User login
Obstructive sleep apnea (OSA) is recognized primarily as a problem of the upper airway. Although narrowing or actual obstruction of the airway during the night can be found in only 1 or 2 areas of the upper airway, most often sleep apnea involves the entire pharyngeal upper airway passages. Three regions are considered to be of major concern: the nasal cavity region, the retropalatal region, and the retrolingual region. As the level of these 3 regions descends, the volume of tissue from the nose to the base of the tongue increases significantly. This leads to increased difficulty treating OSA with each descending region as well as to a lower success rate overall. Sometimes, the problem causing OSA is limited to only 1 region but may involve 2 or even all 3 regions.1
Continuous positive airway pressure (CPAP) therapy and other positive airway pressure (PAP) therapies have been considered a safe and effective treatment for OSA. Unfortunately, compliance rates, even among patients who use it to successfully eliminate their symptoms, can vary from 50% to 70%. Complaints about using CPAP and other PAP therapies range from skin irritation, discomfort to the nose or nasal passages, and eye problems to claustrophobia from wearing a mask. Patients who are unable or unwilling to use CPAP therapy can be candidates for surgical treatment of OSA.2
This article discusses surgical options for adult patients who have OSA who choose not to use CPAP therapy, the reasons to perform surgery for adults who have OSA, and the desired outcomes of surgery for OSA with a focus on minimally invasive procedures. Interventions for pediatric patients are not addressed.
Treatment Options
Treatments other than CPAP can help lessen the severity of OSA. Noninvasive treatments include weight loss, positional treatment (avoiding sleeping on one’s back), and oral appliances. Practice parameters from the American Academy of Sleep Medicine (AASM) for the treatment of OSA include dietary weight loss in addition to the primary therapy. An improvement in the apnea-hypopnea index (AHI) may occur with dietary weight loss.3
Major weight loss through the use of bariatric surgery has been shown to be effective in treating OSA and obesity hypoventilation syndrome (OHS), which is defined as daytime hypercapnia and hypoxemia (PaCO2 > 45 mm Hg and PaO2 < 70 mm Hg at sea level) in an obese patient (body mass index [BMI] > 30 kg/m2) with sleep-disordered breathing in the absence of any other cause of hypoventilation.4 Some individuals may have both conditions. However, CPAP therapy should not be discontinued even when major weight loss occurs until repeat polysomnography has been performed. Major weight loss may cure OHS and help improve the severity of OSA but will not totally resolve the condition.4 According to Woodson, sleep apnea in patients who are morbidly obese may be different from traditional OSA as rapid eye movement (REM), REM-related apneas, and hypoventilation occur more often in this population. Although weight loss is strongly recommended for patients with OSA, bariatric surgery is not recommended as the sole treatment for traditional OSA.5
Positional therapy, wherein the patient avoids the supine position, can be effective as a secondary or supplemental therapy in addition to treatment with CPAP. Guidelines recommend this primarily for individuals who have a lower AHI when in the nonsupine position than when lying on their backs. Patients who benefit from positional therapy tend to be younger, less obese, and with a less severe condition. The AASM practice parameter was based on 3 level II studies—randomized trials with high alpha and beta levels.6 One of the level II studies compared supine with an upright position, stating: “Because not all patients normalize AHI when non-supine, the committee’s opinion is that correction of OSA by position should be documented with an appropriate test.” Special pillows have been described in 2 papers reviewed by AASM, which seemed to improve OSA.6
The AASM guidelines state that oral appliances are indicated for some patients, although they are not as efficacious in treating OSA as CPAP therapy. Oral appliances are recommended for patients with mild to moderate OSA who have not responded to CPAP therapy, cannot tolerate CPAP therapy, are not appropriate for treatment with CPAP for some reason, or fail treatment with CPAP along with other behavioral measures, such as weight loss or positional therapy.6
In addition, the AASM guidelines recommend that patients with severe OSA, “should have an initial trial of nasal CPAP because greater effectiveness has been shown with this intervention than with the use of oral appliances. Upper airway surgery (including tonsillectomy and adenoidectomy, craniofacial operations, and tracheostomy) may also supersede use of oral appliances in patients for whom these operations are predicted to be highly effective in treating sleep apnea.”6
Nasal expiratory positive end pressure (EPAP) devices may be helpful in treating OSA in some patients. These devices contain a mechanical valve with very low inspiratory resistance but high expiratory resistance. The device has an adhesive and is applied by the patient to create a seal. Exhalation causes a high expiratory resistance that splints the upper airway open. This increases the resistance of the airway to close on inspiration.7 An EPAP device is recommended for potential use in mild to moderate OSA for patients who either have an intolerance to CPAP therapy or have failed to respond to it.8
Finally, there are major surgeries performed by oral surgeons that can benefit some patients with OSA. One of these is maxillomandibular advancement (MMA). According to the AASM practice parameter: MMA “involves simultaneous advancement of the maxilla and mandible through sagittal split osteotomies. It provides enlargement of the retrolingual airway and some advancement of the retropalatal airway.”9 It is indicated as a surgical treatment for patients with severe OSA who are either unwilling or do not tolerate CPAP treatment. These individuals would not benefit from an oral appliance (recommended for mild to moderate OSA) or would find it undesirable.9
There is also stepwise or multilevel surgery (MLS) that can be performed. These include a number of combined procedures, which address multiple sites with narrowing in the upper airway. Frequently, MLS will consist of
2 phases: the first involves use of the uvulopalatopharyngoplasty (UPPP) procedure “and or genioglossus advancement and hyoid myotomy (GAHM). The second phase surgeries consist of utilizing maxillary and mandibular advancement osteotomy (MMO), offered to those failing Phase I surgeries.”9
OSA Surgical Procedures
Tracheostomies are first estimated to have been performed in 2000 BC.10 Performing a tracheostomy to bypass the upper airway was used in the 1960s and 1970s for the treatment of OSA and for many years was the only treatment available for people with Pickwickian syndrome (OHS) and nocturnal upper airway obstruction. The procedure was generally not tolerated or accepted by patients, even though it improved their quality of life and added to their life expectancy. Once CPAP treatment proved successful for OSA, tracheostomy has rarely been necessary.11
Uvulopalatopharyngoplasty surgery was introduced in 1981. The aim of this surgery is to decrease snoring and treat OSA by removing obstructive tissues, enlarging the cross-sectional portion of the upper airway, and bypassing the upper airway. Tissue that is removed includes the tonsils, uvula, and the distal portion of the soft palate.12
Woodson considers surgery for OSA to be the third-line of treatment. The first-line treatment would be CPAP therapy, and second-line therapy would include oral appliances to enlarge the airway or retain the tongue (if the individual has no dentition). The intent of surgery falls into 3 categories: curative, salvage, and ancillary.
In a chronic disease such as OSA, “many may question whether a cure exists.” Instead of eliminating OSA, the curative intent is definitively to reduce symptoms and disease morbidity for long periods. The criteria for defining a responder or cure to surgery for OSA are found in Table 1.5
Surgery for salvage aims at treating patients who have failed CPAP therapy. Successful treatment with the intent of salvage can occur with a lessening of disease severity, including morbidity and mortality, but not necessarily totally eliminating the symptoms. Finally, ancillary surgery for OSA aims to combine a surgical procedure with the first-line therapy (positive pressure) to add an additional therapeutic benefit. The combination of CPAP and ancillary procedures may be of the most benefit to patients with OSA.5
As mentioned previously, the AASM has developed practice parameters for the treatment of OSA, including surgery. Desired outcomes of treatment for OSA include the resolution of symptoms and clinical signs, normalization of the quality of sleep, AHI, and levels of oxyhemoglobin saturation. It is recognized that normalization of the AHI may not reverse all the components of OSA, and up to 22% of patients continue to have residual hypersomnia with CPAP therapy.9
Despite this, most studies that show significant benefits in lowering cardiovascular risk, mortality rates, symptoms, and neurocognitive effects have also shown significant reductions in the AHI.9 Therefore, the AASM puts a high value on treating OSA with the goal of normalization of the AHI. There exists a lack of quality studies and good evidence regarding the effectiveness of surgical procedures of the upper airway as treatment for OSA. Despite this, the AASM recommendation is that “all reasonable treatment alternatives for OSA be discussed in a manner that allows the patient to make an informed decision.”9
Types of OSA Surgeries
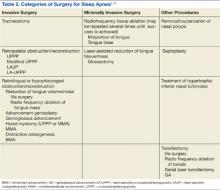
Surgery for OSA can be divided into 2 categories, invasive surgery and minimally invasive surgery (Table 2). Invasive surgeries for OSA have a higher risk of complications and postoperative pain.9
Invasive Surgery
The UPPP surgery is perhaps the best-known invasive procedure for treating OSA. A meta-analysis of 15 studies on UPPP outcomes found overall improvement in AHI of 33% but with postoperative AHI remaining elevated at an average of 29.8 events per hour.13
Adverse effects for the procedure include changes in taste, dysphagia, nasal regurgitation, and voice alterations. Seven of the 15 studies reported at least 1 death following the UPPP procedure. The effects of UPPP surgery on systemic and cardiovascular sequelae of OSA are not fully known and need to be further researched. 13
Another invasive procedure that is similar to the UPPP is the laser-assisted uvulopalatoplasty (LAUP) procedure. First reported in 1990, LAUP was developed as a procedure to eliminate snoring and was found to be effective. Other studies have been conducted since then, but there are inconsistencies in the specific types of procedures performed and a lack of detailed data analysis.
Adverse effects of the LAUP procedure include minor bleeding, globus sensation, local infection, minor dysphagia and dysphonia, and transient velopharyngeal insufficiency.14 However, studies have also shown that about one-quarter of the participants developed persistent dysphagia or mild to moderate scar fibrosis. Postoperative swelling can reduce an already narrow airway, and the use of sedatives or narcotics can make this problem worse. Some studies have noted structural changes following this procedure that lower airway resistance, resulting in the collapse of the upper airway and the narrowing of the airway during inhalation with worsening OSA.13,14
Practice parameters for the LAUP procedure were developed by the AASM in 1994. The AASM recommended against using LAUP to treat OSA (and other sleep-related breathing disorders) and against substituting LAUP for UPPP surgery. The AASM also suggested criteria for choosing candidates for LAUP and urged that patients be given full information about the procedure and a preoperative evaluation from their provider.5,14
Minimally Invasive Surgery
Radiofrequency surgery and soft palate implants are considered minimally invasive procedures, according to the criteria established in a study by Maurer.11 Various nasal surgeries (eg, septoplasty, adenoidectomy, and polyp removal) could also be considered minimally invasive and are often performed in patients with OSA to improve tolerance of CPAP by improving the ability to breathe nasally. However, nasal surgery with improvement of nasal breathing has not been found to have a significant impact on adults who have OSA. An advantage of nasal surgery is that some studies have shown longer use of CPAP therapy, up to 2 hours longer per night.5
With radiofrequency of the tongue base, high-frequency radio waves, either monopolar or bipolar, are used to coagulate the interstitial tissue at the base of the tongue. This, in turn, leads to necrosis and scarring, which then leads to stiffening of the tissue and in some cases, volume reduction. The surgeon controls the temperature to avoid overdosing and tissue carbonization. A number of lesions are produced during a session and the number of sessions necessary for full treatment varies per surgeon and the technical system used in procedures.
Although radiofrequency ablation (RFA) has been used on the tonsils and soft palate, RFA is currently available only to patients with OSA on the tongue base. A reduction in AHI of 33% on average was achieved in a review of 6 studies, and the reduction was stable over a 2-year period.11 The average presurgery AHI was 39.5, and the average postsurgery AHI was 28.7. In this same review, the average Epworth Sleepiness Scale (ESS) (a commonly used subjective tool to measure levels of daytime sleepiness) score was 10.4 presurgery and 4.5 postsurgery.11
A Chinese study looked at complications from RFA of the tongue base. According to the abstract (the article was written in Chinese), 1 patient (n = 193) developed a fatal arrhythmia. The authors’ recommendation was for close monitoring after surgery until the swelling subsided.15
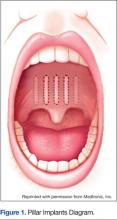
With soft palate implants, small cylinders of woven polyester (“polyethylene terephthalate, commonly marketed as Dacron polyester”) yarn, 18 mm long by 2 mm diameter, are inserted at the junction of the hard palate and soft palate. Placed into the proximal soft palate tissue, these implants are designed to stiffen the soft palate “through fibrotic tissue encapsulation and tissue ingrowth over time.” Many patients with only snoring or mild to moderate OSA have found improvement with this procedure. Improved reduction of snoring was found in 1 study (n = 79), which looked at the use of Pillar implants to reduce snoring (Figure 1). This reduction of snoring occurred in patients with lower AHI levels before the procedure.16 Mauer reviewed 3 studies of the palatal implants and found a pooled reduction of 26% in AHI after placement.11
Criteria for Surgery
The mechanism that causes collapse of a patient’s airway will vary from one person to the next. Variables include age, body weight, anatomy, and body position. Thus, different findings will require different surgical techniques, and there is no one-size-fits-all surgery for OSA.11
A thorough physical examination of the upper airway is important. Maurer recommends considering the use of videoendoscopy under sedation, which allows a view of the mechanism and site of obstruction, with pressure catheters inserted to collect data. This procedure was developed in the 1990s to improve the analysis of obstruction within the upper airway. Unfortunately, the data are unclear about whether this aids in the surgical outcome.11 Powell stated that sleep endoscopy is currently considered investigational because medication-induced sleep may differ from natural sleep without medication.1
Nasopharyngoscopy, using fiberoptics, and lateral cephalometric analysis have been used for several years as primary diagnostic tools for patients being considered for a surgical procedure for OSA. Some new imaging procedures (eg, 3-D imaging coupled with advanced software programs) have been developed that may be helpful to assess regions that are constricted as well as provide a more exact measurement of the airway from the nose to the larynx.1
Risks and Complications of Surgery
The higher risk of anesthesia-related complications during or after surgery related to OSA is one reason that surgery is usually considered to be the third-line of treatment. Patients with OSA commonly have hypertension and other cardiovascular disease, which adds to the risk of anesthesia-related complications. Patients who have anatomical abnormalities of the jaw or portions of the upper airway will also be at a higher risk. Initial intubation and providing anesthesia during procedures may be more difficult for patients with these jaw or airway abnormalities. Postsurgical pain management with opioids contributes to the higher rate of apnea. After extubation, the incidence of apnea has been found to be higher in the population with OSA as well.17
A study published in 2001 used a case-control matched population methodology to examine patients who were undergoing elective surgeries for either hip or knee joint replacements. Significant differences in overall complication rates were found among the patients with OSA (n = 101) compared with those of the control group (n = 101). These differences were not related to the type of anesthesia, narcotic use after surgery, or the type of operation performed. The OSA group had a significantly higher number of patients who required higher flow rates of oxygen postoperatively and for a longer period. Serious complications, including the reintubation of 2 patients and unplanned intensive care unit (ICU) transfers, were noted in the patients with OSA (24%) compared with the control group (P = .004). The mean length of hospital stay was significantly longer for patients with OSA (6.8 + 2.8 days) compared with those in the control group (5.1 + 4.1 days, P = .007).18
The OSA group in the aforementioned study was divided into 2 types during this investigation: one group (n = 36) included patients undiagnosed with OSA until after their surgery (mean 1.8 years); the other group (n = 65) had a confirmed diagnosis at the time of surgery. All 36 in the first group and 32 from the second group with diagnosed OSA did not use CPAP therapy at home; a total of 68 patients (67.3%) who had not received therapy before the surgery. The 33 patients with diagnosed OSA (32.7%) who did use CPAP therapy before surgery had lower complication rates, including shorter hospital stays (6.0 + 2.1 days) compared with their counterparts with untreated OSA (7.2 + 3.1 days). The authors surmised that there might be a carryover protective effect at least for the first postoperative day.18
A retrospective study examined patients with OSA who had outpatient surgical procedures performed under either major regional anesthesia (central neuraxial) or general anesthesia. The study looked at the first outpatient surgical procedure for the patient with OSA following the diagnosis except for otorhinolaryngologic surgeries, which were excluded from the study. The 234 patients with OSA were then matched to the same number of control patients who had also had outpatient surgical procedures (excluding the otorhinolaryngologic procedures). The researchers noted a higher incidence of endotracheal intubation in the OSA group (79.9% vs 73.9% in the control group, P = .017).
The OSA group was less likely to have a laryngeal mask airway used during surgery for their airway management (5.1% vs 10.7% in the control group, P = .017). The only significantly different complication between the 2 groups was unplanned admissions to an ICU in the postoperative period (although numbers/percentages were not listed in the article). However, there was no difference in the overall unplanned hospital admission rate between the 2 groups. One limitation to this study was that the control group had not been tested for OSA, and therefore, it was possible that some in the control group might have had undiagnosed OSA.19
It is recommended that the surgeon and anesthesiologist ask about a diagnosis of OSA or sleep apnea symptoms during the preanesthesia assessment for any surgery. The provider performing the preoperative physical examination should pay attention to the circumference of the neck, chin-throat length, Mallampati classification, mandible position, and BMI. Any findings that suggest undiagnosed OSA should prompt further evaluation before any elective surgery.
Those who are at risk of OSA anesthesia-related complications should be offered regional anesthesia if possible. It is undesirable to premedicate with sedatives or opioids for this population. The sniffing position during surgery has been found to reduce the collapsibility of the airway and improve its dimensions. All patients who use CPAP therapy at home should have this therapy available for use in the recovery room with the lateral position preferred.17
Outpatient surgery using sedation and local anesthetics can also be a higher risk for patients with OSA. The unprotected, potentially problematic airway is still a serious concern. Patients requiring short procedures may do well with titrated sedation if well positioned and appropriately selected for this before the procedure. Clinicians must be prepared to handle any complications that occur. One complication that occurs commonly in obese patients related to a smaller functional residual capacity is rapid oxygen desaturation.17
A Chinese study evaluated complications in patients who had received tongue base reduction through RFA. Complications, both intraoperative and postoperative, included hematoma of the tongue base, bleeding, altered taste, an abscess at the base of the tongue, speech dysfunction, numbness, and deviation of the tongue extension movement. One cardiac death occurred 37 hours following surgery related to swelling of the tongue base and pain, which aggravated sleep apnea and nocturnal hypoxemia and induced a fatal arrhythmia.15
The Future of OSA Treatments
Research is underway to evaluate the effectiveness of an implanted system to stimulate the hypoglossal nerve with the intent of activating the upper airway musculature. A small study of 8 patients found improvement in the degree of upper airway collapsibility and the severity of OSA. Continued research on the device is focusing on the parameters for the nerve stimulation. The criteria for patient selection are also being established.20
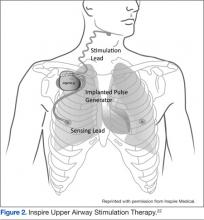
Another study of hypoglossal nerve stimulation (HNS) evaluated 21 patients who were unable to tolerate CPAP therapy. The researchers found significant improvement in AHI (43.1 + 17.5 to 19.5 + 16.7); Functional Outcomes of Sleep Questionnaire (14.4 + 2.0 to 16.7 + 2.2); ESS (12.1 + 4.7 to 8.1 + 4.4); Beck Depression Inventory (15.8 + 9.0 to 9.7 + 7.6); and the Calgary Sleep Apnea Quality of Life Index (3.2 + 1.0 to 4.9 to 4.9 + 1.3). The researchers concluded HNS decreased OSA-related symptoms and the severity of OSA.21 At least one of these devices, the Inspire device, has been approved for use in Europe (Figure 2).22 In addition, the FDA has approve the Inspire Upper Airway Stimulation therapy for use in a subset of patients with moderate to severe OSA who are unable to use CPAP.23
A recent study investigated the effect of HNS on the severity of OSA among patients who had moderate to severe OSA and had not had a response to CPAP therapy. The HNS was associated with significant improvement in reducing the frequency of respiratory events. The median AHI at 12 months decreased from 29.3 events per hour to 9.0 events per hour (P < .001), and the number of times per hour of sleep that the blood oxygen level dropped by ≥ 4% from the baseline (oxygen desaturation index score) decreased from 25.4 to 7.4 events per hour (P < .001).24
Another area being studied is the development of a device to advance the tongue. Forty-two patients had mandibular bone anchors inserted with a flexible tether connected to a surgically inserted anchor in the posterior tongue. Unfortunately, 31% of the participants developed tissue anchor barb fractures that were asymptomatic and detected through radiography. At this time, the failure rate does not warrant clinical use. However, some patients showed significant improvement in multiple measures for sleep apnea, and more research is ongoing with the device.25
Similarly, a surgically inserted tongue pin that connects to an oral device during sleep has been studied. This pin prevents the tongue from moving back and obstructing the upper airway. Polysomnographies were performed both before and after and showed an increased rate of apnea in the 10 subjects. Visualization with magnetic resonance imaging showed that the upper airway was not kept open by this fixation device. Additional research with a modified design for the device is recommended.26
Reshaping of the epiglottis using a CO2 laser has been studied with cadaver specimens. The researchers believe scar formation following the reshaping would aid in retraction of the epiglottis anteriorly. Studies with animals are being conducted with different laser types. Future studies involving selected patients with OSA who have epiglottis malformations would be the next step.27
More studies about both minimally invasive and invasive surgeries for OSA are needed. There are many unanswered questions, including the timing of follow-up assessments, how long to observe a patient after surgery, whether the use of multilevel surgery affects the rate of relapse, and what are the best follow-up tools to use. Research needs to be performed in diverse populations, including gender, race/ethnicity, and age groups. Complications of surgical procedures need to be studied and clarified over both short- and long-term periods.9
Conclusions
Although there have been some successes in using surgical procedures to treat OSA, CPAP therapy remains the first-line treatment. Invasive surgeries have higher rates of complications and risks than do those of minimally invasive procedures. There are also additional risks related to the use of anesthesia both during and after surgery. Referrals for surgery should include information about a diagnosis of OSA, and patients should be instructed to discuss this with the anesthesiologist or certified nurse anesthetist.
With careful selection by the surgeon and sleep providers, patients who do not tolerate CPAP therapy or respond to other noninvasive interventions can benefit from a surgical procedure. Minimally invasive surgeries are less risky for the patient and should be considered.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
WEB EXCLUSIVE CASE STUDY
A Physical Examination of the Upper Airway by an Ear, Nose, and Throat Physician
1. Powell NB. Contemporary surgery for obstructive sleep apnea syndrome. Clin Exp Otorhinolaryngol. 2009;2(3):107-114.
2. Shine NP, Lewis RH. Transpalatal advancement paryngoplasty for obstructive sleep apnea syndrome: Results and analysis of failures. Arch Otolaryngol Head Neck Surg. 2009;135(5): 434-438.
3. Morgenthaler TI, Kapen S, Lee-Chiong T; American Academy of Sleep Medicine. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8):1031-1035.
4. Aigner MJ, Sikka P, Whitfield P. Obesity hypoventilation syndrome: What is it? How is it treated? Is there a cure? Bariatric Nursing and Surgical Patient Care. 2009;4(2):109-113.
5. Woodson BT. Non-pressure therapies for obstructive sleep apnea: Surgery and oral appliances. Respir Care. 2010;55(10):1314-1321.
6. Kushida CA, Morgenthaler TI, Littner MR, et al; American Academy of Sleep Medicine. Practice parameters for the treatment of snoring and Obstructive Sleep Apnea with oral appliances: An update for 2005. Sleep. 2006;29(2):240-243.
7. Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: A randomized controlled trial. Sleep. 2011;34(4):479-485.
8. Kryger MH, Berry RB, Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment of obstructive sleep apnea (OSA). J Clin Sleep Med. 2011;7(5):449-453.
9. Aurora RN, Casey KR, Kristo D, et al; American Academy of Sleep Medicine. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33(10):1408-1413.
10. Szmuk P, Ezri T, Evron S, Roth Y, Katz J. A brief history of tracheostomy and tracheal intubation, from the Bronze Age to the space age. Intensive Care Med. 2008;34(2):222-228.
11. Maurer JT. Update on surgical treatment for sleep apnoea. Swiss Med Wkly. 2009;139(43-44):624-629.
12. Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obsructive sleep apnea—a systematic review. Sleep. 2009;32(1):27-36.
13. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: A systematic review and meta-analysis. Sleep. 2010; 33(10):1396-1407.
14. Littner M, Kushida CA, Hartse K, et al. Practice parameters for the use of laser-assisted uvulopalatoplasty: An update for 2000. Sleep. 2001;24(5):603-619.
15. Chen JH, Luo ZH, Yang XL, Zhu MW, Tao ZZ. Complications of tongue base reduction with radiofrequency tissue ablation on obstructive sleep apnea hypopnea syndrome. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45(7):574-577. (Abstract, full text in Chinese.)
16. Gillespie MB, Smith JE, Clarke J, Nguyen SA. Effectiveness of Pillar palatal implants for snoring management. Otolaryngol Head Neck Surg. 2009;140(3):363-368.
17. Ephros HD, Madani M, Yalamanchili SC. Surgical treatment of snoring and obstructive sleep apnoea. Indian J Med Res. 2010;131:267-276.
18. Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: A case-control study. Mayo Clin Proc. 2001;76(9):897-905.
19. Sabers C, Plevak DJ, Schroeder DR, Warner DO. The diagnosis of obstructive sleep apnea as a risk factor for unanticipated admissions in outpatient surgery. Anesth Analg. 2003;96(5):1328-1335.
20. Kerzirian EJ, Boudewyns A, Eisele DW, et al. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev. 2010;14(5):299-305.
21. Eastwood PR, Barnes M, Walsh JH, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34(11):1479-1486.
22. Inspire Medical. STAR Trial. Inspire Medical Website. http://www.inspiresleep.com/star-trial.php. Accessed May 7, 2014.
23. FDA approves Inspire Upper Airway Stimulation (UAS) therapy for obstructive sleep apnea [press release]. Inspire Medical Website. http://www.inspiresleep.com/pdf/FDA-Approval-Press-Release-final-20140501.pdf.
Accessed May 29, 2014.
24. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149.
25. Woodson BT, Steward DL, Mickelson S, Huntley T, Goldberg A. Multicenter study of a novel adjustable tongue-advancement device for obstructive sleep apnea. Otolaryngol Head Neck Surg. 2010;143(4):585-590.
26. Punke C, Schöentag C, Hortian B, et al. Tongue fixation system for therapy of sleeping disorders. A feasibility study. HNO. 2010;58(12):1184-1189. (Abstract, full text in German).
27. Bourolinas C, Hajiioannou J, Sobol E, Velegrakis G, Helidonis E. Epiglottis reshaping using CO2: A minimally invasive technique and its potential applications. Head Face Med. 2008;4:15.
Obstructive sleep apnea (OSA) is recognized primarily as a problem of the upper airway. Although narrowing or actual obstruction of the airway during the night can be found in only 1 or 2 areas of the upper airway, most often sleep apnea involves the entire pharyngeal upper airway passages. Three regions are considered to be of major concern: the nasal cavity region, the retropalatal region, and the retrolingual region. As the level of these 3 regions descends, the volume of tissue from the nose to the base of the tongue increases significantly. This leads to increased difficulty treating OSA with each descending region as well as to a lower success rate overall. Sometimes, the problem causing OSA is limited to only 1 region but may involve 2 or even all 3 regions.1
Continuous positive airway pressure (CPAP) therapy and other positive airway pressure (PAP) therapies have been considered a safe and effective treatment for OSA. Unfortunately, compliance rates, even among patients who use it to successfully eliminate their symptoms, can vary from 50% to 70%. Complaints about using CPAP and other PAP therapies range from skin irritation, discomfort to the nose or nasal passages, and eye problems to claustrophobia from wearing a mask. Patients who are unable or unwilling to use CPAP therapy can be candidates for surgical treatment of OSA.2
This article discusses surgical options for adult patients who have OSA who choose not to use CPAP therapy, the reasons to perform surgery for adults who have OSA, and the desired outcomes of surgery for OSA with a focus on minimally invasive procedures. Interventions for pediatric patients are not addressed.
Treatment Options
Treatments other than CPAP can help lessen the severity of OSA. Noninvasive treatments include weight loss, positional treatment (avoiding sleeping on one’s back), and oral appliances. Practice parameters from the American Academy of Sleep Medicine (AASM) for the treatment of OSA include dietary weight loss in addition to the primary therapy. An improvement in the apnea-hypopnea index (AHI) may occur with dietary weight loss.3
Major weight loss through the use of bariatric surgery has been shown to be effective in treating OSA and obesity hypoventilation syndrome (OHS), which is defined as daytime hypercapnia and hypoxemia (PaCO2 > 45 mm Hg and PaO2 < 70 mm Hg at sea level) in an obese patient (body mass index [BMI] > 30 kg/m2) with sleep-disordered breathing in the absence of any other cause of hypoventilation.4 Some individuals may have both conditions. However, CPAP therapy should not be discontinued even when major weight loss occurs until repeat polysomnography has been performed. Major weight loss may cure OHS and help improve the severity of OSA but will not totally resolve the condition.4 According to Woodson, sleep apnea in patients who are morbidly obese may be different from traditional OSA as rapid eye movement (REM), REM-related apneas, and hypoventilation occur more often in this population. Although weight loss is strongly recommended for patients with OSA, bariatric surgery is not recommended as the sole treatment for traditional OSA.5
Positional therapy, wherein the patient avoids the supine position, can be effective as a secondary or supplemental therapy in addition to treatment with CPAP. Guidelines recommend this primarily for individuals who have a lower AHI when in the nonsupine position than when lying on their backs. Patients who benefit from positional therapy tend to be younger, less obese, and with a less severe condition. The AASM practice parameter was based on 3 level II studies—randomized trials with high alpha and beta levels.6 One of the level II studies compared supine with an upright position, stating: “Because not all patients normalize AHI when non-supine, the committee’s opinion is that correction of OSA by position should be documented with an appropriate test.” Special pillows have been described in 2 papers reviewed by AASM, which seemed to improve OSA.6
The AASM guidelines state that oral appliances are indicated for some patients, although they are not as efficacious in treating OSA as CPAP therapy. Oral appliances are recommended for patients with mild to moderate OSA who have not responded to CPAP therapy, cannot tolerate CPAP therapy, are not appropriate for treatment with CPAP for some reason, or fail treatment with CPAP along with other behavioral measures, such as weight loss or positional therapy.6
In addition, the AASM guidelines recommend that patients with severe OSA, “should have an initial trial of nasal CPAP because greater effectiveness has been shown with this intervention than with the use of oral appliances. Upper airway surgery (including tonsillectomy and adenoidectomy, craniofacial operations, and tracheostomy) may also supersede use of oral appliances in patients for whom these operations are predicted to be highly effective in treating sleep apnea.”6
Nasal expiratory positive end pressure (EPAP) devices may be helpful in treating OSA in some patients. These devices contain a mechanical valve with very low inspiratory resistance but high expiratory resistance. The device has an adhesive and is applied by the patient to create a seal. Exhalation causes a high expiratory resistance that splints the upper airway open. This increases the resistance of the airway to close on inspiration.7 An EPAP device is recommended for potential use in mild to moderate OSA for patients who either have an intolerance to CPAP therapy or have failed to respond to it.8
Finally, there are major surgeries performed by oral surgeons that can benefit some patients with OSA. One of these is maxillomandibular advancement (MMA). According to the AASM practice parameter: MMA “involves simultaneous advancement of the maxilla and mandible through sagittal split osteotomies. It provides enlargement of the retrolingual airway and some advancement of the retropalatal airway.”9 It is indicated as a surgical treatment for patients with severe OSA who are either unwilling or do not tolerate CPAP treatment. These individuals would not benefit from an oral appliance (recommended for mild to moderate OSA) or would find it undesirable.9
There is also stepwise or multilevel surgery (MLS) that can be performed. These include a number of combined procedures, which address multiple sites with narrowing in the upper airway. Frequently, MLS will consist of
2 phases: the first involves use of the uvulopalatopharyngoplasty (UPPP) procedure “and or genioglossus advancement and hyoid myotomy (GAHM). The second phase surgeries consist of utilizing maxillary and mandibular advancement osteotomy (MMO), offered to those failing Phase I surgeries.”9
OSA Surgical Procedures
Tracheostomies are first estimated to have been performed in 2000 BC.10 Performing a tracheostomy to bypass the upper airway was used in the 1960s and 1970s for the treatment of OSA and for many years was the only treatment available for people with Pickwickian syndrome (OHS) and nocturnal upper airway obstruction. The procedure was generally not tolerated or accepted by patients, even though it improved their quality of life and added to their life expectancy. Once CPAP treatment proved successful for OSA, tracheostomy has rarely been necessary.11
Uvulopalatopharyngoplasty surgery was introduced in 1981. The aim of this surgery is to decrease snoring and treat OSA by removing obstructive tissues, enlarging the cross-sectional portion of the upper airway, and bypassing the upper airway. Tissue that is removed includes the tonsils, uvula, and the distal portion of the soft palate.12
Woodson considers surgery for OSA to be the third-line of treatment. The first-line treatment would be CPAP therapy, and second-line therapy would include oral appliances to enlarge the airway or retain the tongue (if the individual has no dentition). The intent of surgery falls into 3 categories: curative, salvage, and ancillary.
In a chronic disease such as OSA, “many may question whether a cure exists.” Instead of eliminating OSA, the curative intent is definitively to reduce symptoms and disease morbidity for long periods. The criteria for defining a responder or cure to surgery for OSA are found in Table 1.5
Surgery for salvage aims at treating patients who have failed CPAP therapy. Successful treatment with the intent of salvage can occur with a lessening of disease severity, including morbidity and mortality, but not necessarily totally eliminating the symptoms. Finally, ancillary surgery for OSA aims to combine a surgical procedure with the first-line therapy (positive pressure) to add an additional therapeutic benefit. The combination of CPAP and ancillary procedures may be of the most benefit to patients with OSA.5
As mentioned previously, the AASM has developed practice parameters for the treatment of OSA, including surgery. Desired outcomes of treatment for OSA include the resolution of symptoms and clinical signs, normalization of the quality of sleep, AHI, and levels of oxyhemoglobin saturation. It is recognized that normalization of the AHI may not reverse all the components of OSA, and up to 22% of patients continue to have residual hypersomnia with CPAP therapy.9
Despite this, most studies that show significant benefits in lowering cardiovascular risk, mortality rates, symptoms, and neurocognitive effects have also shown significant reductions in the AHI.9 Therefore, the AASM puts a high value on treating OSA with the goal of normalization of the AHI. There exists a lack of quality studies and good evidence regarding the effectiveness of surgical procedures of the upper airway as treatment for OSA. Despite this, the AASM recommendation is that “all reasonable treatment alternatives for OSA be discussed in a manner that allows the patient to make an informed decision.”9
Types of OSA Surgeries
Surgery for OSA can be divided into 2 categories, invasive surgery and minimally invasive surgery (Table 2). Invasive surgeries for OSA have a higher risk of complications and postoperative pain.9
Invasive Surgery
The UPPP surgery is perhaps the best-known invasive procedure for treating OSA. A meta-analysis of 15 studies on UPPP outcomes found overall improvement in AHI of 33% but with postoperative AHI remaining elevated at an average of 29.8 events per hour.13
Adverse effects for the procedure include changes in taste, dysphagia, nasal regurgitation, and voice alterations. Seven of the 15 studies reported at least 1 death following the UPPP procedure. The effects of UPPP surgery on systemic and cardiovascular sequelae of OSA are not fully known and need to be further researched. 13
Another invasive procedure that is similar to the UPPP is the laser-assisted uvulopalatoplasty (LAUP) procedure. First reported in 1990, LAUP was developed as a procedure to eliminate snoring and was found to be effective. Other studies have been conducted since then, but there are inconsistencies in the specific types of procedures performed and a lack of detailed data analysis.
Adverse effects of the LAUP procedure include minor bleeding, globus sensation, local infection, minor dysphagia and dysphonia, and transient velopharyngeal insufficiency.14 However, studies have also shown that about one-quarter of the participants developed persistent dysphagia or mild to moderate scar fibrosis. Postoperative swelling can reduce an already narrow airway, and the use of sedatives or narcotics can make this problem worse. Some studies have noted structural changes following this procedure that lower airway resistance, resulting in the collapse of the upper airway and the narrowing of the airway during inhalation with worsening OSA.13,14
Practice parameters for the LAUP procedure were developed by the AASM in 1994. The AASM recommended against using LAUP to treat OSA (and other sleep-related breathing disorders) and against substituting LAUP for UPPP surgery. The AASM also suggested criteria for choosing candidates for LAUP and urged that patients be given full information about the procedure and a preoperative evaluation from their provider.5,14
Minimally Invasive Surgery
Radiofrequency surgery and soft palate implants are considered minimally invasive procedures, according to the criteria established in a study by Maurer.11 Various nasal surgeries (eg, septoplasty, adenoidectomy, and polyp removal) could also be considered minimally invasive and are often performed in patients with OSA to improve tolerance of CPAP by improving the ability to breathe nasally. However, nasal surgery with improvement of nasal breathing has not been found to have a significant impact on adults who have OSA. An advantage of nasal surgery is that some studies have shown longer use of CPAP therapy, up to 2 hours longer per night.5
With radiofrequency of the tongue base, high-frequency radio waves, either monopolar or bipolar, are used to coagulate the interstitial tissue at the base of the tongue. This, in turn, leads to necrosis and scarring, which then leads to stiffening of the tissue and in some cases, volume reduction. The surgeon controls the temperature to avoid overdosing and tissue carbonization. A number of lesions are produced during a session and the number of sessions necessary for full treatment varies per surgeon and the technical system used in procedures.
Although radiofrequency ablation (RFA) has been used on the tonsils and soft palate, RFA is currently available only to patients with OSA on the tongue base. A reduction in AHI of 33% on average was achieved in a review of 6 studies, and the reduction was stable over a 2-year period.11 The average presurgery AHI was 39.5, and the average postsurgery AHI was 28.7. In this same review, the average Epworth Sleepiness Scale (ESS) (a commonly used subjective tool to measure levels of daytime sleepiness) score was 10.4 presurgery and 4.5 postsurgery.11
A Chinese study looked at complications from RFA of the tongue base. According to the abstract (the article was written in Chinese), 1 patient (n = 193) developed a fatal arrhythmia. The authors’ recommendation was for close monitoring after surgery until the swelling subsided.15
With soft palate implants, small cylinders of woven polyester (“polyethylene terephthalate, commonly marketed as Dacron polyester”) yarn, 18 mm long by 2 mm diameter, are inserted at the junction of the hard palate and soft palate. Placed into the proximal soft palate tissue, these implants are designed to stiffen the soft palate “through fibrotic tissue encapsulation and tissue ingrowth over time.” Many patients with only snoring or mild to moderate OSA have found improvement with this procedure. Improved reduction of snoring was found in 1 study (n = 79), which looked at the use of Pillar implants to reduce snoring (Figure 1). This reduction of snoring occurred in patients with lower AHI levels before the procedure.16 Mauer reviewed 3 studies of the palatal implants and found a pooled reduction of 26% in AHI after placement.11
Criteria for Surgery
The mechanism that causes collapse of a patient’s airway will vary from one person to the next. Variables include age, body weight, anatomy, and body position. Thus, different findings will require different surgical techniques, and there is no one-size-fits-all surgery for OSA.11
A thorough physical examination of the upper airway is important. Maurer recommends considering the use of videoendoscopy under sedation, which allows a view of the mechanism and site of obstruction, with pressure catheters inserted to collect data. This procedure was developed in the 1990s to improve the analysis of obstruction within the upper airway. Unfortunately, the data are unclear about whether this aids in the surgical outcome.11 Powell stated that sleep endoscopy is currently considered investigational because medication-induced sleep may differ from natural sleep without medication.1
Nasopharyngoscopy, using fiberoptics, and lateral cephalometric analysis have been used for several years as primary diagnostic tools for patients being considered for a surgical procedure for OSA. Some new imaging procedures (eg, 3-D imaging coupled with advanced software programs) have been developed that may be helpful to assess regions that are constricted as well as provide a more exact measurement of the airway from the nose to the larynx.1
Risks and Complications of Surgery
The higher risk of anesthesia-related complications during or after surgery related to OSA is one reason that surgery is usually considered to be the third-line of treatment. Patients with OSA commonly have hypertension and other cardiovascular disease, which adds to the risk of anesthesia-related complications. Patients who have anatomical abnormalities of the jaw or portions of the upper airway will also be at a higher risk. Initial intubation and providing anesthesia during procedures may be more difficult for patients with these jaw or airway abnormalities. Postsurgical pain management with opioids contributes to the higher rate of apnea. After extubation, the incidence of apnea has been found to be higher in the population with OSA as well.17
A study published in 2001 used a case-control matched population methodology to examine patients who were undergoing elective surgeries for either hip or knee joint replacements. Significant differences in overall complication rates were found among the patients with OSA (n = 101) compared with those of the control group (n = 101). These differences were not related to the type of anesthesia, narcotic use after surgery, or the type of operation performed. The OSA group had a significantly higher number of patients who required higher flow rates of oxygen postoperatively and for a longer period. Serious complications, including the reintubation of 2 patients and unplanned intensive care unit (ICU) transfers, were noted in the patients with OSA (24%) compared with the control group (P = .004). The mean length of hospital stay was significantly longer for patients with OSA (6.8 + 2.8 days) compared with those in the control group (5.1 + 4.1 days, P = .007).18
The OSA group in the aforementioned study was divided into 2 types during this investigation: one group (n = 36) included patients undiagnosed with OSA until after their surgery (mean 1.8 years); the other group (n = 65) had a confirmed diagnosis at the time of surgery. All 36 in the first group and 32 from the second group with diagnosed OSA did not use CPAP therapy at home; a total of 68 patients (67.3%) who had not received therapy before the surgery. The 33 patients with diagnosed OSA (32.7%) who did use CPAP therapy before surgery had lower complication rates, including shorter hospital stays (6.0 + 2.1 days) compared with their counterparts with untreated OSA (7.2 + 3.1 days). The authors surmised that there might be a carryover protective effect at least for the first postoperative day.18
A retrospective study examined patients with OSA who had outpatient surgical procedures performed under either major regional anesthesia (central neuraxial) or general anesthesia. The study looked at the first outpatient surgical procedure for the patient with OSA following the diagnosis except for otorhinolaryngologic surgeries, which were excluded from the study. The 234 patients with OSA were then matched to the same number of control patients who had also had outpatient surgical procedures (excluding the otorhinolaryngologic procedures). The researchers noted a higher incidence of endotracheal intubation in the OSA group (79.9% vs 73.9% in the control group, P = .017).
The OSA group was less likely to have a laryngeal mask airway used during surgery for their airway management (5.1% vs 10.7% in the control group, P = .017). The only significantly different complication between the 2 groups was unplanned admissions to an ICU in the postoperative period (although numbers/percentages were not listed in the article). However, there was no difference in the overall unplanned hospital admission rate between the 2 groups. One limitation to this study was that the control group had not been tested for OSA, and therefore, it was possible that some in the control group might have had undiagnosed OSA.19
It is recommended that the surgeon and anesthesiologist ask about a diagnosis of OSA or sleep apnea symptoms during the preanesthesia assessment for any surgery. The provider performing the preoperative physical examination should pay attention to the circumference of the neck, chin-throat length, Mallampati classification, mandible position, and BMI. Any findings that suggest undiagnosed OSA should prompt further evaluation before any elective surgery.
Those who are at risk of OSA anesthesia-related complications should be offered regional anesthesia if possible. It is undesirable to premedicate with sedatives or opioids for this population. The sniffing position during surgery has been found to reduce the collapsibility of the airway and improve its dimensions. All patients who use CPAP therapy at home should have this therapy available for use in the recovery room with the lateral position preferred.17
Outpatient surgery using sedation and local anesthetics can also be a higher risk for patients with OSA. The unprotected, potentially problematic airway is still a serious concern. Patients requiring short procedures may do well with titrated sedation if well positioned and appropriately selected for this before the procedure. Clinicians must be prepared to handle any complications that occur. One complication that occurs commonly in obese patients related to a smaller functional residual capacity is rapid oxygen desaturation.17
A Chinese study evaluated complications in patients who had received tongue base reduction through RFA. Complications, both intraoperative and postoperative, included hematoma of the tongue base, bleeding, altered taste, an abscess at the base of the tongue, speech dysfunction, numbness, and deviation of the tongue extension movement. One cardiac death occurred 37 hours following surgery related to swelling of the tongue base and pain, which aggravated sleep apnea and nocturnal hypoxemia and induced a fatal arrhythmia.15
The Future of OSA Treatments
Research is underway to evaluate the effectiveness of an implanted system to stimulate the hypoglossal nerve with the intent of activating the upper airway musculature. A small study of 8 patients found improvement in the degree of upper airway collapsibility and the severity of OSA. Continued research on the device is focusing on the parameters for the nerve stimulation. The criteria for patient selection are also being established.20
Another study of hypoglossal nerve stimulation (HNS) evaluated 21 patients who were unable to tolerate CPAP therapy. The researchers found significant improvement in AHI (43.1 + 17.5 to 19.5 + 16.7); Functional Outcomes of Sleep Questionnaire (14.4 + 2.0 to 16.7 + 2.2); ESS (12.1 + 4.7 to 8.1 + 4.4); Beck Depression Inventory (15.8 + 9.0 to 9.7 + 7.6); and the Calgary Sleep Apnea Quality of Life Index (3.2 + 1.0 to 4.9 to 4.9 + 1.3). The researchers concluded HNS decreased OSA-related symptoms and the severity of OSA.21 At least one of these devices, the Inspire device, has been approved for use in Europe (Figure 2).22 In addition, the FDA has approve the Inspire Upper Airway Stimulation therapy for use in a subset of patients with moderate to severe OSA who are unable to use CPAP.23
A recent study investigated the effect of HNS on the severity of OSA among patients who had moderate to severe OSA and had not had a response to CPAP therapy. The HNS was associated with significant improvement in reducing the frequency of respiratory events. The median AHI at 12 months decreased from 29.3 events per hour to 9.0 events per hour (P < .001), and the number of times per hour of sleep that the blood oxygen level dropped by ≥ 4% from the baseline (oxygen desaturation index score) decreased from 25.4 to 7.4 events per hour (P < .001).24
Another area being studied is the development of a device to advance the tongue. Forty-two patients had mandibular bone anchors inserted with a flexible tether connected to a surgically inserted anchor in the posterior tongue. Unfortunately, 31% of the participants developed tissue anchor barb fractures that were asymptomatic and detected through radiography. At this time, the failure rate does not warrant clinical use. However, some patients showed significant improvement in multiple measures for sleep apnea, and more research is ongoing with the device.25
Similarly, a surgically inserted tongue pin that connects to an oral device during sleep has been studied. This pin prevents the tongue from moving back and obstructing the upper airway. Polysomnographies were performed both before and after and showed an increased rate of apnea in the 10 subjects. Visualization with magnetic resonance imaging showed that the upper airway was not kept open by this fixation device. Additional research with a modified design for the device is recommended.26
Reshaping of the epiglottis using a CO2 laser has been studied with cadaver specimens. The researchers believe scar formation following the reshaping would aid in retraction of the epiglottis anteriorly. Studies with animals are being conducted with different laser types. Future studies involving selected patients with OSA who have epiglottis malformations would be the next step.27
More studies about both minimally invasive and invasive surgeries for OSA are needed. There are many unanswered questions, including the timing of follow-up assessments, how long to observe a patient after surgery, whether the use of multilevel surgery affects the rate of relapse, and what are the best follow-up tools to use. Research needs to be performed in diverse populations, including gender, race/ethnicity, and age groups. Complications of surgical procedures need to be studied and clarified over both short- and long-term periods.9
Conclusions
Although there have been some successes in using surgical procedures to treat OSA, CPAP therapy remains the first-line treatment. Invasive surgeries have higher rates of complications and risks than do those of minimally invasive procedures. There are also additional risks related to the use of anesthesia both during and after surgery. Referrals for surgery should include information about a diagnosis of OSA, and patients should be instructed to discuss this with the anesthesiologist or certified nurse anesthetist.
With careful selection by the surgeon and sleep providers, patients who do not tolerate CPAP therapy or respond to other noninvasive interventions can benefit from a surgical procedure. Minimally invasive surgeries are less risky for the patient and should be considered.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
WEB EXCLUSIVE CASE STUDY
A Physical Examination of the Upper Airway by an Ear, Nose, and Throat Physician
Obstructive sleep apnea (OSA) is recognized primarily as a problem of the upper airway. Although narrowing or actual obstruction of the airway during the night can be found in only 1 or 2 areas of the upper airway, most often sleep apnea involves the entire pharyngeal upper airway passages. Three regions are considered to be of major concern: the nasal cavity region, the retropalatal region, and the retrolingual region. As the level of these 3 regions descends, the volume of tissue from the nose to the base of the tongue increases significantly. This leads to increased difficulty treating OSA with each descending region as well as to a lower success rate overall. Sometimes, the problem causing OSA is limited to only 1 region but may involve 2 or even all 3 regions.1
Continuous positive airway pressure (CPAP) therapy and other positive airway pressure (PAP) therapies have been considered a safe and effective treatment for OSA. Unfortunately, compliance rates, even among patients who use it to successfully eliminate their symptoms, can vary from 50% to 70%. Complaints about using CPAP and other PAP therapies range from skin irritation, discomfort to the nose or nasal passages, and eye problems to claustrophobia from wearing a mask. Patients who are unable or unwilling to use CPAP therapy can be candidates for surgical treatment of OSA.2
This article discusses surgical options for adult patients who have OSA who choose not to use CPAP therapy, the reasons to perform surgery for adults who have OSA, and the desired outcomes of surgery for OSA with a focus on minimally invasive procedures. Interventions for pediatric patients are not addressed.
Treatment Options
Treatments other than CPAP can help lessen the severity of OSA. Noninvasive treatments include weight loss, positional treatment (avoiding sleeping on one’s back), and oral appliances. Practice parameters from the American Academy of Sleep Medicine (AASM) for the treatment of OSA include dietary weight loss in addition to the primary therapy. An improvement in the apnea-hypopnea index (AHI) may occur with dietary weight loss.3
Major weight loss through the use of bariatric surgery has been shown to be effective in treating OSA and obesity hypoventilation syndrome (OHS), which is defined as daytime hypercapnia and hypoxemia (PaCO2 > 45 mm Hg and PaO2 < 70 mm Hg at sea level) in an obese patient (body mass index [BMI] > 30 kg/m2) with sleep-disordered breathing in the absence of any other cause of hypoventilation.4 Some individuals may have both conditions. However, CPAP therapy should not be discontinued even when major weight loss occurs until repeat polysomnography has been performed. Major weight loss may cure OHS and help improve the severity of OSA but will not totally resolve the condition.4 According to Woodson, sleep apnea in patients who are morbidly obese may be different from traditional OSA as rapid eye movement (REM), REM-related apneas, and hypoventilation occur more often in this population. Although weight loss is strongly recommended for patients with OSA, bariatric surgery is not recommended as the sole treatment for traditional OSA.5
Positional therapy, wherein the patient avoids the supine position, can be effective as a secondary or supplemental therapy in addition to treatment with CPAP. Guidelines recommend this primarily for individuals who have a lower AHI when in the nonsupine position than when lying on their backs. Patients who benefit from positional therapy tend to be younger, less obese, and with a less severe condition. The AASM practice parameter was based on 3 level II studies—randomized trials with high alpha and beta levels.6 One of the level II studies compared supine with an upright position, stating: “Because not all patients normalize AHI when non-supine, the committee’s opinion is that correction of OSA by position should be documented with an appropriate test.” Special pillows have been described in 2 papers reviewed by AASM, which seemed to improve OSA.6
The AASM guidelines state that oral appliances are indicated for some patients, although they are not as efficacious in treating OSA as CPAP therapy. Oral appliances are recommended for patients with mild to moderate OSA who have not responded to CPAP therapy, cannot tolerate CPAP therapy, are not appropriate for treatment with CPAP for some reason, or fail treatment with CPAP along with other behavioral measures, such as weight loss or positional therapy.6
In addition, the AASM guidelines recommend that patients with severe OSA, “should have an initial trial of nasal CPAP because greater effectiveness has been shown with this intervention than with the use of oral appliances. Upper airway surgery (including tonsillectomy and adenoidectomy, craniofacial operations, and tracheostomy) may also supersede use of oral appliances in patients for whom these operations are predicted to be highly effective in treating sleep apnea.”6
Nasal expiratory positive end pressure (EPAP) devices may be helpful in treating OSA in some patients. These devices contain a mechanical valve with very low inspiratory resistance but high expiratory resistance. The device has an adhesive and is applied by the patient to create a seal. Exhalation causes a high expiratory resistance that splints the upper airway open. This increases the resistance of the airway to close on inspiration.7 An EPAP device is recommended for potential use in mild to moderate OSA for patients who either have an intolerance to CPAP therapy or have failed to respond to it.8
Finally, there are major surgeries performed by oral surgeons that can benefit some patients with OSA. One of these is maxillomandibular advancement (MMA). According to the AASM practice parameter: MMA “involves simultaneous advancement of the maxilla and mandible through sagittal split osteotomies. It provides enlargement of the retrolingual airway and some advancement of the retropalatal airway.”9 It is indicated as a surgical treatment for patients with severe OSA who are either unwilling or do not tolerate CPAP treatment. These individuals would not benefit from an oral appliance (recommended for mild to moderate OSA) or would find it undesirable.9
There is also stepwise or multilevel surgery (MLS) that can be performed. These include a number of combined procedures, which address multiple sites with narrowing in the upper airway. Frequently, MLS will consist of
2 phases: the first involves use of the uvulopalatopharyngoplasty (UPPP) procedure “and or genioglossus advancement and hyoid myotomy (GAHM). The second phase surgeries consist of utilizing maxillary and mandibular advancement osteotomy (MMO), offered to those failing Phase I surgeries.”9
OSA Surgical Procedures
Tracheostomies are first estimated to have been performed in 2000 BC.10 Performing a tracheostomy to bypass the upper airway was used in the 1960s and 1970s for the treatment of OSA and for many years was the only treatment available for people with Pickwickian syndrome (OHS) and nocturnal upper airway obstruction. The procedure was generally not tolerated or accepted by patients, even though it improved their quality of life and added to their life expectancy. Once CPAP treatment proved successful for OSA, tracheostomy has rarely been necessary.11
Uvulopalatopharyngoplasty surgery was introduced in 1981. The aim of this surgery is to decrease snoring and treat OSA by removing obstructive tissues, enlarging the cross-sectional portion of the upper airway, and bypassing the upper airway. Tissue that is removed includes the tonsils, uvula, and the distal portion of the soft palate.12
Woodson considers surgery for OSA to be the third-line of treatment. The first-line treatment would be CPAP therapy, and second-line therapy would include oral appliances to enlarge the airway or retain the tongue (if the individual has no dentition). The intent of surgery falls into 3 categories: curative, salvage, and ancillary.
In a chronic disease such as OSA, “many may question whether a cure exists.” Instead of eliminating OSA, the curative intent is definitively to reduce symptoms and disease morbidity for long periods. The criteria for defining a responder or cure to surgery for OSA are found in Table 1.5
Surgery for salvage aims at treating patients who have failed CPAP therapy. Successful treatment with the intent of salvage can occur with a lessening of disease severity, including morbidity and mortality, but not necessarily totally eliminating the symptoms. Finally, ancillary surgery for OSA aims to combine a surgical procedure with the first-line therapy (positive pressure) to add an additional therapeutic benefit. The combination of CPAP and ancillary procedures may be of the most benefit to patients with OSA.5
As mentioned previously, the AASM has developed practice parameters for the treatment of OSA, including surgery. Desired outcomes of treatment for OSA include the resolution of symptoms and clinical signs, normalization of the quality of sleep, AHI, and levels of oxyhemoglobin saturation. It is recognized that normalization of the AHI may not reverse all the components of OSA, and up to 22% of patients continue to have residual hypersomnia with CPAP therapy.9
Despite this, most studies that show significant benefits in lowering cardiovascular risk, mortality rates, symptoms, and neurocognitive effects have also shown significant reductions in the AHI.9 Therefore, the AASM puts a high value on treating OSA with the goal of normalization of the AHI. There exists a lack of quality studies and good evidence regarding the effectiveness of surgical procedures of the upper airway as treatment for OSA. Despite this, the AASM recommendation is that “all reasonable treatment alternatives for OSA be discussed in a manner that allows the patient to make an informed decision.”9
Types of OSA Surgeries
Surgery for OSA can be divided into 2 categories, invasive surgery and minimally invasive surgery (Table 2). Invasive surgeries for OSA have a higher risk of complications and postoperative pain.9
Invasive Surgery
The UPPP surgery is perhaps the best-known invasive procedure for treating OSA. A meta-analysis of 15 studies on UPPP outcomes found overall improvement in AHI of 33% but with postoperative AHI remaining elevated at an average of 29.8 events per hour.13
Adverse effects for the procedure include changes in taste, dysphagia, nasal regurgitation, and voice alterations. Seven of the 15 studies reported at least 1 death following the UPPP procedure. The effects of UPPP surgery on systemic and cardiovascular sequelae of OSA are not fully known and need to be further researched. 13
Another invasive procedure that is similar to the UPPP is the laser-assisted uvulopalatoplasty (LAUP) procedure. First reported in 1990, LAUP was developed as a procedure to eliminate snoring and was found to be effective. Other studies have been conducted since then, but there are inconsistencies in the specific types of procedures performed and a lack of detailed data analysis.
Adverse effects of the LAUP procedure include minor bleeding, globus sensation, local infection, minor dysphagia and dysphonia, and transient velopharyngeal insufficiency.14 However, studies have also shown that about one-quarter of the participants developed persistent dysphagia or mild to moderate scar fibrosis. Postoperative swelling can reduce an already narrow airway, and the use of sedatives or narcotics can make this problem worse. Some studies have noted structural changes following this procedure that lower airway resistance, resulting in the collapse of the upper airway and the narrowing of the airway during inhalation with worsening OSA.13,14
Practice parameters for the LAUP procedure were developed by the AASM in 1994. The AASM recommended against using LAUP to treat OSA (and other sleep-related breathing disorders) and against substituting LAUP for UPPP surgery. The AASM also suggested criteria for choosing candidates for LAUP and urged that patients be given full information about the procedure and a preoperative evaluation from their provider.5,14
Minimally Invasive Surgery
Radiofrequency surgery and soft palate implants are considered minimally invasive procedures, according to the criteria established in a study by Maurer.11 Various nasal surgeries (eg, septoplasty, adenoidectomy, and polyp removal) could also be considered minimally invasive and are often performed in patients with OSA to improve tolerance of CPAP by improving the ability to breathe nasally. However, nasal surgery with improvement of nasal breathing has not been found to have a significant impact on adults who have OSA. An advantage of nasal surgery is that some studies have shown longer use of CPAP therapy, up to 2 hours longer per night.5
With radiofrequency of the tongue base, high-frequency radio waves, either monopolar or bipolar, are used to coagulate the interstitial tissue at the base of the tongue. This, in turn, leads to necrosis and scarring, which then leads to stiffening of the tissue and in some cases, volume reduction. The surgeon controls the temperature to avoid overdosing and tissue carbonization. A number of lesions are produced during a session and the number of sessions necessary for full treatment varies per surgeon and the technical system used in procedures.
Although radiofrequency ablation (RFA) has been used on the tonsils and soft palate, RFA is currently available only to patients with OSA on the tongue base. A reduction in AHI of 33% on average was achieved in a review of 6 studies, and the reduction was stable over a 2-year period.11 The average presurgery AHI was 39.5, and the average postsurgery AHI was 28.7. In this same review, the average Epworth Sleepiness Scale (ESS) (a commonly used subjective tool to measure levels of daytime sleepiness) score was 10.4 presurgery and 4.5 postsurgery.11
A Chinese study looked at complications from RFA of the tongue base. According to the abstract (the article was written in Chinese), 1 patient (n = 193) developed a fatal arrhythmia. The authors’ recommendation was for close monitoring after surgery until the swelling subsided.15
With soft palate implants, small cylinders of woven polyester (“polyethylene terephthalate, commonly marketed as Dacron polyester”) yarn, 18 mm long by 2 mm diameter, are inserted at the junction of the hard palate and soft palate. Placed into the proximal soft palate tissue, these implants are designed to stiffen the soft palate “through fibrotic tissue encapsulation and tissue ingrowth over time.” Many patients with only snoring or mild to moderate OSA have found improvement with this procedure. Improved reduction of snoring was found in 1 study (n = 79), which looked at the use of Pillar implants to reduce snoring (Figure 1). This reduction of snoring occurred in patients with lower AHI levels before the procedure.16 Mauer reviewed 3 studies of the palatal implants and found a pooled reduction of 26% in AHI after placement.11
Criteria for Surgery
The mechanism that causes collapse of a patient’s airway will vary from one person to the next. Variables include age, body weight, anatomy, and body position. Thus, different findings will require different surgical techniques, and there is no one-size-fits-all surgery for OSA.11
A thorough physical examination of the upper airway is important. Maurer recommends considering the use of videoendoscopy under sedation, which allows a view of the mechanism and site of obstruction, with pressure catheters inserted to collect data. This procedure was developed in the 1990s to improve the analysis of obstruction within the upper airway. Unfortunately, the data are unclear about whether this aids in the surgical outcome.11 Powell stated that sleep endoscopy is currently considered investigational because medication-induced sleep may differ from natural sleep without medication.1
Nasopharyngoscopy, using fiberoptics, and lateral cephalometric analysis have been used for several years as primary diagnostic tools for patients being considered for a surgical procedure for OSA. Some new imaging procedures (eg, 3-D imaging coupled with advanced software programs) have been developed that may be helpful to assess regions that are constricted as well as provide a more exact measurement of the airway from the nose to the larynx.1
Risks and Complications of Surgery
The higher risk of anesthesia-related complications during or after surgery related to OSA is one reason that surgery is usually considered to be the third-line of treatment. Patients with OSA commonly have hypertension and other cardiovascular disease, which adds to the risk of anesthesia-related complications. Patients who have anatomical abnormalities of the jaw or portions of the upper airway will also be at a higher risk. Initial intubation and providing anesthesia during procedures may be more difficult for patients with these jaw or airway abnormalities. Postsurgical pain management with opioids contributes to the higher rate of apnea. After extubation, the incidence of apnea has been found to be higher in the population with OSA as well.17
A study published in 2001 used a case-control matched population methodology to examine patients who were undergoing elective surgeries for either hip or knee joint replacements. Significant differences in overall complication rates were found among the patients with OSA (n = 101) compared with those of the control group (n = 101). These differences were not related to the type of anesthesia, narcotic use after surgery, or the type of operation performed. The OSA group had a significantly higher number of patients who required higher flow rates of oxygen postoperatively and for a longer period. Serious complications, including the reintubation of 2 patients and unplanned intensive care unit (ICU) transfers, were noted in the patients with OSA (24%) compared with the control group (P = .004). The mean length of hospital stay was significantly longer for patients with OSA (6.8 + 2.8 days) compared with those in the control group (5.1 + 4.1 days, P = .007).18
The OSA group in the aforementioned study was divided into 2 types during this investigation: one group (n = 36) included patients undiagnosed with OSA until after their surgery (mean 1.8 years); the other group (n = 65) had a confirmed diagnosis at the time of surgery. All 36 in the first group and 32 from the second group with diagnosed OSA did not use CPAP therapy at home; a total of 68 patients (67.3%) who had not received therapy before the surgery. The 33 patients with diagnosed OSA (32.7%) who did use CPAP therapy before surgery had lower complication rates, including shorter hospital stays (6.0 + 2.1 days) compared with their counterparts with untreated OSA (7.2 + 3.1 days). The authors surmised that there might be a carryover protective effect at least for the first postoperative day.18
A retrospective study examined patients with OSA who had outpatient surgical procedures performed under either major regional anesthesia (central neuraxial) or general anesthesia. The study looked at the first outpatient surgical procedure for the patient with OSA following the diagnosis except for otorhinolaryngologic surgeries, which were excluded from the study. The 234 patients with OSA were then matched to the same number of control patients who had also had outpatient surgical procedures (excluding the otorhinolaryngologic procedures). The researchers noted a higher incidence of endotracheal intubation in the OSA group (79.9% vs 73.9% in the control group, P = .017).
The OSA group was less likely to have a laryngeal mask airway used during surgery for their airway management (5.1% vs 10.7% in the control group, P = .017). The only significantly different complication between the 2 groups was unplanned admissions to an ICU in the postoperative period (although numbers/percentages were not listed in the article). However, there was no difference in the overall unplanned hospital admission rate between the 2 groups. One limitation to this study was that the control group had not been tested for OSA, and therefore, it was possible that some in the control group might have had undiagnosed OSA.19
It is recommended that the surgeon and anesthesiologist ask about a diagnosis of OSA or sleep apnea symptoms during the preanesthesia assessment for any surgery. The provider performing the preoperative physical examination should pay attention to the circumference of the neck, chin-throat length, Mallampati classification, mandible position, and BMI. Any findings that suggest undiagnosed OSA should prompt further evaluation before any elective surgery.
Those who are at risk of OSA anesthesia-related complications should be offered regional anesthesia if possible. It is undesirable to premedicate with sedatives or opioids for this population. The sniffing position during surgery has been found to reduce the collapsibility of the airway and improve its dimensions. All patients who use CPAP therapy at home should have this therapy available for use in the recovery room with the lateral position preferred.17
Outpatient surgery using sedation and local anesthetics can also be a higher risk for patients with OSA. The unprotected, potentially problematic airway is still a serious concern. Patients requiring short procedures may do well with titrated sedation if well positioned and appropriately selected for this before the procedure. Clinicians must be prepared to handle any complications that occur. One complication that occurs commonly in obese patients related to a smaller functional residual capacity is rapid oxygen desaturation.17
A Chinese study evaluated complications in patients who had received tongue base reduction through RFA. Complications, both intraoperative and postoperative, included hematoma of the tongue base, bleeding, altered taste, an abscess at the base of the tongue, speech dysfunction, numbness, and deviation of the tongue extension movement. One cardiac death occurred 37 hours following surgery related to swelling of the tongue base and pain, which aggravated sleep apnea and nocturnal hypoxemia and induced a fatal arrhythmia.15
The Future of OSA Treatments
Research is underway to evaluate the effectiveness of an implanted system to stimulate the hypoglossal nerve with the intent of activating the upper airway musculature. A small study of 8 patients found improvement in the degree of upper airway collapsibility and the severity of OSA. Continued research on the device is focusing on the parameters for the nerve stimulation. The criteria for patient selection are also being established.20
Another study of hypoglossal nerve stimulation (HNS) evaluated 21 patients who were unable to tolerate CPAP therapy. The researchers found significant improvement in AHI (43.1 + 17.5 to 19.5 + 16.7); Functional Outcomes of Sleep Questionnaire (14.4 + 2.0 to 16.7 + 2.2); ESS (12.1 + 4.7 to 8.1 + 4.4); Beck Depression Inventory (15.8 + 9.0 to 9.7 + 7.6); and the Calgary Sleep Apnea Quality of Life Index (3.2 + 1.0 to 4.9 to 4.9 + 1.3). The researchers concluded HNS decreased OSA-related symptoms and the severity of OSA.21 At least one of these devices, the Inspire device, has been approved for use in Europe (Figure 2).22 In addition, the FDA has approve the Inspire Upper Airway Stimulation therapy for use in a subset of patients with moderate to severe OSA who are unable to use CPAP.23
A recent study investigated the effect of HNS on the severity of OSA among patients who had moderate to severe OSA and had not had a response to CPAP therapy. The HNS was associated with significant improvement in reducing the frequency of respiratory events. The median AHI at 12 months decreased from 29.3 events per hour to 9.0 events per hour (P < .001), and the number of times per hour of sleep that the blood oxygen level dropped by ≥ 4% from the baseline (oxygen desaturation index score) decreased from 25.4 to 7.4 events per hour (P < .001).24
Another area being studied is the development of a device to advance the tongue. Forty-two patients had mandibular bone anchors inserted with a flexible tether connected to a surgically inserted anchor in the posterior tongue. Unfortunately, 31% of the participants developed tissue anchor barb fractures that were asymptomatic and detected through radiography. At this time, the failure rate does not warrant clinical use. However, some patients showed significant improvement in multiple measures for sleep apnea, and more research is ongoing with the device.25
Similarly, a surgically inserted tongue pin that connects to an oral device during sleep has been studied. This pin prevents the tongue from moving back and obstructing the upper airway. Polysomnographies were performed both before and after and showed an increased rate of apnea in the 10 subjects. Visualization with magnetic resonance imaging showed that the upper airway was not kept open by this fixation device. Additional research with a modified design for the device is recommended.26
Reshaping of the epiglottis using a CO2 laser has been studied with cadaver specimens. The researchers believe scar formation following the reshaping would aid in retraction of the epiglottis anteriorly. Studies with animals are being conducted with different laser types. Future studies involving selected patients with OSA who have epiglottis malformations would be the next step.27
More studies about both minimally invasive and invasive surgeries for OSA are needed. There are many unanswered questions, including the timing of follow-up assessments, how long to observe a patient after surgery, whether the use of multilevel surgery affects the rate of relapse, and what are the best follow-up tools to use. Research needs to be performed in diverse populations, including gender, race/ethnicity, and age groups. Complications of surgical procedures need to be studied and clarified over both short- and long-term periods.9
Conclusions
Although there have been some successes in using surgical procedures to treat OSA, CPAP therapy remains the first-line treatment. Invasive surgeries have higher rates of complications and risks than do those of minimally invasive procedures. There are also additional risks related to the use of anesthesia both during and after surgery. Referrals for surgery should include information about a diagnosis of OSA, and patients should be instructed to discuss this with the anesthesiologist or certified nurse anesthetist.
With careful selection by the surgeon and sleep providers, patients who do not tolerate CPAP therapy or respond to other noninvasive interventions can benefit from a surgical procedure. Minimally invasive surgeries are less risky for the patient and should be considered.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
WEB EXCLUSIVE CASE STUDY
A Physical Examination of the Upper Airway by an Ear, Nose, and Throat Physician
1. Powell NB. Contemporary surgery for obstructive sleep apnea syndrome. Clin Exp Otorhinolaryngol. 2009;2(3):107-114.
2. Shine NP, Lewis RH. Transpalatal advancement paryngoplasty for obstructive sleep apnea syndrome: Results and analysis of failures. Arch Otolaryngol Head Neck Surg. 2009;135(5): 434-438.
3. Morgenthaler TI, Kapen S, Lee-Chiong T; American Academy of Sleep Medicine. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8):1031-1035.
4. Aigner MJ, Sikka P, Whitfield P. Obesity hypoventilation syndrome: What is it? How is it treated? Is there a cure? Bariatric Nursing and Surgical Patient Care. 2009;4(2):109-113.
5. Woodson BT. Non-pressure therapies for obstructive sleep apnea: Surgery and oral appliances. Respir Care. 2010;55(10):1314-1321.
6. Kushida CA, Morgenthaler TI, Littner MR, et al; American Academy of Sleep Medicine. Practice parameters for the treatment of snoring and Obstructive Sleep Apnea with oral appliances: An update for 2005. Sleep. 2006;29(2):240-243.
7. Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: A randomized controlled trial. Sleep. 2011;34(4):479-485.
8. Kryger MH, Berry RB, Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment of obstructive sleep apnea (OSA). J Clin Sleep Med. 2011;7(5):449-453.
9. Aurora RN, Casey KR, Kristo D, et al; American Academy of Sleep Medicine. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33(10):1408-1413.
10. Szmuk P, Ezri T, Evron S, Roth Y, Katz J. A brief history of tracheostomy and tracheal intubation, from the Bronze Age to the space age. Intensive Care Med. 2008;34(2):222-228.
11. Maurer JT. Update on surgical treatment for sleep apnoea. Swiss Med Wkly. 2009;139(43-44):624-629.
12. Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obsructive sleep apnea—a systematic review. Sleep. 2009;32(1):27-36.
13. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: A systematic review and meta-analysis. Sleep. 2010; 33(10):1396-1407.
14. Littner M, Kushida CA, Hartse K, et al. Practice parameters for the use of laser-assisted uvulopalatoplasty: An update for 2000. Sleep. 2001;24(5):603-619.
15. Chen JH, Luo ZH, Yang XL, Zhu MW, Tao ZZ. Complications of tongue base reduction with radiofrequency tissue ablation on obstructive sleep apnea hypopnea syndrome. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45(7):574-577. (Abstract, full text in Chinese.)
16. Gillespie MB, Smith JE, Clarke J, Nguyen SA. Effectiveness of Pillar palatal implants for snoring management. Otolaryngol Head Neck Surg. 2009;140(3):363-368.
17. Ephros HD, Madani M, Yalamanchili SC. Surgical treatment of snoring and obstructive sleep apnoea. Indian J Med Res. 2010;131:267-276.
18. Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: A case-control study. Mayo Clin Proc. 2001;76(9):897-905.
19. Sabers C, Plevak DJ, Schroeder DR, Warner DO. The diagnosis of obstructive sleep apnea as a risk factor for unanticipated admissions in outpatient surgery. Anesth Analg. 2003;96(5):1328-1335.
20. Kerzirian EJ, Boudewyns A, Eisele DW, et al. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev. 2010;14(5):299-305.
21. Eastwood PR, Barnes M, Walsh JH, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34(11):1479-1486.
22. Inspire Medical. STAR Trial. Inspire Medical Website. http://www.inspiresleep.com/star-trial.php. Accessed May 7, 2014.
23. FDA approves Inspire Upper Airway Stimulation (UAS) therapy for obstructive sleep apnea [press release]. Inspire Medical Website. http://www.inspiresleep.com/pdf/FDA-Approval-Press-Release-final-20140501.pdf.
Accessed May 29, 2014.
24. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149.
25. Woodson BT, Steward DL, Mickelson S, Huntley T, Goldberg A. Multicenter study of a novel adjustable tongue-advancement device for obstructive sleep apnea. Otolaryngol Head Neck Surg. 2010;143(4):585-590.
26. Punke C, Schöentag C, Hortian B, et al. Tongue fixation system for therapy of sleeping disorders. A feasibility study. HNO. 2010;58(12):1184-1189. (Abstract, full text in German).
27. Bourolinas C, Hajiioannou J, Sobol E, Velegrakis G, Helidonis E. Epiglottis reshaping using CO2: A minimally invasive technique and its potential applications. Head Face Med. 2008;4:15.
1. Powell NB. Contemporary surgery for obstructive sleep apnea syndrome. Clin Exp Otorhinolaryngol. 2009;2(3):107-114.
2. Shine NP, Lewis RH. Transpalatal advancement paryngoplasty for obstructive sleep apnea syndrome: Results and analysis of failures. Arch Otolaryngol Head Neck Surg. 2009;135(5): 434-438.
3. Morgenthaler TI, Kapen S, Lee-Chiong T; American Academy of Sleep Medicine. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8):1031-1035.
4. Aigner MJ, Sikka P, Whitfield P. Obesity hypoventilation syndrome: What is it? How is it treated? Is there a cure? Bariatric Nursing and Surgical Patient Care. 2009;4(2):109-113.
5. Woodson BT. Non-pressure therapies for obstructive sleep apnea: Surgery and oral appliances. Respir Care. 2010;55(10):1314-1321.
6. Kushida CA, Morgenthaler TI, Littner MR, et al; American Academy of Sleep Medicine. Practice parameters for the treatment of snoring and Obstructive Sleep Apnea with oral appliances: An update for 2005. Sleep. 2006;29(2):240-243.
7. Berry RB, Kryger MH, Massie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: A randomized controlled trial. Sleep. 2011;34(4):479-485.
8. Kryger MH, Berry RB, Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment of obstructive sleep apnea (OSA). J Clin Sleep Med. 2011;7(5):449-453.
9. Aurora RN, Casey KR, Kristo D, et al; American Academy of Sleep Medicine. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33(10):1408-1413.
10. Szmuk P, Ezri T, Evron S, Roth Y, Katz J. A brief history of tracheostomy and tracheal intubation, from the Bronze Age to the space age. Intensive Care Med. 2008;34(2):222-228.
11. Maurer JT. Update on surgical treatment for sleep apnoea. Swiss Med Wkly. 2009;139(43-44):624-629.
12. Franklin KA, Anttila H, Axelsson S, et al. Effects and side-effects of surgery for snoring and obsructive sleep apnea—a systematic review. Sleep. 2009;32(1):27-36.
13. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: A systematic review and meta-analysis. Sleep. 2010; 33(10):1396-1407.
14. Littner M, Kushida CA, Hartse K, et al. Practice parameters for the use of laser-assisted uvulopalatoplasty: An update for 2000. Sleep. 2001;24(5):603-619.
15. Chen JH, Luo ZH, Yang XL, Zhu MW, Tao ZZ. Complications of tongue base reduction with radiofrequency tissue ablation on obstructive sleep apnea hypopnea syndrome. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45(7):574-577. (Abstract, full text in Chinese.)
16. Gillespie MB, Smith JE, Clarke J, Nguyen SA. Effectiveness of Pillar palatal implants for snoring management. Otolaryngol Head Neck Surg. 2009;140(3):363-368.
17. Ephros HD, Madani M, Yalamanchili SC. Surgical treatment of snoring and obstructive sleep apnoea. Indian J Med Res. 2010;131:267-276.
18. Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: A case-control study. Mayo Clin Proc. 2001;76(9):897-905.
19. Sabers C, Plevak DJ, Schroeder DR, Warner DO. The diagnosis of obstructive sleep apnea as a risk factor for unanticipated admissions in outpatient surgery. Anesth Analg. 2003;96(5):1328-1335.
20. Kerzirian EJ, Boudewyns A, Eisele DW, et al. Electrical stimulation of the hypoglossal nerve in the treatment of obstructive sleep apnea. Sleep Med Rev. 2010;14(5):299-305.
21. Eastwood PR, Barnes M, Walsh JH, et al. Treating obstructive sleep apnea with hypoglossal nerve stimulation. Sleep. 2011;34(11):1479-1486.
22. Inspire Medical. STAR Trial. Inspire Medical Website. http://www.inspiresleep.com/star-trial.php. Accessed May 7, 2014.
23. FDA approves Inspire Upper Airway Stimulation (UAS) therapy for obstructive sleep apnea [press release]. Inspire Medical Website. http://www.inspiresleep.com/pdf/FDA-Approval-Press-Release-final-20140501.pdf.
Accessed May 29, 2014.
24. Strollo PJ Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139-149.
25. Woodson BT, Steward DL, Mickelson S, Huntley T, Goldberg A. Multicenter study of a novel adjustable tongue-advancement device for obstructive sleep apnea. Otolaryngol Head Neck Surg. 2010;143(4):585-590.
26. Punke C, Schöentag C, Hortian B, et al. Tongue fixation system for therapy of sleeping disorders. A feasibility study. HNO. 2010;58(12):1184-1189. (Abstract, full text in German).
27. Bourolinas C, Hajiioannou J, Sobol E, Velegrakis G, Helidonis E. Epiglottis reshaping using CO2: A minimally invasive technique and its potential applications. Head Face Med. 2008;4:15.