular infiltrates of histiocytes with small lymphocytes without atypia, eosinophils, and plasma cells. Small lymphocytes with cerebriform nuclei and larger lymphocytes with hyperconvoluted nuclei also may be seen, in addition to multinucleated histiocytic giant cells. Although MF commonly manifests with epidermotropism, it typically is absent in granulomatous MF (GMF).10 Granulomatous MF may manifest similarly to LL. Noduloulcerative lesions and infiltration of atypical lymphocytes into the epidermis (epidermotropism) are much more common in GMF than in LL; however, although ulcerative nodules are not a common feature in patients with leprosy (except during reactional states [ie, Lucio phenomenon]) or secondary to neuropathies, they also can occur in LL.11 In GMF, the infiltrate does not follow a specific pattern, whereas LL infiltrates tend to follow a nerve distribution. Treatment for MF is determined by disease severity.12 First-line therapy includes local corticosteroids and phototherapy with UVB irradiation.

Sarcoidosis is a multisystem disease that demonstrates nonspecific clinical manifestations affecting the lungs, eyes, liver, and skin.13 Environmental exposures to silica and inorganic matter have been linked to an increased risk for sarcoidosis, with patients presenting with fatigue, fever, and arthralgia.13 Skin manifestations include subcutaneous nodules, polymorphous plaques, and erythema nodosum—nodosum—the most common cutaneous presentation of sarcoidosis. Erythema nodosum manifests as symmetrically distributed, nonulcerative, painful red nodules on the skin, especially the lower legs. The histopathology of sarcoidosis shows noncaseating granulomas with activated T-lymphocytes, epithelioid cells, and multinucleated giant cells (Figure 4). Although granulomas occur in both LL and sarcoidosis, those in sarcoidosis typically consist of epithelioid cells surrounded by a rim of lymphocytes, whereas LL granulomas contain foamy histiocytes and multinucleated giant cells. Treatment of sarcoidosis depends on disease progression and generally involves oral corticosteroids, followed by corticosteroid-sparing regimens.

Subacute cutaneous lupus erythematosus is a chronic autoimmune disease that predominantly affects younger women. Common findings in SCLE include red scaly plaques and ring-shaped lesions on sun-exposed areas of the skin.14 Subacute cutaneous lupus erythematosus primarily is characterized by a photosensitive rash, often with arthralgia, myalgia, and/or oral ulcers; less commonly, a small percentage of patients can experience central nervous system involvement, vasculitis, or nephritis. The histologic findings of SCLE include hydropic degeneration of the basal cell layer and periadnexal infiltrates (Figure 5). The incidence of SCLE often is associated with anti-Ro (SSA) and anti-La (SSB) antibodies.15 Treatment of SCLE focuses on managing skin symptoms with corticosteroids, antimalarials, and sun protection.

THE DIAGNOSIS: Lepromatous Leprosy
Histopathology showed collections of epithelioid to sarcoidal granulomas throughout the dermis and clustered around nerve bundles with a grenz zone at the dermoepidermal junction. Fite stain was positive for acid-fast bacteria, which were confirmed to be Mycobacterium leprae by by the National Hansen’s Disease program. Based on these findings, a diagnosis of lepromatous leprosy (LL) was made. The patient was treated by the infectious disease department with multidrug therapy that included monthly rifampin, moxifloxacin, and minocycline; weekly methotrexate with daily folic acid; and an extended prednisone taper with prophylactic cholecalciferol.
Lepromatous leprosy is characterized by high antibody titers to the acid-fast, gram-positive bacillus Mycobacterium leprae as well as a high bacillary load.1 Patients typically present with muscle weakness, anesthetic skin patches, and claw hands. Patients also may present with foot drop, ulcerations of the hands and feet, autonomic dysfunction with anhidrosis or impaired sweating, and localized alopecia.2 Over months to years, LL may progress to extensive sensory loss and indurated lesions that infiltrate the skin and cause thickening, especially on the face (known as leonine facies). Furthermore, LL is characterized by extensive bilaterally symmetric cutaneous lesions with poorly defined borders and raised indurated centers.3
Lepromatous leprosy transmission is not fully understood but is thought to occur via airborne droplets from coughing/sneezing and nasal secretions.2 Histopathology generally shows a dense and diffuse granulomatous infiltrate that involves the dermis but is separated from the epidermis by a zone of collagen (grenz zone).3 Histology is characterized by the presence of lymphocytes and numerous foamy macrophages (lepra or Virchow cells) containing M leprae organisms. In persistent lesions, the high density of uncleared bacilli forms spherical cytoplasmic clumps known as globi within enlarged foamy histiocytes (Figure 1).4 The macrophages form granulomatous lesions in the skin and around nerve bundles, resulting in tissue damage and decreased sensation. The current standard of care for LL is a multidrug combination of dapsone, rifampin, and clofazimine. Early diagnosis and complete treatment of LL is crucial, as this approach typically leads to complete cure of the disease.

The differential diagnosis for LL includes granuloma annulare (GA), mycosis fungoides (MF), sarcoidosis, and subacute cutaneous lupus erythematosus (SCLE). Granuloma annulare is a noninfectious inflammatory granulomatous skin disease that manifests in a localized, generalized, or subcutaneous pattern. Localized GA is the most common form and manifests as self-resolving, flesh-colored or erythematous papules or plaques limited to the extremities.5,6 Generalized GA is defined by more than 10 widespread annular plaques involving the trunk and extremities and can persist for decades.6 This form can be associated with hyperlipidemia, diabetes, autoimmune disease and immunodeficiency (eg, HIV), and rarely with lymphoma or solid tumors. On histology, GA shows necrobiosis surrounded by palisading histiocytes and mucin (palisading GA) or patchy interstitial histiocytes and lymphocytes (interstitial GA)(Figure 2).6 This palisading pattern differs from the histiocytes in LL, which contain numerous acid-fast bacilli and bacterial clumps. Topical and intralesional corticosteroids are first-line therapies for GA.

Mycosis fungoides is a cutaneous T-cell lymphoma characterized by proliferation of CD4+ T cells.7 In the early stages of MF, patients may present with multiple erythematous and scaly patches, plaques, or nodules that most commonly develop on unexposed areas of the skin, but specific variants frequently may cause lesions on the face or scalp.8 Tumors may be solitary, localized, or generalized and may be observed alongside patches and plaques or in the absence of cutaneous lesions.7 The pathologic features of MF include fibrosis of the papillary dermis, individual haloed atypical lymphocytes in the epidermis, and atypical lymphoid cells with cerebriform nuclei (Figure 3).9 Granulomatous MF is characterized by diffuse nodular and perivascular infiltrates of histiocytes with small lymphocytes without atypia, eosinophils, and plasma cells. Small lymphocytes with cerebriform nuclei and larger lymphocytes with hyperconvoluted nuclei also may be seen, in addition to multinucleated histiocytic giant cells. Although MF commonly manifests with epidermotropism, it typically is absent in granulomatous MF (GMF).10 Granulomatous MF may manifest similarly to LL. Noduloulcerative lesions and infiltration of atypical lymphocytes into the epidermis (epidermotropism) are much more common in GMF than in LL; however, although ulcerative nodules are not a common feature in patients with leprosy (except during reactional states [ie, Lucio phenomenon]) or secondary to neuropathies, they also can occur in LL.11 In GMF, the infiltrate does not follow a specific pattern, whereas LL infiltrates tend to follow a nerve distribution. Treatment for MF is determined by disease severity.12 First-line therapy includes local corticosteroids and phototherapy with UVB irradiation.

Sarcoidosis is a multisystem disease that demonstrates nonspecific clinical manifestations affecting the lungs, eyes, liver, and skin.13 Environmental exposures to silica and inorganic matter have been linked to an increased risk for sarcoidosis, with patients presenting with fatigue, fever, and arthralgia.13 Skin manifestations include subcutaneous nodules, polymorphous plaques, and erythema nodosum—nodosum—the most common cutaneous presentation of sarcoidosis. Erythema nodosum manifests as symmetrically distributed, nonulcerative, painful red nodules on the skin, especially the lower legs. The histopathology of sarcoidosis shows noncaseating granulomas with activated T-lymphocytes, epithelioid cells, and multinucleated giant cells (Figure 4). Although granulomas occur in both LL and sarcoidosis, those in sarcoidosis typically consist of epithelioid cells surrounded by a rim of lymphocytes, whereas LL granulomas contain foamy histiocytes and multinucleated giant cells. Treatment of sarcoidosis depends on disease progression and generally involves oral corticosteroids, followed by corticosteroid-sparing regimens.

Subacute cutaneous lupus erythematosus is a chronic autoimmune disease that predominantly affects younger women. Common findings in SCLE include red scaly plaques and ring-shaped lesions on sun-exposed areas of the skin.14 Subacute cutaneous lupus erythematosus primarily is characterized by a photosensitive rash, often with arthralgia, myalgia, and/or oral ulcers; less commonly, a small percentage of patients can experience central nervous system involvement, vasculitis, or nephritis. The histologic findings of SCLE include hydropic degeneration of the basal cell layer and periadnexal infiltrates (Figure 5). The incidence of SCLE often is associated with anti-Ro (SSA) and anti-La (SSB) antibodies.15 Treatment of SCLE focuses on managing skin symptoms with corticosteroids, antimalarials, and sun protection.

- Bobosha K, Wilson L, van Meijgaarden KE, et al. T-cell regulation in lepromatous leprosy. PLoS Negl Trop Dis. 2014;8:E2773. doi:10.1371 /journal.pntd.0002773
- Fischer M. Leprosy–an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827. doi:10.1111/ddg.13301
- Jolly M, Pickard SA, Mikolaitis RA, et al. Lupus QoL-US benchmarks for US patients with systemic lupus erythematosus. J Rheumatol. 2010;37:1828-1833. doi:10.3899/jrheum.091443
- Chan MMF, Smoller BR. Overview of the histopathology and other laboratory investigations in leprosy. Curr Trop Med Rep. 2016;3:131-137. doi:10.1007/s40475-016-0086-y
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016; 75:457-465. doi:10.1016/j.jaad.2015.03.054
- Lukács J, Schliemann S, Elsner P. Treatment of generalized granuloma annulare–a systematic review. J Eur Acad Dermatol Venereol. 2015;29:1467-1480. doi:10.1111/jdv.12976
- Zinzani PL, Ferreri AJM, Cerroni L. Mycosis fungoides. Crit Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Ahn CS, ALSayyah A, Sangüeza OP. Mycosis fungoides: an updated review of clinicopathologic variants. Am J Dermatopathol. 2014;36:933- 951. doi:10.1097/DAD.0000000000000207
- Gutte R, Kharkar V, Mahajan S, et al. Granulomatous mycosis fungoides with hypohidrosis mimicking lepromatous leprosy. Indian J Dermatol Venereol Leprol. 2010;76:686. doi:10.4103/0378-6323.72470
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the cutaneous lymphoma histopathology task force group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001 /archdermatol.2008.46
- Miyashiro D, Cardona C, Valente N, et al. Ulcers in leprosy patients, an unrecognized clinical manifestation: a report of 8 cases. BMC Infect Dis. 2019;19:1013. doi:10.1186/s12879-019-4639-2
- Cerroni L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37:2-10. doi:10.12788/j.sder.2018.002
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390 /jcm9041081
- Zÿ ychowska M, Reich A. Dermoscopic features of acute, subacute, chronic and intermittent subtypes of cutaneous lupus erythematosus in Caucasians. J Clin Med. 2022;11:4088. doi:10.3390/jcm11144088
- Lazar AL. Subacute cutaneous lupus erythematosus: a facultative paraneoplastic dermatosis. Clin Dermatol. 2022;40:728-742. doi:10.1016 /j.clindermatol.2022.07.007
- Bobosha K, Wilson L, van Meijgaarden KE, et al. T-cell regulation in lepromatous leprosy. PLoS Negl Trop Dis. 2014;8:E2773. doi:10.1371 /journal.pntd.0002773
- Fischer M. Leprosy–an overview of clinical features, diagnosis, and treatment. J Dtsch Dermatol Ges. 2017;15:801-827. doi:10.1111/ddg.13301
- Jolly M, Pickard SA, Mikolaitis RA, et al. Lupus QoL-US benchmarks for US patients with systemic lupus erythematosus. J Rheumatol. 2010;37:1828-1833. doi:10.3899/jrheum.091443
- Chan MMF, Smoller BR. Overview of the histopathology and other laboratory investigations in leprosy. Curr Trop Med Rep. 2016;3:131-137. doi:10.1007/s40475-016-0086-y
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016; 75:457-465. doi:10.1016/j.jaad.2015.03.054
- Lukács J, Schliemann S, Elsner P. Treatment of generalized granuloma annulare–a systematic review. J Eur Acad Dermatol Venereol. 2015;29:1467-1480. doi:10.1111/jdv.12976
- Zinzani PL, Ferreri AJM, Cerroni L. Mycosis fungoides. Crit Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Ahn CS, ALSayyah A, Sangüeza OP. Mycosis fungoides: an updated review of clinicopathologic variants. Am J Dermatopathol. 2014;36:933- 951. doi:10.1097/DAD.0000000000000207
- Gutte R, Kharkar V, Mahajan S, et al. Granulomatous mycosis fungoides with hypohidrosis mimicking lepromatous leprosy. Indian J Dermatol Venereol Leprol. 2010;76:686. doi:10.4103/0378-6323.72470
- Kempf W, Ostheeren-Michaelis S, Paulli M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the cutaneous lymphoma histopathology task force group of the European Organization for Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144:1609-1617. doi:10.1001 /archdermatol.2008.46
- Miyashiro D, Cardona C, Valente N, et al. Ulcers in leprosy patients, an unrecognized clinical manifestation: a report of 8 cases. BMC Infect Dis. 2019;19:1013. doi:10.1186/s12879-019-4639-2
- Cerroni L. Mycosis fungoides-clinical and histopathologic features, differential diagnosis, and treatment. Semin Cutan Med Surg. 2018;37:2-10. doi:10.12788/j.sder.2018.002
- Jain R, Yadav D, Puranik N, et al. Sarcoidosis: causes, diagnosis, clinical features, and treatments. J Clin Med. 2020;9:1081. doi:10.3390 /jcm9041081
- Zÿ ychowska M, Reich A. Dermoscopic features of acute, subacute, chronic and intermittent subtypes of cutaneous lupus erythematosus in Caucasians. J Clin Med. 2022;11:4088. doi:10.3390/jcm11144088
- Lazar AL. Subacute cutaneous lupus erythematosus: a facultative paraneoplastic dermatosis. Clin Dermatol. 2022;40:728-742. doi:10.1016 /j.clindermatol.2022.07.007
Smooth Symmetric Plaques on the Face, Trunk, and Extremities
Smooth Symmetric Plaques on the Face, Trunk, and Extremities
A 44-year-old woman presented to the dermatology clinic with a widespread red, itchy, bumpy rash of 1 year’s duration. Physical examination revealed smooth, coalescing, erythematous and edematous plaques on the face (notably the forehead, malar cheeks, and nose), back, arms, and legs. Several plaques on the back had central hypopigmentation. The patient also reported numbness and weakness in the fingers and toes, and hypoesthesia within the lesions was noted. A biopsy of one of the lesions on the left ventral forearm was performed.
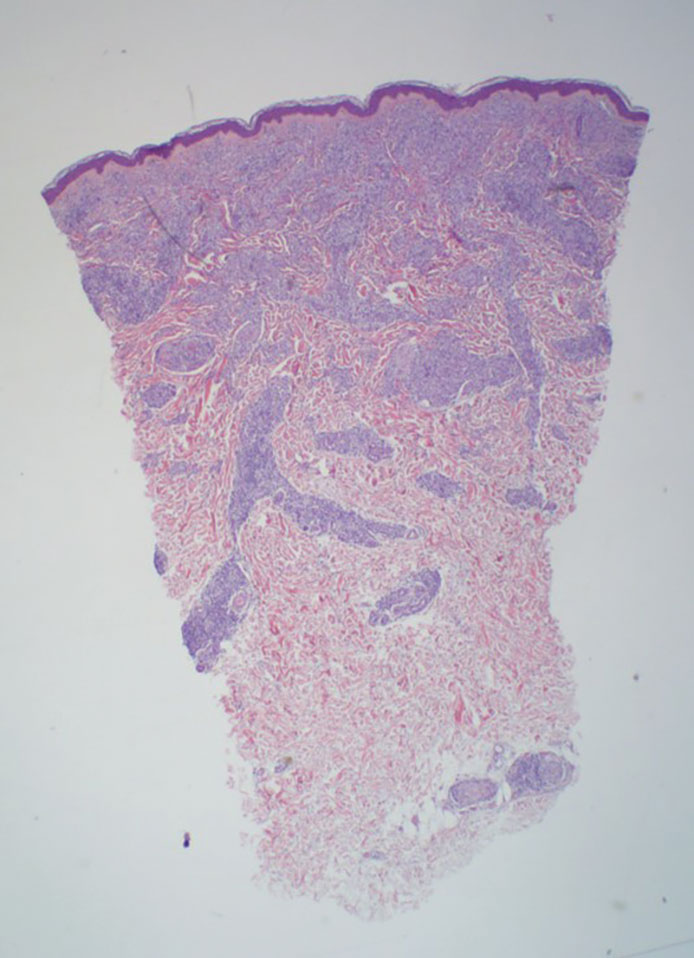
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.
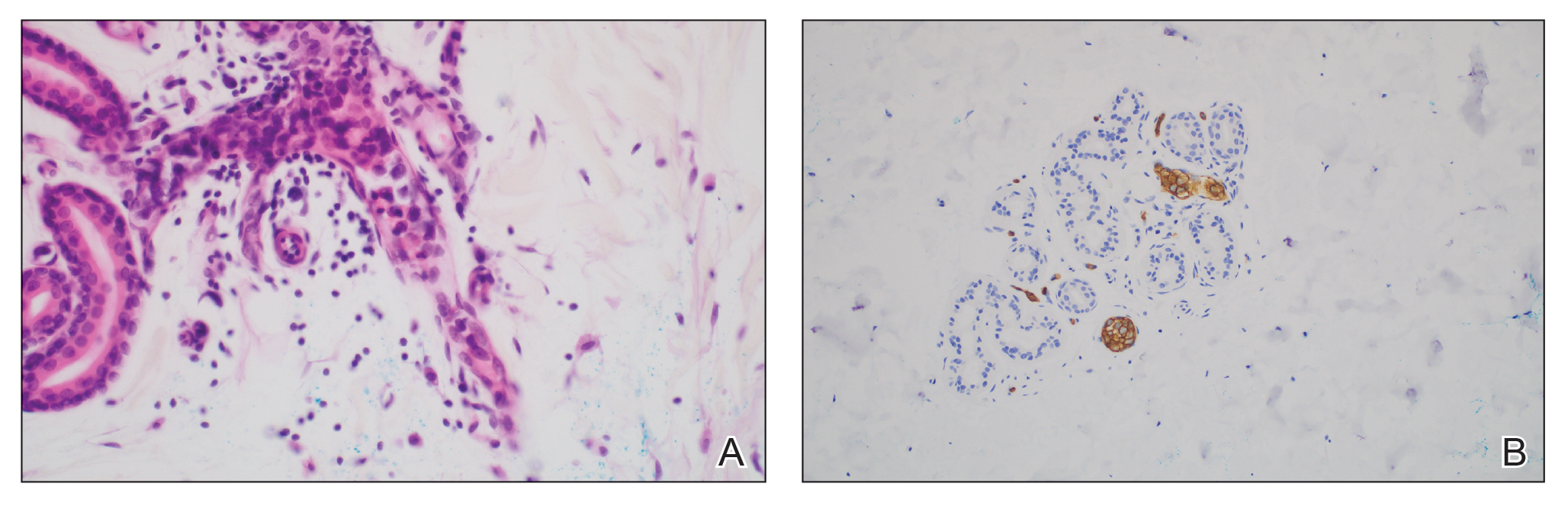
Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).
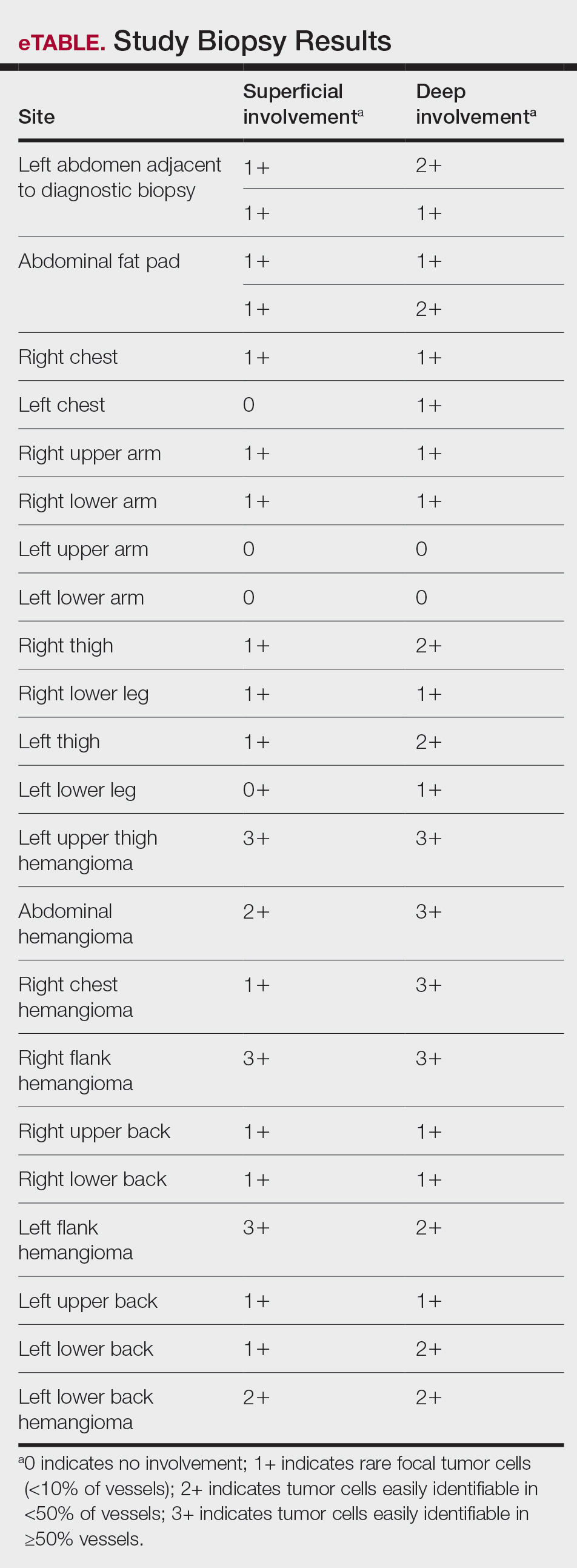
Results
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).
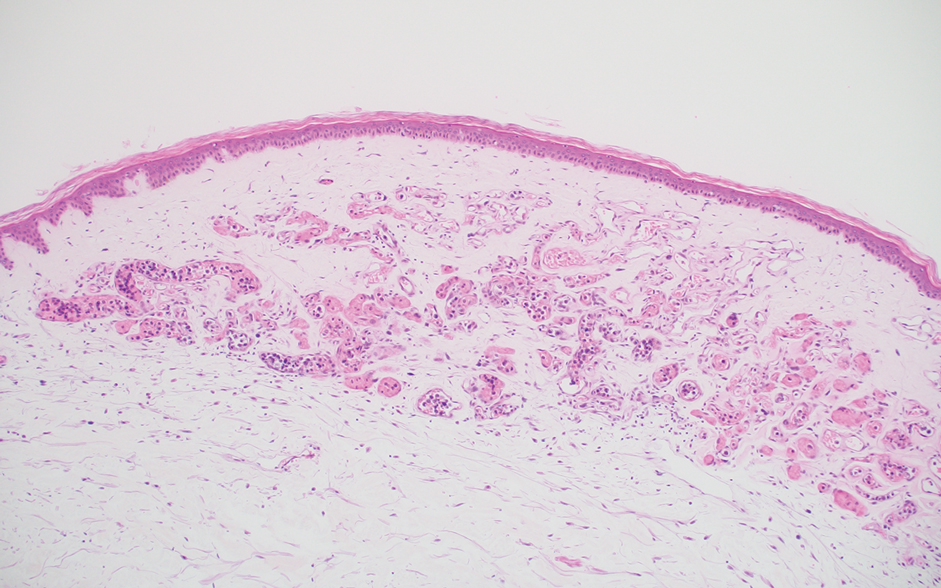
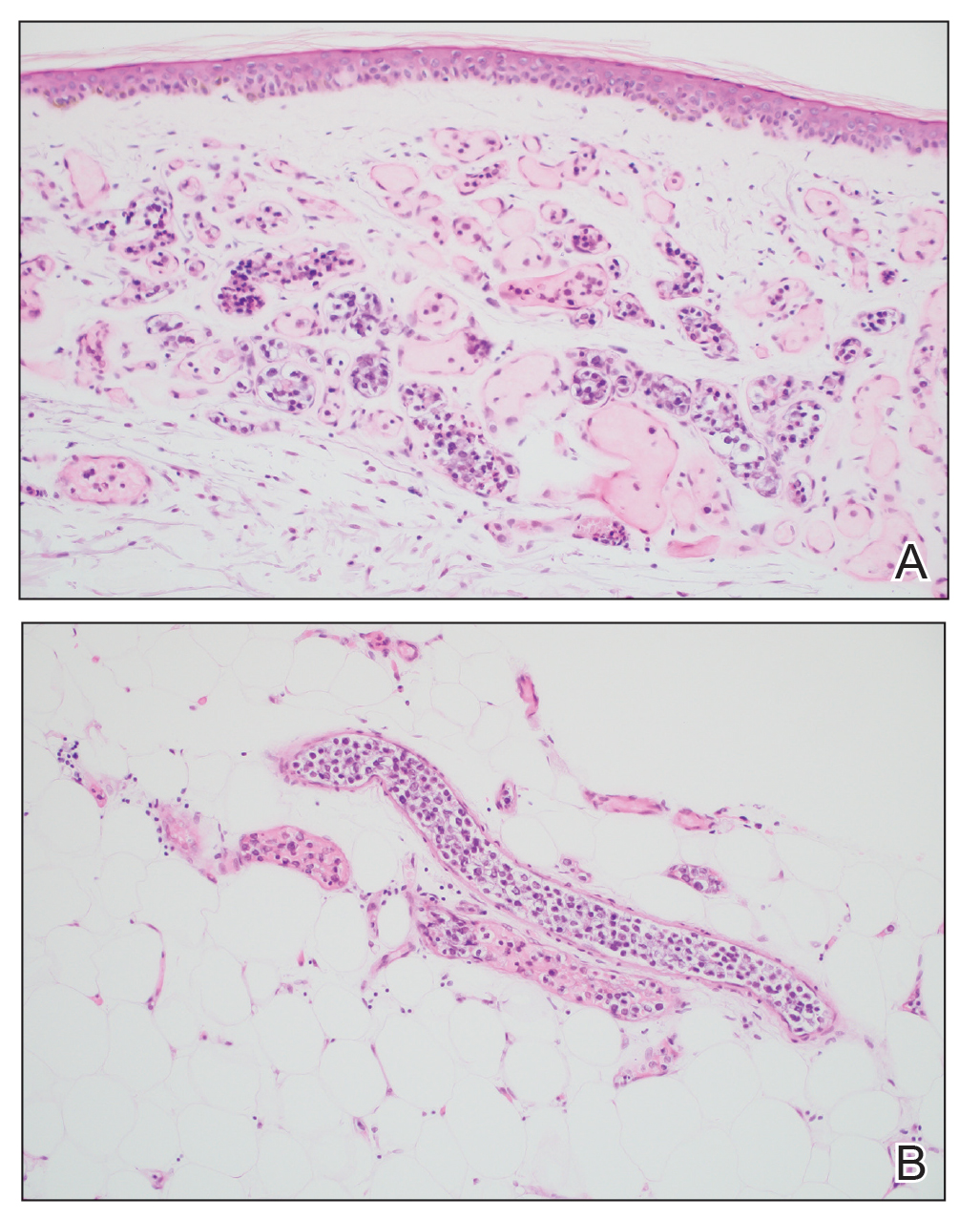
Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.

Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).

Results
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).


Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
Intravascular large B-cell lymphoma (IVBCL) is an exceedingly rare aggressive form of non-Hodgkin lymphoma with tumor cells growing selectively in vascular lumina.1 The annual incidence of IVBCL is fewer than 0.5 cases per 1,000,000 individuals worldwide.2 Only about 500 known cases of IVBCL have been recorded in the literature,3 and it accounts for less than 1% of all lymphomas. It generally affects middle-aged to elderly individuals, with an average age at diagnosis of 70 years.2 It has a predilection for men and commonly develops in individuals who are immunosuppressed.3,4
Multiple variants of IVBCL have been described in the literature, with central nervous system and cutaneous involvement being the most classic findings.5 Bone marrow involvement with hepatosplenomegaly also has been noted in the literature.6,7 Diagnosis of IVBCL and its variants requires a high index of suspicion, as the clinical manifestations and tissues involved typically are nonspecific and highly variable. Even in the classic variant of IVBCL, skin involvement is only reported in approximately half of cases.3 When present, cutaneous manifestations can range from nodules and violaceous plaques to induration and telangiectasias.3 Lymphadenopathy and lymphoma (leukemic) cells are not seen on a peripheral blood smear.2,8,9
The lack of lymphadenopathy or identifiable leukemic cells in the peripheral blood presents a diagnostic dilemma, as sufficient information for accurate diagnosis must be obtained while minimizing invasive procedures and resource expenditure. Because IVBCL cells can reside in the vascular lumina of various organs, numerous biopsy sites have been proposed for diagnosis of lymphoma, including the bone marrow, skin, prostate, adrenal gland, brain, liver, and kidneys.10 While some studies have reported that the optimal diagnostic site is the bone marrow, skin biopsies are more routinely carried out, as they represent a convenient and cost-effective alternative to other more invasive techniques.6,7,10 Studies have shown biopsy sensitivity values ranging from 77.8% to 83.3% for detection of IVBCL in normal-appearing skin, which is comparable to the sensitivities of a bone marrow biopsy.7,8 Although skin biopsy of random sites has shown diagnostic efficacy, some studies have proposed that biopsies taken from hemangiomas and other hypervascular lesions can further improve diagnostic yield, as lymphoma cells often are present in capillaries of subcutaneous adipose tissue.6,10,11 However, no obvious clinicopathologic differences were observed between IVBCL with and without involvement of a cutaneous hemangioma.11
The purpose of this study was to determine the diagnostic efficacy of skin biopsies for detecting IVBCL at various body sites and to establish whether biopsies from hemangiomas yield higher diagnostic value.
Methods
A 66-year-old man recently died at our institution secondary to IVBCL. His disease course was characterized by multiple hospital admissions in a 6-month period for fever of unknown origin and tachycardia unresponsive to broad-spectrum antibiotics and systemic steroids. The patient declined over the course of 3 to 4 weeks with findings suggestive of lymphoma and tumor lysis syndrome, and he eventually developed shock, hypoxic respiratory failure, and acute renal failure. As imaging studies and examinations had not shown lymphadenopathy, bone marrow biopsy was performed, and dermatology was consulted to perform skin biopsies to evaluate for IVBCL. Both bone marrow biopsies and random skin biopsies from the abdomen showed large and atypical CD20+ B cells within select vascular lumina (Figure). No extravascular lymphoma cells were seen. Based on the bone marrow and skin biopsies, a diagnosis of IVBCL was made. Unfortunately, no progress was made clinically, and the patient was transitioned to comfort measures. Upon the patient’s death, his family expressed interest in participating in IVBCL research and agreed to a limited autopsy consisting of numerous skin biopsies to evaluate different body sites and biopsy types (normal skin vs hemangiomas) to ascertain whether diagnostic yield could be increased by performing selective biopsies of hemangiomas if IVBCL was suspected.

Twenty-four postmortem 4-mm punch biopsies containing subcutaneous adipose tissue were taken within 24 hours of the death of the patient before embalming. The biopsies were taken from all regions of the body except the head and neck for cosmetic preservation of the decedent. Eighteen of the biopsies were taken from random sites of normal-appearing skin; the remaining 6 were taken from clinically identifiable cherry hemangiomas (5 on the trunk and 1 on the thigh). There was a variable degree of livor mortis in the dependent areas of the body, which was included in the random biopsies from the back to ensure any pooling of dependent blood would not alter the findings.
A histopathologic examination by a board-certified dermatopathologist (M.P.) on a single hematoxylin-eosin–stained level was performed to evaluate each biopsy for superficial involvement and deep involvement by IVBCL. Superficial involvement was defined as dermal involvement at or above the level of the eccrine sweat glands; deep involvement was defined as dermal involvement beneath the eccrine sweat glands and all subcutaneous fat present. Skin and bone marrow biopsies used to make the original diagnosis prior to the patient’s death were reviewed, including CD20 immunohistochemistry for morphologic comparison to the study slides. Involvement was graded as 0 to 3+ (eTable).

Results
Results from all 24 biopsies are shown in the eTable. Twenty-two (91.7%) biopsies showed at least focal involvement by IVBCL. Nine (37.5%) biopsies showed more deep vs superficial involvement of the same site. On average, the 6 biopsies from clinically detected hemangiomas showed more involvement by IVBCL than the random biopsies (eFigures 1 and 2A). The superficial involvement of skin with a hemangioma showed an average score of 2.33 v 0.78 when compared with the superficial aspect of the random biopsies; the deep involvement of skin with a hemangioma showed an average score of 2.67 vs 1.16 when compared with the deep aspect of the random biopsies (eFigure 2B).


Comment
Intravascular large B-cell lymphoma is an aggressive malignancy that traditionally is difficult to diagnose. Many efforts have been made to improve detection and early diagnosis. As cutaneous involvement is common and sometimes the only sign of disease, dermatologists may be called upon to evaluate and biopsy patients with this suspected diagnosis. The purpose of our study was to improve diagnostic efficacy by methodically performing numerous biopsies and assessing the level of involvement of the superficial and deep skin as well as involvement of hemangiomas. The goal of this meticulous approach was to identify the highest-yield areas for biopsy with minimal impact on the patient. Our results showed that random skin biopsies are an effective way to identify IVBCL. Twenty-two (91.7%) biopsies contained at least focal lymphoma cells. Although the 2 biopsies that showed no tumor cells at all happened to both be from the left arm, this is believed to be coincidental. No discernable pattern was identified regarding involvement and anatomic region. Even though 20 (83.3%) biopsies showed superficial involvement, deep biopsy is essential, as 9 (37.5%) biopsies showed increased deep involvement compared to superficial involvement. Therefore, a deep punch biopsy is essential for maximum sensitivity.
Hemangiomas provide a potential target that could increase the sensitivity of a biopsy in the absence of clinical findings, when the disease in question is exclusively intravascular. The data gathered in this study support this idea, as biopsies from hemangiomas showed increased involvement compared to random biopsies, both superficially and deep (2.33 vs 0.78 and 2.67 vs 1.16, respectively). Interestingly, the hemangioma biopsy sites showed increased deep and superficial involvement, despite these typical cherry hemangiomas only involving the superficial dermis. One possible explanation for this is that the hemangiomas have larger-caliber feeder vessels with increased blood flow beneath them. It would then follow that this increased vasculature would increase the chances of identifying intravascular lymphoma cells. This finding further accentuates the need for a deep punch biopsy containing subcutaneous fat.
Completing the study in the setting of an autopsy provided the advantage of being able to take numerous biopsies without increased harm to the patient. This extensive set of biopsies would not be reasonable to complete on a living patient. This study also has limitations. Although this patient did fall within the typical demographics for patients with IVBCL, the data were still limited to 1 patient. This autopsy format (on a patient whose cause of death was indeed IVBCL) also implies terminal disease, which may mean the patient had a larger disease burden than a living patient who would typically be biopsied. Although this increased disease burden may have increased the sensitivity of finding IVBCL in the biopsies of this study, this further emphasizes the importance of trying to determine any factors that could increase sensitivity in a living patient with a lower disease burden.
Conclusion
Skin biopsies can provide a sensitive, low-cost, and low-morbidity method to assess a patient for IVBCL. Though random skin biopsies can yield valuable information, deep, 4-mm punch biopsies of clinically identifiable hemangiomas may provide the highest sensitivity for IVBCL.
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
- Ponzoni M, Campo E, Nakamura S. Intravascular large B-cell lymphoma: a chameleon with multiple faces and many masks. Blood. 2018;132:1561-1567. doi:10.1182/blood-2017-04-737445
- Roy AM, Pandey Y, Middleton D, et al. Intravascular large B-cell lymphoma: a diagnostic dilemma. Cureus. 2021;13:e16459. doi:10.7759/cureus.16459
- Bayçelebi D, Yildiz L, S?entürk N. A case report and literature review of cutaneous intravascular large B-cell lymphoma presenting clinically as panniculitis: a difficult diagnosis, but a good prognosis. An Bras Dermatol. 2021;96:72-75. doi:10.1016/j.abd.2020.08.004
- Orwat DE, Batalis NI. Intravascular large B-cell lymphoma. Arch Pathol Lab Med. 2012;136:333-338. doi:10.5858/arpa.2010-0747-RS
- Breakell T, Waibel H, Schliep S, et al. Intravascular large B-cell lymphoma: a review with a focus on the prognostic value of skininvolvement. Curr Oncol. 2022;29:2909-2919. doi:10.3390/curroncol29050237
- Oppegard L, O’Donnell M, Piro K, et al. Going skin deep: excavating a diagnosis of intravascular large B cell lymphoma. J Gen Intern Med. 2020;35:3368-3371. doi:10.1007/s11606-020-06141-1
- Barker JL, Swarup O, Puliyayil A. Intravascular large B-cell lymphoma: representative cases and approach to diagnosis. BMJ Case Rep. 2021;14:e244069. doi:10.1136/bcr-2021-244069
- Matsue K, Asada N, Odawara J, et al. Random skin biopsy and bone marrow biopsy for diagnosis of intravascular large B cell lymphoma. Ann Hematol. 2011;90:417-421. doi:10.1007/s00277-010-1101-3
- Shimada K, Kinoshita T, Naoe T, et al. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895-902. doi:10.1016/S1470-2045(09)70140-8
- Adachi Y, Kosami K, Mizuta N, et al. Benefits of skin biopsy of senile hemangioma in intravascular large B-cell lymphoma: a case report and review of the literature. Oncol Lett. 2014;7:2003-2006. doi:10.3892/ol.2014.2017
- Ishida M, Hodohara K, Yoshida T, et al. Intravascular large B-cell lymphoma colonizing in senile hemangioma: a case report and proposal of possible diagnostic strategy for intravascular lymphoma. Pathol Int. 2011;61:555-557. doi:10.1111/j.1440-1827.2011.02697.x
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Diagnostic Value of Deep Punch Biopsies in Intravascular Large B-cell Lymphoma
Practice Points
- Skin biopsy is an effective method for identifying intravascular large B-cell lymphoma (IVBCL).
- Deep punch biopsies of sites involving hemangiomas may further heighten sensitivity for detection of IVBCL, as these lesions may harbor increased numbers of intravascular lymphoma cells.
- Deep and strategically placed skin biopsies offer potential improvements in timely diagnosis and outcomes of patients with IVBCL.
Exophytic Papule on the Chin of a Child
Exophytic Papule on the Chin of a Child
THE DIAGNOSIS: Rhabdomyomatous Mesenchymal Hamartoma
Histopathologic examination of the excised tissue revealed haphazardly arranged bundles of mature striated muscle within the dermis and subcutaneous tissue admixed with adipose tissue, adnexal structures, blood vessels, and nerves. The presence of the lesion since birth, midline clinical presentation, and histologic findings were consistent with a diagnosis of rhabdomyomatous mesenchymal hamartoma (RMH).
Also referred to as striated muscle hamartoma, RMH is a rare benign lesion thought to have embryonic origin due to its midline presentation.1 The etiology of RMH is unknown but is hypothesized to be due to abnormal migration or growth of embryonic mesenchymal tissue. Rhabdomyomatous mesenchymal hamartoma typically manifests in infancy or early childhood as a solitary midline papule on the head or neck, although there have been rare reports of development in adulthood.2-4 Lesions often are polypoid or exophytic but may manifest as smooth papules or subcutaneous nodules.2 Although benign, RMH may be associated with other congenital abnormalities and conditions, such as Delleman syndrome, which is caused by a sporadic genetic abnormality and results in defects of the eye, central nervous system, and skin.5 Treatment for RMH is not needed, but surgical excision for cosmetic purposes can be performed with low risk for recurrence. Histologically, RMH demonstrates a normal epidermis overlying disorganized bundles of skeletal muscle accompanied by varying amounts of other mature dermal and subcutaneous tissues including nerves, blood vessels, adipose tissue, and other adnexal structures.2,6 Myoglobin and desmin are positive within the skeletal muscle bundles.7
Fibrous hamartoma of infancy (FHI) often manifests as a movable, ill-defined nodule within the subcutaneous tissue. While also occurring in young children—typically within the first 2 years of life—FHI primarily is found on the upper arms, back, and axillae, in contrast to FHI.8 The classic histopathologic presentation of FHI consists of a triphasic morphology consisting of undifferentiated mesenchymal cells and dense fibroblastic/myofibroblastic tissue with mature adipose tissue woven throughout in islands (Figure 1).9 Skeletal muscle is not a component of this tumor.
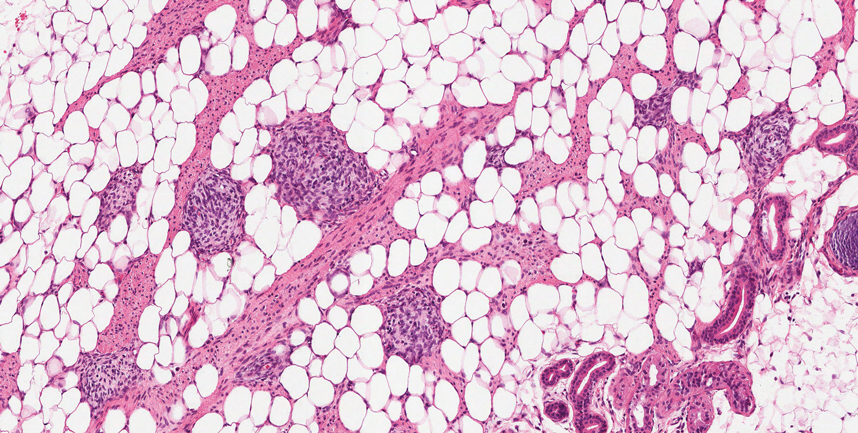
Neurofibromas also may manifest clinically as papules or nodules and arise from the peripheral nerve sheath. There are 3 major subtypes of neurofibromas—localized, diffuse, and plexiform—with the last being strongly associated with neurofibromatosis type 1.10 The plexiform type has a rare risk for malignant transformation. The typical histopathologic finding of a localized cutaneous neurofibroma is a dermal proliferation of spindle cells with wavy nuclei within a variably myxoid stroma (Figure 2).11 Interspersed mast cells also can be seen. A plexiform neurofibroma typically involves multiple nerve fascicles and comprises multinodular or tortuous bundles of cytologically bland spindle cells. Compared to RMH, skeletal muscle is not a component of this tumor.
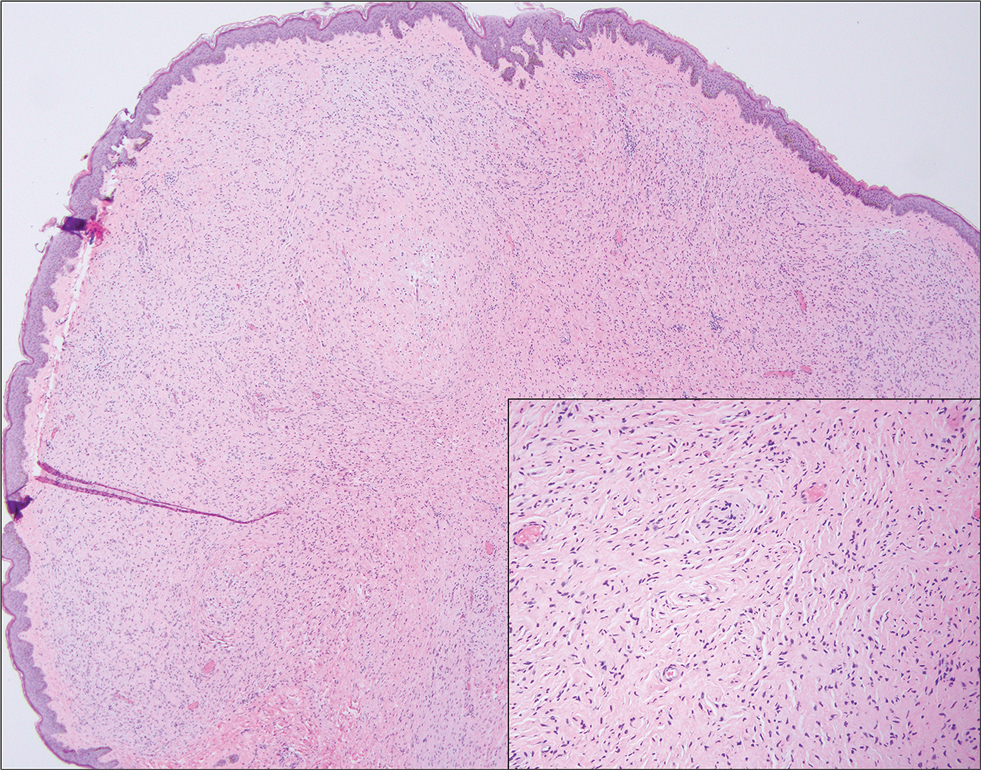
Nevus lipomatosus superficialis is a benign hamartoma that can manifest as a pedunculated or exophytic papule. The lesions may be solitary or multiple and, unlike RMH, are most common on the buttocks, upper thighs, and trunk.12 The histopathologic features of nevus lipomatosus superficialis include clusters of mature adipose tissue in the superficial dermis admixed with collagen fibers and variably increased vasculature (Figure 3).13 Nevus lipomatosus superficialis does not contain skeletal muscle within the tumor in comparison to RMH.
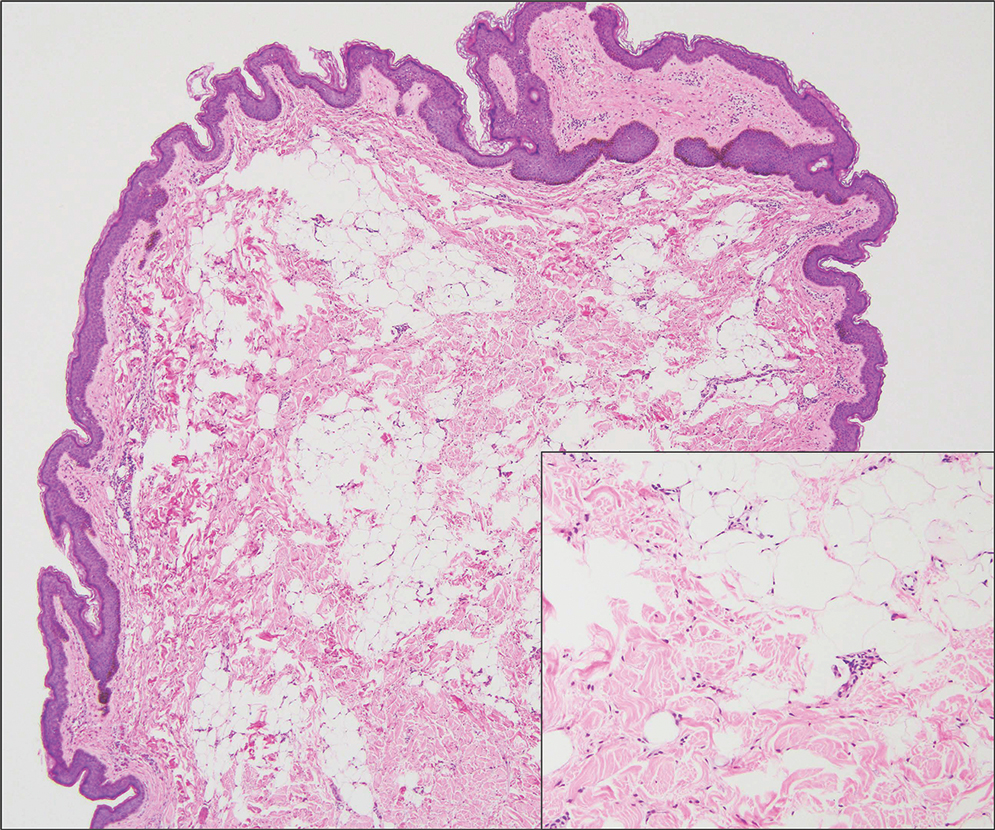
It is important to distinguish rhabdomyosarcoma (RMS) from RMH, as it is associated with increased mortality and morbidity. Rhabdomyosarcoma is the most common soft-tissue sarcoma in children and is derived from mesenchyme with variable degrees of skeletal muscle differentiation.14 Due to its mesenchymal origin, these tumors can manifest in a variety of places but most commonly on the head and neck and in the genital region.15 The most common subtype is embryonal rhabdomyosarcoma. Histologically, embryonal RMS shows a moderately cellular tumor composed of sheets of spindle-shaped or round cells with scant or eosinophilic cytoplasm (Figure 4). The absence of genetic translocation in the paired box-forkhead box protein 01 (PAX-FOXO1) gene helps distinguish it from solid alveolar RMS, the second most common and more aggressive subtype.12 Positive immunohistochemical staining for desmin, myoblast determination protein 1 (MyoD1), and myogenin supports myogenic differentiation.14
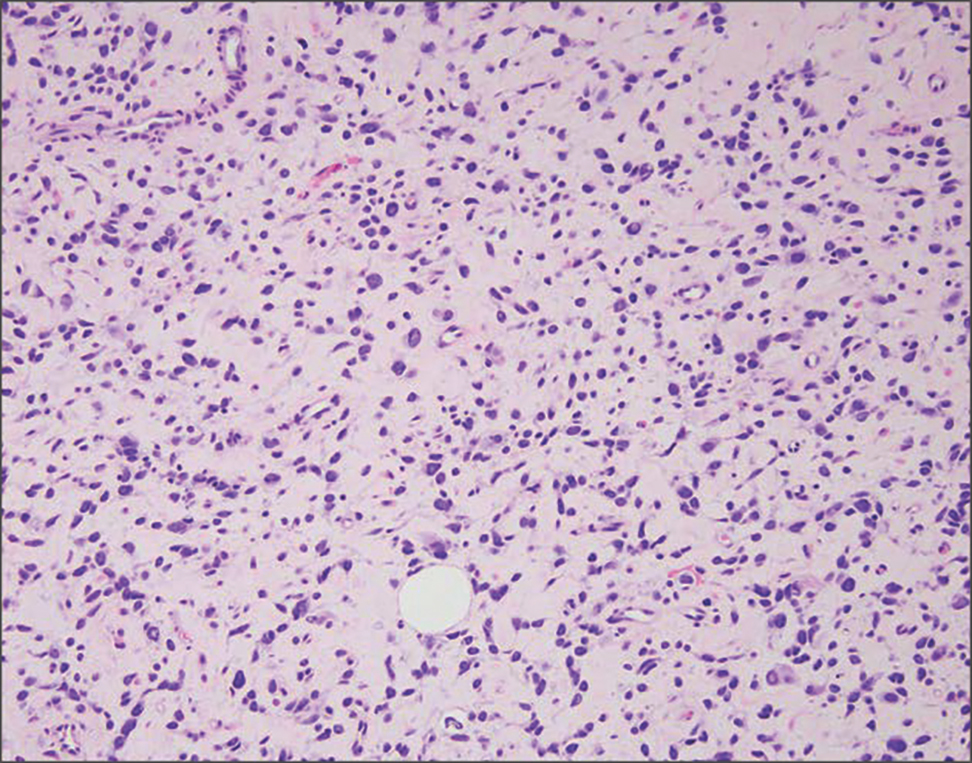
- Bernal-Mañas CM, Isaac-Montero MA, Vargas-Uribe MC, et al. Hamartoma mesenquimal rabdomiomatoso [rhabdomyomatous mesenchymal hamartoma]. An Pediatr (Barc). 2013;78:260-262. doi:10.1016/j.anpedi.2012.08.005
- Al Amri R, De Stefano DV, Wang Q, et al. Morphologic spectrum of rhabdomyomatous mesenchymal hamartomas (striated muscle hamartomas) in pediatric dermatopathology. Am J Dermatopathol. 2022;44:170-173. doi:10.1097/DAD.0000000000002062
- Carboni A, Fomin D. A rare adult presentation of a congenital tumor discovered incidentally after trauma. JAAD Case Rep. 2022;31:121-123. doi:10.1016/j.jdcr.2022.10.024
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005; 21(4):185-188. doi:10.1016/S1607-551X(09)70299-2
- Bahmani M, Naseri R, Iraniparast A, et al. Oculocerebrocutaneous syndrome (Delleman syndrome): a case with a novel presentation of orbital involvement. Case Rep Pediatr. 2021;2021:5524131. doi:10.1155/2021/5524131
- Kim H, Chung JH, Sung HM, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a midline mass on a chin. Arch Craniofac Surg. 2017;18:292-295. doi:10.7181/acfs.2017.18.4.292.
- Lin CP, Nguyen JM, Aboutalebi S, et al. Incidental rhabdomyomatous mesenchymal hamartoma. Proc (Bayl Univ Med Cent). 2020;34:161-162. doi:10.1080/08998280.2020.1801087
- Ji Y, Hu P, Zhang C, et al. Fibrous hamartoma of infancy: radiologic features and literature review. BMC Musculoskelet Disord. 2019;20:356. doi:10.1186/s12891-019-2743-5
- Yu G, Wang Y, Wang G, et al. Fibrous hamartoma of infancy: a clinical pathological analysis of seventeen cases. Int J Clin Exp Pathol. 2015;8:3374-3377.
- Ferner RE, O’Doherty MJ. Neurofibroma and schwannoma. Curr Opin Neurol. 2002;15:679-684. doi:10.1097/01.wco.0000044763.39452.aa
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1-10. doi:10.1016/j.humpath.2017.05.010
- Kim RH, Stevenson ML, Hale CS, et al. Nevus lipomatosus superficialis. Dermatol Online J. 2014;20:13030/qt2cb3c5t3.
- Singh P, Anandani GM. Nevus lipomatosus superficialis, an unusual case report. J Family Med Prim Care. 2022;11:4045-4047. doi:10.4103/jfmpc.jfmpc_2352_21
- Shern JF, Yohe ME, Khan J. Pediatric rhabdomyosarcoma. Crit Rev Oncog. 2015;20:227-243. doi:10.1615/critrevoncog.2015013800
- Rogers TN, Dasgupta R. Management of rhabdomyosarcoma in pediatric patients. Surg Oncol Clin N Am. 2021;30:339-353. doi:10.1016/j.soc.2020.11.003
- Machado I, Mayordomo-Aranda E, Giner F, et al. The role of immunohistochemistry in rhabdomyosarcoma diagnosis using tissue microarray technology and a xenograft model. Fetal Pediatr Pathol. 2015;34:271-281. doi:10.3109/15513815.2015.1042604
THE DIAGNOSIS: Rhabdomyomatous Mesenchymal Hamartoma
Histopathologic examination of the excised tissue revealed haphazardly arranged bundles of mature striated muscle within the dermis and subcutaneous tissue admixed with adipose tissue, adnexal structures, blood vessels, and nerves. The presence of the lesion since birth, midline clinical presentation, and histologic findings were consistent with a diagnosis of rhabdomyomatous mesenchymal hamartoma (RMH).
Also referred to as striated muscle hamartoma, RMH is a rare benign lesion thought to have embryonic origin due to its midline presentation.1 The etiology of RMH is unknown but is hypothesized to be due to abnormal migration or growth of embryonic mesenchymal tissue. Rhabdomyomatous mesenchymal hamartoma typically manifests in infancy or early childhood as a solitary midline papule on the head or neck, although there have been rare reports of development in adulthood.2-4 Lesions often are polypoid or exophytic but may manifest as smooth papules or subcutaneous nodules.2 Although benign, RMH may be associated with other congenital abnormalities and conditions, such as Delleman syndrome, which is caused by a sporadic genetic abnormality and results in defects of the eye, central nervous system, and skin.5 Treatment for RMH is not needed, but surgical excision for cosmetic purposes can be performed with low risk for recurrence. Histologically, RMH demonstrates a normal epidermis overlying disorganized bundles of skeletal muscle accompanied by varying amounts of other mature dermal and subcutaneous tissues including nerves, blood vessels, adipose tissue, and other adnexal structures.2,6 Myoglobin and desmin are positive within the skeletal muscle bundles.7
Fibrous hamartoma of infancy (FHI) often manifests as a movable, ill-defined nodule within the subcutaneous tissue. While also occurring in young children—typically within the first 2 years of life—FHI primarily is found on the upper arms, back, and axillae, in contrast to FHI.8 The classic histopathologic presentation of FHI consists of a triphasic morphology consisting of undifferentiated mesenchymal cells and dense fibroblastic/myofibroblastic tissue with mature adipose tissue woven throughout in islands (Figure 1).9 Skeletal muscle is not a component of this tumor.

Neurofibromas also may manifest clinically as papules or nodules and arise from the peripheral nerve sheath. There are 3 major subtypes of neurofibromas—localized, diffuse, and plexiform—with the last being strongly associated with neurofibromatosis type 1.10 The plexiform type has a rare risk for malignant transformation. The typical histopathologic finding of a localized cutaneous neurofibroma is a dermal proliferation of spindle cells with wavy nuclei within a variably myxoid stroma (Figure 2).11 Interspersed mast cells also can be seen. A plexiform neurofibroma typically involves multiple nerve fascicles and comprises multinodular or tortuous bundles of cytologically bland spindle cells. Compared to RMH, skeletal muscle is not a component of this tumor.

Nevus lipomatosus superficialis is a benign hamartoma that can manifest as a pedunculated or exophytic papule. The lesions may be solitary or multiple and, unlike RMH, are most common on the buttocks, upper thighs, and trunk.12 The histopathologic features of nevus lipomatosus superficialis include clusters of mature adipose tissue in the superficial dermis admixed with collagen fibers and variably increased vasculature (Figure 3).13 Nevus lipomatosus superficialis does not contain skeletal muscle within the tumor in comparison to RMH.

It is important to distinguish rhabdomyosarcoma (RMS) from RMH, as it is associated with increased mortality and morbidity. Rhabdomyosarcoma is the most common soft-tissue sarcoma in children and is derived from mesenchyme with variable degrees of skeletal muscle differentiation.14 Due to its mesenchymal origin, these tumors can manifest in a variety of places but most commonly on the head and neck and in the genital region.15 The most common subtype is embryonal rhabdomyosarcoma. Histologically, embryonal RMS shows a moderately cellular tumor composed of sheets of spindle-shaped or round cells with scant or eosinophilic cytoplasm (Figure 4). The absence of genetic translocation in the paired box-forkhead box protein 01 (PAX-FOXO1) gene helps distinguish it from solid alveolar RMS, the second most common and more aggressive subtype.12 Positive immunohistochemical staining for desmin, myoblast determination protein 1 (MyoD1), and myogenin supports myogenic differentiation.14

THE DIAGNOSIS: Rhabdomyomatous Mesenchymal Hamartoma
Histopathologic examination of the excised tissue revealed haphazardly arranged bundles of mature striated muscle within the dermis and subcutaneous tissue admixed with adipose tissue, adnexal structures, blood vessels, and nerves. The presence of the lesion since birth, midline clinical presentation, and histologic findings were consistent with a diagnosis of rhabdomyomatous mesenchymal hamartoma (RMH).
Also referred to as striated muscle hamartoma, RMH is a rare benign lesion thought to have embryonic origin due to its midline presentation.1 The etiology of RMH is unknown but is hypothesized to be due to abnormal migration or growth of embryonic mesenchymal tissue. Rhabdomyomatous mesenchymal hamartoma typically manifests in infancy or early childhood as a solitary midline papule on the head or neck, although there have been rare reports of development in adulthood.2-4 Lesions often are polypoid or exophytic but may manifest as smooth papules or subcutaneous nodules.2 Although benign, RMH may be associated with other congenital abnormalities and conditions, such as Delleman syndrome, which is caused by a sporadic genetic abnormality and results in defects of the eye, central nervous system, and skin.5 Treatment for RMH is not needed, but surgical excision for cosmetic purposes can be performed with low risk for recurrence. Histologically, RMH demonstrates a normal epidermis overlying disorganized bundles of skeletal muscle accompanied by varying amounts of other mature dermal and subcutaneous tissues including nerves, blood vessels, adipose tissue, and other adnexal structures.2,6 Myoglobin and desmin are positive within the skeletal muscle bundles.7
Fibrous hamartoma of infancy (FHI) often manifests as a movable, ill-defined nodule within the subcutaneous tissue. While also occurring in young children—typically within the first 2 years of life—FHI primarily is found on the upper arms, back, and axillae, in contrast to FHI.8 The classic histopathologic presentation of FHI consists of a triphasic morphology consisting of undifferentiated mesenchymal cells and dense fibroblastic/myofibroblastic tissue with mature adipose tissue woven throughout in islands (Figure 1).9 Skeletal muscle is not a component of this tumor.

Neurofibromas also may manifest clinically as papules or nodules and arise from the peripheral nerve sheath. There are 3 major subtypes of neurofibromas—localized, diffuse, and plexiform—with the last being strongly associated with neurofibromatosis type 1.10 The plexiform type has a rare risk for malignant transformation. The typical histopathologic finding of a localized cutaneous neurofibroma is a dermal proliferation of spindle cells with wavy nuclei within a variably myxoid stroma (Figure 2).11 Interspersed mast cells also can be seen. A plexiform neurofibroma typically involves multiple nerve fascicles and comprises multinodular or tortuous bundles of cytologically bland spindle cells. Compared to RMH, skeletal muscle is not a component of this tumor.

Nevus lipomatosus superficialis is a benign hamartoma that can manifest as a pedunculated or exophytic papule. The lesions may be solitary or multiple and, unlike RMH, are most common on the buttocks, upper thighs, and trunk.12 The histopathologic features of nevus lipomatosus superficialis include clusters of mature adipose tissue in the superficial dermis admixed with collagen fibers and variably increased vasculature (Figure 3).13 Nevus lipomatosus superficialis does not contain skeletal muscle within the tumor in comparison to RMH.

It is important to distinguish rhabdomyosarcoma (RMS) from RMH, as it is associated with increased mortality and morbidity. Rhabdomyosarcoma is the most common soft-tissue sarcoma in children and is derived from mesenchyme with variable degrees of skeletal muscle differentiation.14 Due to its mesenchymal origin, these tumors can manifest in a variety of places but most commonly on the head and neck and in the genital region.15 The most common subtype is embryonal rhabdomyosarcoma. Histologically, embryonal RMS shows a moderately cellular tumor composed of sheets of spindle-shaped or round cells with scant or eosinophilic cytoplasm (Figure 4). The absence of genetic translocation in the paired box-forkhead box protein 01 (PAX-FOXO1) gene helps distinguish it from solid alveolar RMS, the second most common and more aggressive subtype.12 Positive immunohistochemical staining for desmin, myoblast determination protein 1 (MyoD1), and myogenin supports myogenic differentiation.14

- Bernal-Mañas CM, Isaac-Montero MA, Vargas-Uribe MC, et al. Hamartoma mesenquimal rabdomiomatoso [rhabdomyomatous mesenchymal hamartoma]. An Pediatr (Barc). 2013;78:260-262. doi:10.1016/j.anpedi.2012.08.005
- Al Amri R, De Stefano DV, Wang Q, et al. Morphologic spectrum of rhabdomyomatous mesenchymal hamartomas (striated muscle hamartomas) in pediatric dermatopathology. Am J Dermatopathol. 2022;44:170-173. doi:10.1097/DAD.0000000000002062
- Carboni A, Fomin D. A rare adult presentation of a congenital tumor discovered incidentally after trauma. JAAD Case Rep. 2022;31:121-123. doi:10.1016/j.jdcr.2022.10.024
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005; 21(4):185-188. doi:10.1016/S1607-551X(09)70299-2
- Bahmani M, Naseri R, Iraniparast A, et al. Oculocerebrocutaneous syndrome (Delleman syndrome): a case with a novel presentation of orbital involvement. Case Rep Pediatr. 2021;2021:5524131. doi:10.1155/2021/5524131
- Kim H, Chung JH, Sung HM, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a midline mass on a chin. Arch Craniofac Surg. 2017;18:292-295. doi:10.7181/acfs.2017.18.4.292.
- Lin CP, Nguyen JM, Aboutalebi S, et al. Incidental rhabdomyomatous mesenchymal hamartoma. Proc (Bayl Univ Med Cent). 2020;34:161-162. doi:10.1080/08998280.2020.1801087
- Ji Y, Hu P, Zhang C, et al. Fibrous hamartoma of infancy: radiologic features and literature review. BMC Musculoskelet Disord. 2019;20:356. doi:10.1186/s12891-019-2743-5
- Yu G, Wang Y, Wang G, et al. Fibrous hamartoma of infancy: a clinical pathological analysis of seventeen cases. Int J Clin Exp Pathol. 2015;8:3374-3377.
- Ferner RE, O’Doherty MJ. Neurofibroma and schwannoma. Curr Opin Neurol. 2002;15:679-684. doi:10.1097/01.wco.0000044763.39452.aa
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1-10. doi:10.1016/j.humpath.2017.05.010
- Kim RH, Stevenson ML, Hale CS, et al. Nevus lipomatosus superficialis. Dermatol Online J. 2014;20:13030/qt2cb3c5t3.
- Singh P, Anandani GM. Nevus lipomatosus superficialis, an unusual case report. J Family Med Prim Care. 2022;11:4045-4047. doi:10.4103/jfmpc.jfmpc_2352_21
- Shern JF, Yohe ME, Khan J. Pediatric rhabdomyosarcoma. Crit Rev Oncog. 2015;20:227-243. doi:10.1615/critrevoncog.2015013800
- Rogers TN, Dasgupta R. Management of rhabdomyosarcoma in pediatric patients. Surg Oncol Clin N Am. 2021;30:339-353. doi:10.1016/j.soc.2020.11.003
- Machado I, Mayordomo-Aranda E, Giner F, et al. The role of immunohistochemistry in rhabdomyosarcoma diagnosis using tissue microarray technology and a xenograft model. Fetal Pediatr Pathol. 2015;34:271-281. doi:10.3109/15513815.2015.1042604
- Bernal-Mañas CM, Isaac-Montero MA, Vargas-Uribe MC, et al. Hamartoma mesenquimal rabdomiomatoso [rhabdomyomatous mesenchymal hamartoma]. An Pediatr (Barc). 2013;78:260-262. doi:10.1016/j.anpedi.2012.08.005
- Al Amri R, De Stefano DV, Wang Q, et al. Morphologic spectrum of rhabdomyomatous mesenchymal hamartomas (striated muscle hamartomas) in pediatric dermatopathology. Am J Dermatopathol. 2022;44:170-173. doi:10.1097/DAD.0000000000002062
- Carboni A, Fomin D. A rare adult presentation of a congenital tumor discovered incidentally after trauma. JAAD Case Rep. 2022;31:121-123. doi:10.1016/j.jdcr.2022.10.024
- Chang CP, Chen GS. Rhabdomyomatous mesenchymal hamartoma: a plaque-type variant in an adult. Kaohsiung J Med Sci. 2005; 21(4):185-188. doi:10.1016/S1607-551X(09)70299-2
- Bahmani M, Naseri R, Iraniparast A, et al. Oculocerebrocutaneous syndrome (Delleman syndrome): a case with a novel presentation of orbital involvement. Case Rep Pediatr. 2021;2021:5524131. doi:10.1155/2021/5524131
- Kim H, Chung JH, Sung HM, et al. Rhabdomyomatous mesenchymal hamartoma presenting as a midline mass on a chin. Arch Craniofac Surg. 2017;18:292-295. doi:10.7181/acfs.2017.18.4.292.
- Lin CP, Nguyen JM, Aboutalebi S, et al. Incidental rhabdomyomatous mesenchymal hamartoma. Proc (Bayl Univ Med Cent). 2020;34:161-162. doi:10.1080/08998280.2020.1801087
- Ji Y, Hu P, Zhang C, et al. Fibrous hamartoma of infancy: radiologic features and literature review. BMC Musculoskelet Disord. 2019;20:356. doi:10.1186/s12891-019-2743-5
- Yu G, Wang Y, Wang G, et al. Fibrous hamartoma of infancy: a clinical pathological analysis of seventeen cases. Int J Clin Exp Pathol. 2015;8:3374-3377.
- Ferner RE, O’Doherty MJ. Neurofibroma and schwannoma. Curr Opin Neurol. 2002;15:679-684. doi:10.1097/01.wco.0000044763.39452.aa
- Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol. 2017;67:1-10. doi:10.1016/j.humpath.2017.05.010
- Kim RH, Stevenson ML, Hale CS, et al. Nevus lipomatosus superficialis. Dermatol Online J. 2014;20:13030/qt2cb3c5t3.
- Singh P, Anandani GM. Nevus lipomatosus superficialis, an unusual case report. J Family Med Prim Care. 2022;11:4045-4047. doi:10.4103/jfmpc.jfmpc_2352_21
- Shern JF, Yohe ME, Khan J. Pediatric rhabdomyosarcoma. Crit Rev Oncog. 2015;20:227-243. doi:10.1615/critrevoncog.2015013800
- Rogers TN, Dasgupta R. Management of rhabdomyosarcoma in pediatric patients. Surg Oncol Clin N Am. 2021;30:339-353. doi:10.1016/j.soc.2020.11.003
- Machado I, Mayordomo-Aranda E, Giner F, et al. The role of immunohistochemistry in rhabdomyosarcoma diagnosis using tissue microarray technology and a xenograft model. Fetal Pediatr Pathol. 2015;34:271-281. doi:10.3109/15513815.2015.1042604
Exophytic Papule on the Chin of a Child
Exophytic Papule on the Chin of a Child
A 3-year-old boy presented to the dermatology department for evaluation of an asymptomatic papule on the chin that had been present since birth. His medical history was otherwise unremarkable. Physical examination revealed a 4×2-mm, flesh-colored, exophytic papule on the midline chin. An excisional biopsy was performed.
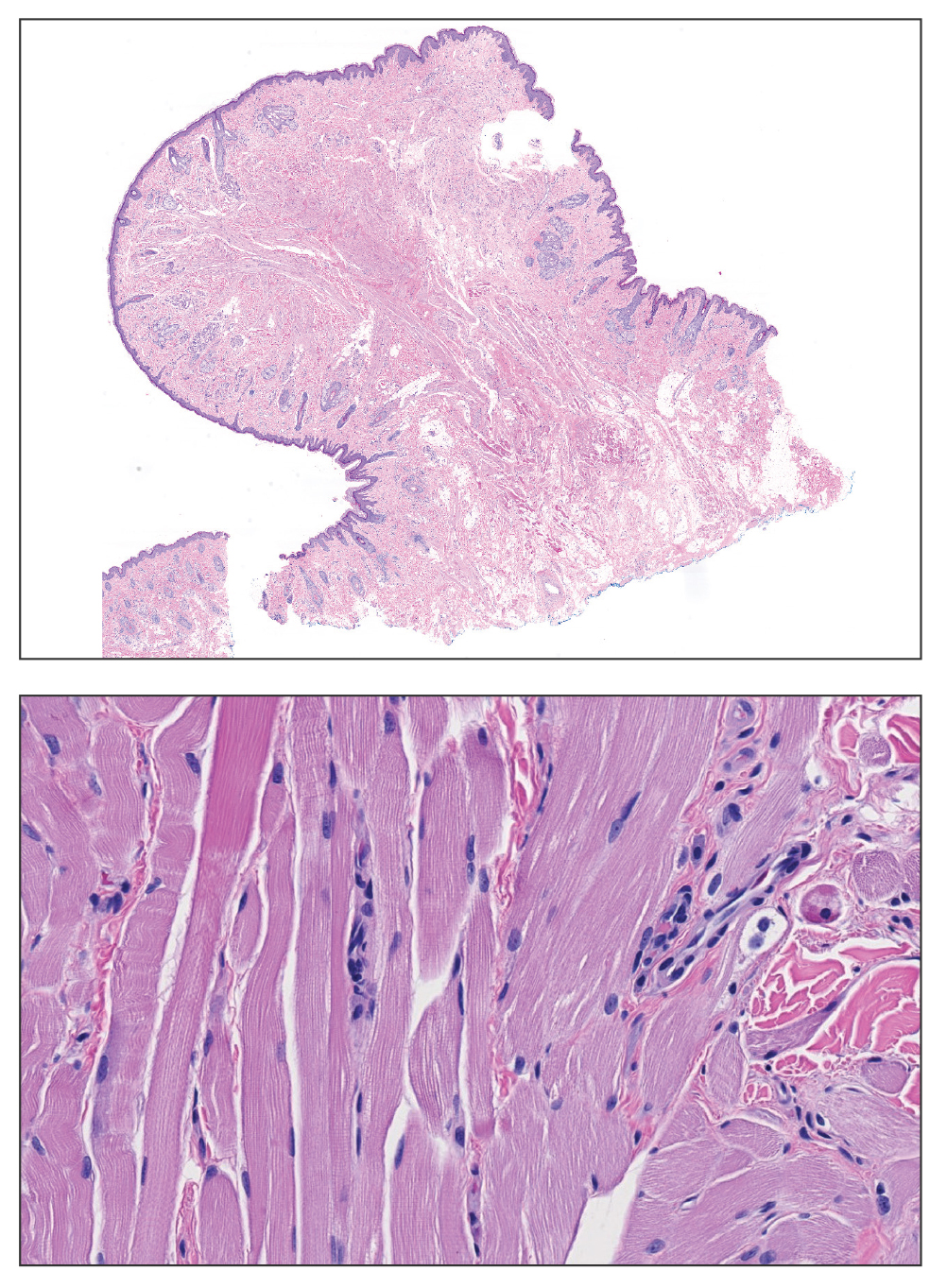
Growing Pink Nodule on the Ankle
Growing Pink Nodule on the Ankle
THE DIAGNOSIS: Epithelioid Fibrous Histiocytoma
In our patient, immunohistochemical stains for Factor XIIIa, CD68, and anaplastic lymphoma kinase (ALK) 1 confirmed the diagnosis of epithelioid fibrous histiocytoma (EFH). The location and relatively large size of the lesion led to a joint decision by the patient and physician to perform a complete excision, which was done with no complications.
Once considered a rare variant of dermatofibroma, EFH most commonly manifests as a solitary, vascular-appearing or flesh-colored papule or nodule on the legs. It often develops in the fifth decade of life with greater prevalence in men.1-5 Our patient is one of the few known cases of EFH in children that have been reported in the literature.3,6 Although EFH is benign, complete excision typically is performed due to the rarity of the lesion.3
The overexpression of ALK distinguishes EFH from other fibrohistiocytic lesions (Figure 1).5 The most common fusion partners are sequestosome 1 and vinculin (VCL), which account for more than 70% of cases.3,5,7 Interestingly, VCL-ALK fusions have been reported to occur in a subset of pediatric renal cell carcinomas and recently in an ovoid spindle cell neoplasm considered to be a low-grade sarcoma.3,7-9 Further studies have identified less common fusion partners, including the dynactin subunit 1, ETS variant transcription factor 6, protein-tyrosine phosphatase, receptor-type, F polypeptide-interacting protein-binding protein 1, sperm antigen with calponin homology and coiled-coil domains 1, tropomyosin 3, protein kinase cAMP-dependent type II regulatory subunit alpha, melanophilin, and Echinoderm microtubule-associated protein-like 4 genes.3,8
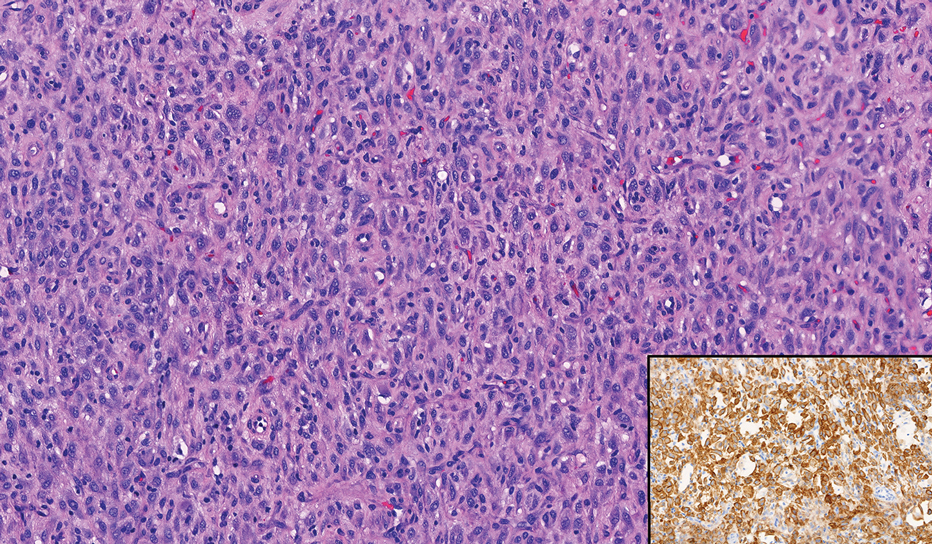
In contrast to benign fibrous histiocytomas, EFHs primarily consist of epithelioid cells, have well-defined borders, exhibit prominent vascularity, usually are situated close to the epidermis, and lack multinucleated cells or histiocytes laden with lipids or hemosiderin.2 The characteristic histopathologic finding is rounded or angulated epithelioid cells, with eosinophilic cytoplasm accounting for more than 50% of the tumor cell population.1-3,5 The nuclei of the epithelioid cells are rounded and vesicular with small eosinophilic nucleoli and low mitotic activity. Common clinical features include an exophytic nodule with a classic epidermal collarette and an epidermis that exhibits variable degrees of hyperplasia.1-3,5 Epithelioid fibrous histiocytomas often are confined to the superficial dermis and rarely extend to the subcutaneous layer. The stroma is collagenous with prominent vascularity, although older lesions can become more hyalinized and sclerotic.3 Histopathologically, these tumors can be a diagnostic challenge, as they often are mistaken for other fibrohistiocytic or melanocytic lesions.
Atypical fibroxanthoma (AFX) manifests as a dome-shaped exophytic nodule that can rapidly grow to 1 to 2 cm. Historically, it was thought to be a pseudomalignancy, but most investigators consider it within the spectrum of pleomorphic dermal sarcoma and undifferentiated pleomorphic sarcoma. Atypical fibroxanthoma usually occurs on the head and neck in elderly patients with sun-damaged skin. Histopathologically, the neoplastic cells of AFX range from atypical spindle cells and pleomorphic round to polygonal epithelioid cells to large, irregularly shaped multinucleated cells, some with foamy cytoplasm (Figure 2). The atypical spindle cells stain diffusely positive for CD10 and vimentin, while small subpopulations stain positively for CD68 or CD163 and procollagen 1. Smooth muscle actin inconsistently stains the tumor, and when it does, the staining typically is faint and patchy. Atypical fibroxanthomas usually do not stain positively for melanocytic, skeletal muscle, or keratinocytic markers.
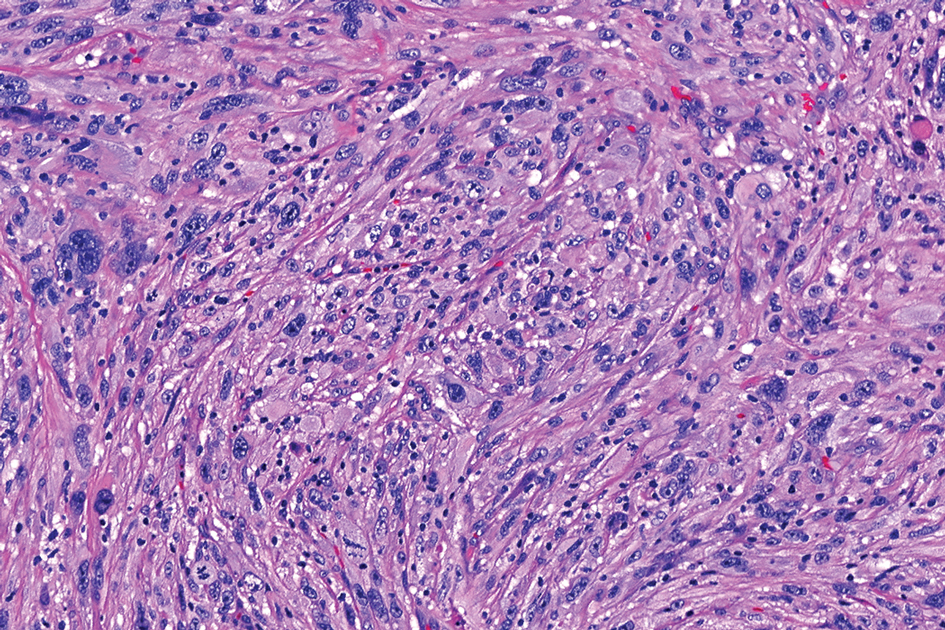
Cellular dermatofibroma typically manifests as small, dome-shaped papules on the arms and legs that normally range from a few millimeters to 1 cm but occasionally measure up to 2 cm. Histopathologically, there are interweaving fascicles of spindle cells with hyperchromatic nuclei and peripheral splaying of the plump spindle cells that wrap around collagen bundles, known as collagen trapping (Figure 3). Unlike EFH, multinucleated cells and histiocytes with abundant lipids and hemosiderin often accompany the spindle cells in cellular dermatofibromas, which stain strongly positive for CD10 and vimentin, similar to AFX and EFH. The smooth muscle actin–staining pattern usually is faint and patchy, and in some cases, cellular dermatofibroma may not stain at all. Factor XIIIa and CD68 highlight the 2 populations of cells—fibroblasts and histiocytes—that make up the lesion.4
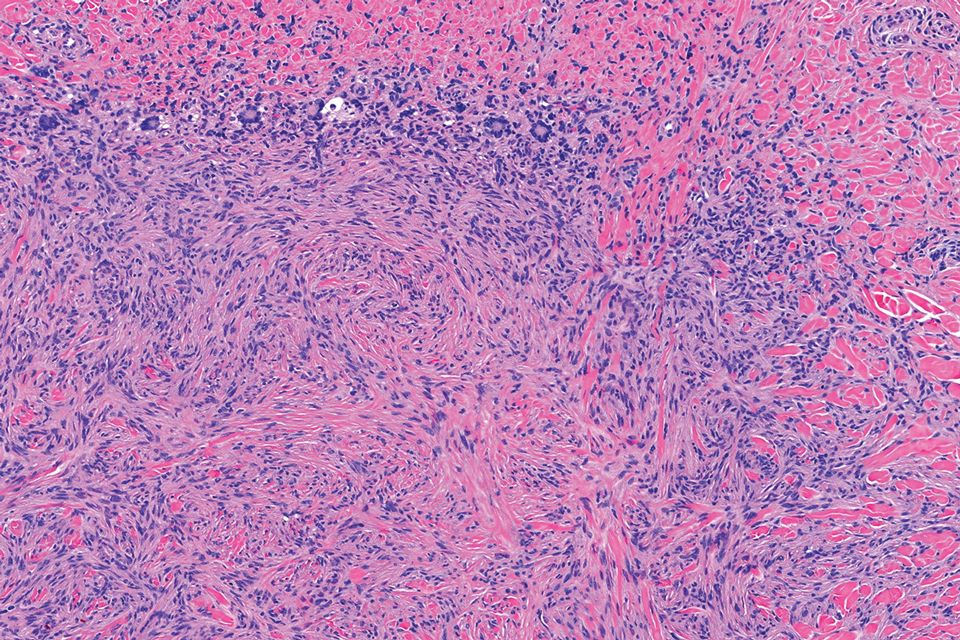
Epithelioid sarcoma comprises 2 types: distal (or conventional) type occurring on the distal arms and legs, particularly the hands and fingers of young adults, and proximal type occurring on the trunk and proximal extremities, including the upper arms and thighs.10 Epithelioid sarcoma is a rare aggressive malignancy that usually manifests as a firm nodule, sometimes with ulceration depending on the size. Histopathologically, diffuse dermal proliferation of ovoid to polygonal epithelioid cells arranged in short fascicles and nodular aggregations is observed (Figure 4). Spindle cells may be observed at the periphery of the lesion. Areas of necrosis are a frequent finding and a helpful diagnostic clue. Nearly all cases stain positively for pancytokeratin, CAM5.2, epithelial membrane antigen, and vimentin, and approximately half stain positively for CD34; there are variable expressions of ERG and smooth muscle actin.10 In most cases, epithelioid sarcoma does not stain positively for S100 or CD68. The majority (90%) of cases harbor a mutation in the SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 gene, resulting in the loss of INI1 protein expression, which can be demonstrated by immunohistochemistry. 10 As the cytologic atypia usually is minimal, epithelioid sarcoma may be misdiagnosed as a necrotizing granuloma and benign fibrous lesions, particularly when superficial or small partial biopsies are performed.
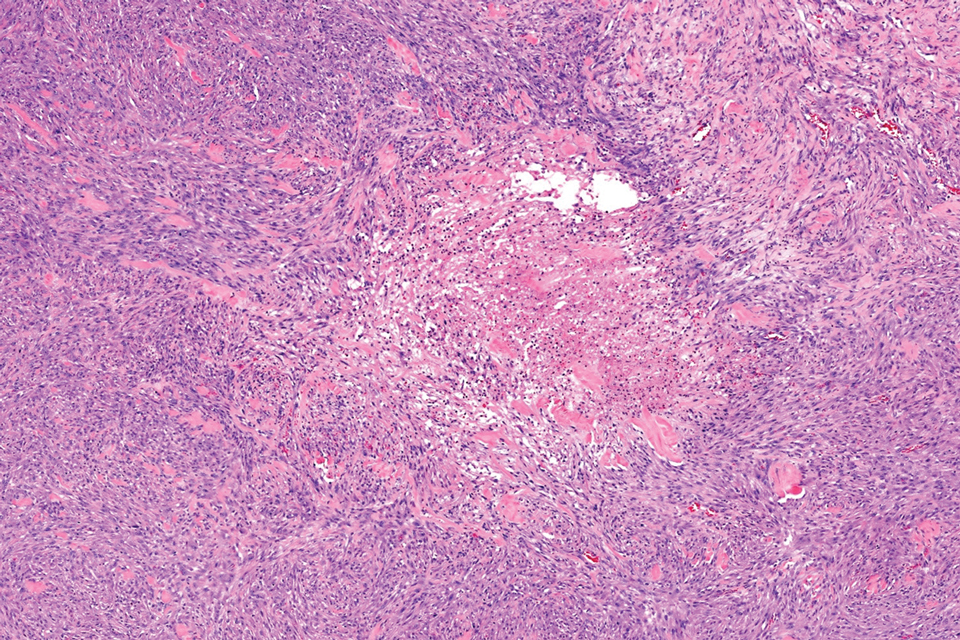
Intradermal Spitz nevi can measure from a few millimeters to more than 2 cm and can range from pink to brown to black. The most common locations are the lower extremities as well as the head and neck. Histopathologically, intradermal Spitz nevi have nests of large epithelioid melanocytes with large nuclei and abundant cytoplasm (eFigure). Nuclear pseudo-inclusions, which are cytoplasmic invaginations into the nucleus, are frequent. Unlike the other conditions in the differential, these entities stain positively for melanocytic markers—S100, SOX10, and Melan-A—but not CD68 or CD163.11 A variety of kinase fusions are observed in Spitz nevi, including the ALK gene, neurotrophic tyrosine receptor kinase, ROS proto-oncogene 1, megakaryocyte-erythroid progenitor, and v-raf murine sarcoma viral oncogene homolog B1 genes.12
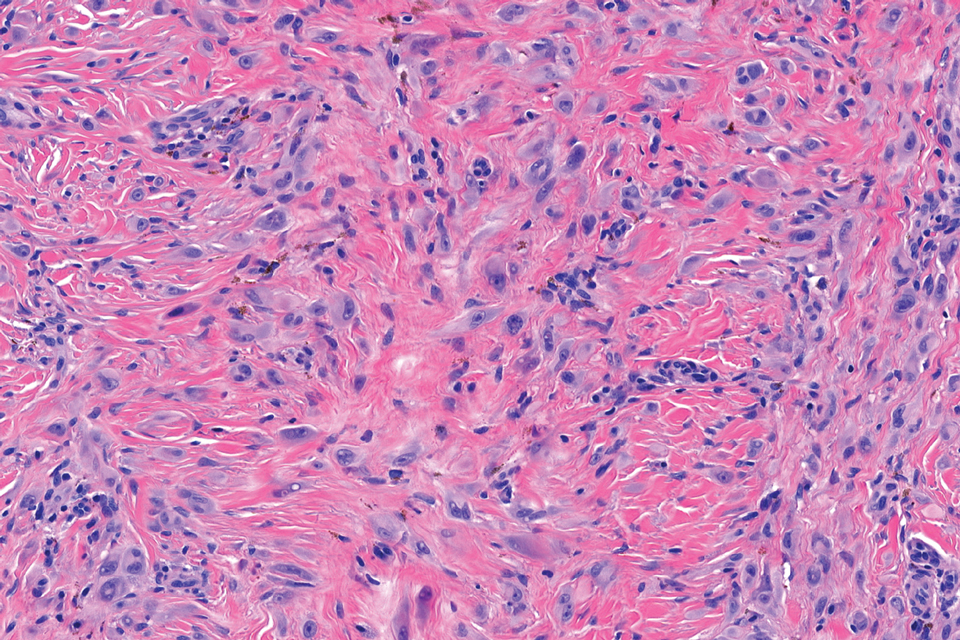
- Jones EW, Cerio R, Smith NP. Epithelioid cell histiocytoma: a new entity. Br J Dermatol. 1989;120:185-195.
- Glusac EJ, McNiff JM. Epithelioid cell histiocytoma: a simulant of vascular and melanocytic neoplasms. Am J Dermatopathol. 1999;21:1-7.
- Felty CC, Linos K. Epithelioid fibrous histiocytoma: a concise review [published correction appears in Am J Dermatopathol. 2020 Aug;42(8):628]. Am J Dermatopathol. 2019;41:879-883.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165. doi:10.1111/j.1365-2559.2009.03447.x
- Doyle LA, Mariño-Enriquez A, Fletcher CD, et al. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol. 2015;28:904-912.
- Singh Gomez C, Calonje E, Fletcher CD. Epithelioid benign fibrous histiocytoma of skin: clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994;24:123-129.
- Jedrych J, Nikiforova M, Kennedy TF, et al. Epithelioid cell histiocytoma of the skin with clonal ALK gene rearrangement resulting in VCL- and SQSTM1-ALK gene fusions. Br J Dermatol. 2015;172: 1427-1429.
- Dickson BC, Swanson D, Charames GS, et al. Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Mod Pathol. 2018;31:753-762.
- Helm M, Chang A, Fanburg-Smith JC, et al. Cutaneous VCL::ALK fusion ovoid-spindle cell neoplasm. J Cutan Pathol. 2023;50:405-409.
- Thway K, Jones RL, Noujaim J, et al. Epithelioid sarcoma: diagnostic features and genetics. Adv Anat Pathol. 2016;23:41-49.
- Bolognia JL, Jorizzo JJ, Schaffer JV et al. Dermatology, 4th ed. Philadelphia: Elsevier; 2018.
- Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116.
THE DIAGNOSIS: Epithelioid Fibrous Histiocytoma
In our patient, immunohistochemical stains for Factor XIIIa, CD68, and anaplastic lymphoma kinase (ALK) 1 confirmed the diagnosis of epithelioid fibrous histiocytoma (EFH). The location and relatively large size of the lesion led to a joint decision by the patient and physician to perform a complete excision, which was done with no complications.
Once considered a rare variant of dermatofibroma, EFH most commonly manifests as a solitary, vascular-appearing or flesh-colored papule or nodule on the legs. It often develops in the fifth decade of life with greater prevalence in men.1-5 Our patient is one of the few known cases of EFH in children that have been reported in the literature.3,6 Although EFH is benign, complete excision typically is performed due to the rarity of the lesion.3
The overexpression of ALK distinguishes EFH from other fibrohistiocytic lesions (Figure 1).5 The most common fusion partners are sequestosome 1 and vinculin (VCL), which account for more than 70% of cases.3,5,7 Interestingly, VCL-ALK fusions have been reported to occur in a subset of pediatric renal cell carcinomas and recently in an ovoid spindle cell neoplasm considered to be a low-grade sarcoma.3,7-9 Further studies have identified less common fusion partners, including the dynactin subunit 1, ETS variant transcription factor 6, protein-tyrosine phosphatase, receptor-type, F polypeptide-interacting protein-binding protein 1, sperm antigen with calponin homology and coiled-coil domains 1, tropomyosin 3, protein kinase cAMP-dependent type II regulatory subunit alpha, melanophilin, and Echinoderm microtubule-associated protein-like 4 genes.3,8

In contrast to benign fibrous histiocytomas, EFHs primarily consist of epithelioid cells, have well-defined borders, exhibit prominent vascularity, usually are situated close to the epidermis, and lack multinucleated cells or histiocytes laden with lipids or hemosiderin.2 The characteristic histopathologic finding is rounded or angulated epithelioid cells, with eosinophilic cytoplasm accounting for more than 50% of the tumor cell population.1-3,5 The nuclei of the epithelioid cells are rounded and vesicular with small eosinophilic nucleoli and low mitotic activity. Common clinical features include an exophytic nodule with a classic epidermal collarette and an epidermis that exhibits variable degrees of hyperplasia.1-3,5 Epithelioid fibrous histiocytomas often are confined to the superficial dermis and rarely extend to the subcutaneous layer. The stroma is collagenous with prominent vascularity, although older lesions can become more hyalinized and sclerotic.3 Histopathologically, these tumors can be a diagnostic challenge, as they often are mistaken for other fibrohistiocytic or melanocytic lesions.
Atypical fibroxanthoma (AFX) manifests as a dome-shaped exophytic nodule that can rapidly grow to 1 to 2 cm. Historically, it was thought to be a pseudomalignancy, but most investigators consider it within the spectrum of pleomorphic dermal sarcoma and undifferentiated pleomorphic sarcoma. Atypical fibroxanthoma usually occurs on the head and neck in elderly patients with sun-damaged skin. Histopathologically, the neoplastic cells of AFX range from atypical spindle cells and pleomorphic round to polygonal epithelioid cells to large, irregularly shaped multinucleated cells, some with foamy cytoplasm (Figure 2). The atypical spindle cells stain diffusely positive for CD10 and vimentin, while small subpopulations stain positively for CD68 or CD163 and procollagen 1. Smooth muscle actin inconsistently stains the tumor, and when it does, the staining typically is faint and patchy. Atypical fibroxanthomas usually do not stain positively for melanocytic, skeletal muscle, or keratinocytic markers.

Cellular dermatofibroma typically manifests as small, dome-shaped papules on the arms and legs that normally range from a few millimeters to 1 cm but occasionally measure up to 2 cm. Histopathologically, there are interweaving fascicles of spindle cells with hyperchromatic nuclei and peripheral splaying of the plump spindle cells that wrap around collagen bundles, known as collagen trapping (Figure 3). Unlike EFH, multinucleated cells and histiocytes with abundant lipids and hemosiderin often accompany the spindle cells in cellular dermatofibromas, which stain strongly positive for CD10 and vimentin, similar to AFX and EFH. The smooth muscle actin–staining pattern usually is faint and patchy, and in some cases, cellular dermatofibroma may not stain at all. Factor XIIIa and CD68 highlight the 2 populations of cells—fibroblasts and histiocytes—that make up the lesion.4

Epithelioid sarcoma comprises 2 types: distal (or conventional) type occurring on the distal arms and legs, particularly the hands and fingers of young adults, and proximal type occurring on the trunk and proximal extremities, including the upper arms and thighs.10 Epithelioid sarcoma is a rare aggressive malignancy that usually manifests as a firm nodule, sometimes with ulceration depending on the size. Histopathologically, diffuse dermal proliferation of ovoid to polygonal epithelioid cells arranged in short fascicles and nodular aggregations is observed (Figure 4). Spindle cells may be observed at the periphery of the lesion. Areas of necrosis are a frequent finding and a helpful diagnostic clue. Nearly all cases stain positively for pancytokeratin, CAM5.2, epithelial membrane antigen, and vimentin, and approximately half stain positively for CD34; there are variable expressions of ERG and smooth muscle actin.10 In most cases, epithelioid sarcoma does not stain positively for S100 or CD68. The majority (90%) of cases harbor a mutation in the SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 gene, resulting in the loss of INI1 protein expression, which can be demonstrated by immunohistochemistry. 10 As the cytologic atypia usually is minimal, epithelioid sarcoma may be misdiagnosed as a necrotizing granuloma and benign fibrous lesions, particularly when superficial or small partial biopsies are performed.

Intradermal Spitz nevi can measure from a few millimeters to more than 2 cm and can range from pink to brown to black. The most common locations are the lower extremities as well as the head and neck. Histopathologically, intradermal Spitz nevi have nests of large epithelioid melanocytes with large nuclei and abundant cytoplasm (eFigure). Nuclear pseudo-inclusions, which are cytoplasmic invaginations into the nucleus, are frequent. Unlike the other conditions in the differential, these entities stain positively for melanocytic markers—S100, SOX10, and Melan-A—but not CD68 or CD163.11 A variety of kinase fusions are observed in Spitz nevi, including the ALK gene, neurotrophic tyrosine receptor kinase, ROS proto-oncogene 1, megakaryocyte-erythroid progenitor, and v-raf murine sarcoma viral oncogene homolog B1 genes.12

THE DIAGNOSIS: Epithelioid Fibrous Histiocytoma
In our patient, immunohistochemical stains for Factor XIIIa, CD68, and anaplastic lymphoma kinase (ALK) 1 confirmed the diagnosis of epithelioid fibrous histiocytoma (EFH). The location and relatively large size of the lesion led to a joint decision by the patient and physician to perform a complete excision, which was done with no complications.
Once considered a rare variant of dermatofibroma, EFH most commonly manifests as a solitary, vascular-appearing or flesh-colored papule or nodule on the legs. It often develops in the fifth decade of life with greater prevalence in men.1-5 Our patient is one of the few known cases of EFH in children that have been reported in the literature.3,6 Although EFH is benign, complete excision typically is performed due to the rarity of the lesion.3
The overexpression of ALK distinguishes EFH from other fibrohistiocytic lesions (Figure 1).5 The most common fusion partners are sequestosome 1 and vinculin (VCL), which account for more than 70% of cases.3,5,7 Interestingly, VCL-ALK fusions have been reported to occur in a subset of pediatric renal cell carcinomas and recently in an ovoid spindle cell neoplasm considered to be a low-grade sarcoma.3,7-9 Further studies have identified less common fusion partners, including the dynactin subunit 1, ETS variant transcription factor 6, protein-tyrosine phosphatase, receptor-type, F polypeptide-interacting protein-binding protein 1, sperm antigen with calponin homology and coiled-coil domains 1, tropomyosin 3, protein kinase cAMP-dependent type II regulatory subunit alpha, melanophilin, and Echinoderm microtubule-associated protein-like 4 genes.3,8

In contrast to benign fibrous histiocytomas, EFHs primarily consist of epithelioid cells, have well-defined borders, exhibit prominent vascularity, usually are situated close to the epidermis, and lack multinucleated cells or histiocytes laden with lipids or hemosiderin.2 The characteristic histopathologic finding is rounded or angulated epithelioid cells, with eosinophilic cytoplasm accounting for more than 50% of the tumor cell population.1-3,5 The nuclei of the epithelioid cells are rounded and vesicular with small eosinophilic nucleoli and low mitotic activity. Common clinical features include an exophytic nodule with a classic epidermal collarette and an epidermis that exhibits variable degrees of hyperplasia.1-3,5 Epithelioid fibrous histiocytomas often are confined to the superficial dermis and rarely extend to the subcutaneous layer. The stroma is collagenous with prominent vascularity, although older lesions can become more hyalinized and sclerotic.3 Histopathologically, these tumors can be a diagnostic challenge, as they often are mistaken for other fibrohistiocytic or melanocytic lesions.
Atypical fibroxanthoma (AFX) manifests as a dome-shaped exophytic nodule that can rapidly grow to 1 to 2 cm. Historically, it was thought to be a pseudomalignancy, but most investigators consider it within the spectrum of pleomorphic dermal sarcoma and undifferentiated pleomorphic sarcoma. Atypical fibroxanthoma usually occurs on the head and neck in elderly patients with sun-damaged skin. Histopathologically, the neoplastic cells of AFX range from atypical spindle cells and pleomorphic round to polygonal epithelioid cells to large, irregularly shaped multinucleated cells, some with foamy cytoplasm (Figure 2). The atypical spindle cells stain diffusely positive for CD10 and vimentin, while small subpopulations stain positively for CD68 or CD163 and procollagen 1. Smooth muscle actin inconsistently stains the tumor, and when it does, the staining typically is faint and patchy. Atypical fibroxanthomas usually do not stain positively for melanocytic, skeletal muscle, or keratinocytic markers.

Cellular dermatofibroma typically manifests as small, dome-shaped papules on the arms and legs that normally range from a few millimeters to 1 cm but occasionally measure up to 2 cm. Histopathologically, there are interweaving fascicles of spindle cells with hyperchromatic nuclei and peripheral splaying of the plump spindle cells that wrap around collagen bundles, known as collagen trapping (Figure 3). Unlike EFH, multinucleated cells and histiocytes with abundant lipids and hemosiderin often accompany the spindle cells in cellular dermatofibromas, which stain strongly positive for CD10 and vimentin, similar to AFX and EFH. The smooth muscle actin–staining pattern usually is faint and patchy, and in some cases, cellular dermatofibroma may not stain at all. Factor XIIIa and CD68 highlight the 2 populations of cells—fibroblasts and histiocytes—that make up the lesion.4

Epithelioid sarcoma comprises 2 types: distal (or conventional) type occurring on the distal arms and legs, particularly the hands and fingers of young adults, and proximal type occurring on the trunk and proximal extremities, including the upper arms and thighs.10 Epithelioid sarcoma is a rare aggressive malignancy that usually manifests as a firm nodule, sometimes with ulceration depending on the size. Histopathologically, diffuse dermal proliferation of ovoid to polygonal epithelioid cells arranged in short fascicles and nodular aggregations is observed (Figure 4). Spindle cells may be observed at the periphery of the lesion. Areas of necrosis are a frequent finding and a helpful diagnostic clue. Nearly all cases stain positively for pancytokeratin, CAM5.2, epithelial membrane antigen, and vimentin, and approximately half stain positively for CD34; there are variable expressions of ERG and smooth muscle actin.10 In most cases, epithelioid sarcoma does not stain positively for S100 or CD68. The majority (90%) of cases harbor a mutation in the SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 gene, resulting in the loss of INI1 protein expression, which can be demonstrated by immunohistochemistry. 10 As the cytologic atypia usually is minimal, epithelioid sarcoma may be misdiagnosed as a necrotizing granuloma and benign fibrous lesions, particularly when superficial or small partial biopsies are performed.

Intradermal Spitz nevi can measure from a few millimeters to more than 2 cm and can range from pink to brown to black. The most common locations are the lower extremities as well as the head and neck. Histopathologically, intradermal Spitz nevi have nests of large epithelioid melanocytes with large nuclei and abundant cytoplasm (eFigure). Nuclear pseudo-inclusions, which are cytoplasmic invaginations into the nucleus, are frequent. Unlike the other conditions in the differential, these entities stain positively for melanocytic markers—S100, SOX10, and Melan-A—but not CD68 or CD163.11 A variety of kinase fusions are observed in Spitz nevi, including the ALK gene, neurotrophic tyrosine receptor kinase, ROS proto-oncogene 1, megakaryocyte-erythroid progenitor, and v-raf murine sarcoma viral oncogene homolog B1 genes.12

- Jones EW, Cerio R, Smith NP. Epithelioid cell histiocytoma: a new entity. Br J Dermatol. 1989;120:185-195.
- Glusac EJ, McNiff JM. Epithelioid cell histiocytoma: a simulant of vascular and melanocytic neoplasms. Am J Dermatopathol. 1999;21:1-7.
- Felty CC, Linos K. Epithelioid fibrous histiocytoma: a concise review [published correction appears in Am J Dermatopathol. 2020 Aug;42(8):628]. Am J Dermatopathol. 2019;41:879-883.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165. doi:10.1111/j.1365-2559.2009.03447.x
- Doyle LA, Mariño-Enriquez A, Fletcher CD, et al. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol. 2015;28:904-912.
- Singh Gomez C, Calonje E, Fletcher CD. Epithelioid benign fibrous histiocytoma of skin: clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994;24:123-129.
- Jedrych J, Nikiforova M, Kennedy TF, et al. Epithelioid cell histiocytoma of the skin with clonal ALK gene rearrangement resulting in VCL- and SQSTM1-ALK gene fusions. Br J Dermatol. 2015;172: 1427-1429.
- Dickson BC, Swanson D, Charames GS, et al. Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Mod Pathol. 2018;31:753-762.
- Helm M, Chang A, Fanburg-Smith JC, et al. Cutaneous VCL::ALK fusion ovoid-spindle cell neoplasm. J Cutan Pathol. 2023;50:405-409.
- Thway K, Jones RL, Noujaim J, et al. Epithelioid sarcoma: diagnostic features and genetics. Adv Anat Pathol. 2016;23:41-49.
- Bolognia JL, Jorizzo JJ, Schaffer JV et al. Dermatology, 4th ed. Philadelphia: Elsevier; 2018.
- Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116.
- Jones EW, Cerio R, Smith NP. Epithelioid cell histiocytoma: a new entity. Br J Dermatol. 1989;120:185-195.
- Glusac EJ, McNiff JM. Epithelioid cell histiocytoma: a simulant of vascular and melanocytic neoplasms. Am J Dermatopathol. 1999;21:1-7.
- Felty CC, Linos K. Epithelioid fibrous histiocytoma: a concise review [published correction appears in Am J Dermatopathol. 2020 Aug;42(8):628]. Am J Dermatopathol. 2019;41:879-883.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165. doi:10.1111/j.1365-2559.2009.03447.x
- Doyle LA, Mariño-Enriquez A, Fletcher CD, et al. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol. 2015;28:904-912.
- Singh Gomez C, Calonje E, Fletcher CD. Epithelioid benign fibrous histiocytoma of skin: clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994;24:123-129.
- Jedrych J, Nikiforova M, Kennedy TF, et al. Epithelioid cell histiocytoma of the skin with clonal ALK gene rearrangement resulting in VCL- and SQSTM1-ALK gene fusions. Br J Dermatol. 2015;172: 1427-1429.
- Dickson BC, Swanson D, Charames GS, et al. Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Mod Pathol. 2018;31:753-762.
- Helm M, Chang A, Fanburg-Smith JC, et al. Cutaneous VCL::ALK fusion ovoid-spindle cell neoplasm. J Cutan Pathol. 2023;50:405-409.
- Thway K, Jones RL, Noujaim J, et al. Epithelioid sarcoma: diagnostic features and genetics. Adv Anat Pathol. 2016;23:41-49.
- Bolognia JL, Jorizzo JJ, Schaffer JV et al. Dermatology, 4th ed. Philadelphia: Elsevier; 2018.
- Wiesner T, He J, Yelensky R, et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat Commun. 2014;5:3116.
Growing Pink Nodule on the Ankle
Growing Pink Nodule on the Ankle
A 17-year-old girl presented to the dermatology department with a slow-growing lesion on the right lower leg that progressed in size over 1 year. The patient reported that the lesion occasionally bled but denied any other associated symptoms or a personal or family history of skin cancer. Physical examination revealed a solitary, well-circumscribed, circular, pink nodule on the right lateral upper ankle. Dermoscopy showed an amorphous mixture of pale and pink areas. A shave biopsy revealed a proliferation of epithelioid cells that diffusely stained positive for Factor XIIIa and anaplastic lymphoma kinase 1 and stained negatively for pancytokeratin, Melan A, CD34, ERG, CD31, SOX10, smooth muscle actin, desmin, and CD30. Next-generation sequencing revealed a vinculin and anaplastic lymphoma kinase gene fusion.
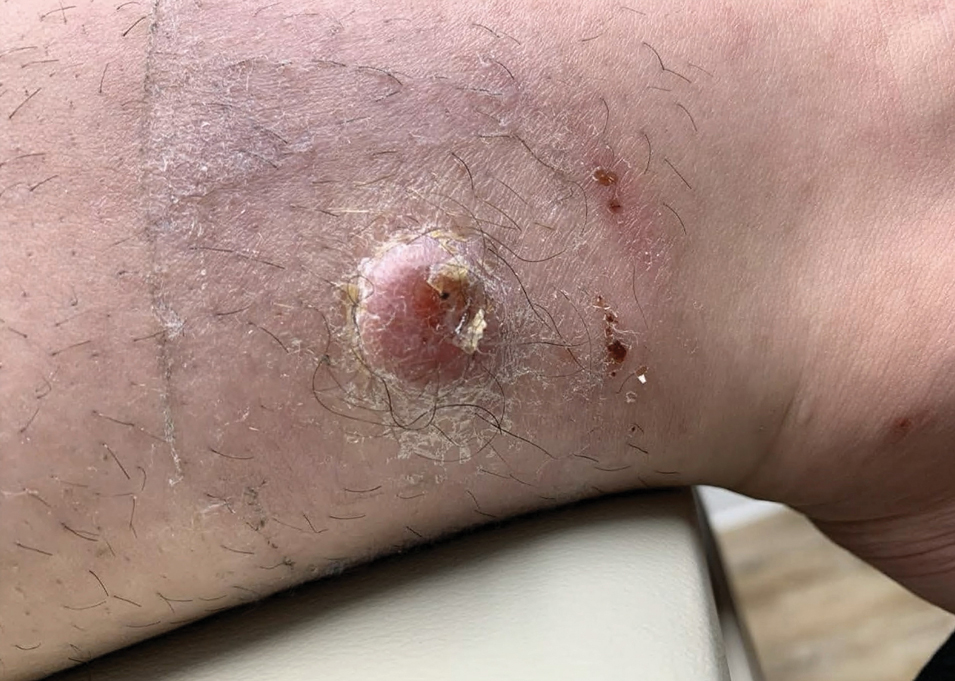
Painful Flesh-Colored Nodule on the Shoulder
Painful Flesh-Colored Nodule on the Shoulder
THE DIAGNOSIS: Dermatofibrosarcoma Protuberans
The histologic findings showed fascicular proliferation of relatively monomorphic spindle cells with extensive entrapment of collagen and adipocytes. Immunohistochemical staining showed that the lesional cells were diffusely positive for CD34 and negative for SOX10, S100, desmin, and factor XIIIa. The decision was made to perform cytogenetic testing with fluorescence in situ hybridization to evaluate for the presence of platelet-derived growth factor receptor beta (PDGFB) polypeptide rearrangement, a key biomarker known to be positive in most patients with dermatofibrosarcoma protuberans (DFSP).1 This rearrangement results in overproduction of PDGFB, continuous activation of platelet-derived growth factor receptor beta, cellular proliferation, and tumor formation.2 In our patient, results were positive for the PDGFB polypeptide rearrangement, which confirmed suspected diagnosis of DFSP with fibrous histiocytoma like morphology. The patient was referred for Mohs micrographic surgery for proper management.
Dermatofibrosarcoma protuberans is a rare soft-tissue tumor that involves the dermis, subcutaneous fat, and sometimes muscle and fascia.2 Dermatofibrosarcoma protuberans primarily affects young to middle-aged adults, with a slight predilection for individuals in the third to fifth decades of life.3 Lesions preferentially involve the trunk, particularly the shoulder and chest regions, and manifest as poorly circumscribed, locally aggressive mesenchymal neoplasms with a high local recurrence rate but low metastatic potential.4,5 Clinically, the lesions appear as flesh-colored, rubbery plaques or nodules. A diagnosis of DFSP requires a high index of clinical suspicion, and histologic, immunohistochemical, and molecular testing usually are required for confirmation.
On histopathologic examination, DFSP classically demonstrates uniform, spindle-shaped cells that traditionally are arranged in an intersecting pattern and primarily are based in the dermis (Figure 1).5 Infiltration into the underlying tissue is a common feature, with neoplastic extensions causing a classic honeycomb pattern6 that also can be seen in diffuse neurofibroma and may cause diagnostic challenges; however, the immunohistology staining of neurofibroma differs from DFSP in that it stains positive for CD34, SOX-100, and S100, while DFSP has strong and diffuse CD34 immunoreactivity with negative immunostaining for SOX10, S100, desmin, and factor XIIIa.2,6
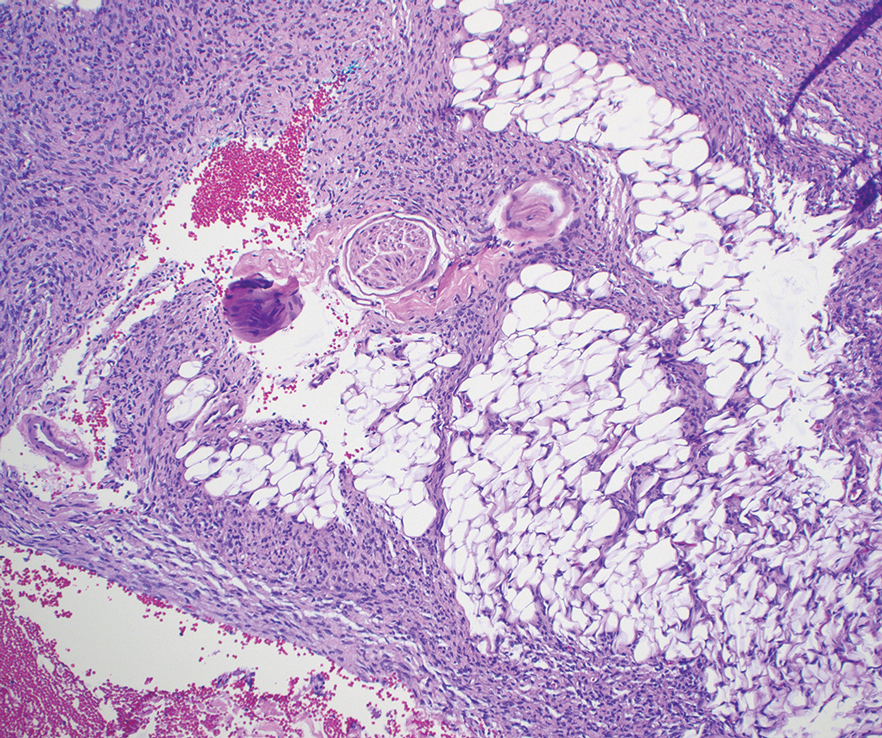
Dermatofibrosarcoma protuberans can cause considerable fat infiltration compared to other soft-tissue neoplasms, making this finding suspicious for—if not characteristic of—DFSP. Collagen trapping also can be observed; however, this is more pathognomonic in cellular fibrous histiocytoma, which is a distinct clinical variant of dermatofibromas. Due to its similarity to other lesions, histopathologic examination along with immunostaining can assist in differentiating and accurately diagnosing DFSP.6
Cellular fibrous histiocytoma (CFH), a distinct clinical variant of dermatofibromas, is a benign tumor of mesenchymal origin that occurs more commonly on the trunk, arms, and legs. On histologic examination, CFH is composed of spindle-shaped cells with variable amounts of eosinophilic cytoplasm and small, oval-shaped eosinophilic nuclei and collagen trapping (Figure 2).7,8 Most CFHs occupy the superficial dermis but can extend into the deep reticular dermis, thus mimicking the honeycomb pattern seen in DFSP. This neoplasm can show a similar architecture to DFSP, which is why further investigation including cytogenetics and immunohistochemical staining can help differentiate the two conditions. Cellular fibrous histiocytoma typically stains negative for CD34 and positive for factor XIIIa.9 However, CD34 can be positive in a subset of CFHs, with a considerable subset showing peripheral CD34 positivity and a smaller subset showing central CD34 the positivity.10 This suggests that CD34 cannot be the only factor differentiating these 2 lesions in making a proper dermatopathologic diagnosis.
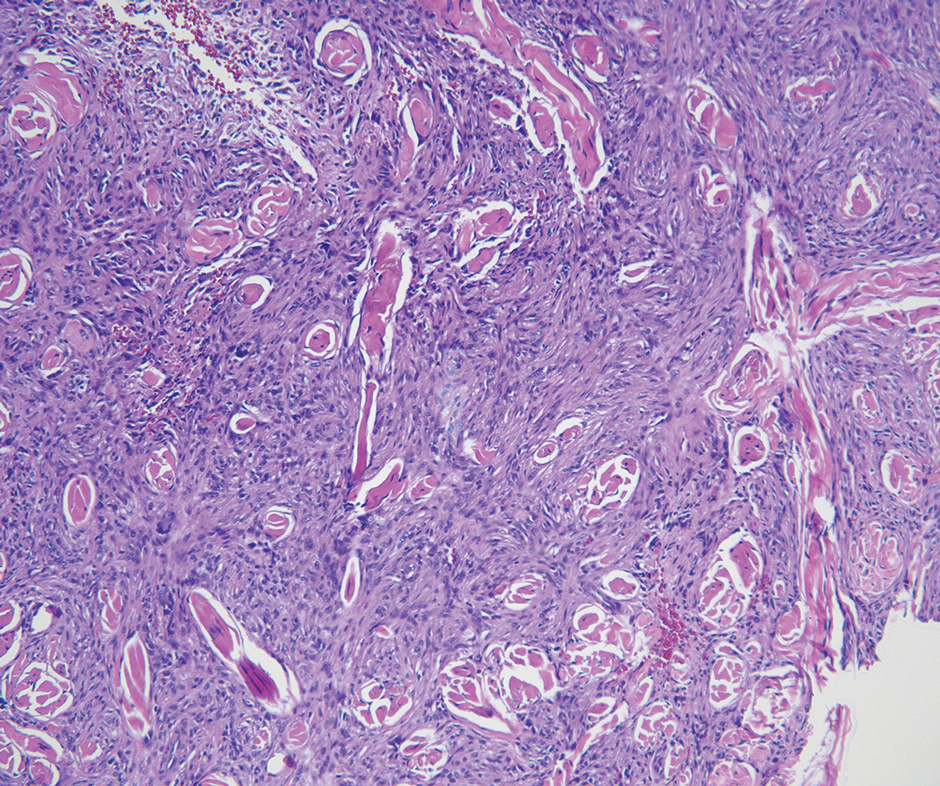
Solitary fibrous tumor (SFT) is a rare mesenchymal tumor that can occur anywhere on the body and typically manifests as a deep, painless, enlarging mass in adults aged 50 to 60 years.11 On histologic examination, SFT consists of randomly arranged cells with a spindle or ovoid shape within a collagenous stroma intermixed with blood vessels with a characteristic staghorn shape (Figure 3).11 Low-grade SFT shows a patternless arrangement with spindle cells, a low number of mitotic figures, and vessels with a staghorn appearance compared to high-grade SFT, which shows hypercellularity with nuclear pleomorphism and a high number of mitotic figures.11 Solitary fibrous tumors are positive for CD34 and STAT-6 and negative for CD31 and typically demonstrate NGFI-A binding protein 2 (NAB2)—signal transducer and activator of transcription 6 (STAT 6) gene fusion.11
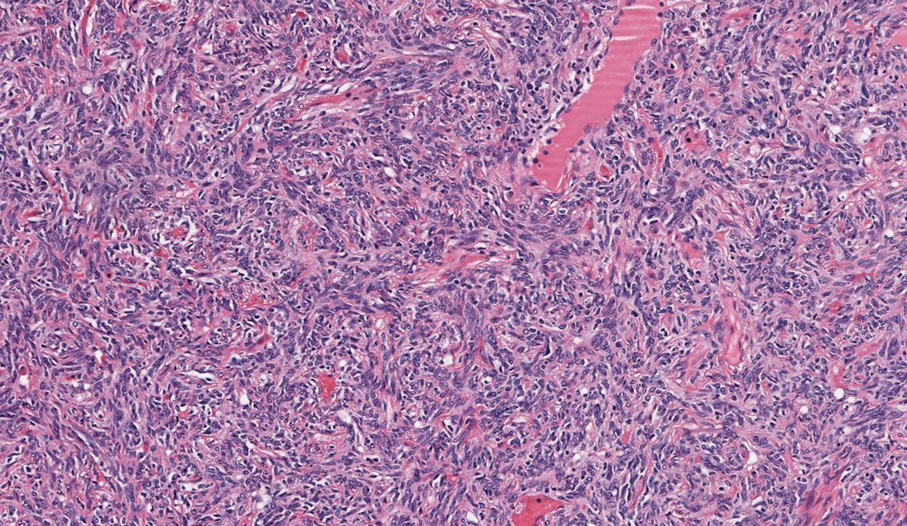
Spindle-cell lipomas are rare, benign, slow-growing, lipomatous tumors that typically manifest in men aged 40 to 70 years.12 These lesions originate most frequently in the subcutaneous tissue of the upper back, posterior neck, and shoulders. The histologic growth pattern of spindle-cell lipomas can mimic other spindle-cell and myxoid tumors, which is why cytogenetic analysis is crucial for differentiating these lesions. On histologic examination, spindle-cell lipomas exhibit a mixture of mature adipocytes, uniform spindle cells, and collagen bundles (eFigure). Spindle-cell lipoma stains positive for CD34 but negative for S100.13 In addition, spindle-cell lipomas tend to show structural rearrangements (mainly deletions) of the long arm of chromosome 13 or even losses of whole chromosome 13, which contains the retinoblastoma (RB1) gene.13
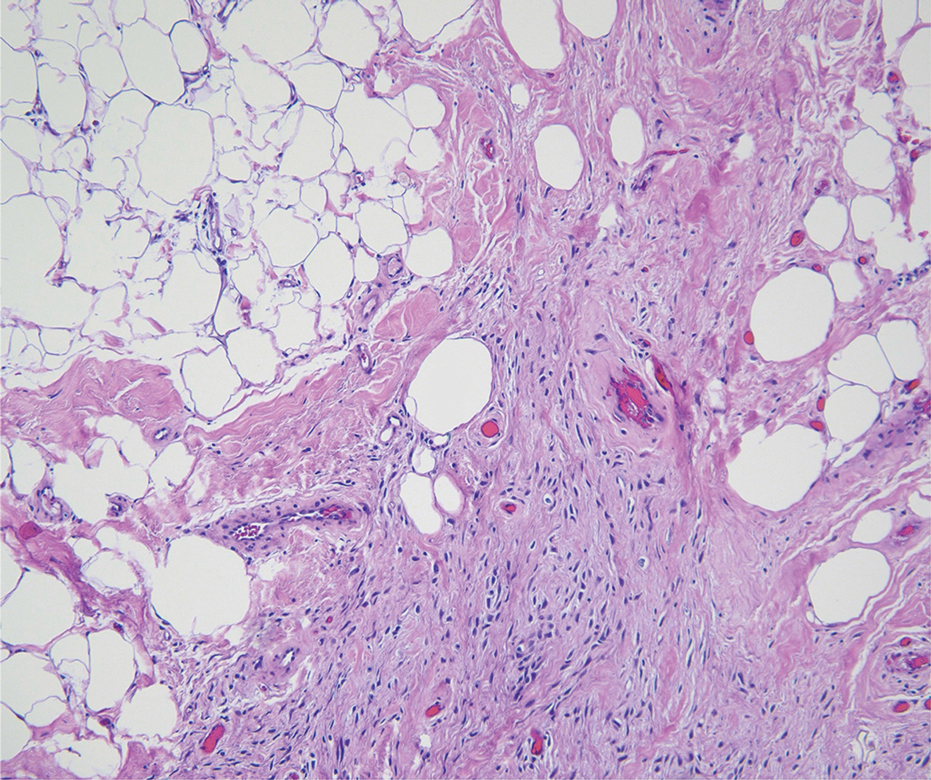
Pleomorphic dermal sarcoma is a rare mesenchymal tumor that can appear clinically and histologically similar to atypical fibroxanthoma.14 This lesion often manifests in elderly patients and is strongly associated with chronic sun exposure.15 Pleomorphic dermal sarcoma is a locally aggressive tumor with metastatic potential to the skin or lymph nodes. On histologic examination, these tumors exhibit pleomorphic atypical epithelioid or spindle cells as well as multinucleated tumor giant cells with possible tumor necrosis, lymphovascular invasion, or perineural infiltration (Figure 4). Pleomorphic dermal sarcoma, typically a diagnosis of exclusion, requires immunohistochemistry to aid in proper identification.16 These lesions stain positive for CD10 and negative for cytokeratins, desmin, HMB45, CD34, p63, p40, SOX10, and S100.15,16
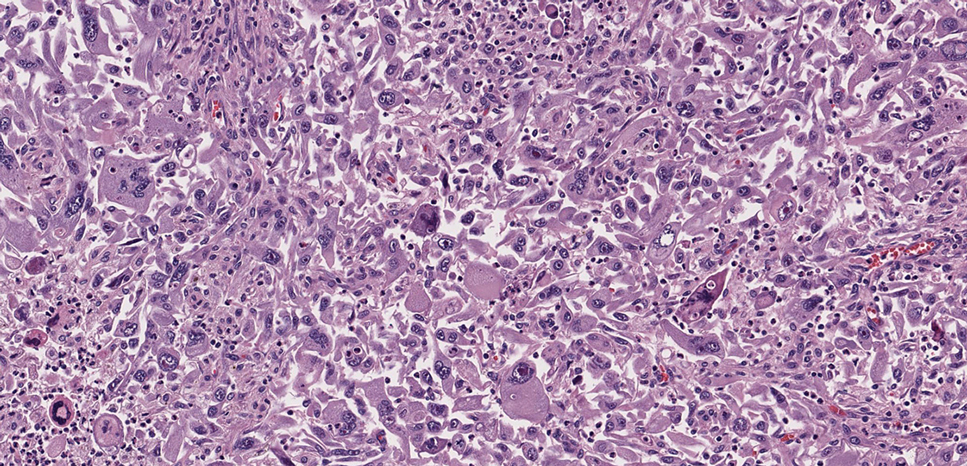
- Ugurel S, Kortmann R, Mohr P, et al. S1 guidelines for dermatofibrosarcoma protuberans (DFSP)—update 2018. J Dtsch Dermatol Ges. 2019;17:663-668. doi:10.1111/ddg.13849
- Brooks J, Ramsey ML. Dermatofibrosarcoma protuberans. StatPearls Publishing; 2024. Updated April 18, 2024. Accessed April 30, 2025.
- Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88:2711-2720.
- Lim SX, Ramaiya A, Levell NJ, et al. Review of dermatofibrosarcoma protuberans. Clin Exp Dermatol. 2022;48:297-302. doi:10.1093/ced/llac111
- Trinidad CM, Wangsiricharoen S, Prieto VG, et al. Rare variants of dermatofibrosarcoma protuberans: clinical, histologic, and molecular features and diagnostic pitfalls. Dermatopathology. 2023;10:54-62. doi:10.3390/dermatopathology10010008
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Tsunoda K, Oikawa H, Maeda F, et al. A case of cellular fibrous histiocytoma on the right elbow with repeated relapse within a short period. Case Rep Dermatol. 2015;7:10–16. https://doi.org/10.1159/000371790
- Calonje E, Mentzel T, Fletcher CD. Cellular benign fibrous histiocytoma. Clinicopathologic analysis of 74 cases of a distinctive variant of cutaneous fibrous histiocytoma with frequent recurrence. Am J Surg Pathol. 1994;18:668-676.
- Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathology. 1997;19:147-153. doi:10.1097/00000372-199704000-00008
- Volpicelli ER, Fletcher CD. Desmin and CD34 positivity in cellular fibrous histiocytoma: an immunohistochemical analysis of 100 cases. J Cutan Pathol. 2012;39:747-752. doi:10.1111/j.1600-0560.2012.01944.x
- Martin-Broto J, Mondaza-Hernandez JL, Moura DS, et al. A comprehensive review on solitary fibrous tumor: new insights for new horizons. Cancers (Basel). 2021;13:2913. doi:10.3390/cancers13122913
- Machol JA, Cusic JG, O’Connor EA, et al. Spindle cell lipoma of the neck: review of the literature and case report. Plast Reconstr Surg Glob Open. 2015;3:E550. doi:10.1097/GOX.0000000000000405
- Domanski HA, Carlén B, Jonsson K, et al. Distinct cytologic features of spindle cell lipoma. a cytologic-histologic study with clinical, radiologic, electron microscopic, and cytogenetic correlations. Cancer. 2001;93:381-389. doi:10.1002/cncr.10142
- Devine RL, Cameron A, Holden AM, et al. The pleomorphic dermal sarcoma: its management, follow-up and the need for more guidance. Adv Oral Maxillofac Surg. 2021;2:100046. doi:10.1016 /j.adoms.2021.100046
- Seretis K, Klaroudas A, Galani V, et al. Pleomorphic dermal sarcoma: it might be rare but it exists [published online August 4, 2023]. J Surg Case Rep. doi:10.1093/jscr/rjad374
- Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma. Am J Surg Pathol. 2012;36:1317-1326. doi:10.1097/pas.0b013e31825359e1
THE DIAGNOSIS: Dermatofibrosarcoma Protuberans
The histologic findings showed fascicular proliferation of relatively monomorphic spindle cells with extensive entrapment of collagen and adipocytes. Immunohistochemical staining showed that the lesional cells were diffusely positive for CD34 and negative for SOX10, S100, desmin, and factor XIIIa. The decision was made to perform cytogenetic testing with fluorescence in situ hybridization to evaluate for the presence of platelet-derived growth factor receptor beta (PDGFB) polypeptide rearrangement, a key biomarker known to be positive in most patients with dermatofibrosarcoma protuberans (DFSP).1 This rearrangement results in overproduction of PDGFB, continuous activation of platelet-derived growth factor receptor beta, cellular proliferation, and tumor formation.2 In our patient, results were positive for the PDGFB polypeptide rearrangement, which confirmed suspected diagnosis of DFSP with fibrous histiocytoma like morphology. The patient was referred for Mohs micrographic surgery for proper management.
Dermatofibrosarcoma protuberans is a rare soft-tissue tumor that involves the dermis, subcutaneous fat, and sometimes muscle and fascia.2 Dermatofibrosarcoma protuberans primarily affects young to middle-aged adults, with a slight predilection for individuals in the third to fifth decades of life.3 Lesions preferentially involve the trunk, particularly the shoulder and chest regions, and manifest as poorly circumscribed, locally aggressive mesenchymal neoplasms with a high local recurrence rate but low metastatic potential.4,5 Clinically, the lesions appear as flesh-colored, rubbery plaques or nodules. A diagnosis of DFSP requires a high index of clinical suspicion, and histologic, immunohistochemical, and molecular testing usually are required for confirmation.
On histopathologic examination, DFSP classically demonstrates uniform, spindle-shaped cells that traditionally are arranged in an intersecting pattern and primarily are based in the dermis (Figure 1).5 Infiltration into the underlying tissue is a common feature, with neoplastic extensions causing a classic honeycomb pattern6 that also can be seen in diffuse neurofibroma and may cause diagnostic challenges; however, the immunohistology staining of neurofibroma differs from DFSP in that it stains positive for CD34, SOX-100, and S100, while DFSP has strong and diffuse CD34 immunoreactivity with negative immunostaining for SOX10, S100, desmin, and factor XIIIa.2,6

Dermatofibrosarcoma protuberans can cause considerable fat infiltration compared to other soft-tissue neoplasms, making this finding suspicious for—if not characteristic of—DFSP. Collagen trapping also can be observed; however, this is more pathognomonic in cellular fibrous histiocytoma, which is a distinct clinical variant of dermatofibromas. Due to its similarity to other lesions, histopathologic examination along with immunostaining can assist in differentiating and accurately diagnosing DFSP.6
Cellular fibrous histiocytoma (CFH), a distinct clinical variant of dermatofibromas, is a benign tumor of mesenchymal origin that occurs more commonly on the trunk, arms, and legs. On histologic examination, CFH is composed of spindle-shaped cells with variable amounts of eosinophilic cytoplasm and small, oval-shaped eosinophilic nuclei and collagen trapping (Figure 2).7,8 Most CFHs occupy the superficial dermis but can extend into the deep reticular dermis, thus mimicking the honeycomb pattern seen in DFSP. This neoplasm can show a similar architecture to DFSP, which is why further investigation including cytogenetics and immunohistochemical staining can help differentiate the two conditions. Cellular fibrous histiocytoma typically stains negative for CD34 and positive for factor XIIIa.9 However, CD34 can be positive in a subset of CFHs, with a considerable subset showing peripheral CD34 positivity and a smaller subset showing central CD34 the positivity.10 This suggests that CD34 cannot be the only factor differentiating these 2 lesions in making a proper dermatopathologic diagnosis.

Solitary fibrous tumor (SFT) is a rare mesenchymal tumor that can occur anywhere on the body and typically manifests as a deep, painless, enlarging mass in adults aged 50 to 60 years.11 On histologic examination, SFT consists of randomly arranged cells with a spindle or ovoid shape within a collagenous stroma intermixed with blood vessels with a characteristic staghorn shape (Figure 3).11 Low-grade SFT shows a patternless arrangement with spindle cells, a low number of mitotic figures, and vessels with a staghorn appearance compared to high-grade SFT, which shows hypercellularity with nuclear pleomorphism and a high number of mitotic figures.11 Solitary fibrous tumors are positive for CD34 and STAT-6 and negative for CD31 and typically demonstrate NGFI-A binding protein 2 (NAB2)—signal transducer and activator of transcription 6 (STAT 6) gene fusion.11

Spindle-cell lipomas are rare, benign, slow-growing, lipomatous tumors that typically manifest in men aged 40 to 70 years.12 These lesions originate most frequently in the subcutaneous tissue of the upper back, posterior neck, and shoulders. The histologic growth pattern of spindle-cell lipomas can mimic other spindle-cell and myxoid tumors, which is why cytogenetic analysis is crucial for differentiating these lesions. On histologic examination, spindle-cell lipomas exhibit a mixture of mature adipocytes, uniform spindle cells, and collagen bundles (eFigure). Spindle-cell lipoma stains positive for CD34 but negative for S100.13 In addition, spindle-cell lipomas tend to show structural rearrangements (mainly deletions) of the long arm of chromosome 13 or even losses of whole chromosome 13, which contains the retinoblastoma (RB1) gene.13

Pleomorphic dermal sarcoma is a rare mesenchymal tumor that can appear clinically and histologically similar to atypical fibroxanthoma.14 This lesion often manifests in elderly patients and is strongly associated with chronic sun exposure.15 Pleomorphic dermal sarcoma is a locally aggressive tumor with metastatic potential to the skin or lymph nodes. On histologic examination, these tumors exhibit pleomorphic atypical epithelioid or spindle cells as well as multinucleated tumor giant cells with possible tumor necrosis, lymphovascular invasion, or perineural infiltration (Figure 4). Pleomorphic dermal sarcoma, typically a diagnosis of exclusion, requires immunohistochemistry to aid in proper identification.16 These lesions stain positive for CD10 and negative for cytokeratins, desmin, HMB45, CD34, p63, p40, SOX10, and S100.15,16

THE DIAGNOSIS: Dermatofibrosarcoma Protuberans
The histologic findings showed fascicular proliferation of relatively monomorphic spindle cells with extensive entrapment of collagen and adipocytes. Immunohistochemical staining showed that the lesional cells were diffusely positive for CD34 and negative for SOX10, S100, desmin, and factor XIIIa. The decision was made to perform cytogenetic testing with fluorescence in situ hybridization to evaluate for the presence of platelet-derived growth factor receptor beta (PDGFB) polypeptide rearrangement, a key biomarker known to be positive in most patients with dermatofibrosarcoma protuberans (DFSP).1 This rearrangement results in overproduction of PDGFB, continuous activation of platelet-derived growth factor receptor beta, cellular proliferation, and tumor formation.2 In our patient, results were positive for the PDGFB polypeptide rearrangement, which confirmed suspected diagnosis of DFSP with fibrous histiocytoma like morphology. The patient was referred for Mohs micrographic surgery for proper management.
Dermatofibrosarcoma protuberans is a rare soft-tissue tumor that involves the dermis, subcutaneous fat, and sometimes muscle and fascia.2 Dermatofibrosarcoma protuberans primarily affects young to middle-aged adults, with a slight predilection for individuals in the third to fifth decades of life.3 Lesions preferentially involve the trunk, particularly the shoulder and chest regions, and manifest as poorly circumscribed, locally aggressive mesenchymal neoplasms with a high local recurrence rate but low metastatic potential.4,5 Clinically, the lesions appear as flesh-colored, rubbery plaques or nodules. A diagnosis of DFSP requires a high index of clinical suspicion, and histologic, immunohistochemical, and molecular testing usually are required for confirmation.
On histopathologic examination, DFSP classically demonstrates uniform, spindle-shaped cells that traditionally are arranged in an intersecting pattern and primarily are based in the dermis (Figure 1).5 Infiltration into the underlying tissue is a common feature, with neoplastic extensions causing a classic honeycomb pattern6 that also can be seen in diffuse neurofibroma and may cause diagnostic challenges; however, the immunohistology staining of neurofibroma differs from DFSP in that it stains positive for CD34, SOX-100, and S100, while DFSP has strong and diffuse CD34 immunoreactivity with negative immunostaining for SOX10, S100, desmin, and factor XIIIa.2,6

Dermatofibrosarcoma protuberans can cause considerable fat infiltration compared to other soft-tissue neoplasms, making this finding suspicious for—if not characteristic of—DFSP. Collagen trapping also can be observed; however, this is more pathognomonic in cellular fibrous histiocytoma, which is a distinct clinical variant of dermatofibromas. Due to its similarity to other lesions, histopathologic examination along with immunostaining can assist in differentiating and accurately diagnosing DFSP.6
Cellular fibrous histiocytoma (CFH), a distinct clinical variant of dermatofibromas, is a benign tumor of mesenchymal origin that occurs more commonly on the trunk, arms, and legs. On histologic examination, CFH is composed of spindle-shaped cells with variable amounts of eosinophilic cytoplasm and small, oval-shaped eosinophilic nuclei and collagen trapping (Figure 2).7,8 Most CFHs occupy the superficial dermis but can extend into the deep reticular dermis, thus mimicking the honeycomb pattern seen in DFSP. This neoplasm can show a similar architecture to DFSP, which is why further investigation including cytogenetics and immunohistochemical staining can help differentiate the two conditions. Cellular fibrous histiocytoma typically stains negative for CD34 and positive for factor XIIIa.9 However, CD34 can be positive in a subset of CFHs, with a considerable subset showing peripheral CD34 positivity and a smaller subset showing central CD34 the positivity.10 This suggests that CD34 cannot be the only factor differentiating these 2 lesions in making a proper dermatopathologic diagnosis.

Solitary fibrous tumor (SFT) is a rare mesenchymal tumor that can occur anywhere on the body and typically manifests as a deep, painless, enlarging mass in adults aged 50 to 60 years.11 On histologic examination, SFT consists of randomly arranged cells with a spindle or ovoid shape within a collagenous stroma intermixed with blood vessels with a characteristic staghorn shape (Figure 3).11 Low-grade SFT shows a patternless arrangement with spindle cells, a low number of mitotic figures, and vessels with a staghorn appearance compared to high-grade SFT, which shows hypercellularity with nuclear pleomorphism and a high number of mitotic figures.11 Solitary fibrous tumors are positive for CD34 and STAT-6 and negative for CD31 and typically demonstrate NGFI-A binding protein 2 (NAB2)—signal transducer and activator of transcription 6 (STAT 6) gene fusion.11

Spindle-cell lipomas are rare, benign, slow-growing, lipomatous tumors that typically manifest in men aged 40 to 70 years.12 These lesions originate most frequently in the subcutaneous tissue of the upper back, posterior neck, and shoulders. The histologic growth pattern of spindle-cell lipomas can mimic other spindle-cell and myxoid tumors, which is why cytogenetic analysis is crucial for differentiating these lesions. On histologic examination, spindle-cell lipomas exhibit a mixture of mature adipocytes, uniform spindle cells, and collagen bundles (eFigure). Spindle-cell lipoma stains positive for CD34 but negative for S100.13 In addition, spindle-cell lipomas tend to show structural rearrangements (mainly deletions) of the long arm of chromosome 13 or even losses of whole chromosome 13, which contains the retinoblastoma (RB1) gene.13

Pleomorphic dermal sarcoma is a rare mesenchymal tumor that can appear clinically and histologically similar to atypical fibroxanthoma.14 This lesion often manifests in elderly patients and is strongly associated with chronic sun exposure.15 Pleomorphic dermal sarcoma is a locally aggressive tumor with metastatic potential to the skin or lymph nodes. On histologic examination, these tumors exhibit pleomorphic atypical epithelioid or spindle cells as well as multinucleated tumor giant cells with possible tumor necrosis, lymphovascular invasion, or perineural infiltration (Figure 4). Pleomorphic dermal sarcoma, typically a diagnosis of exclusion, requires immunohistochemistry to aid in proper identification.16 These lesions stain positive for CD10 and negative for cytokeratins, desmin, HMB45, CD34, p63, p40, SOX10, and S100.15,16

- Ugurel S, Kortmann R, Mohr P, et al. S1 guidelines for dermatofibrosarcoma protuberans (DFSP)—update 2018. J Dtsch Dermatol Ges. 2019;17:663-668. doi:10.1111/ddg.13849
- Brooks J, Ramsey ML. Dermatofibrosarcoma protuberans. StatPearls Publishing; 2024. Updated April 18, 2024. Accessed April 30, 2025.
- Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88:2711-2720.
- Lim SX, Ramaiya A, Levell NJ, et al. Review of dermatofibrosarcoma protuberans. Clin Exp Dermatol. 2022;48:297-302. doi:10.1093/ced/llac111
- Trinidad CM, Wangsiricharoen S, Prieto VG, et al. Rare variants of dermatofibrosarcoma protuberans: clinical, histologic, and molecular features and diagnostic pitfalls. Dermatopathology. 2023;10:54-62. doi:10.3390/dermatopathology10010008
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Tsunoda K, Oikawa H, Maeda F, et al. A case of cellular fibrous histiocytoma on the right elbow with repeated relapse within a short period. Case Rep Dermatol. 2015;7:10–16. https://doi.org/10.1159/000371790
- Calonje E, Mentzel T, Fletcher CD. Cellular benign fibrous histiocytoma. Clinicopathologic analysis of 74 cases of a distinctive variant of cutaneous fibrous histiocytoma with frequent recurrence. Am J Surg Pathol. 1994;18:668-676.
- Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathology. 1997;19:147-153. doi:10.1097/00000372-199704000-00008
- Volpicelli ER, Fletcher CD. Desmin and CD34 positivity in cellular fibrous histiocytoma: an immunohistochemical analysis of 100 cases. J Cutan Pathol. 2012;39:747-752. doi:10.1111/j.1600-0560.2012.01944.x
- Martin-Broto J, Mondaza-Hernandez JL, Moura DS, et al. A comprehensive review on solitary fibrous tumor: new insights for new horizons. Cancers (Basel). 2021;13:2913. doi:10.3390/cancers13122913
- Machol JA, Cusic JG, O’Connor EA, et al. Spindle cell lipoma of the neck: review of the literature and case report. Plast Reconstr Surg Glob Open. 2015;3:E550. doi:10.1097/GOX.0000000000000405
- Domanski HA, Carlén B, Jonsson K, et al. Distinct cytologic features of spindle cell lipoma. a cytologic-histologic study with clinical, radiologic, electron microscopic, and cytogenetic correlations. Cancer. 2001;93:381-389. doi:10.1002/cncr.10142
- Devine RL, Cameron A, Holden AM, et al. The pleomorphic dermal sarcoma: its management, follow-up and the need for more guidance. Adv Oral Maxillofac Surg. 2021;2:100046. doi:10.1016 /j.adoms.2021.100046
- Seretis K, Klaroudas A, Galani V, et al. Pleomorphic dermal sarcoma: it might be rare but it exists [published online August 4, 2023]. J Surg Case Rep. doi:10.1093/jscr/rjad374
- Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma. Am J Surg Pathol. 2012;36:1317-1326. doi:10.1097/pas.0b013e31825359e1
- Ugurel S, Kortmann R, Mohr P, et al. S1 guidelines for dermatofibrosarcoma protuberans (DFSP)—update 2018. J Dtsch Dermatol Ges. 2019;17:663-668. doi:10.1111/ddg.13849
- Brooks J, Ramsey ML. Dermatofibrosarcoma protuberans. StatPearls Publishing; 2024. Updated April 18, 2024. Accessed April 30, 2025.
- Bowne WB, Antonescu CR, Leung DH, et al. Dermatofibrosarcoma protuberans: a clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88:2711-2720.
- Lim SX, Ramaiya A, Levell NJ, et al. Review of dermatofibrosarcoma protuberans. Clin Exp Dermatol. 2022;48:297-302. doi:10.1093/ced/llac111
- Trinidad CM, Wangsiricharoen S, Prieto VG, et al. Rare variants of dermatofibrosarcoma protuberans: clinical, histologic, and molecular features and diagnostic pitfalls. Dermatopathology. 2023;10:54-62. doi:10.3390/dermatopathology10010008
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752. doi:10.3390/jcm9061752
- Tsunoda K, Oikawa H, Maeda F, et al. A case of cellular fibrous histiocytoma on the right elbow with repeated relapse within a short period. Case Rep Dermatol. 2015;7:10–16. https://doi.org/10.1159/000371790
- Calonje E, Mentzel T, Fletcher CD. Cellular benign fibrous histiocytoma. Clinicopathologic analysis of 74 cases of a distinctive variant of cutaneous fibrous histiocytoma with frequent recurrence. Am J Surg Pathol. 1994;18:668-676.
- Goldblum JR, Tuthill RJ. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am J Dermatopathology. 1997;19:147-153. doi:10.1097/00000372-199704000-00008
- Volpicelli ER, Fletcher CD. Desmin and CD34 positivity in cellular fibrous histiocytoma: an immunohistochemical analysis of 100 cases. J Cutan Pathol. 2012;39:747-752. doi:10.1111/j.1600-0560.2012.01944.x
- Martin-Broto J, Mondaza-Hernandez JL, Moura DS, et al. A comprehensive review on solitary fibrous tumor: new insights for new horizons. Cancers (Basel). 2021;13:2913. doi:10.3390/cancers13122913
- Machol JA, Cusic JG, O’Connor EA, et al. Spindle cell lipoma of the neck: review of the literature and case report. Plast Reconstr Surg Glob Open. 2015;3:E550. doi:10.1097/GOX.0000000000000405
- Domanski HA, Carlén B, Jonsson K, et al. Distinct cytologic features of spindle cell lipoma. a cytologic-histologic study with clinical, radiologic, electron microscopic, and cytogenetic correlations. Cancer. 2001;93:381-389. doi:10.1002/cncr.10142
- Devine RL, Cameron A, Holden AM, et al. The pleomorphic dermal sarcoma: its management, follow-up and the need for more guidance. Adv Oral Maxillofac Surg. 2021;2:100046. doi:10.1016 /j.adoms.2021.100046
- Seretis K, Klaroudas A, Galani V, et al. Pleomorphic dermal sarcoma: it might be rare but it exists [published online August 4, 2023]. J Surg Case Rep. doi:10.1093/jscr/rjad374
- Miller K, Goodlad JR, Brenn T. Pleomorphic dermal sarcoma. Am J Surg Pathol. 2012;36:1317-1326. doi:10.1097/pas.0b013e31825359e1
Painful Flesh-Colored Nodule on the Shoulder
Painful Flesh-Colored Nodule on the Shoulder
A 26-year-old man with no notable medical history presented to the dermatology clinic with an inconspicuous, painful, raised lesion on the right posterior shoulder of 6 months’ duration. The patient reported that the lesion was tender to light palpation and bothersome in his daily activities. Physical examination revealed a firm, flesh-colored, 1.8-cm nodule with no erythema or pigmentation on the right shoulder. An elliptical excisional biopsy was performed and submitted for histologic evaluation.
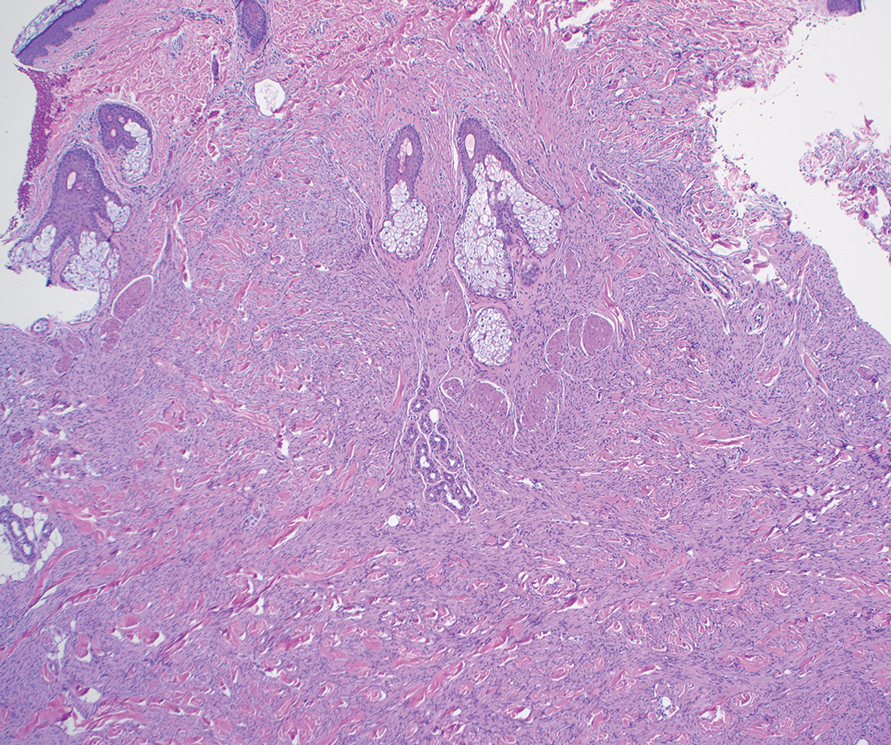
Plaque With Central Ulceration on the Abdomen
Plaque With Central Ulceration on the Abdomen
THE DIAGNOSIS: Plaquelike Myofibroblastic Tumor
An incisional biopsy of the plaque demonstrated a hypercellular proliferation of bland spindle cells in the dermis that infiltrated the subcutis. The overlying epidermis was mildly acanthotic with both ulceration and follicular induction. There was trapping of individual adipocytes in a honeycomb pattern with foci of erythrocyte extravasation, microvesiculation, and widened fibrous septa (Figure 1). Immunohistochemistry was positive for vimentin, actin, and smooth muscle actin (SMA)(Figure 2A). Variable positivity for Factor XIIIa antibodies was noted. CD68 staining was focal positive, suggesting fibrohistiocytic lineage. Expression of CD31, CD34, S100, and anaplastic lymphoma kinase was negative, and Ki-67 was present in less than 10% of cells (Figure 2B).
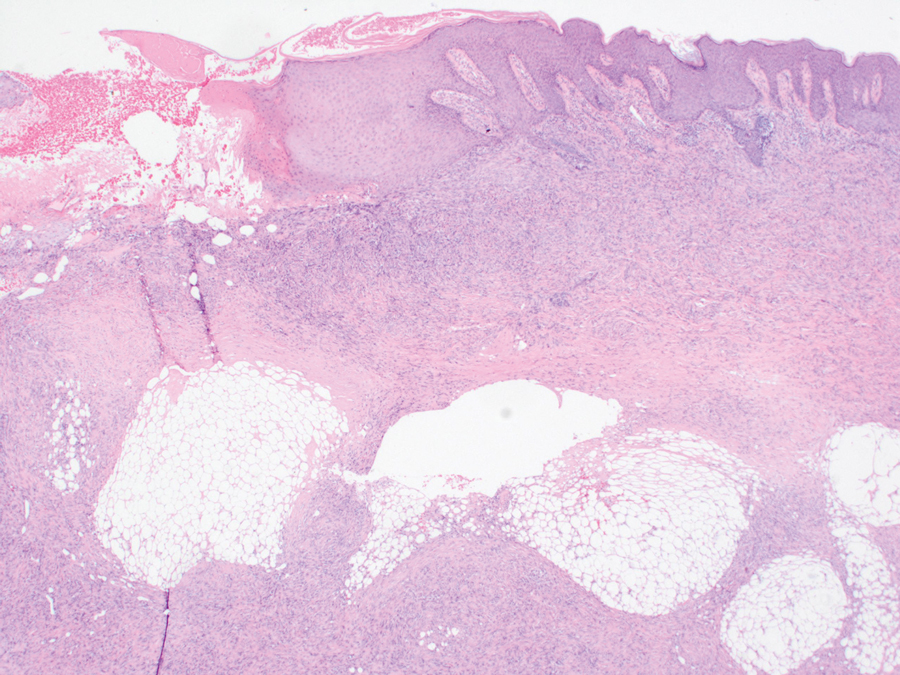
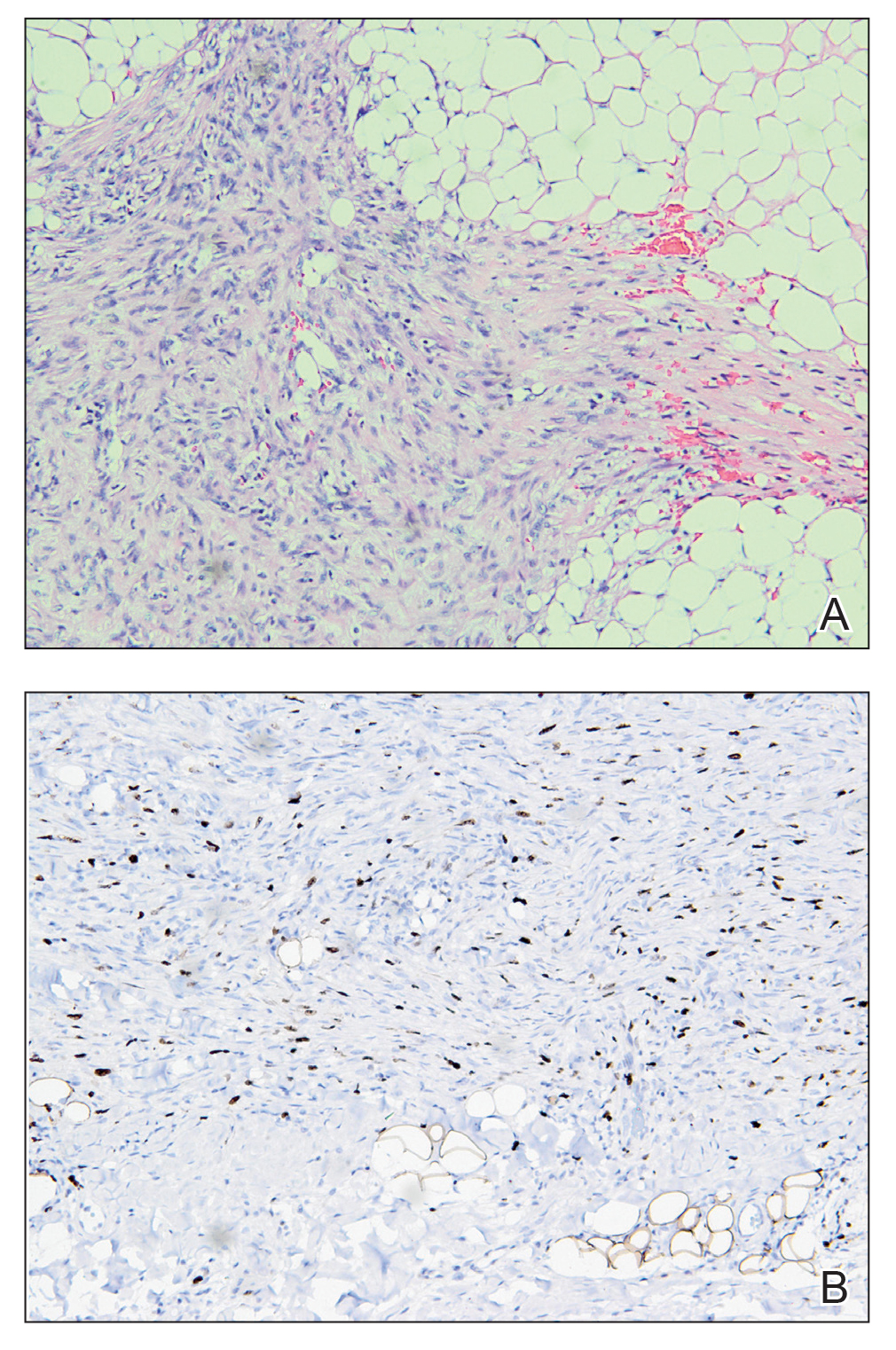
We reviewed the case in conjunction with a soft-tissue pathologist (Y.L.), and based on the clinical and immunophenotypic features, a diagnosis of plaquelike myofibroblastic tumor (PLMT) was made. The patient’s parents refused further treatment, and there was no sign of disease progression at 6-month follow-up.
Plaquelike myofibroblastic tumor is an unusual pediatric dermal tumor that was first described by Clarke et al1 in 2007. Clinical manifestation of PLMT on the right abdomen was unique in our patient, as the lesions typically present as indurated plaques on the lower back, but the central ulceration in our case resembled a report by Marqueling et al.2 Ulceration and induration of PLMT developing at 8 months of age can suggest an aggressive disease course corresponding with deep infiltration and is seen mostly in children.
The histopathologic features of PLMT include an acanthotic epidermis and follicular induction, which also are characteristic of dermatofibroma (DF). The proliferation of spindle cells extended deep into the fat with foci of erythrocyte extravasation and microvesiculation of the stroma similar to nodular fasciitis and proliferative fasciitis. The presentation of infiltrating and expanding fibrous septae and trapping of individual adipocytes in a honeycomb pattern is similar to dermatofibrosarcoma protuberans (DFSP). Most cases of PLMT are positive for SMA. Factor XIIIa typically is variably positive, and in one report, 31% (4/13) of cases showed positive staining for calponin.3 Rapid growth, ulceration, and recurrence emphasize that PLMT can be locally aggressive, similar to DFSP.4
The main differential diagnoses include DF and its variants, dermatomyofibroma, DFSP, and proliferative fasciitis.3,5 In the cases mentioned above, microscopic features were similar with a relatively well-circumscribed proliferation of spindle cells arranged in short fascicles through the entire reticular dermis, and the overlying epidermis was acanthotic.
Dermatofibroma commonly manifests in adults as a minor nodular lesion (commonly <1 cm), and usually is located on the legs. It has several clinical and histologic variants, including multiple clustered DF (MCDF)—a rare condition that has been reported in children and young adults and generally appears in the first and second decades of life. Of the reported cases of MCDF, immunohistochemical staining for SMA was performed in 8 cases. All these cases showed negative or minimal staining.3-5 Smooth muscle actin staining in DFs is negative, or weak and patchy, unlike in PLMT where it is diffuse, uniform, and strong.
Dermatofibrosarcoma protuberans typically occurs in young adults and manifests as dermal and subcutaneous nodular/multinodular or plaquelike masses, with rare congenital cases. Immunohistochemical staining for CD34, which typically is firmly and diffusely positive, is the most reliable marker of DFSP.6 Factor XIIIA in DFSP typically is negative for focal staining, mainly at periphery or in scattered dendritic cells. The prognosis of DFSP generally is excellent, with local recurrences in up to 30% of cases and extremely low metastatic potential (essentially only in cases with fibrosarcomatous transformation).6 Dermatomyofibroma is another rare benign dermal myofibroblastic tumor that typically manifests with indurated hyperpigmented or erythematous plaques or nodules on the shoulders and torso.6 This condition occurs mainly in adolescents and young adults, unlike PLMT. The most striking features of dermatomyofibroma are the horizontal orientation of the spindle cell nuclei and the pattern of the proliferation concerning the adnexal structures, especially hair follicles. The hair follicles have a normal appearance, and the proliferation extends up to each follicle, then continues to the other side without any displacement of the follicle. Tumor cells are variably positive for SMA in dermatomyofibromas and are negative for muscle-specific actin, desmin, S100, CD34, and Factor XIIIA.6
Immunohistochemistry can be very useful in differentiating PLMT from other conditions. Neoplastic cells stain positively for CD34 but not for Factor XIIIa and SMA in cases of DFSP. Dermatofibroma and its variants always present with collagen trapping at the periphery of the lesions and may demonstrate foamy macrophages, hemosiderin, or plasma cells FXIIIA(+), CD34(-), and variable SMA reactivity. This positivity usually is less prominent in DF than in PLMT. Neoplastic cells in dermatomyofibroma often stain positive for calponin, but only focally for SMA. The clinical features of dermatomyofibroma include early onset, large size, multiple nodules, and plaquelike morphology. Moulonguet et al4 hypothesized that, although MCDF and PLMT appear to show some distinctive clinical and histologic features, they also show similarities that could suggest they form part of the myofibroblastic spectrum. Furthermore, Moradi et al7 also considered them as part of the same disease spectrum because of their overlapping clinical, histologic, and immunohistochemical features.
The microscopic features in our case are notable, as the lesion demonstrated overlying acanthosis and follicular induction, resembling DF. The stroma contained microvesicular changes and erythrocyte extravasation, characteristic of nodular or proliferative fasciitis. Additionally, densely packed spindle cells infiltrated deep into the subcutaneous adipose tissue, similar to DFSP.2,3 Our findings expand on the reported histopathologic spectrum of this tumor to date.
- Clarke JT, Clarke LE, Miller C, et al. Plaque-like myofibroblastic tumor of infancy. Pediatr Dermatol. 2007;24:E83-E87. doi:10.1111 /j.1525-1470.2007.00449.x
- Marqueling AL, Dasher D, Friedlander SF, et al. Plaque-like myofibroblastic tumor: report of three cases. Pediatr Dermatol. 2013;30:600-607. doi:10.1111/pde.12185
- Sekar T, Mushtaq J, AlBadry W, et al. Plaque-like myofibroblastic tumor: a series of 2 cases of this unusual dermal tumor which occurs in infancy and early childhood. Pediatr Dev Pathol. 2018;21:444-448. doi: 10.1177/1093526617746807
- Moulonguet I, Biaggi A, Eschard C, et al. Plaque-like myofibroblastic tumor: report of 4 cases. Am J Dermatopathol. 2017;39:767-772. doi: 10.1097/DAD.0000000000000869
- Virdi A, Baraldi C, Barisani A, et al. Plaque-like myofibroblastic tumor, a rare entity of childhood: possible pitfalls in differential diagnosis. J Cutan Pathol. 2019;46:389-392. doi:10.1111/cup.13441
- Cassarino DS. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2021.
- Moradi S, Mnayer L, Earle J, et al. Plaque-like dermatofibroma: case report of a rare entity. Dermatopathology (Basel). 2021;8:337-341. doi:10.3390/dermatopathology8030038
THE DIAGNOSIS: Plaquelike Myofibroblastic Tumor
An incisional biopsy of the plaque demonstrated a hypercellular proliferation of bland spindle cells in the dermis that infiltrated the subcutis. The overlying epidermis was mildly acanthotic with both ulceration and follicular induction. There was trapping of individual adipocytes in a honeycomb pattern with foci of erythrocyte extravasation, microvesiculation, and widened fibrous septa (Figure 1). Immunohistochemistry was positive for vimentin, actin, and smooth muscle actin (SMA)(Figure 2A). Variable positivity for Factor XIIIa antibodies was noted. CD68 staining was focal positive, suggesting fibrohistiocytic lineage. Expression of CD31, CD34, S100, and anaplastic lymphoma kinase was negative, and Ki-67 was present in less than 10% of cells (Figure 2B).


We reviewed the case in conjunction with a soft-tissue pathologist (Y.L.), and based on the clinical and immunophenotypic features, a diagnosis of plaquelike myofibroblastic tumor (PLMT) was made. The patient’s parents refused further treatment, and there was no sign of disease progression at 6-month follow-up.
Plaquelike myofibroblastic tumor is an unusual pediatric dermal tumor that was first described by Clarke et al1 in 2007. Clinical manifestation of PLMT on the right abdomen was unique in our patient, as the lesions typically present as indurated plaques on the lower back, but the central ulceration in our case resembled a report by Marqueling et al.2 Ulceration and induration of PLMT developing at 8 months of age can suggest an aggressive disease course corresponding with deep infiltration and is seen mostly in children.
The histopathologic features of PLMT include an acanthotic epidermis and follicular induction, which also are characteristic of dermatofibroma (DF). The proliferation of spindle cells extended deep into the fat with foci of erythrocyte extravasation and microvesiculation of the stroma similar to nodular fasciitis and proliferative fasciitis. The presentation of infiltrating and expanding fibrous septae and trapping of individual adipocytes in a honeycomb pattern is similar to dermatofibrosarcoma protuberans (DFSP). Most cases of PLMT are positive for SMA. Factor XIIIa typically is variably positive, and in one report, 31% (4/13) of cases showed positive staining for calponin.3 Rapid growth, ulceration, and recurrence emphasize that PLMT can be locally aggressive, similar to DFSP.4
The main differential diagnoses include DF and its variants, dermatomyofibroma, DFSP, and proliferative fasciitis.3,5 In the cases mentioned above, microscopic features were similar with a relatively well-circumscribed proliferation of spindle cells arranged in short fascicles through the entire reticular dermis, and the overlying epidermis was acanthotic.
Dermatofibroma commonly manifests in adults as a minor nodular lesion (commonly <1 cm), and usually is located on the legs. It has several clinical and histologic variants, including multiple clustered DF (MCDF)—a rare condition that has been reported in children and young adults and generally appears in the first and second decades of life. Of the reported cases of MCDF, immunohistochemical staining for SMA was performed in 8 cases. All these cases showed negative or minimal staining.3-5 Smooth muscle actin staining in DFs is negative, or weak and patchy, unlike in PLMT where it is diffuse, uniform, and strong.
Dermatofibrosarcoma protuberans typically occurs in young adults and manifests as dermal and subcutaneous nodular/multinodular or plaquelike masses, with rare congenital cases. Immunohistochemical staining for CD34, which typically is firmly and diffusely positive, is the most reliable marker of DFSP.6 Factor XIIIA in DFSP typically is negative for focal staining, mainly at periphery or in scattered dendritic cells. The prognosis of DFSP generally is excellent, with local recurrences in up to 30% of cases and extremely low metastatic potential (essentially only in cases with fibrosarcomatous transformation).6 Dermatomyofibroma is another rare benign dermal myofibroblastic tumor that typically manifests with indurated hyperpigmented or erythematous plaques or nodules on the shoulders and torso.6 This condition occurs mainly in adolescents and young adults, unlike PLMT. The most striking features of dermatomyofibroma are the horizontal orientation of the spindle cell nuclei and the pattern of the proliferation concerning the adnexal structures, especially hair follicles. The hair follicles have a normal appearance, and the proliferation extends up to each follicle, then continues to the other side without any displacement of the follicle. Tumor cells are variably positive for SMA in dermatomyofibromas and are negative for muscle-specific actin, desmin, S100, CD34, and Factor XIIIA.6
Immunohistochemistry can be very useful in differentiating PLMT from other conditions. Neoplastic cells stain positively for CD34 but not for Factor XIIIa and SMA in cases of DFSP. Dermatofibroma and its variants always present with collagen trapping at the periphery of the lesions and may demonstrate foamy macrophages, hemosiderin, or plasma cells FXIIIA(+), CD34(-), and variable SMA reactivity. This positivity usually is less prominent in DF than in PLMT. Neoplastic cells in dermatomyofibroma often stain positive for calponin, but only focally for SMA. The clinical features of dermatomyofibroma include early onset, large size, multiple nodules, and plaquelike morphology. Moulonguet et al4 hypothesized that, although MCDF and PLMT appear to show some distinctive clinical and histologic features, they also show similarities that could suggest they form part of the myofibroblastic spectrum. Furthermore, Moradi et al7 also considered them as part of the same disease spectrum because of their overlapping clinical, histologic, and immunohistochemical features.
The microscopic features in our case are notable, as the lesion demonstrated overlying acanthosis and follicular induction, resembling DF. The stroma contained microvesicular changes and erythrocyte extravasation, characteristic of nodular or proliferative fasciitis. Additionally, densely packed spindle cells infiltrated deep into the subcutaneous adipose tissue, similar to DFSP.2,3 Our findings expand on the reported histopathologic spectrum of this tumor to date.
THE DIAGNOSIS: Plaquelike Myofibroblastic Tumor
An incisional biopsy of the plaque demonstrated a hypercellular proliferation of bland spindle cells in the dermis that infiltrated the subcutis. The overlying epidermis was mildly acanthotic with both ulceration and follicular induction. There was trapping of individual adipocytes in a honeycomb pattern with foci of erythrocyte extravasation, microvesiculation, and widened fibrous septa (Figure 1). Immunohistochemistry was positive for vimentin, actin, and smooth muscle actin (SMA)(Figure 2A). Variable positivity for Factor XIIIa antibodies was noted. CD68 staining was focal positive, suggesting fibrohistiocytic lineage. Expression of CD31, CD34, S100, and anaplastic lymphoma kinase was negative, and Ki-67 was present in less than 10% of cells (Figure 2B).


We reviewed the case in conjunction with a soft-tissue pathologist (Y.L.), and based on the clinical and immunophenotypic features, a diagnosis of plaquelike myofibroblastic tumor (PLMT) was made. The patient’s parents refused further treatment, and there was no sign of disease progression at 6-month follow-up.
Plaquelike myofibroblastic tumor is an unusual pediatric dermal tumor that was first described by Clarke et al1 in 2007. Clinical manifestation of PLMT on the right abdomen was unique in our patient, as the lesions typically present as indurated plaques on the lower back, but the central ulceration in our case resembled a report by Marqueling et al.2 Ulceration and induration of PLMT developing at 8 months of age can suggest an aggressive disease course corresponding with deep infiltration and is seen mostly in children.
The histopathologic features of PLMT include an acanthotic epidermis and follicular induction, which also are characteristic of dermatofibroma (DF). The proliferation of spindle cells extended deep into the fat with foci of erythrocyte extravasation and microvesiculation of the stroma similar to nodular fasciitis and proliferative fasciitis. The presentation of infiltrating and expanding fibrous septae and trapping of individual adipocytes in a honeycomb pattern is similar to dermatofibrosarcoma protuberans (DFSP). Most cases of PLMT are positive for SMA. Factor XIIIa typically is variably positive, and in one report, 31% (4/13) of cases showed positive staining for calponin.3 Rapid growth, ulceration, and recurrence emphasize that PLMT can be locally aggressive, similar to DFSP.4
The main differential diagnoses include DF and its variants, dermatomyofibroma, DFSP, and proliferative fasciitis.3,5 In the cases mentioned above, microscopic features were similar with a relatively well-circumscribed proliferation of spindle cells arranged in short fascicles through the entire reticular dermis, and the overlying epidermis was acanthotic.
Dermatofibroma commonly manifests in adults as a minor nodular lesion (commonly <1 cm), and usually is located on the legs. It has several clinical and histologic variants, including multiple clustered DF (MCDF)—a rare condition that has been reported in children and young adults and generally appears in the first and second decades of life. Of the reported cases of MCDF, immunohistochemical staining for SMA was performed in 8 cases. All these cases showed negative or minimal staining.3-5 Smooth muscle actin staining in DFs is negative, or weak and patchy, unlike in PLMT where it is diffuse, uniform, and strong.
Dermatofibrosarcoma protuberans typically occurs in young adults and manifests as dermal and subcutaneous nodular/multinodular or plaquelike masses, with rare congenital cases. Immunohistochemical staining for CD34, which typically is firmly and diffusely positive, is the most reliable marker of DFSP.6 Factor XIIIA in DFSP typically is negative for focal staining, mainly at periphery or in scattered dendritic cells. The prognosis of DFSP generally is excellent, with local recurrences in up to 30% of cases and extremely low metastatic potential (essentially only in cases with fibrosarcomatous transformation).6 Dermatomyofibroma is another rare benign dermal myofibroblastic tumor that typically manifests with indurated hyperpigmented or erythematous plaques or nodules on the shoulders and torso.6 This condition occurs mainly in adolescents and young adults, unlike PLMT. The most striking features of dermatomyofibroma are the horizontal orientation of the spindle cell nuclei and the pattern of the proliferation concerning the adnexal structures, especially hair follicles. The hair follicles have a normal appearance, and the proliferation extends up to each follicle, then continues to the other side without any displacement of the follicle. Tumor cells are variably positive for SMA in dermatomyofibromas and are negative for muscle-specific actin, desmin, S100, CD34, and Factor XIIIA.6
Immunohistochemistry can be very useful in differentiating PLMT from other conditions. Neoplastic cells stain positively for CD34 but not for Factor XIIIa and SMA in cases of DFSP. Dermatofibroma and its variants always present with collagen trapping at the periphery of the lesions and may demonstrate foamy macrophages, hemosiderin, or plasma cells FXIIIA(+), CD34(-), and variable SMA reactivity. This positivity usually is less prominent in DF than in PLMT. Neoplastic cells in dermatomyofibroma often stain positive for calponin, but only focally for SMA. The clinical features of dermatomyofibroma include early onset, large size, multiple nodules, and plaquelike morphology. Moulonguet et al4 hypothesized that, although MCDF and PLMT appear to show some distinctive clinical and histologic features, they also show similarities that could suggest they form part of the myofibroblastic spectrum. Furthermore, Moradi et al7 also considered them as part of the same disease spectrum because of their overlapping clinical, histologic, and immunohistochemical features.
The microscopic features in our case are notable, as the lesion demonstrated overlying acanthosis and follicular induction, resembling DF. The stroma contained microvesicular changes and erythrocyte extravasation, characteristic of nodular or proliferative fasciitis. Additionally, densely packed spindle cells infiltrated deep into the subcutaneous adipose tissue, similar to DFSP.2,3 Our findings expand on the reported histopathologic spectrum of this tumor to date.
- Clarke JT, Clarke LE, Miller C, et al. Plaque-like myofibroblastic tumor of infancy. Pediatr Dermatol. 2007;24:E83-E87. doi:10.1111 /j.1525-1470.2007.00449.x
- Marqueling AL, Dasher D, Friedlander SF, et al. Plaque-like myofibroblastic tumor: report of three cases. Pediatr Dermatol. 2013;30:600-607. doi:10.1111/pde.12185
- Sekar T, Mushtaq J, AlBadry W, et al. Plaque-like myofibroblastic tumor: a series of 2 cases of this unusual dermal tumor which occurs in infancy and early childhood. Pediatr Dev Pathol. 2018;21:444-448. doi: 10.1177/1093526617746807
- Moulonguet I, Biaggi A, Eschard C, et al. Plaque-like myofibroblastic tumor: report of 4 cases. Am J Dermatopathol. 2017;39:767-772. doi: 10.1097/DAD.0000000000000869
- Virdi A, Baraldi C, Barisani A, et al. Plaque-like myofibroblastic tumor, a rare entity of childhood: possible pitfalls in differential diagnosis. J Cutan Pathol. 2019;46:389-392. doi:10.1111/cup.13441
- Cassarino DS. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2021.
- Moradi S, Mnayer L, Earle J, et al. Plaque-like dermatofibroma: case report of a rare entity. Dermatopathology (Basel). 2021;8:337-341. doi:10.3390/dermatopathology8030038
- Clarke JT, Clarke LE, Miller C, et al. Plaque-like myofibroblastic tumor of infancy. Pediatr Dermatol. 2007;24:E83-E87. doi:10.1111 /j.1525-1470.2007.00449.x
- Marqueling AL, Dasher D, Friedlander SF, et al. Plaque-like myofibroblastic tumor: report of three cases. Pediatr Dermatol. 2013;30:600-607. doi:10.1111/pde.12185
- Sekar T, Mushtaq J, AlBadry W, et al. Plaque-like myofibroblastic tumor: a series of 2 cases of this unusual dermal tumor which occurs in infancy and early childhood. Pediatr Dev Pathol. 2018;21:444-448. doi: 10.1177/1093526617746807
- Moulonguet I, Biaggi A, Eschard C, et al. Plaque-like myofibroblastic tumor: report of 4 cases. Am J Dermatopathol. 2017;39:767-772. doi: 10.1097/DAD.0000000000000869
- Virdi A, Baraldi C, Barisani A, et al. Plaque-like myofibroblastic tumor, a rare entity of childhood: possible pitfalls in differential diagnosis. J Cutan Pathol. 2019;46:389-392. doi:10.1111/cup.13441
- Cassarino DS. Diagnostic Pathology: Neoplastic Dermatopathology. 2nd ed. Elsevier; 2021.
- Moradi S, Mnayer L, Earle J, et al. Plaque-like dermatofibroma: case report of a rare entity. Dermatopathology (Basel). 2021;8:337-341. doi:10.3390/dermatopathology8030038
Plaque With Central Ulceration on the Abdomen
Plaque With Central Ulceration on the Abdomen
A 14-month-old girl presented to the dermatology department with a firm asymptomatic lesion on the abdomen of 6 months’ duration. The lesion started as a flesh-colored papule and developed slowly into an indurated plaque that darkened in color. The patient had no history of trauma to the area. Physical examination revealed a dark reddish–brown, indurated, irregularly shaped plaque with central ulceration and elevated borders on the right abdomen. The plaque measured 2×3 cm with a few smaller satellite nodules distributed along the periphery. Abdominal ultrasonography revealed a multinodular proliferation in the dermis and subcutis of the right abdomen.
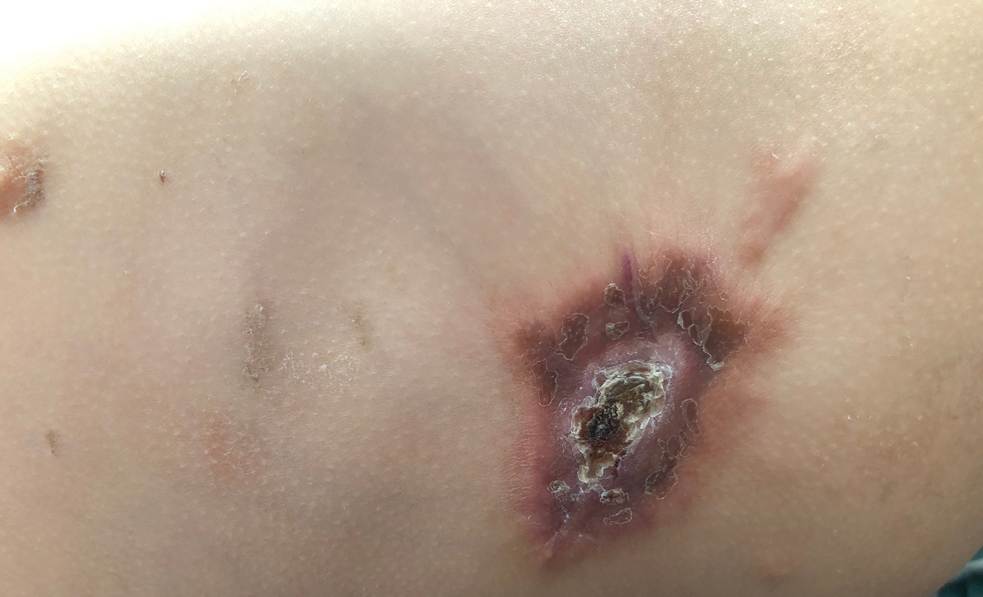
Pseudoverrucous Papules and Nodules Around a Surgical Stoma
Pseudoverrucous Papules and Nodules Around a Surgical Stoma
To the Editor:
A 22-year-old man was referred to our dermatology outpatient department for wartlike growths that gradually developed around a postoperative enteroatmospheric fistula and stoma over the past 4 months. The patient presented for an emergency exploratory laparotomy with a history of perforation peritonitis 1.5 years prior to the current presentation. He also had a small bowel obstruction 5 months prior to the current presentation that resulted in the resection of a large segment of the small bowel. He underwent a diverting loop ileostomy when the abdominal closure was not achieved because of bowel edema, following which he developed a postoperative enteroatmospheric fistula. In addition, the stoma retracted and was followed by dermal dehiscence, which led to notable leakage and resulted in heavy fecal contamination of the midline wound.
At the current presentation, physical examination revealed multiple grayish-white, dome-shaped, moist papules coalescing to form a peristomal pseudoverrucous mass on the lower side of the stoma (Figure 1). The patient experienced mild itching. The lesion showed no signs of erosion, bleeding, or purulent discharge, and there were no nearby lumps or enlarged lymph nodes. The differential diagnosis included peristomal pyoderma gangrenosum, human papillomavirus (HPV) infection, pseudoverrucous papules and nodules (PPNs), squamous cell carcinoma, and exuberant granulation tissue. A skin biopsy was performed, and histopathology revealed hyperkeratosis, moderate papillomatosis, and marked acanthotic hyperplasia seen as downgrowths into the dermis (Figure 2). No koilocytes, atypia, or mitotic figures were present. Abundant neutrophils and few eosinophils were seen in the dermal infiltrate. A final diagnosis of PPN was made based on clinicopathologic correlation. The patient was advised to use a smaller stoma bag and to change the collection pouch frequently to reduce skin contact with fecal matter.
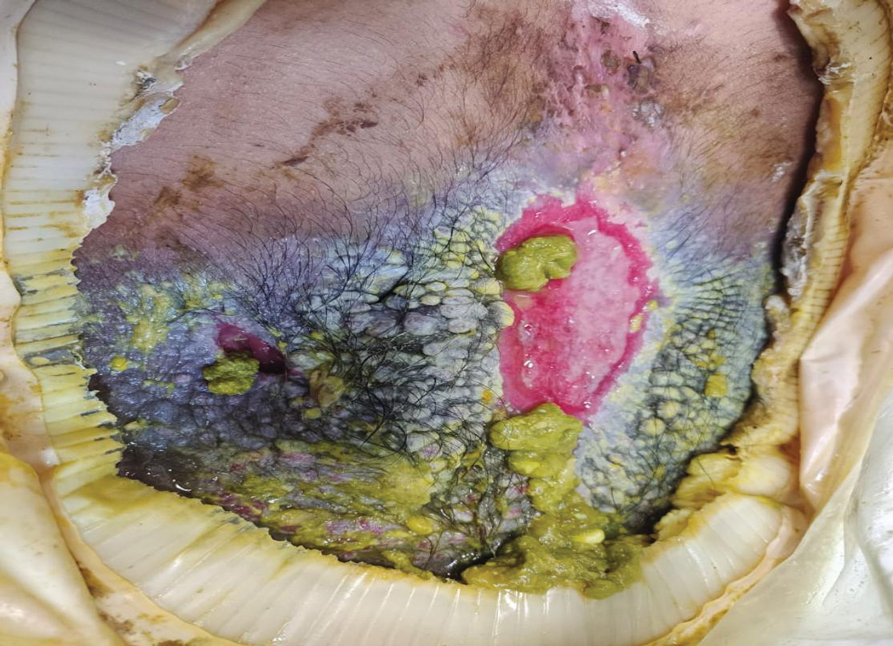
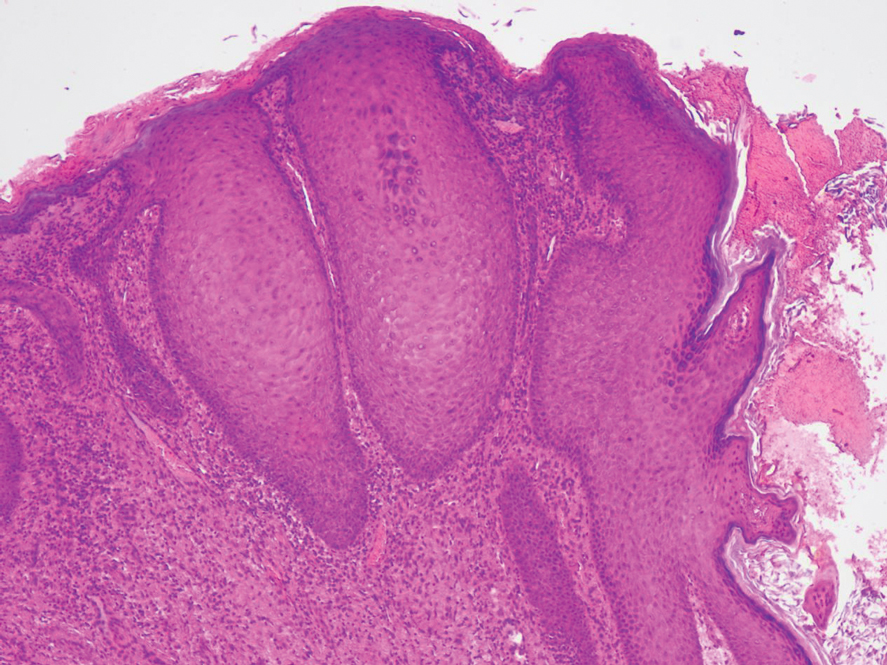
Peristomal skin conditions are reported in 18% to 55% of patients with stomas and include allergic contact dermatitis, mechanical dermatitis, infections, pyoderma gangrenosum, and irritant contact dermatitis.1,2 Pseudoverrucous papules (also called chronic papillomatous dermatitis or pseudoverrucous lesions) is a rare dermatologic complication found on the skin around stomas,3 most commonly around urostomy stomas. The presence of PPNs around colostomy stomas and the perianal region is extremely rare.2,4 This condition is the result of chronic irritant dermatitis from frequent exposure to urine or feces, leading to maceration and epidermal hyperplasia. It occurs because of improper sizing of the stoma bag or incorrect positioning or construction of the stoma.5
the overuse of topical benzocaine-resorcinol, leading to chronic irritation.6 It is clinically characterized by multiple grayish-white, wartlike, confluent papulonodules around areas chronically exposed to moisture. Differential diagnoses such as secondary neoplasms, HPV infection, exuberant granulation tissue, and candidal infections should be considered.3 Final diagnosis is based on clinicopathologic findings, similar to our case. Epidermal growth factor and transforming growth factor are thought to play a role in the pathophysiology of pseudoepitheliomatous hyperplasia. Increased expression of these mediators leads to proliferation of the epidermis into the dermis.7 The role of HPV in PPN remains unclear, as not all PPN lesions are positive for HPV and the cutaneous lesions resolve once the source of irritation is removed. Recommended treatment includes local skin care; stoma refitting; and, in severe cases, excision and revision of the stoma.2 Dermatologists must be aware of this often-underdiagnosed condition.
- Alslaim F, Al Farajat F, Alslaim HS, et al. Etiology and management of peristomal pseudoepitheliomatous hyperplasia. Cureus. 2021;13 :E20196. doi:10.7759/cureus.20196
- Rambhia PH, Conic RZ, Honda K, et al. Chronic papillomatous dermatitis in a patient with a urinary ileal diversion: a case report and review of the literature. Dermatol Arch. 2017;1:47-50. doi:10.36959/661/297
- Latour-Álvarez I, García-Peris E, Pestana-Eliche MM, et al. Nodular peristomal lesions. Actas Dermosifiliogr. 2016;108:363-364. doi:10.1016/j.ad.2016.02.018
- Dandale A, Dhurat R, Ghate S. Perianal pseudoverrucous papules and nodules. Indian J Sex Transm Dis AIDS. 2013;34:44-46. doi:10.4103/0253-7184.112939
- Brogna L. Prevention and management of pseudoverrucous lesions: a review and case scenarios. Adv Skin Wound Care. 2021;34:461-471. doi:10.1097/01.ASW.0000758620.93518.39
- Robson KJ, Maughan JA, Purcell SD, et al. Erosive papulonodular dermatosis associated with topical benzocaine: a report of two cases and evidence that granuloma gluteale, pseudoverrucous papules, and Jacquet’s erosive dermatitis are a disease spectrum. J Am Acad Dermatol. 2006;55(5 suppl):S74-S80. doi:10.1016/j .jaad.2005.12.025
- Oğuz ID, Vural S, Cinar E, et al. Peristomal pseudoverrucous lesions: a rare skin complication of colostomy. Cureus. 2023;15:E38068. doi:10.7759/cureus.38068
To the Editor:
A 22-year-old man was referred to our dermatology outpatient department for wartlike growths that gradually developed around a postoperative enteroatmospheric fistula and stoma over the past 4 months. The patient presented for an emergency exploratory laparotomy with a history of perforation peritonitis 1.5 years prior to the current presentation. He also had a small bowel obstruction 5 months prior to the current presentation that resulted in the resection of a large segment of the small bowel. He underwent a diverting loop ileostomy when the abdominal closure was not achieved because of bowel edema, following which he developed a postoperative enteroatmospheric fistula. In addition, the stoma retracted and was followed by dermal dehiscence, which led to notable leakage and resulted in heavy fecal contamination of the midline wound.
At the current presentation, physical examination revealed multiple grayish-white, dome-shaped, moist papules coalescing to form a peristomal pseudoverrucous mass on the lower side of the stoma (Figure 1). The patient experienced mild itching. The lesion showed no signs of erosion, bleeding, or purulent discharge, and there were no nearby lumps or enlarged lymph nodes. The differential diagnosis included peristomal pyoderma gangrenosum, human papillomavirus (HPV) infection, pseudoverrucous papules and nodules (PPNs), squamous cell carcinoma, and exuberant granulation tissue. A skin biopsy was performed, and histopathology revealed hyperkeratosis, moderate papillomatosis, and marked acanthotic hyperplasia seen as downgrowths into the dermis (Figure 2). No koilocytes, atypia, or mitotic figures were present. Abundant neutrophils and few eosinophils were seen in the dermal infiltrate. A final diagnosis of PPN was made based on clinicopathologic correlation. The patient was advised to use a smaller stoma bag and to change the collection pouch frequently to reduce skin contact with fecal matter.


Peristomal skin conditions are reported in 18% to 55% of patients with stomas and include allergic contact dermatitis, mechanical dermatitis, infections, pyoderma gangrenosum, and irritant contact dermatitis.1,2 Pseudoverrucous papules (also called chronic papillomatous dermatitis or pseudoverrucous lesions) is a rare dermatologic complication found on the skin around stomas,3 most commonly around urostomy stomas. The presence of PPNs around colostomy stomas and the perianal region is extremely rare.2,4 This condition is the result of chronic irritant dermatitis from frequent exposure to urine or feces, leading to maceration and epidermal hyperplasia. It occurs because of improper sizing of the stoma bag or incorrect positioning or construction of the stoma.5
the overuse of topical benzocaine-resorcinol, leading to chronic irritation.6 It is clinically characterized by multiple grayish-white, wartlike, confluent papulonodules around areas chronically exposed to moisture. Differential diagnoses such as secondary neoplasms, HPV infection, exuberant granulation tissue, and candidal infections should be considered.3 Final diagnosis is based on clinicopathologic findings, similar to our case. Epidermal growth factor and transforming growth factor are thought to play a role in the pathophysiology of pseudoepitheliomatous hyperplasia. Increased expression of these mediators leads to proliferation of the epidermis into the dermis.7 The role of HPV in PPN remains unclear, as not all PPN lesions are positive for HPV and the cutaneous lesions resolve once the source of irritation is removed. Recommended treatment includes local skin care; stoma refitting; and, in severe cases, excision and revision of the stoma.2 Dermatologists must be aware of this often-underdiagnosed condition.
To the Editor:
A 22-year-old man was referred to our dermatology outpatient department for wartlike growths that gradually developed around a postoperative enteroatmospheric fistula and stoma over the past 4 months. The patient presented for an emergency exploratory laparotomy with a history of perforation peritonitis 1.5 years prior to the current presentation. He also had a small bowel obstruction 5 months prior to the current presentation that resulted in the resection of a large segment of the small bowel. He underwent a diverting loop ileostomy when the abdominal closure was not achieved because of bowel edema, following which he developed a postoperative enteroatmospheric fistula. In addition, the stoma retracted and was followed by dermal dehiscence, which led to notable leakage and resulted in heavy fecal contamination of the midline wound.
At the current presentation, physical examination revealed multiple grayish-white, dome-shaped, moist papules coalescing to form a peristomal pseudoverrucous mass on the lower side of the stoma (Figure 1). The patient experienced mild itching. The lesion showed no signs of erosion, bleeding, or purulent discharge, and there were no nearby lumps or enlarged lymph nodes. The differential diagnosis included peristomal pyoderma gangrenosum, human papillomavirus (HPV) infection, pseudoverrucous papules and nodules (PPNs), squamous cell carcinoma, and exuberant granulation tissue. A skin biopsy was performed, and histopathology revealed hyperkeratosis, moderate papillomatosis, and marked acanthotic hyperplasia seen as downgrowths into the dermis (Figure 2). No koilocytes, atypia, or mitotic figures were present. Abundant neutrophils and few eosinophils were seen in the dermal infiltrate. A final diagnosis of PPN was made based on clinicopathologic correlation. The patient was advised to use a smaller stoma bag and to change the collection pouch frequently to reduce skin contact with fecal matter.


Peristomal skin conditions are reported in 18% to 55% of patients with stomas and include allergic contact dermatitis, mechanical dermatitis, infections, pyoderma gangrenosum, and irritant contact dermatitis.1,2 Pseudoverrucous papules (also called chronic papillomatous dermatitis or pseudoverrucous lesions) is a rare dermatologic complication found on the skin around stomas,3 most commonly around urostomy stomas. The presence of PPNs around colostomy stomas and the perianal region is extremely rare.2,4 This condition is the result of chronic irritant dermatitis from frequent exposure to urine or feces, leading to maceration and epidermal hyperplasia. It occurs because of improper sizing of the stoma bag or incorrect positioning or construction of the stoma.5
the overuse of topical benzocaine-resorcinol, leading to chronic irritation.6 It is clinically characterized by multiple grayish-white, wartlike, confluent papulonodules around areas chronically exposed to moisture. Differential diagnoses such as secondary neoplasms, HPV infection, exuberant granulation tissue, and candidal infections should be considered.3 Final diagnosis is based on clinicopathologic findings, similar to our case. Epidermal growth factor and transforming growth factor are thought to play a role in the pathophysiology of pseudoepitheliomatous hyperplasia. Increased expression of these mediators leads to proliferation of the epidermis into the dermis.7 The role of HPV in PPN remains unclear, as not all PPN lesions are positive for HPV and the cutaneous lesions resolve once the source of irritation is removed. Recommended treatment includes local skin care; stoma refitting; and, in severe cases, excision and revision of the stoma.2 Dermatologists must be aware of this often-underdiagnosed condition.
- Alslaim F, Al Farajat F, Alslaim HS, et al. Etiology and management of peristomal pseudoepitheliomatous hyperplasia. Cureus. 2021;13 :E20196. doi:10.7759/cureus.20196
- Rambhia PH, Conic RZ, Honda K, et al. Chronic papillomatous dermatitis in a patient with a urinary ileal diversion: a case report and review of the literature. Dermatol Arch. 2017;1:47-50. doi:10.36959/661/297
- Latour-Álvarez I, García-Peris E, Pestana-Eliche MM, et al. Nodular peristomal lesions. Actas Dermosifiliogr. 2016;108:363-364. doi:10.1016/j.ad.2016.02.018
- Dandale A, Dhurat R, Ghate S. Perianal pseudoverrucous papules and nodules. Indian J Sex Transm Dis AIDS. 2013;34:44-46. doi:10.4103/0253-7184.112939
- Brogna L. Prevention and management of pseudoverrucous lesions: a review and case scenarios. Adv Skin Wound Care. 2021;34:461-471. doi:10.1097/01.ASW.0000758620.93518.39
- Robson KJ, Maughan JA, Purcell SD, et al. Erosive papulonodular dermatosis associated with topical benzocaine: a report of two cases and evidence that granuloma gluteale, pseudoverrucous papules, and Jacquet’s erosive dermatitis are a disease spectrum. J Am Acad Dermatol. 2006;55(5 suppl):S74-S80. doi:10.1016/j .jaad.2005.12.025
- Oğuz ID, Vural S, Cinar E, et al. Peristomal pseudoverrucous lesions: a rare skin complication of colostomy. Cureus. 2023;15:E38068. doi:10.7759/cureus.38068
- Alslaim F, Al Farajat F, Alslaim HS, et al. Etiology and management of peristomal pseudoepitheliomatous hyperplasia. Cureus. 2021;13 :E20196. doi:10.7759/cureus.20196
- Rambhia PH, Conic RZ, Honda K, et al. Chronic papillomatous dermatitis in a patient with a urinary ileal diversion: a case report and review of the literature. Dermatol Arch. 2017;1:47-50. doi:10.36959/661/297
- Latour-Álvarez I, García-Peris E, Pestana-Eliche MM, et al. Nodular peristomal lesions. Actas Dermosifiliogr. 2016;108:363-364. doi:10.1016/j.ad.2016.02.018
- Dandale A, Dhurat R, Ghate S. Perianal pseudoverrucous papules and nodules. Indian J Sex Transm Dis AIDS. 2013;34:44-46. doi:10.4103/0253-7184.112939
- Brogna L. Prevention and management of pseudoverrucous lesions: a review and case scenarios. Adv Skin Wound Care. 2021;34:461-471. doi:10.1097/01.ASW.0000758620.93518.39
- Robson KJ, Maughan JA, Purcell SD, et al. Erosive papulonodular dermatosis associated with topical benzocaine: a report of two cases and evidence that granuloma gluteale, pseudoverrucous papules, and Jacquet’s erosive dermatitis are a disease spectrum. J Am Acad Dermatol. 2006;55(5 suppl):S74-S80. doi:10.1016/j .jaad.2005.12.025
- Oğuz ID, Vural S, Cinar E, et al. Peristomal pseudoverrucous lesions: a rare skin complication of colostomy. Cureus. 2023;15:E38068. doi:10.7759/cureus.38068
Pseudoverrucous Papules and Nodules Around a Surgical Stoma
Pseudoverrucous Papules and Nodules Around a Surgical Stoma
PRACTICE POINTS
- Pseudoverrucous papules and nodules (PPNs) can develop around stomas due to chronic irritant dermatitis from fecal or urinary exposure.
- Proper stoma management, including the use of appropriately sized stoma bags and frequent changes, is essential to prevent skin complications such as PPN.
- When evaluating peristomal lesions, consider a broad differential diagnosis, including infections, neoplasms, and dermatitis, and ensure thorough clinicopathologic correlation for accurate diagnosis and treatment.
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents
Dermatopathology education, which comprises approximately 30% of the dermatology residency curriculum, is crucial for the holistic training of dermatology residents to diagnose and manage a range of dermatologic conditions.1 Additionally, dermatopathology is the topic of one of the 4 American Board of Dermatology CORE Exam modules, further highlighting the need for comprehensive education in this area. A variety of resources including virtual dermatopathology and conventional microscopy training currently are used in residency programs for dermatopathology education.2,3 Although used less frequently, social media platforms such as Instagram also are used to aid in dermatopathology education for a wider audience.4 Other online resources, including the American Society of Dermatopathology website (www.asdp.org) and DermpathAtlas.com, are excellent tools for medical students, residents, and fellows to develop their knowledge.5 While these resources are accessible, they must be directly sought out by the student and utilized on their own time. Additionally, if medical students do not have a strong understanding of the basics of dermatopathology, they may not have the foundation required to benefit from these resources.
Dermatopathology education is critical for the overall practice of dermatology, yet most dermatology residency programs may not be incorporating dermatopathology education early enough in training. One study evaluating the timing and length of dermatopathology education during residency reported that fewer than 40% (20/51) of dermatology residency programs allocate 3 or more weeks to dermatopathology education during the second postgraduate year.1 Despite Ackerman6 advocating for early dermatopathology exposure to best prepare medical students to recognize and manage certain dermatologic conditions, the majority of exposure still seems to occur during postgraduate year 4.1 Furthermore, current primary care residents feel that their medical school training did not sufficiently prepare them to diagnose and manage dermatologic conditions, with only 37% (93/252) reporting feeling adequately prepared.7,8 Medical students also reported a lack of confidence in overall dermatology knowledge, with 89% (72/81) reporting they felt neutral, slightly confident, or not at all confident when asked to diagnose skin lesions.9 In the same study, the average score was 46.6% (7/15 questions answered correctly) when 74 participants were assessed via a multiple choice quiz on dermatologic diagnosis and treatment, further demonstrating the lack of general dermatology comfort among medical students.9 This likely stems from limited dermatology curriculum in medical schools, demonstrating the need for further dermatology education as a whole in medical school.10
Ensuring robust dermatopathology education in medical school and the first year of dermatology residency has the potential to better prepare medical students for the transition into dermatology residency and clinical practice. We created an introductory dermatopathology lecture and presented it to medical students and first year dermatology residents to improve dermatopathology knowledge and confidence in learners early in their dermatology training.
Structure of the Lecture
Participants included first-year dermatology residents and fourth-year medical students rotating with the Wayne State University Department of Dermatology (Detroit, Michigan). The same facilitator (H.O.) taught each of the lectures, and all lectures were conducted via Zoom at the beginning of the month from May 2024 through November 2024. A total of 7 lectures were given. The lecture was formatted so that a histologic image was shown, then learners expressed their thoughts about what the image was showing before the answer was given. This format allowed participants to view the images on their own device screen and allowed the facilitator to annotate the images. The lecture was divided into 3 sections: (1) cell types and basic structures, (2) anatomic slides, and (3) common diagnoses. Each session lasted approximately 45 minutes.
Section 1: Cell Types and Basic Structures—The first section covered the fundamental cell types (neutrophils, lymphocytes, plasma cells, melanocytes, and eosinophils) along with glandular structures (apocrine, eccrine, and sebaceous). The session was designed to follow a retention and allow learners to think through each slide. First, participants were shown histologic images of each cell type and were asked to identify what type of cell was being shown. On the following slide, key features of each cell type were highlighted. Next, participants similarly were shown images of the glandular structures followed by key features of each. The section concluded with a review of the layers of the skin (stratum corneum, stratum granulosum, stratum lucidum, stratum spinosum, and stratum basale). A histologic image was shown, and the facilitator discussed how to distinguish the layers.
Section 2: Anatomic Sites—This section focused on key pathologic features for differentiating body surfaces, including the scalp, face, eyelids, ears, areolae, palms and soles, and mucosae. Participants initially were shown an image of a hematoxylin and eosin–stained slide from a specific body surface and then were asked to identify structures that may serve as a clue to the anatomic location. If the participants were not sure, they were given hints; for example, when participants were shown an image of the ear and were unsure of the location, the facilitator circled cartilage and asked them to identify the structure. In most cases, once participants named this structure, they were able to recognize that the location was the ear.
Section 3: Common Diagnoses—This section addressed frequently encountered diagnoses in dermatopathology, including basal cell carcinoma, squamous cell carcinoma, squamous cell carcinoma in situ, epidermoid cyst, pilar cyst, seborrheic keratosis, solar lentigo, melanocytic nevus, melanoma, verruca vulgaris, spongiotic dermatitis, psoriasis, and lichen planus. It followed the same format of the first section: participants were shown an hemotoxyllin and eosin–stained image and then were asked to discuss what the diagnosis could be and why. Hints were given if participants struggled to come up with the correct diagnosis. A few slides also were dedicated to distinguishing benign nevi, dysplastic nevi, and melanoma.
Pretest and Posttest Results
Residents participated in the lecture as part of their first-year orientation, and medical students participated during their dermatology rotation. All participants were invited to complete a pretest and a posttest before and after the lecture, respectively. Both assessments were optional and anonymous. The pretest was completed electronically and consisted of 10 knowledge-based, multiple-choice questions that included a histopathologic image and asked, “What is the most likely diagnosis?,” “What is the predominant cell type?,” and “Where was this specimen taken from?” In addition to the knowledge-based questions, participants also were asked to rate their confidence in dermatopathology on a 5-point Likert scale ranging from 1 (not confident at all) to 5 (extremely confident). Participants completed the entire pretest before any information on the topic was provided. After the lecture, participants were asked to complete a posttest identical to the pretest and to rate their confidence in dermatopathology again on the same scale. The posttest included an additional question asking participants to rate the helpfulness of the lecture on a Likert scale ranging from 1 (not helpful at all) to 5 (extremely helpful). Participants completed the posttest within 48 hours of the lecture.
Overall, 15 learners participated in the pretest and 12 in the posttest. Of the 15 pretest participants, 3 were first-year residents and 12 were medical students. Similarly, in the posttest, 2 respondents were first-year residents and 10 were medical students. All responses contained complete pretests and posttests. The mean score on the pretest was 62%, whereas the mean score on the posttest was 75%. A paired t test indicated a statistically significant improvement (P=.017). In addition, the mean rating for confidence in dermatopathology knowledge before the lecture was 1.5 prior to the lecture and 2.6 after the lecture. A paired t test demonstrated statistical significance (P=.010). The mean rating of the helpfulness of the lecture was 4.67. The majority (91.7% [11/12]) of the participants gave a rating of 4 or 5.
Impact of the Lecture on Dermatopathology Knowledge
There is a gap in dermatopathology education early in medical training. Our introductory lecture led to higher post test scores and increased confidence in dermatopathology among medical students and dermatology residents, demonstrating the effectiveness of this kind of program in bridging this education gap. The majority of participants in our lecture said they found the session helpful. A previously published article called for early implementation of dermatology education as a whole in the medical curriculum due to lack of knowledge and confidence, and our introductory lecture may help to bridge this gap.8 Increasing dermatopathology content for medical students and first-year dermatology residents can expand knowledge, as shown by the increased scores on the posttest, and better supports learners transitioning to dermatology residency, where dermatopathology constitutes a large part of the overall curriculum.2 More comprehensive knowledge of dermatopathology early in dermatology training also may help to better prepare residents to accurately diagnose and manage dermatologic conditions.
Pretest scores showed that the average confidence rating in dermatopathology among participants in our lecture was 1.5, which is rather low. This is consistent with prior studies that have found that residents feel that medical school inadequately prepared them for dermatology residency.7,8 More than 87% (71/81) of medical students surveyed felt they received inadequate general dermatology training in medical school.9 This supports the proposed educational gap that is impacting confidence in overall dermatology knowledge, which includes dermatopathology. In our study, the average confidence rating increased by 1.1 points after the lecture, which was statistically significant (P=.010) and demonstrates that an introductory lecture serves as a feasible intervention to improve confidence in this area.
The feedback we received from participants in our lecture shows the benefits of an introductory interactive lecture with virtual dermatopathology images. Ngo et al2 highlighted how residents perceive virtual images to be superior to conventional microscopy for dermatopathology, which we utilized in our lecture. This method is not only cost effective but also provides a simple way for learners and facilitators to point out key findings on histopathology slides.2
Final Thoughts
Overall, implementing dermatopathology education early in training has a measurable impact on dermatopathology knowledge and confidence among medical students and first-year dermatology residents. An interactive lecture with virtual images similar to the one we describe here may better prepare learners for the transition to dermatology residency by addressing the educational gap in dermatopathology early in training.
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826, vii.
- Ngo TB, Niu W, Fang Z, et al. Dermatology residents’ perspectives on virtual dermatopathology education. J Cutan Pathol. 2024;51:530-537.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Hubbard G, Saal R, Wintringham J, et al. Utilizing Instagram as a novel method for dermatopathology instruction. Clin Exp Dermatol. 2023;49:89-91.
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14.
- Ackerman AB. Training residents in dermatopathology: why, when, where, and how. J Am Acad Dermatol. 1990;22(6 Pt 1):1104-1106.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.e1.
- Murase JE. Understanding the importance of dermatology training in undergraduate medical education. Dermatol Pract Concept. 2015;5:95-96.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
Dermatopathology education, which comprises approximately 30% of the dermatology residency curriculum, is crucial for the holistic training of dermatology residents to diagnose and manage a range of dermatologic conditions.1 Additionally, dermatopathology is the topic of one of the 4 American Board of Dermatology CORE Exam modules, further highlighting the need for comprehensive education in this area. A variety of resources including virtual dermatopathology and conventional microscopy training currently are used in residency programs for dermatopathology education.2,3 Although used less frequently, social media platforms such as Instagram also are used to aid in dermatopathology education for a wider audience.4 Other online resources, including the American Society of Dermatopathology website (www.asdp.org) and DermpathAtlas.com, are excellent tools for medical students, residents, and fellows to develop their knowledge.5 While these resources are accessible, they must be directly sought out by the student and utilized on their own time. Additionally, if medical students do not have a strong understanding of the basics of dermatopathology, they may not have the foundation required to benefit from these resources.
Dermatopathology education is critical for the overall practice of dermatology, yet most dermatology residency programs may not be incorporating dermatopathology education early enough in training. One study evaluating the timing and length of dermatopathology education during residency reported that fewer than 40% (20/51) of dermatology residency programs allocate 3 or more weeks to dermatopathology education during the second postgraduate year.1 Despite Ackerman6 advocating for early dermatopathology exposure to best prepare medical students to recognize and manage certain dermatologic conditions, the majority of exposure still seems to occur during postgraduate year 4.1 Furthermore, current primary care residents feel that their medical school training did not sufficiently prepare them to diagnose and manage dermatologic conditions, with only 37% (93/252) reporting feeling adequately prepared.7,8 Medical students also reported a lack of confidence in overall dermatology knowledge, with 89% (72/81) reporting they felt neutral, slightly confident, or not at all confident when asked to diagnose skin lesions.9 In the same study, the average score was 46.6% (7/15 questions answered correctly) when 74 participants were assessed via a multiple choice quiz on dermatologic diagnosis and treatment, further demonstrating the lack of general dermatology comfort among medical students.9 This likely stems from limited dermatology curriculum in medical schools, demonstrating the need for further dermatology education as a whole in medical school.10
Ensuring robust dermatopathology education in medical school and the first year of dermatology residency has the potential to better prepare medical students for the transition into dermatology residency and clinical practice. We created an introductory dermatopathology lecture and presented it to medical students and first year dermatology residents to improve dermatopathology knowledge and confidence in learners early in their dermatology training.
Structure of the Lecture
Participants included first-year dermatology residents and fourth-year medical students rotating with the Wayne State University Department of Dermatology (Detroit, Michigan). The same facilitator (H.O.) taught each of the lectures, and all lectures were conducted via Zoom at the beginning of the month from May 2024 through November 2024. A total of 7 lectures were given. The lecture was formatted so that a histologic image was shown, then learners expressed their thoughts about what the image was showing before the answer was given. This format allowed participants to view the images on their own device screen and allowed the facilitator to annotate the images. The lecture was divided into 3 sections: (1) cell types and basic structures, (2) anatomic slides, and (3) common diagnoses. Each session lasted approximately 45 minutes.
Section 1: Cell Types and Basic Structures—The first section covered the fundamental cell types (neutrophils, lymphocytes, plasma cells, melanocytes, and eosinophils) along with glandular structures (apocrine, eccrine, and sebaceous). The session was designed to follow a retention and allow learners to think through each slide. First, participants were shown histologic images of each cell type and were asked to identify what type of cell was being shown. On the following slide, key features of each cell type were highlighted. Next, participants similarly were shown images of the glandular structures followed by key features of each. The section concluded with a review of the layers of the skin (stratum corneum, stratum granulosum, stratum lucidum, stratum spinosum, and stratum basale). A histologic image was shown, and the facilitator discussed how to distinguish the layers.
Section 2: Anatomic Sites—This section focused on key pathologic features for differentiating body surfaces, including the scalp, face, eyelids, ears, areolae, palms and soles, and mucosae. Participants initially were shown an image of a hematoxylin and eosin–stained slide from a specific body surface and then were asked to identify structures that may serve as a clue to the anatomic location. If the participants were not sure, they were given hints; for example, when participants were shown an image of the ear and were unsure of the location, the facilitator circled cartilage and asked them to identify the structure. In most cases, once participants named this structure, they were able to recognize that the location was the ear.
Section 3: Common Diagnoses—This section addressed frequently encountered diagnoses in dermatopathology, including basal cell carcinoma, squamous cell carcinoma, squamous cell carcinoma in situ, epidermoid cyst, pilar cyst, seborrheic keratosis, solar lentigo, melanocytic nevus, melanoma, verruca vulgaris, spongiotic dermatitis, psoriasis, and lichen planus. It followed the same format of the first section: participants were shown an hemotoxyllin and eosin–stained image and then were asked to discuss what the diagnosis could be and why. Hints were given if participants struggled to come up with the correct diagnosis. A few slides also were dedicated to distinguishing benign nevi, dysplastic nevi, and melanoma.
Pretest and Posttest Results
Residents participated in the lecture as part of their first-year orientation, and medical students participated during their dermatology rotation. All participants were invited to complete a pretest and a posttest before and after the lecture, respectively. Both assessments were optional and anonymous. The pretest was completed electronically and consisted of 10 knowledge-based, multiple-choice questions that included a histopathologic image and asked, “What is the most likely diagnosis?,” “What is the predominant cell type?,” and “Where was this specimen taken from?” In addition to the knowledge-based questions, participants also were asked to rate their confidence in dermatopathology on a 5-point Likert scale ranging from 1 (not confident at all) to 5 (extremely confident). Participants completed the entire pretest before any information on the topic was provided. After the lecture, participants were asked to complete a posttest identical to the pretest and to rate their confidence in dermatopathology again on the same scale. The posttest included an additional question asking participants to rate the helpfulness of the lecture on a Likert scale ranging from 1 (not helpful at all) to 5 (extremely helpful). Participants completed the posttest within 48 hours of the lecture.
Overall, 15 learners participated in the pretest and 12 in the posttest. Of the 15 pretest participants, 3 were first-year residents and 12 were medical students. Similarly, in the posttest, 2 respondents were first-year residents and 10 were medical students. All responses contained complete pretests and posttests. The mean score on the pretest was 62%, whereas the mean score on the posttest was 75%. A paired t test indicated a statistically significant improvement (P=.017). In addition, the mean rating for confidence in dermatopathology knowledge before the lecture was 1.5 prior to the lecture and 2.6 after the lecture. A paired t test demonstrated statistical significance (P=.010). The mean rating of the helpfulness of the lecture was 4.67. The majority (91.7% [11/12]) of the participants gave a rating of 4 or 5.
Impact of the Lecture on Dermatopathology Knowledge
There is a gap in dermatopathology education early in medical training. Our introductory lecture led to higher post test scores and increased confidence in dermatopathology among medical students and dermatology residents, demonstrating the effectiveness of this kind of program in bridging this education gap. The majority of participants in our lecture said they found the session helpful. A previously published article called for early implementation of dermatology education as a whole in the medical curriculum due to lack of knowledge and confidence, and our introductory lecture may help to bridge this gap.8 Increasing dermatopathology content for medical students and first-year dermatology residents can expand knowledge, as shown by the increased scores on the posttest, and better supports learners transitioning to dermatology residency, where dermatopathology constitutes a large part of the overall curriculum.2 More comprehensive knowledge of dermatopathology early in dermatology training also may help to better prepare residents to accurately diagnose and manage dermatologic conditions.
Pretest scores showed that the average confidence rating in dermatopathology among participants in our lecture was 1.5, which is rather low. This is consistent with prior studies that have found that residents feel that medical school inadequately prepared them for dermatology residency.7,8 More than 87% (71/81) of medical students surveyed felt they received inadequate general dermatology training in medical school.9 This supports the proposed educational gap that is impacting confidence in overall dermatology knowledge, which includes dermatopathology. In our study, the average confidence rating increased by 1.1 points after the lecture, which was statistically significant (P=.010) and demonstrates that an introductory lecture serves as a feasible intervention to improve confidence in this area.
The feedback we received from participants in our lecture shows the benefits of an introductory interactive lecture with virtual dermatopathology images. Ngo et al2 highlighted how residents perceive virtual images to be superior to conventional microscopy for dermatopathology, which we utilized in our lecture. This method is not only cost effective but also provides a simple way for learners and facilitators to point out key findings on histopathology slides.2
Final Thoughts
Overall, implementing dermatopathology education early in training has a measurable impact on dermatopathology knowledge and confidence among medical students and first-year dermatology residents. An interactive lecture with virtual images similar to the one we describe here may better prepare learners for the transition to dermatology residency by addressing the educational gap in dermatopathology early in training.
Dermatopathology education, which comprises approximately 30% of the dermatology residency curriculum, is crucial for the holistic training of dermatology residents to diagnose and manage a range of dermatologic conditions.1 Additionally, dermatopathology is the topic of one of the 4 American Board of Dermatology CORE Exam modules, further highlighting the need for comprehensive education in this area. A variety of resources including virtual dermatopathology and conventional microscopy training currently are used in residency programs for dermatopathology education.2,3 Although used less frequently, social media platforms such as Instagram also are used to aid in dermatopathology education for a wider audience.4 Other online resources, including the American Society of Dermatopathology website (www.asdp.org) and DermpathAtlas.com, are excellent tools for medical students, residents, and fellows to develop their knowledge.5 While these resources are accessible, they must be directly sought out by the student and utilized on their own time. Additionally, if medical students do not have a strong understanding of the basics of dermatopathology, they may not have the foundation required to benefit from these resources.
Dermatopathology education is critical for the overall practice of dermatology, yet most dermatology residency programs may not be incorporating dermatopathology education early enough in training. One study evaluating the timing and length of dermatopathology education during residency reported that fewer than 40% (20/51) of dermatology residency programs allocate 3 or more weeks to dermatopathology education during the second postgraduate year.1 Despite Ackerman6 advocating for early dermatopathology exposure to best prepare medical students to recognize and manage certain dermatologic conditions, the majority of exposure still seems to occur during postgraduate year 4.1 Furthermore, current primary care residents feel that their medical school training did not sufficiently prepare them to diagnose and manage dermatologic conditions, with only 37% (93/252) reporting feeling adequately prepared.7,8 Medical students also reported a lack of confidence in overall dermatology knowledge, with 89% (72/81) reporting they felt neutral, slightly confident, or not at all confident when asked to diagnose skin lesions.9 In the same study, the average score was 46.6% (7/15 questions answered correctly) when 74 participants were assessed via a multiple choice quiz on dermatologic diagnosis and treatment, further demonstrating the lack of general dermatology comfort among medical students.9 This likely stems from limited dermatology curriculum in medical schools, demonstrating the need for further dermatology education as a whole in medical school.10
Ensuring robust dermatopathology education in medical school and the first year of dermatology residency has the potential to better prepare medical students for the transition into dermatology residency and clinical practice. We created an introductory dermatopathology lecture and presented it to medical students and first year dermatology residents to improve dermatopathology knowledge and confidence in learners early in their dermatology training.
Structure of the Lecture
Participants included first-year dermatology residents and fourth-year medical students rotating with the Wayne State University Department of Dermatology (Detroit, Michigan). The same facilitator (H.O.) taught each of the lectures, and all lectures were conducted via Zoom at the beginning of the month from May 2024 through November 2024. A total of 7 lectures were given. The lecture was formatted so that a histologic image was shown, then learners expressed their thoughts about what the image was showing before the answer was given. This format allowed participants to view the images on their own device screen and allowed the facilitator to annotate the images. The lecture was divided into 3 sections: (1) cell types and basic structures, (2) anatomic slides, and (3) common diagnoses. Each session lasted approximately 45 minutes.
Section 1: Cell Types and Basic Structures—The first section covered the fundamental cell types (neutrophils, lymphocytes, plasma cells, melanocytes, and eosinophils) along with glandular structures (apocrine, eccrine, and sebaceous). The session was designed to follow a retention and allow learners to think through each slide. First, participants were shown histologic images of each cell type and were asked to identify what type of cell was being shown. On the following slide, key features of each cell type were highlighted. Next, participants similarly were shown images of the glandular structures followed by key features of each. The section concluded with a review of the layers of the skin (stratum corneum, stratum granulosum, stratum lucidum, stratum spinosum, and stratum basale). A histologic image was shown, and the facilitator discussed how to distinguish the layers.
Section 2: Anatomic Sites—This section focused on key pathologic features for differentiating body surfaces, including the scalp, face, eyelids, ears, areolae, palms and soles, and mucosae. Participants initially were shown an image of a hematoxylin and eosin–stained slide from a specific body surface and then were asked to identify structures that may serve as a clue to the anatomic location. If the participants were not sure, they were given hints; for example, when participants were shown an image of the ear and were unsure of the location, the facilitator circled cartilage and asked them to identify the structure. In most cases, once participants named this structure, they were able to recognize that the location was the ear.
Section 3: Common Diagnoses—This section addressed frequently encountered diagnoses in dermatopathology, including basal cell carcinoma, squamous cell carcinoma, squamous cell carcinoma in situ, epidermoid cyst, pilar cyst, seborrheic keratosis, solar lentigo, melanocytic nevus, melanoma, verruca vulgaris, spongiotic dermatitis, psoriasis, and lichen planus. It followed the same format of the first section: participants were shown an hemotoxyllin and eosin–stained image and then were asked to discuss what the diagnosis could be and why. Hints were given if participants struggled to come up with the correct diagnosis. A few slides also were dedicated to distinguishing benign nevi, dysplastic nevi, and melanoma.
Pretest and Posttest Results
Residents participated in the lecture as part of their first-year orientation, and medical students participated during their dermatology rotation. All participants were invited to complete a pretest and a posttest before and after the lecture, respectively. Both assessments were optional and anonymous. The pretest was completed electronically and consisted of 10 knowledge-based, multiple-choice questions that included a histopathologic image and asked, “What is the most likely diagnosis?,” “What is the predominant cell type?,” and “Where was this specimen taken from?” In addition to the knowledge-based questions, participants also were asked to rate their confidence in dermatopathology on a 5-point Likert scale ranging from 1 (not confident at all) to 5 (extremely confident). Participants completed the entire pretest before any information on the topic was provided. After the lecture, participants were asked to complete a posttest identical to the pretest and to rate their confidence in dermatopathology again on the same scale. The posttest included an additional question asking participants to rate the helpfulness of the lecture on a Likert scale ranging from 1 (not helpful at all) to 5 (extremely helpful). Participants completed the posttest within 48 hours of the lecture.
Overall, 15 learners participated in the pretest and 12 in the posttest. Of the 15 pretest participants, 3 were first-year residents and 12 were medical students. Similarly, in the posttest, 2 respondents were first-year residents and 10 were medical students. All responses contained complete pretests and posttests. The mean score on the pretest was 62%, whereas the mean score on the posttest was 75%. A paired t test indicated a statistically significant improvement (P=.017). In addition, the mean rating for confidence in dermatopathology knowledge before the lecture was 1.5 prior to the lecture and 2.6 after the lecture. A paired t test demonstrated statistical significance (P=.010). The mean rating of the helpfulness of the lecture was 4.67. The majority (91.7% [11/12]) of the participants gave a rating of 4 or 5.
Impact of the Lecture on Dermatopathology Knowledge
There is a gap in dermatopathology education early in medical training. Our introductory lecture led to higher post test scores and increased confidence in dermatopathology among medical students and dermatology residents, demonstrating the effectiveness of this kind of program in bridging this education gap. The majority of participants in our lecture said they found the session helpful. A previously published article called for early implementation of dermatology education as a whole in the medical curriculum due to lack of knowledge and confidence, and our introductory lecture may help to bridge this gap.8 Increasing dermatopathology content for medical students and first-year dermatology residents can expand knowledge, as shown by the increased scores on the posttest, and better supports learners transitioning to dermatology residency, where dermatopathology constitutes a large part of the overall curriculum.2 More comprehensive knowledge of dermatopathology early in dermatology training also may help to better prepare residents to accurately diagnose and manage dermatologic conditions.
Pretest scores showed that the average confidence rating in dermatopathology among participants in our lecture was 1.5, which is rather low. This is consistent with prior studies that have found that residents feel that medical school inadequately prepared them for dermatology residency.7,8 More than 87% (71/81) of medical students surveyed felt they received inadequate general dermatology training in medical school.9 This supports the proposed educational gap that is impacting confidence in overall dermatology knowledge, which includes dermatopathology. In our study, the average confidence rating increased by 1.1 points after the lecture, which was statistically significant (P=.010) and demonstrates that an introductory lecture serves as a feasible intervention to improve confidence in this area.
The feedback we received from participants in our lecture shows the benefits of an introductory interactive lecture with virtual dermatopathology images. Ngo et al2 highlighted how residents perceive virtual images to be superior to conventional microscopy for dermatopathology, which we utilized in our lecture. This method is not only cost effective but also provides a simple way for learners and facilitators to point out key findings on histopathology slides.2
Final Thoughts
Overall, implementing dermatopathology education early in training has a measurable impact on dermatopathology knowledge and confidence among medical students and first-year dermatology residents. An interactive lecture with virtual images similar to the one we describe here may better prepare learners for the transition to dermatology residency by addressing the educational gap in dermatopathology early in training.
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826, vii.
- Ngo TB, Niu W, Fang Z, et al. Dermatology residents’ perspectives on virtual dermatopathology education. J Cutan Pathol. 2024;51:530-537.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Hubbard G, Saal R, Wintringham J, et al. Utilizing Instagram as a novel method for dermatopathology instruction. Clin Exp Dermatol. 2023;49:89-91.
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14.
- Ackerman AB. Training residents in dermatopathology: why, when, where, and how. J Am Acad Dermatol. 1990;22(6 Pt 1):1104-1106.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.e1.
- Murase JE. Understanding the importance of dermatology training in undergraduate medical education. Dermatol Pract Concept. 2015;5:95-96.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826, vii.
- Ngo TB, Niu W, Fang Z, et al. Dermatology residents’ perspectives on virtual dermatopathology education. J Cutan Pathol. 2024;51:530-537.
- Shahriari N, Grant-Kels J, Murphy MJ. Dermatopathology education in the era of modern technology. J Cutan Pathol. 2017;44:763-771.
- Hubbard G, Saal R, Wintringham J, et al. Utilizing Instagram as a novel method for dermatopathology instruction. Clin Exp Dermatol. 2023;49:89-91.
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14.
- Ackerman AB. Training residents in dermatopathology: why, when, where, and how. J Am Acad Dermatol. 1990;22(6 Pt 1):1104-1106.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.e1.
- Murase JE. Understanding the importance of dermatology training in undergraduate medical education. Dermatol Pract Concept. 2015;5:95-96.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents
Impact of an Introductory Dermatopathology Lecture on Medical Students and First-Year Dermatology Residents