User login
The Food and Drug Administration has approved ranibizumab for treatment of diabetic macular edema.
Ranibizumab injection, or Lucentis, is the first approved drug treatment of diabetic macular edema (DME) to be administered at a dosage of 0.3 mg once a month by a health care professional. The standard therapy for the condition thus far has been focal laser treatment.
"Today’s approval represents a major development for the treatment of people whose vision is impaired by DME as a complication of their disease," said Dr. Renata Albrecht, director of the division of transplant and ophthalmology products in FDA’s Center for Drug Evaluation and Research, in a statement.
Lucentis, developed by Genentech, was previously approved for treatment of neovascular age-related macular degeneration and for macular edema following retinal vein occlusion.
"We developed Lucentis to treat diseases of the eye and are pleased to have received this third U.S. indication to help a new population of people whose eyesight may be affected by diabetes," said Dr. Hal Barron, chief medical officer and head of global product development for Genentech, in a statement.
Roughly 4 million adults with diabetes reporter vision problems in 2010, according to the FDA.
The drug’s approval is based on the results of two clinical studies including 759 patients who were treated and followed for 3 years.
Results showed that 34%-45% of the patients who were treated monthly with 0.3 mg of Lucentis, gained at least three lines of vision compared with 12%-18% of those who didn’t receive the injection, according to the FDA. The higher tested dose of 0.5 mg showed no additional benefit.
The most common side effects of the drug are bleeding of the conjunctiva.
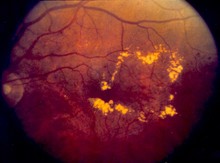
DME is a sight-threatening eye disease, in which fluid leaks to the macula and causes blurred vision. The condition affects people with diabetes and can lead to blindness.
The Food and Drug Administration has approved ranibizumab for treatment of diabetic macular edema.
Ranibizumab injection, or Lucentis, is the first approved drug treatment of diabetic macular edema (DME) to be administered at a dosage of 0.3 mg once a month by a health care professional. The standard therapy for the condition thus far has been focal laser treatment.
"Today’s approval represents a major development for the treatment of people whose vision is impaired by DME as a complication of their disease," said Dr. Renata Albrecht, director of the division of transplant and ophthalmology products in FDA’s Center for Drug Evaluation and Research, in a statement.
Lucentis, developed by Genentech, was previously approved for treatment of neovascular age-related macular degeneration and for macular edema following retinal vein occlusion.
"We developed Lucentis to treat diseases of the eye and are pleased to have received this third U.S. indication to help a new population of people whose eyesight may be affected by diabetes," said Dr. Hal Barron, chief medical officer and head of global product development for Genentech, in a statement.
Roughly 4 million adults with diabetes reporter vision problems in 2010, according to the FDA.
The drug’s approval is based on the results of two clinical studies including 759 patients who were treated and followed for 3 years.
Results showed that 34%-45% of the patients who were treated monthly with 0.3 mg of Lucentis, gained at least three lines of vision compared with 12%-18% of those who didn’t receive the injection, according to the FDA. The higher tested dose of 0.5 mg showed no additional benefit.
The most common side effects of the drug are bleeding of the conjunctiva.
DME is a sight-threatening eye disease, in which fluid leaks to the macula and causes blurred vision. The condition affects people with diabetes and can lead to blindness.
The Food and Drug Administration has approved ranibizumab for treatment of diabetic macular edema.
Ranibizumab injection, or Lucentis, is the first approved drug treatment of diabetic macular edema (DME) to be administered at a dosage of 0.3 mg once a month by a health care professional. The standard therapy for the condition thus far has been focal laser treatment.
"Today’s approval represents a major development for the treatment of people whose vision is impaired by DME as a complication of their disease," said Dr. Renata Albrecht, director of the division of transplant and ophthalmology products in FDA’s Center for Drug Evaluation and Research, in a statement.
Lucentis, developed by Genentech, was previously approved for treatment of neovascular age-related macular degeneration and for macular edema following retinal vein occlusion.
"We developed Lucentis to treat diseases of the eye and are pleased to have received this third U.S. indication to help a new population of people whose eyesight may be affected by diabetes," said Dr. Hal Barron, chief medical officer and head of global product development for Genentech, in a statement.
Roughly 4 million adults with diabetes reporter vision problems in 2010, according to the FDA.
The drug’s approval is based on the results of two clinical studies including 759 patients who were treated and followed for 3 years.
Results showed that 34%-45% of the patients who were treated monthly with 0.3 mg of Lucentis, gained at least three lines of vision compared with 12%-18% of those who didn’t receive the injection, according to the FDA. The higher tested dose of 0.5 mg showed no additional benefit.
The most common side effects of the drug are bleeding of the conjunctiva.
DME is a sight-threatening eye disease, in which fluid leaks to the macula and causes blurred vision. The condition affects people with diabetes and can lead to blindness.