User login
Dermal fillers are considered Class III medical devices by the US Food and Drug Administration (FDA).1 Reports of adverse events (AEs) for medical devices are made public by the FDA to allow for transparent postmarketing surveillance.2The AE trends extracted from these historical data may help distinguish between expected learning curves of new dermal fillers versus unsafe products that may require FDA intervention. Considering that aesthetic treatments are not medically necessary, a low risk profile is paramount and determining what constitutes normal learning curves is important for impartial assessment of AEs as new fillers come on the market. The concept of a 3-year learning curve can be an important tool for safety monitoring going forward, creating a bar for quality that could trigger increased surveillance if a product fails to meet an expected arc of diminished AEs over time. This study serves to evaluate historical AE data and to establish learning curves for FDA-approved dermal fillers.
Methods
We searched the OpenFDA Device Adverse Event Report Browser (http://openfda.shinyapps.io/devicereports/) for reported AEs within the FDA product code LMH (Implant, Dermal, For Aesthetic Use) that were received from January 1, 1983, to December 31, 2017. For each reported AE, information related to the date of the reported event and the device brand name were recorded. Devices implicated in each AE were classified based on primary composition according to the following 5 categories: collagen, hyaluronic acid (HA), hydroxylapatite, poly-L-lactic acid (PLLA), and polymethyl methacrylate (PMMA). Inaccurate entries of reported AEs or those intended for nonaesthetic use were excluded from the study. A total of 8530 AEs were included in the study. To normalize the data, we obtained annual reports for the number of procedures performed by filler type from the American Society of Plastic Surgeons (ASPS) cosmetic procedure trends. 3 We calculated the annual AE rates for each approved filler by dividing the number of AEs by the number of procedures performed that year.
Results
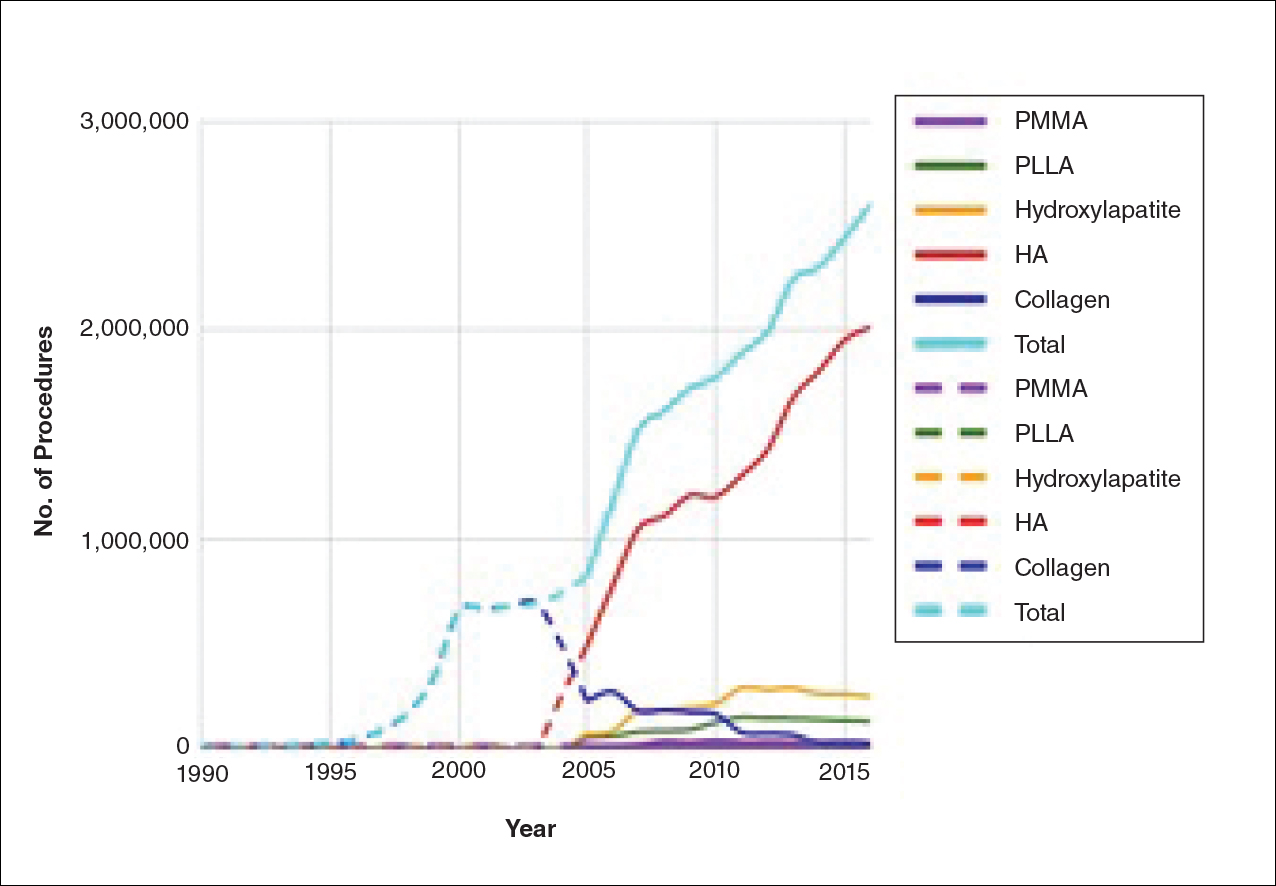
The trends of different filler types depicting the number of procedures performed over time are shown in Figure 1. Data from the ASPS dated back to 2005; therefore, the number of procedures performed prior to that were extrapolated with knowledge of products’ approval dates and market share, indicated by a dotted line. To determine AE rates for each year, we divided the number of AEs by the number of reported procedures for each filler type. The AE rates are displayed graphically in Figures 2 and 3 with superimposed FDA approval dates for each filler.4
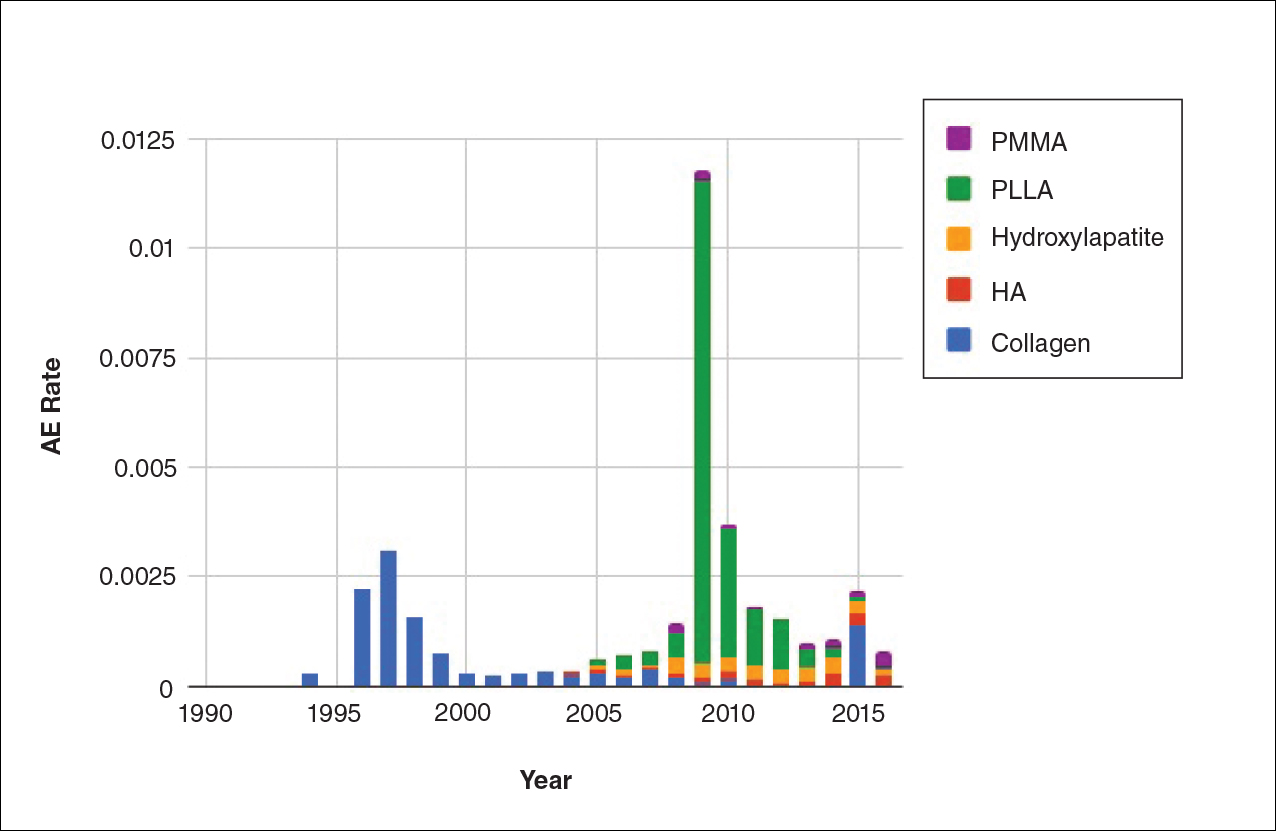
Two major peaks in reported AE rates for all fillers were noted in the late 1990s and late 2000s, mostly associated with collagen and PLLA fillers, respectively (Figure 2). Overall, there has been a low rate of AEs associated with HA fillers since their initial approval in the early 2000s.
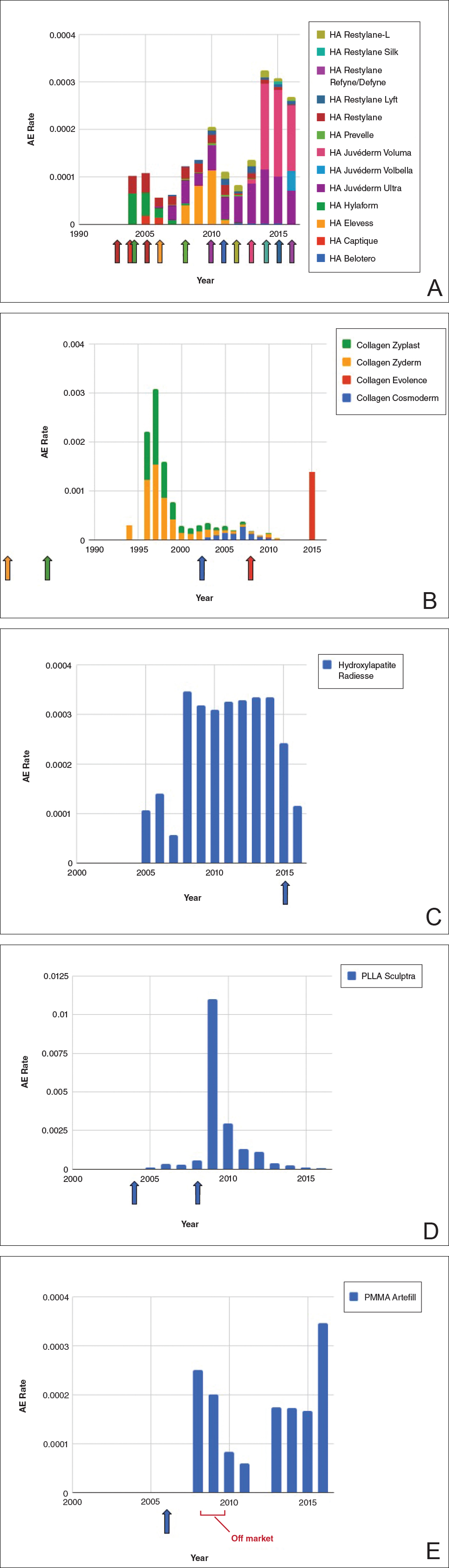
Individual filler AE rates also were analyzed. Hyaluronic acid fillers were associated with an extremely low rate of AEs, ranging from 1 to 4 AEs per 10,000 procedures (Figure 3A). This low AE rate profile underscores the safety of HA fillers, which has spurred their popularity. Adverseevent rates for collagen fillers spiked in the mid- to late 1990s and resolved over the course of the next 3 years (Figure 3B). Hydroxylapatite fillers had a rather uniform AE rate with an early indication of a drop-off after 2015 (Figure 3C). Poly-L-lactic acid fillers showed the steepest learning curve, with a peak of 1 AE per 100 procedures after they were approved in 2008 (Figure 3D); however, there is a comparable 3-year resolution of AE rates. Adverse events for PMMA fillers did not show specific resolution, meaning that they did not follow the 3-year arc that was seen for the other dermal fillers reported in the data set (Figure 3E).
Comment
Our study is unique in that it analyzes reported AE data over a 34-year period for injectable dermal fillers. To our knowledge, this novel method of calculating AE rates across dermal fillers and for individual products is the first of its kind that facilitates usage-normalized comparison of different filler types.
All OpenFDA data are self-reported and therefore have inherent limitations. Anyone can enter information on AEs in this system, including both patients and health care providers, so the quality of the input may be variable. However, this output is the only representation we have for nearly 35 years of AE history for this burgeoning category of popular aesthetic treatments. Another study limitation is that not everyone may know that reporting an AE in the OpenFDA is an option; therefore, we may be missing a portion of AEs due to underreporting. Underreporting may be especially at play in the years before the Internet was prevalent for residential use since access to the Internet would be required to report an AR on the website. However, examining the available data provides an important window into valuable information on complications that have occurred and have been reported for FDA-approved dermal fillers.
An additional challenge in constructing this study was assessing the total number of injectable dermal filler treatments being performed annually across filler types for normalization of the data. Although the absolute numbers of filler use as captured by the ASPS are smaller than the true total filler use across all injectors, the relative use of different filler products will be similar across all specialties because it reflects product popularity. Annual surveys on aesthetic procedures also are conducted by the American Society for Dermatologic Surgery and the American Association for Facial Plastic and Reconstructive Surgery, but neither one captures the relative usage of different filler types. Because individual filler companies do not publish their annual sales numbers by product, the ASPS data give us the best gauge of relative use of fillers by product type given the available information. We conclude that the comparison of AE rates would remain the same even if we had data for total annual filler use across specialties.
Our graphical depiction of the data clearly demonstrates the low AE profile of HA fillers, which is in line with the general consensus of their safety that has contributed to their vast popularity; however, this study represents the first time usage-normalized AE rates are compared to other filler compositions. Hyaluronic acid fillers have the unique feature of being able to be dissolved with the hyaluronidase enzyme, which can limit adverse event potential as compared to other ingredient classes of filler types and may be reflected in their low overall AE profile. The AE rate spike and resolution for collagen fillers represent what we refer to as a “normal learning curve” based on our analysis of the data set as a whole, suggesting an appropriate time course of increased familiarity with the product without inherent issues with the product itself. Multiple sequential anatomic site indications were approved for hydroxylapatite fillers from 2006 through 2015, which may have yielded overlapping learning curves for each approval, resulting in a rather uniform AE rate. The early drop-off in AE rates after the 2015 anatomic site approval may represent the beginning of a normal learning curve, and continued surveillance of AE rates would be of value to confirm this trend. We saw a similar 3-year learning curve for PLLA fillers as the curve for collagen fillers, suggesting a normal learning curve and no out-of-line safety issues. Polymethylmethacrylate fillers were approved in 2006 and were taken off the market for a period in the late 2000s, explaining the drop-off. Once they were back on the market, we do not see a typical learning curve for PMMA, which may warrant surveillance for safety by both clinicians and the FDA.
Conclusion
Our study represents a novel method of evaluating the safety of medical devices, specifically aesthetic fillers. We showed that every AE rate curve for different filler types tells a story. Reactions to AEs for new fillers should be placed in the context of whether they seem to be following the established learning curve.
- Dermal fillers (soft tissue fillers). US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ucm2007470.htm. Updated May 31, 2018. Accessed June 29, 2018.
- Kass-Hout TA, Xu Z, Mohebbi M, et al. OpenFDA: an innovative platform providing access to a wealth of FDA’s publicly available data. J Am Med Inform Assoc. 2016;23:596-600.
- Plastic surgery statistics. American Society of Plastic Surgeons website. https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf. Accessed June 28, 2018.
- Dermal fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Accessed June 28, 2018. Updated March 19, 2018.
Dermal fillers are considered Class III medical devices by the US Food and Drug Administration (FDA).1 Reports of adverse events (AEs) for medical devices are made public by the FDA to allow for transparent postmarketing surveillance.2The AE trends extracted from these historical data may help distinguish between expected learning curves of new dermal fillers versus unsafe products that may require FDA intervention. Considering that aesthetic treatments are not medically necessary, a low risk profile is paramount and determining what constitutes normal learning curves is important for impartial assessment of AEs as new fillers come on the market. The concept of a 3-year learning curve can be an important tool for safety monitoring going forward, creating a bar for quality that could trigger increased surveillance if a product fails to meet an expected arc of diminished AEs over time. This study serves to evaluate historical AE data and to establish learning curves for FDA-approved dermal fillers.
Methods
We searched the OpenFDA Device Adverse Event Report Browser (http://openfda.shinyapps.io/devicereports/) for reported AEs within the FDA product code LMH (Implant, Dermal, For Aesthetic Use) that were received from January 1, 1983, to December 31, 2017. For each reported AE, information related to the date of the reported event and the device brand name were recorded. Devices implicated in each AE were classified based on primary composition according to the following 5 categories: collagen, hyaluronic acid (HA), hydroxylapatite, poly-L-lactic acid (PLLA), and polymethyl methacrylate (PMMA). Inaccurate entries of reported AEs or those intended for nonaesthetic use were excluded from the study. A total of 8530 AEs were included in the study. To normalize the data, we obtained annual reports for the number of procedures performed by filler type from the American Society of Plastic Surgeons (ASPS) cosmetic procedure trends. 3 We calculated the annual AE rates for each approved filler by dividing the number of AEs by the number of procedures performed that year.
Results
The trends of different filler types depicting the number of procedures performed over time are shown in Figure 1. Data from the ASPS dated back to 2005; therefore, the number of procedures performed prior to that were extrapolated with knowledge of products’ approval dates and market share, indicated by a dotted line. To determine AE rates for each year, we divided the number of AEs by the number of reported procedures for each filler type. The AE rates are displayed graphically in Figures 2 and 3 with superimposed FDA approval dates for each filler.4

Two major peaks in reported AE rates for all fillers were noted in the late 1990s and late 2000s, mostly associated with collagen and PLLA fillers, respectively (Figure 2). Overall, there has been a low rate of AEs associated with HA fillers since their initial approval in the early 2000s.

Individual filler AE rates also were analyzed. Hyaluronic acid fillers were associated with an extremely low rate of AEs, ranging from 1 to 4 AEs per 10,000 procedures (Figure 3A). This low AE rate profile underscores the safety of HA fillers, which has spurred their popularity. Adverseevent rates for collagen fillers spiked in the mid- to late 1990s and resolved over the course of the next 3 years (Figure 3B). Hydroxylapatite fillers had a rather uniform AE rate with an early indication of a drop-off after 2015 (Figure 3C). Poly-L-lactic acid fillers showed the steepest learning curve, with a peak of 1 AE per 100 procedures after they were approved in 2008 (Figure 3D); however, there is a comparable 3-year resolution of AE rates. Adverse events for PMMA fillers did not show specific resolution, meaning that they did not follow the 3-year arc that was seen for the other dermal fillers reported in the data set (Figure 3E).

Comment
Our study is unique in that it analyzes reported AE data over a 34-year period for injectable dermal fillers. To our knowledge, this novel method of calculating AE rates across dermal fillers and for individual products is the first of its kind that facilitates usage-normalized comparison of different filler types.
All OpenFDA data are self-reported and therefore have inherent limitations. Anyone can enter information on AEs in this system, including both patients and health care providers, so the quality of the input may be variable. However, this output is the only representation we have for nearly 35 years of AE history for this burgeoning category of popular aesthetic treatments. Another study limitation is that not everyone may know that reporting an AE in the OpenFDA is an option; therefore, we may be missing a portion of AEs due to underreporting. Underreporting may be especially at play in the years before the Internet was prevalent for residential use since access to the Internet would be required to report an AR on the website. However, examining the available data provides an important window into valuable information on complications that have occurred and have been reported for FDA-approved dermal fillers.
An additional challenge in constructing this study was assessing the total number of injectable dermal filler treatments being performed annually across filler types for normalization of the data. Although the absolute numbers of filler use as captured by the ASPS are smaller than the true total filler use across all injectors, the relative use of different filler products will be similar across all specialties because it reflects product popularity. Annual surveys on aesthetic procedures also are conducted by the American Society for Dermatologic Surgery and the American Association for Facial Plastic and Reconstructive Surgery, but neither one captures the relative usage of different filler types. Because individual filler companies do not publish their annual sales numbers by product, the ASPS data give us the best gauge of relative use of fillers by product type given the available information. We conclude that the comparison of AE rates would remain the same even if we had data for total annual filler use across specialties.
Our graphical depiction of the data clearly demonstrates the low AE profile of HA fillers, which is in line with the general consensus of their safety that has contributed to their vast popularity; however, this study represents the first time usage-normalized AE rates are compared to other filler compositions. Hyaluronic acid fillers have the unique feature of being able to be dissolved with the hyaluronidase enzyme, which can limit adverse event potential as compared to other ingredient classes of filler types and may be reflected in their low overall AE profile. The AE rate spike and resolution for collagen fillers represent what we refer to as a “normal learning curve” based on our analysis of the data set as a whole, suggesting an appropriate time course of increased familiarity with the product without inherent issues with the product itself. Multiple sequential anatomic site indications were approved for hydroxylapatite fillers from 2006 through 2015, which may have yielded overlapping learning curves for each approval, resulting in a rather uniform AE rate. The early drop-off in AE rates after the 2015 anatomic site approval may represent the beginning of a normal learning curve, and continued surveillance of AE rates would be of value to confirm this trend. We saw a similar 3-year learning curve for PLLA fillers as the curve for collagen fillers, suggesting a normal learning curve and no out-of-line safety issues. Polymethylmethacrylate fillers were approved in 2006 and were taken off the market for a period in the late 2000s, explaining the drop-off. Once they were back on the market, we do not see a typical learning curve for PMMA, which may warrant surveillance for safety by both clinicians and the FDA.
Conclusion
Our study represents a novel method of evaluating the safety of medical devices, specifically aesthetic fillers. We showed that every AE rate curve for different filler types tells a story. Reactions to AEs for new fillers should be placed in the context of whether they seem to be following the established learning curve.
Dermal fillers are considered Class III medical devices by the US Food and Drug Administration (FDA).1 Reports of adverse events (AEs) for medical devices are made public by the FDA to allow for transparent postmarketing surveillance.2The AE trends extracted from these historical data may help distinguish between expected learning curves of new dermal fillers versus unsafe products that may require FDA intervention. Considering that aesthetic treatments are not medically necessary, a low risk profile is paramount and determining what constitutes normal learning curves is important for impartial assessment of AEs as new fillers come on the market. The concept of a 3-year learning curve can be an important tool for safety monitoring going forward, creating a bar for quality that could trigger increased surveillance if a product fails to meet an expected arc of diminished AEs over time. This study serves to evaluate historical AE data and to establish learning curves for FDA-approved dermal fillers.
Methods
We searched the OpenFDA Device Adverse Event Report Browser (http://openfda.shinyapps.io/devicereports/) for reported AEs within the FDA product code LMH (Implant, Dermal, For Aesthetic Use) that were received from January 1, 1983, to December 31, 2017. For each reported AE, information related to the date of the reported event and the device brand name were recorded. Devices implicated in each AE were classified based on primary composition according to the following 5 categories: collagen, hyaluronic acid (HA), hydroxylapatite, poly-L-lactic acid (PLLA), and polymethyl methacrylate (PMMA). Inaccurate entries of reported AEs or those intended for nonaesthetic use were excluded from the study. A total of 8530 AEs were included in the study. To normalize the data, we obtained annual reports for the number of procedures performed by filler type from the American Society of Plastic Surgeons (ASPS) cosmetic procedure trends. 3 We calculated the annual AE rates for each approved filler by dividing the number of AEs by the number of procedures performed that year.
Results
The trends of different filler types depicting the number of procedures performed over time are shown in Figure 1. Data from the ASPS dated back to 2005; therefore, the number of procedures performed prior to that were extrapolated with knowledge of products’ approval dates and market share, indicated by a dotted line. To determine AE rates for each year, we divided the number of AEs by the number of reported procedures for each filler type. The AE rates are displayed graphically in Figures 2 and 3 with superimposed FDA approval dates for each filler.4

Two major peaks in reported AE rates for all fillers were noted in the late 1990s and late 2000s, mostly associated with collagen and PLLA fillers, respectively (Figure 2). Overall, there has been a low rate of AEs associated with HA fillers since their initial approval in the early 2000s.

Individual filler AE rates also were analyzed. Hyaluronic acid fillers were associated with an extremely low rate of AEs, ranging from 1 to 4 AEs per 10,000 procedures (Figure 3A). This low AE rate profile underscores the safety of HA fillers, which has spurred their popularity. Adverseevent rates for collagen fillers spiked in the mid- to late 1990s and resolved over the course of the next 3 years (Figure 3B). Hydroxylapatite fillers had a rather uniform AE rate with an early indication of a drop-off after 2015 (Figure 3C). Poly-L-lactic acid fillers showed the steepest learning curve, with a peak of 1 AE per 100 procedures after they were approved in 2008 (Figure 3D); however, there is a comparable 3-year resolution of AE rates. Adverse events for PMMA fillers did not show specific resolution, meaning that they did not follow the 3-year arc that was seen for the other dermal fillers reported in the data set (Figure 3E).

Comment
Our study is unique in that it analyzes reported AE data over a 34-year period for injectable dermal fillers. To our knowledge, this novel method of calculating AE rates across dermal fillers and for individual products is the first of its kind that facilitates usage-normalized comparison of different filler types.
All OpenFDA data are self-reported and therefore have inherent limitations. Anyone can enter information on AEs in this system, including both patients and health care providers, so the quality of the input may be variable. However, this output is the only representation we have for nearly 35 years of AE history for this burgeoning category of popular aesthetic treatments. Another study limitation is that not everyone may know that reporting an AE in the OpenFDA is an option; therefore, we may be missing a portion of AEs due to underreporting. Underreporting may be especially at play in the years before the Internet was prevalent for residential use since access to the Internet would be required to report an AR on the website. However, examining the available data provides an important window into valuable information on complications that have occurred and have been reported for FDA-approved dermal fillers.
An additional challenge in constructing this study was assessing the total number of injectable dermal filler treatments being performed annually across filler types for normalization of the data. Although the absolute numbers of filler use as captured by the ASPS are smaller than the true total filler use across all injectors, the relative use of different filler products will be similar across all specialties because it reflects product popularity. Annual surveys on aesthetic procedures also are conducted by the American Society for Dermatologic Surgery and the American Association for Facial Plastic and Reconstructive Surgery, but neither one captures the relative usage of different filler types. Because individual filler companies do not publish their annual sales numbers by product, the ASPS data give us the best gauge of relative use of fillers by product type given the available information. We conclude that the comparison of AE rates would remain the same even if we had data for total annual filler use across specialties.
Our graphical depiction of the data clearly demonstrates the low AE profile of HA fillers, which is in line with the general consensus of their safety that has contributed to their vast popularity; however, this study represents the first time usage-normalized AE rates are compared to other filler compositions. Hyaluronic acid fillers have the unique feature of being able to be dissolved with the hyaluronidase enzyme, which can limit adverse event potential as compared to other ingredient classes of filler types and may be reflected in their low overall AE profile. The AE rate spike and resolution for collagen fillers represent what we refer to as a “normal learning curve” based on our analysis of the data set as a whole, suggesting an appropriate time course of increased familiarity with the product without inherent issues with the product itself. Multiple sequential anatomic site indications were approved for hydroxylapatite fillers from 2006 through 2015, which may have yielded overlapping learning curves for each approval, resulting in a rather uniform AE rate. The early drop-off in AE rates after the 2015 anatomic site approval may represent the beginning of a normal learning curve, and continued surveillance of AE rates would be of value to confirm this trend. We saw a similar 3-year learning curve for PLLA fillers as the curve for collagen fillers, suggesting a normal learning curve and no out-of-line safety issues. Polymethylmethacrylate fillers were approved in 2006 and were taken off the market for a period in the late 2000s, explaining the drop-off. Once they were back on the market, we do not see a typical learning curve for PMMA, which may warrant surveillance for safety by both clinicians and the FDA.
Conclusion
Our study represents a novel method of evaluating the safety of medical devices, specifically aesthetic fillers. We showed that every AE rate curve for different filler types tells a story. Reactions to AEs for new fillers should be placed in the context of whether they seem to be following the established learning curve.
- Dermal fillers (soft tissue fillers). US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ucm2007470.htm. Updated May 31, 2018. Accessed June 29, 2018.
- Kass-Hout TA, Xu Z, Mohebbi M, et al. OpenFDA: an innovative platform providing access to a wealth of FDA’s publicly available data. J Am Med Inform Assoc. 2016;23:596-600.
- Plastic surgery statistics. American Society of Plastic Surgeons website. https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf. Accessed June 28, 2018.
- Dermal fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Accessed June 28, 2018. Updated March 19, 2018.
- Dermal fillers (soft tissue fillers). US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ucm2007470.htm. Updated May 31, 2018. Accessed June 29, 2018.
- Kass-Hout TA, Xu Z, Mohebbi M, et al. OpenFDA: an innovative platform providing access to a wealth of FDA’s publicly available data. J Am Med Inform Assoc. 2016;23:596-600.
- Plastic surgery statistics. American Society of Plastic Surgeons website. https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-full-report-2017.pdf. Accessed June 28, 2018.
- Dermal fillers approved by the Center for Devices and Radiological Health. US Food and Drug Administration website. https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/CosmeticDevices/WrinkleFillers/ucm227749.htm. Accessed June 28, 2018. Updated March 19, 2018.
Resident Pearl
- The US Food and Drug Administration’s (FDA) adverse event database, OpenFDA, provides extensive information regarding safety for a variety of cosmetic devices. Injectable dermal fillers are classified as a medical device by the FDA; therefore, safety studies can be performed using this publicly available database.
