User login
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
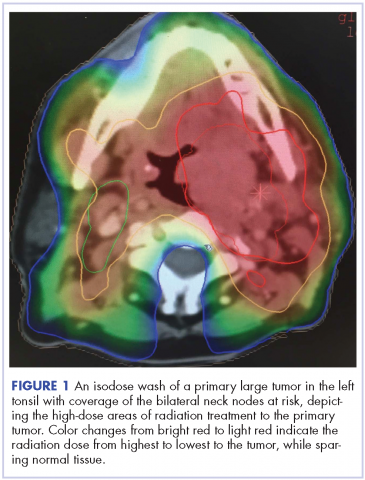
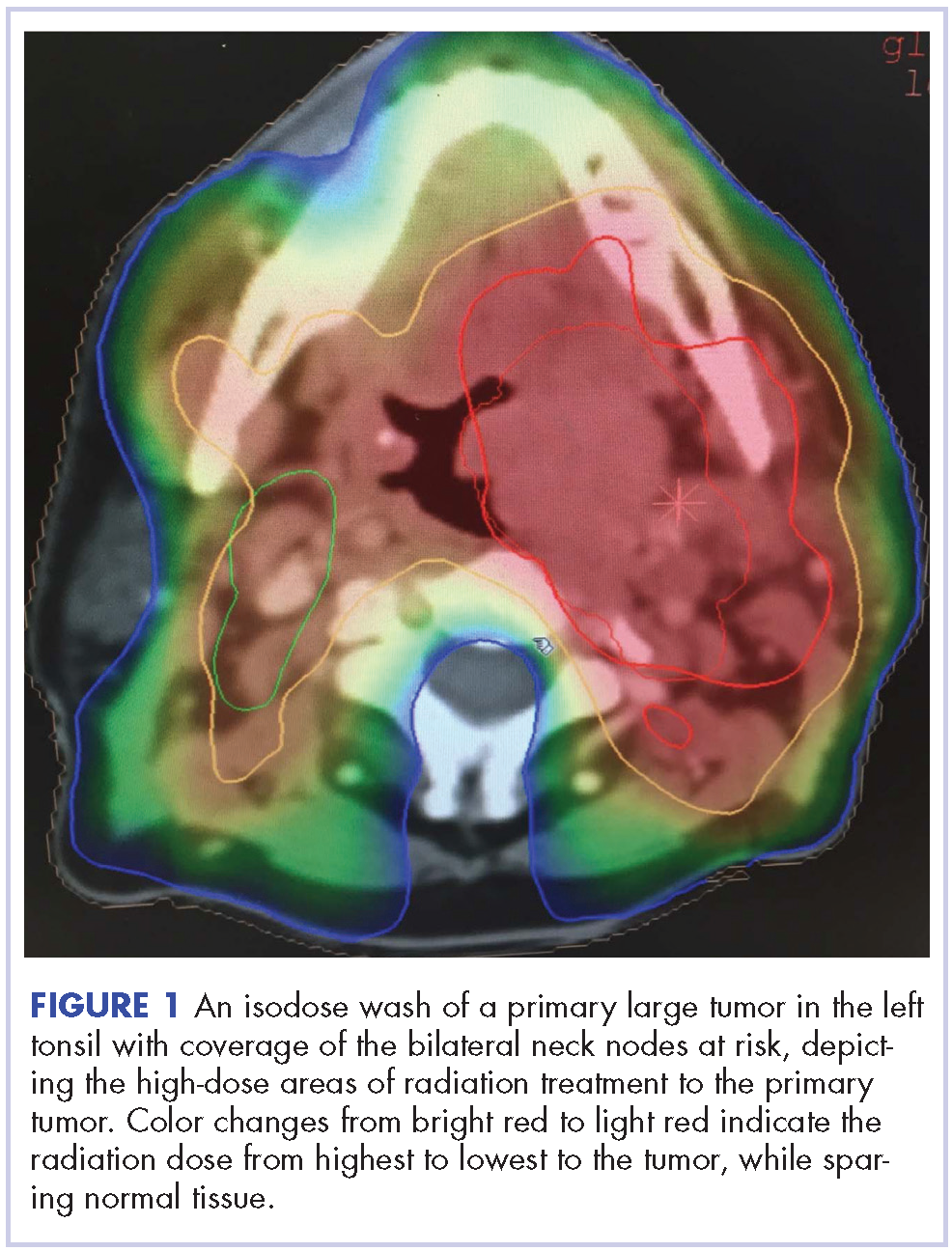
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
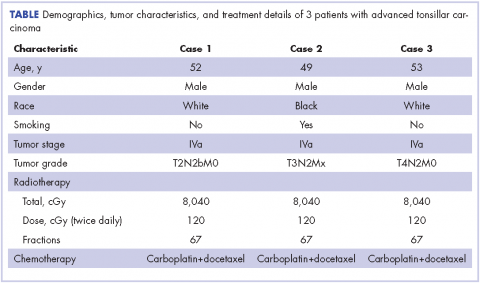
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
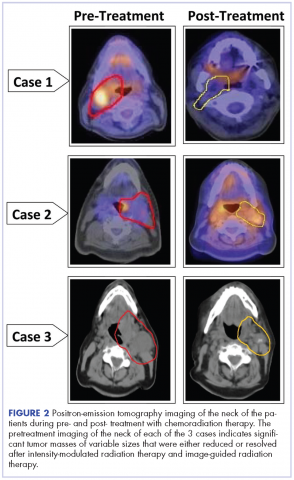
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
Tonsillar carcinoma is the most common of the oropharyngeal malignancies of the head and neck region after thyroid and laryngeal carcinoma. Squamous cell carcinoma is the most frequent histologic type of these tumors.1 Tonsillar tumors may originate in the oral cavity, oropharynx, hypopharynx, or larynx. In the United States, more than 5,000 new cases of oropharynx cancer are diagnosed annually.2 Men are affected three to four times more often than are women, and the rate of incidence increases after the 4th decade of life.3 Surveillance, Epidemiology, and End Results data from 1975-2004 show that tonsillar squamous cell carcinoma has had one of the largest increases in the male-to-female incidence rate ratios.4 The overall incidence of tonsillar carcinoma is increasing, especially in the younger population, and this may be attributed to increasing rates of human papilloma virus.5,6
Squamous cell carcinoma in the head and neck originate from subsites within the oral cavity, oropharynx, hypopharynx, larynx, and nasopharynx.7 Traditionally, alcohol consumption and tobacco use were considered the most significant risk factors for the development of tonsillar cancer.8 More recently, however, the high-risk oncogenic human papilloma virus has emerged as a clinical entity in the pathogenesis of squamous cell carcinoma in the head and neck. Other risk factors include poor oral hygiene, mechanical irritation, chewing of betel quid preparations, and a lack of vegetables and fruits in the diet.9-11 Squamous cell carcinoma of the oropharynx often presents late with lymph node involvement at the time of diagnosis. Nonspecific symptoms such as a sore throat and dysphagia can allow head and neck cancer to evade early detection. Many patients with tonsillar carcinoma present with advanced disease because early lesions are generally asymptomatic when small. This absence of symptoms is responsible for 67%-77% of patients presenting with tumors larger than 2.0 cm and often with regional nodal metastasis. At presentation, 45% of anterior tonsillar pillar lesions and 76% of tonsillar fossa lesions have clinically positive necks.12
Despite significant treatment advances, the management of advanced squamous cell carcinoma of the tonsil remains challenging. Historically, surgery was considered the standard of care for patients with tonsillar carcinoma with or without postoperative adjuvant radiotherapy. In locally advanced tonsillar carcinoma, extensive surgery with major tissue reconstruction was necessary, leading to speech dysfunction, cosmetic deformities, and difficulties in swallowing, all of which are detrimental to patient quality of life.13 Given the critical role of the oropharynx in speech and swallowing, nonsurgical therapy with organ-preserving chemoradiation has gained a greater role in the treatment of tonsil carcinoma.13 Over the past decade, innovations in radiation therapy techniques have led to the introduction of intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT) for the treatment of various cancers including tonsillar carcinoma.14,15 IMRT is an advanced mode of conformal high-precision radiotherapy that uses computer-controlled multiple small radiation beams of varying intensities to deliver precise radiation doses to the target tissues while sparing adjacent healthy tissues.14 By incorporating three-dimensional computed-tomography (CT) or positron-emission–tomography (PET) imaging technology, IMRT allows the radiation dose to conform more precisely to the three-dimensional shape of the tumor while modulating the intensity of the radiation beam and minimizing its dose to those adjacent sensitive and unaffected organs. IGRT uses a range of two-, three-, and four-dimensional imaging techniques that improve the precision and accuracy of the delivery of the radiation dose to the targeted tumor tissue while minimizing the dose to the surrounding normal tissue during the course of radiation therapy (Figure 1). In this report, we present challenging cases of advanced tonsillar carcinoma and describe our experience in managing the disease using a hyperfractionated IMRT-IGRT based three-dimensional conformal radiation therapy protocol with concurrent chemotherapy.
Case presentations and summaries
Case 1
A 52-year-old white, nonsmoking man who worked in a research chemical laboratory, presented with complaints of throat pain and difficulty in swallowing. The patient had a history of asthma and allergies and had been seen by an ear, nose, and throat (ENT) specialist prior to his visit to our oncology center. A biopsy was performed on a right tonsillar mass measuring 2.7 x 3.6 cm. A computed-tomography (CT) scan showed 2 enlarged inhomogeneous lymph nodes measuring 2.9 cm and 1.7 cm. The nodes were well defined with no soft tissue edema. Neoplasm was favored as a diagnosis and biopsy of the mass was carried out. A biopsy specimen measuring 1.0 x 0.4 x 0.3 cm revealed a moderately differentiated infiltrating squamous cell carcinoma, which extended to the edge of the biopsy specimen. The patient’s Karnofsky performance status was 90% (ie, able to carry on normal activity; minor signs or symptoms of disease).
A CT scan of the chest was clear with no evidence of malignant involvement. A subsequent CT scan of the neck revealed a primary neoplasm of the right faucial tonsil measuring 3.3 x 3.0 cm and associated with right level II, level III, and level IV pathological lymphadenopathy. Positron-emission tomography (PET) imaging of the neck revealed a right tonsillar lesion of 2.7 x 3.0 cm involving the right parapharyngeal space (Figure 2, Case 1). The standardized uptake value (SUV) of the PET scan of the primary lesion was measured at 7.3. A cluster of right level II cervical nodes measuring 3.2 x 2.5 cm had an SUV of 3.5. A 1.0-cm right level III jugular node was also seen with an SUV of 1.6, and a right level IV lymph node measuring 1.5 x 1.0 cm was seen with an SUV of 1.8. No other lesions were noted. The tumor stage was T2N2bM0, a stage IVa disease.
The patient had a percutaneous endoscopic gastrostomy (PEG) tube placement before starting radiation. He underwent a course of hyperfractionated intensity-modulated radiation therapy with image guidance (IMRT-IGRT) in 67 fractions of 120 cGy twice a day to a final tumor dose of 8,040 cGy.16 Concurrently, the patient received systemic chemotherapy with carboplatin at a dose of 240 mg weekly. To optimize the treatment, molecular profiling was performed to identify the sensitive genetic targets to systemic chemotherapy drugs.17, 18 Targets sensitive to paclitaxel and docetaxel were identified by molecular profiling of the tumor tissue, then chemotherapy with paclitaxel or docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off) was also administered to the patient.
The follow-up after 41 months indicated that the patient had no evidence of recurrent disease (Figure 2, Case 1). Posttreatment magnetic-resonance imaging (MRI) of the neck also indicated no evidence of residual tonsillar cancer. The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 2
A 49-year-old black male presented with throat pain and a mass seen initially by his family physician. The patient had a history of tobacco use (at least 1 cigar a day) periodically for about 10 years and had quit cigar smoking 15 years prior to developing his disease. An initial evaluation indicated that the patient had a hypopharyngeal mass in the left inferior pole of his tonsil with near occlusion of the hypopharyngeal airway. His larynx could not be visualized because of the obstructive mass. A neck lymph node measuring 3.0 cm in the left jugulodigastric region was also noted. The patient’s Karnofsky performance status was 90%. Subsequently, the patient underwent excision of the right tonsil and left tonsillar region.
The pathology of the right tonsil was found to be benign. Histology of the left tonsil revealed invasive squamous cell carcinoma. The resected tumor size measured 3.7 x 2.7 x 2.5 cm. The tumor was moderately differentiated involving the deep surgical margins. No lymphovascular invasion was seen. A PET scan revealed a mass arising from the left tonsillar pillar measuring 3.6 x 2.6 x 3.3 cm with deviation of the epiglottis posteriorly nearing the left vallecula. In addition, multiple large cervical nodal lesions in the left level II nodal chain were seen, with the largest measuring 3.1 x 3.0 x 4.5 cm with an SUV of 3.4. Displacement of the left submandibular gland with several further enlarged level II lymph nodes was observed. In the region of left vallecula, there was soft tissue thickening with increased activity measuring 2.7 x 1.5 cm, likely crossing the midline with an SUV of 5.5. The rest of the neck was negative for metastatic involvement (Figure 2, Case 2). The tumor stage was T3N2Mx, a stage IVa disease.
The patient had a Port-A-Cath placed, which caused a hemothorax after placement of the port and delayed initiating his treatment. A pretreatment MRI scan of the neck revealed multiple conglomerate hypodense peripherally enhancing nodular areas in the left neck posterior to the left submandibular gland deep to the parotid tail worrisome for necrotic lymphadenopathy. The patient underwent a course of hyperfractionated IMRT-IGRT in 67 fractions of 120 cGy twice daily for a total dose of 8,040 cGy to the primary tumor site.16 The patient had a port and PEG tube prior to initiating his radiation therapy. He received IMRT-IGRT with concurrent chemotherapy that was selected based on the recommendation of his genomic testing.17,18 The chemotherapy regimen used included carboplatin (300 mg weekly) and docetaxel (400 mg weekly). The patient had a treatment break because he was hospitalized for anemia and pancytopenia from his chemotherapy and he received supportive cancer care with epoetin alfa.A post therapy PET scan was negative for evidence of hypermetabolic malignancy; however, a 3.3 x 2.7 cm calcified lesion representing likely level III jugular lymph node exhibited no measurable activity at that time. The follow-up after 40 months indicated that the patient had no reported recurrence of the disease (Figure 2, Case 2). The patient’s demographics, tumor characteristics, and the treatment details are summarized in the Table.
Case 3
A 53-year-old white man, who had no smoking or tobacco history but who was exposed to chemicals including sulfuric acid, hydrogen chloride gas, and glycols at work, presented initially with a sore throat that became more painful over time. His ENT specialist referred him for a CT scan of the neck, which revealed a left-sided neck mass measuring 2.5 cm in diameter posterior to the submandibular gland and lateral to carotid sheath and anterior to the triangle (Figure 2, Case 3). The mass appeared to be encapsulated. There was a lobulated spherical mass in the left supraglottic area with formation of the airway of the pyriform sinus and additional anterior vascular involvement was noted. The mass measured 3.6 cm in transverse diameter.
A left tonsillar biopsy specimen measuring 1.4 x 0.6 x 0.2 cm was obtained, and its pathology revealed that the patient had a metastatic squamous cell carcinoma. The left neck lymph node mass aspiration also revealed the presence of squamous cell carcinoma. A PET-CT scan staging showed a dominant tonsillar fossa mass extending from the soft palate down to the pyriform sinus measuring 4.2 x 3.8 cm, with an SUV uptake of 7.3. There was a dominant left level II necrotic lymph node presence measuring 5.0 x 3.7 cm, with an SUV of 3.0. The patient’s Karnofsky performance status was 90%. The tumor stage was T4N2M0, a stage IVa disease. The patient received a course of conformal hyperfractionated IMRT-IGRT delivered to the primary tumor in 67 fractions at 120 cGy twice daily for a total dose of 8,040 cGy16 and concurrent carboplatin chemotherapy at a weekly dose of 200 mg.
After completion of his radiation therapy, chemotherapy was changed based on genomic testing from single agent to doublet with carboplatin (area under the curve (AUC) dose of 2 or 200 mg, weekly) plus docetaxel (25 mg/m2 weekly for 3 weeks and 1 week off ).17,18 A PET scan after chemoradiation therapy revealed a marked anatomical improvement in the primary neoplastic disease seen in the faucial tonsil. The tonsillar mass noted previously had almost completely resolved over the interval, with only a mild persistent asymmetrical thickening of around 1.5 cm, with a peak SUV of 2.0. A lymph node of 2.8 x 2.0 cm was present anterior to the left sternocleidomastoid muscle exhibiting SUV of only 1.8. No other abnormal lesions were noted (Figure 2, Case 3). The patient continues to do extremely well without local recurrence of the disease 46 months after radiation therapy (see Table for patient demographics, tumor characteristics, and therapy details.)
Discussion
The management of patients with primary squamous cell carcinoma of the oropharyngeal remains controversial. Traditionally, early-stage tonsillar squamous cell carcinoma was managed by a single modality treatment, either by surgery or radiation therapy, each showing similar efficacy and outcomes.19 For late-stage disease, a combined approach using surgery and radiation therapy was found to be superior to single modality treatment. However, surgery in conjugation with radiation therapy has been associated with significant toxicities compared with the radiation therapy alone.13Therefore, the use of radiation therapy without surgery is becoming more common with increasingly sophisticated radiation therapy techniques and organ preservation approach in patients with squamous cell carcinoma of the tonsil.
Findings from several studies have shown that in stage I or II oropharyngeal cancer, single modality treatment with radiation therapy achieves 80%-90% of local control of the disease, but poorer outcomes are reported for locally advanced stages III/IV with a local control rate of 63%-74%.20 These findings and others have led to a shift to evaluate the clinical benefits of radiation therapy given with concurrent chemotherapy for the primary treatment of advanced stage oropharyngeal squamous cell carcinoma.20,21 Findings from a number of studies have since reported comparable efficacy and toxicity outcomes using this regimen with concurrent chemotherapy in patients with locally advanced head and neck squamous cell cancer.22-24 Synchronous carboplatin chemotherapy was used effectively as an alternative to cisplatin with fewer potential adverse effects in the good prognosis group of patients with oropharyngeal squamous cell carcinoma.25,26 For our 3 patients, we used carboplatin-based chemotherapy with concurrent advanced hyperfractionated radiation therapy techniques to successfully manage tonsillar squamous cell carcinoma and reduce renal toxicity and neuropathy.
Advanced radiation therapy techniques such as IMRT-IGRT are used routinely at the University Cancer and Diagnostic Centers in Houston, Texas, to manage a range of malignant cancers.27 These innovative techniques have the potential to deliver highly conformal dose-intense radiation to targeted regions of disease, while sparing adjacent critical nonmalignant tissue. The improved shaping of high-dose distributions with IMRT-IGRT could mitigate treatment-related toxicities. For example, the use of advanced radiation therapy techniques has been associated with increased preservation of parotid salivary flow.28-30 The use of advanced radiation therapy techniques in head and neck squamous cell carcinoma is growing, and early evidence confirms its ability to secure excellent local and regional disease control.31,32 In this study, we have demonstrated that by using hyperfractionated conformal three-dimensional IMRT-IGRT we were able not only to manage advanced tonsillar squamous cell carcinoma and treat the malignant metastasis, but also spare adjacent critical organs that were not involved in the disease, thus reducing many of the detrimental side effects associated with hyperfractionated chemoradiation.
All 3 patients were followed for between 40 and 46 months. They continue to do extremely well without local recurrence of their disease, indicating a 100% disease control and overall survival rate. The disease control and survival outcomes for our patients with stage IVA disease compare favorably to other published reports in the literature.33,34 Findings from a study by Prestwich and colleagues33 of 41 patients with stage IV tonsillar carcinoma showed that the radiation therapy with concurrent chemotherapy achieved local and regional disease control in 91% of complete responders and an overall survival rate of 66% at 3 years. Similarly, Setton and colleagues34 reported on 442 patients – 50% with tonsillar cancer, 46% with base-of-tongue cancer – who underwent IMRT and concurrent chemotherapy and who achieved a 3-year overall survival of 84.9%. Our study findings demonstrate that hyperfractionated conformal three-dimensional IMRT-IGRT with concurrent chemotherapy can be delivered safely and effectively to patients with advanced tonsillar squamous cell carcinoma.
Acknowledgment
The authors thank Ms June Lyliston, LVN, for editing and proofreading the manuscript.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.
1. Stambuk HE, Karimi S, Lee N, Patel SG. Oral cavity and oropharynx tumors. Radiol Clin North Am. 2007;45(1):1-20.
2. Lin DT, Cohen SM, Coppit GL, Burkey BB. Squamous cell carcinoma of the oropharynx and hypopharynx. Otolaryngol Clin North Am. 2005;38(1):59-74, viii.
3. Golas SM. Trends in palatine tonsillar cancer incidence and mortality rates in the United States. Community Dent Oral Epidemiol. 2007;35(2):98-108.
4. Cook MB, Dawsey SM, Freedman ND, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18(4):1174-1182.
5. Enomoto LM, Bann DV, Hollenbeak CS, Goldenberg D. Trends in the Incidence of oropharyngeal cancers in the United States. Otolaryngol Head Neck Surg. 2016.
6. Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the U.S. population ages 20-44 years. Cancer. 2005;103(9):1843-1849.
7. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489-501.
8. Hong AM, Martin A, Chatfield M, et al. Human papillomavirus, smoking status and outcomes in tonsillar squamous cell carcinoma. Int J Cancer. 2013;132(12):2748-2754.
9. Velly AM, Franco EL, Schlecht N, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34(4):284-291.
10. Farrow DC, Vaughan TL, Berwick M, et al. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
11. Freedman ND, Park Y, Subar AF, et al. Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer. 2008;122(10):2330-2336.
12. Guay ME, Lavertu P. Tonsillar carcinoma. Eur Arch Otorhinolaryngol. 1995;252(5):259-264.
13. Parsons JT, Mendenhall WM, Stringer SP, et al. Squamous cell carcinoma of the oropharynx: surgery, radiation therapy, or both. Cancer. 2002;94(11):2967-2980.
14. Yao M, Dornfeld KJ, Buatti JM, et al. Intensity-modulated radiation treatment for head-and-neck squamous cell carcinoma--the University of Iowa experience. Int J Radiat Oncol Biol Phys. 2005;63(2):410-421.
15. Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71(1):29-42.
16. Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2014;89(1):13-20.
17. Tomkiewicz C, Hans S, Mucchielli MH, et al. A head and neck cancer tumor response-specific gene signature for cisplatin, 5-fluorouracil induction chemotherapy fails with added taxanes. PLoS One. 2012;7(10):e47170.
18. Feldman R, Gatalica Z, Knezetic J, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. 2016;38 Suppl 1:E1625-1638.
19. Moose BD, Kelly MD, Levine PA, et al. Definitive radiotherapy for T1 and T2 squamous cell carcinoma of the tonsil. Head Neck. 1995;17(4):334-338.
20. Chen AY, Schrag N, Hao Y, Stewart A, Ward E. Changes in treatment of advanced oropharyngeal cancer, 1985-2001. Laryngoscope. 2007;117(1):16-21.
21. Machtay M, Rosenthal DI, Hershock D, et al. Organ preservation therapy using induction plus concurrent chemoradiation for advanced resectable oropharyngeal carcinoma: a University of Pennsylvania Phase II Trial. J Clin Oncol. 2002;20(19):3964-3971.
22. Jegannathen A, Swindell R, Yap B, et al. Can synchronous chemotherapy be added to accelerated hypofractionated radiotherapy in patients with base of tongue cancer? Clin Oncol (R Coll Radiol). 2010;22(3):185-191.
23. Budach V, Becker ET, Boehmer D, et al. Concurrent hyperfractionated accelerated radiotherapy with 5-FU and once weekly cisplatin in locally advanced head and neck cancer. The 10-year results of a prospective phase II trial. Strahlenther Onkol. 2014;190(3):250-255.
24. Tobias JS, Monson K, Gupta N, et al. Chemoradiotherapy for locally advanced head and neck cancer: 10-year follow-up of the UK Head and Neck (UKHAN1) trial. Lancet Oncol. 2010;11(1):66-74.
25. Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: a matched-pair analysis. Oral Oncol. 2013;49(6):615-619.
26. Benghiat H, Sanghera P, Cashmore1 J, et al. Four week hypofractionated accelerated intensity modulated radiotherapy and synchronous carboplatin or cetuximab in biologically staged oropharyngeal carcinoma. Cancer and Clinical Oncology. 2014;3:1-9.
27. D’Andrea MA, Reddy GK. Management of metastatic malignant thymoma with advanced radiation and chemotherapy techniques: report of a rare case. World J Surg Oncol. 2015;13:77.
28. Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007-1014.
29. Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25(31):4863-4864.
30. Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981-991.
31. Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966-974.
32. Daly ME, Lieskovsky Y, Pawlicki T, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck. 2007;29(3):211-220.
33. Prestwich RJ, Kancherla K, Oksuz DC, et al. A single centre experience with sequential and concomitant chemoradiotherapy in locally advanced stage IV tonsillar cancer. Radiat Oncol. 2010;5:121.
34. Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012;82(1):291-298.