User login
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
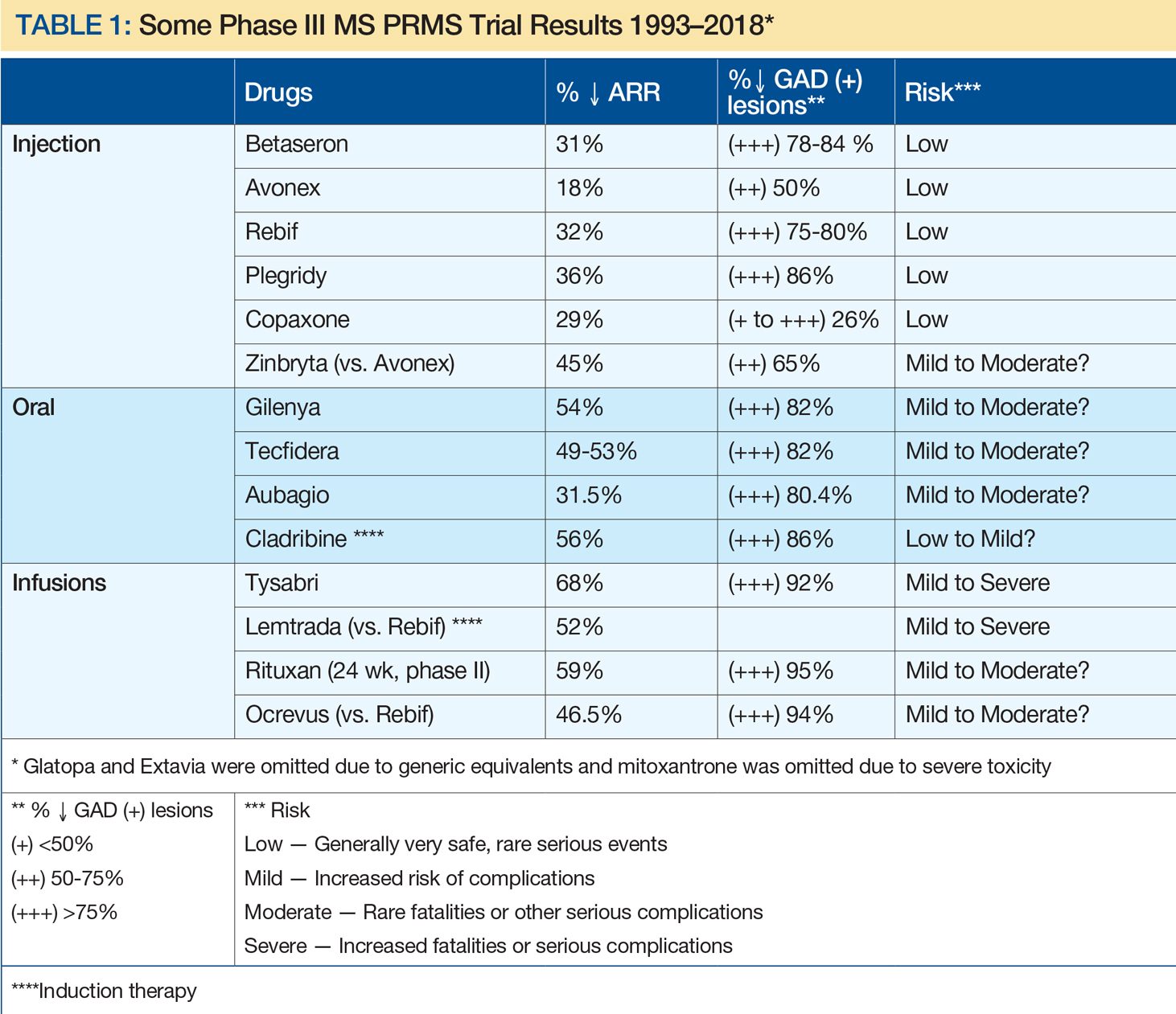
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.
Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.

Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.
Stuart D. Cook, MD, and Abdul Rahman Alchaki
Dr. Cook is the Ruth Dunietz Kushner and Michael Jay Serwitz Professor of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark. Dr. Alchaki is a resident in the Deptartment of Neurology/Neurosciences at Rutgers, the State University of New Jersey, Newark.
Disclosure: Stuart Cook has received honoraria for lectures from Bayer HealthCare and Merck Serono. He has served as a consultant for Merck Serono, Bayer HealthCare, Teva, Novartis, Sanofi-Aventis, Biogen Idec, and Actinobac Biomed. He has served on steering committees for the BEYOND and CLARITY Studies and as a member of Advisory Boards for Merck Serono, Bayer HealthCare, Teva, Biogen Idec, Sanofi Aventis, and Actinobac Biomed.
The Initial Years (1838 to 1930s)
The earliest recognition of MS clinical features and pathology was attributed to Jean-Martin Charcot, Robert Carswell, and Jean Cruveilhier in Europe from 1838 to 1868. Beyond those early descriptions, relatively few MS breakthroughs occurred until the 1930s, when Thomas Rivers discovered experimental autoimmune encephalomyelitis (EAE), a demyelinating disease, in animals. His insightful concepts were widely cited and ultimately contributed to undestanding of the immune mechanisms of MS and acute disseminated encephalomyelitis (ADEM).
Advances in Diagnosis (1965 to 1992)
In 1965, Schumacher et al provided the essential clinical criteria for MS diagnosis. Poser et al refined these criteria in 1983. In 2001, McDonald et al added neuroimaging, CSF analysis, and evoked potentials to further complement MS clinical diagnosis. For the first time, the disease could generally be recognized.
Early Treatments
Various treatments for MS were tried over the years, without great success. However, in 1953, a small descriptive trial by Miller and Gibbons reported clinical benefits in patients using intramuscular (IM) adrenocorticotropic hormone (ACTH) for MS and disseminated encephalomyelitis. This was followed in 1970 by a Cooperative Study of IM ACTH versus placebo by Rose et al, which resulted in ACTH, and subsequently oral corticosteroids, being widely used to treat MS, particularly for acute exacerbations of the disease. However, robust evidence of long-term steroids remain limited, even to the present.
High-Dose Steroids
By 1980, the initial descriptive treatment of high-dose intravenous (IV) steroids for demyelinating diseases, including MS and transverse myelitis, by Dowling et al resulted in rapid clinical improvement in some patients. This result was ultimately confirmed by others. High-dose IV steroids became the gold standard for acute attacks, particularly those aggressive in nature. In the mid 1980s, work by Troiano et al, as well as others, showed that the rapid use of high-dose IV as well as oral steroids showed similar effects, with reduction or elimination of CT contrast-enhancing lesions within as few as eight hours, while lower doses or alternative-day treatments were less effective. In addition, descriptive studies of immune modulatory and immunosuppressive drugs, as well as small randomized studies, were published. These agents did not receive FDA approval.
The Golden Age of Therapy (1993 to 2018)
A remarkable era in MS prognosis and treatment began with immunomodulation injections of Betaseron (INFβ-1b), Avonex (INFβ-1a), and Copaxone (glatiramer acetate). This can be attributed, at least in part, to advances in molecular biology, genetics, and neuroimaging, and support by corporate, private, and public funding. Since the initial FDA approval of INFβ-1b, 15 MS therapies have become clinically available, including eight injectables, three orals, and four infusion treatments (see Table 1). In addition, two other drugs have been FDA approved for uses other than MS: rituximab (approved for lymphoma) and cladribine (for hairy cell leukemia), with the latter now approved by the European Medicines Agency for MS. Table 1 depicts characteristics of these therapies approved by US or European agencies (or for other disorders increasingly used off label for MS) in an attempt to compare annual relapse rates (ARR) and decreases in the percent of gadolinium-enhancing MS lesions versus placebo. This information was chosen because ARR has been uniformly selected and defined for such trials, while percent decrease of gadolinium-enhancing lesions on MRI has been the most sensitive barometer available for assessing acute clinical activity. As a result, risk-benefit considerations have been critical in evaluating these drug treatments, with efficacy improving greatly over time, whereas risks have been more variable.
Disease Categories
In 1996, Lublin and Reingold provided a new classification, not specifically for the diagnosis of MS, but rather for the clinical course of the disease. Initially, there were four categories—relapsing-remitting MS, secondary progressive MS, primary progressive MS, and progressive-relapsing MS—that were universally identified. These were thought to be relatively distinct clinical categories, but over time it became clear that the classification did not fully distinguish MS disease activity within these categories. For that reason, it was subsequently recommended, by Lincoln et al in 2009 and Cook et al in 2012, to include MRI, a vastly more sensitive modality, as well as clinical data in assessing disease activity.
On another note, MS and neuromyelitis optica (NMO), although having similar features, were clearly identified as different diseases by Lennon et al in 2004. Differences in pathology, clinical characteristics, immunology, and therapy separate the two disorders.
MRI in MS
Work by Young et al in 1981 established the central role of MRI brain imaging in MS diagnosis and therapeutic considerations. Since then it has become ubiquitous.
An example of a sensitive and highly productive MRI protocol is the BECOME study of MS and clinically isolated syndrome by Cadavid et al from 2009 to 2017. In this study, IFNβ-1b was compared with glatiramer acetate treatment. Cadavid et al used a 3T scanner with triple-dose gadolinium, performed monthly for as long as 24 consecutive months. This unique study brought about a virtual gold mine of valuable research and clinical information. This included proof that gadolinium-enhancing lesions persisted for six months or more, evidence of a 30:1 ratio of new MRI brain lesions to clinical activity, and documentation that 96% of T2 lesions and black holes derive from prior gadolinium-enhancing lesions. It was further noted that 80% to 90% of acute black holes disappeared with treatment and 75% to 80% of patients taking IFNβ-1b or glatiramer acetate had new MRI lesions despite continuing treatment. Perhaps most interestingly, monthly MRIs could predict relapse and disability in a relatively small number of patients, depending upon the frequency and activity of MRI lesions. In 2017, Brown et al documented that magnetization transfer ratio recovery in MS brain lesions occurred more significantly with glatiramer acetate than with IFNβ-1b, whereas more chronic black hole lesions were found with glatiramer acetate. Also in 2017, Maranzano et al found evidence of acute inflammatory leukocortical lesions, which were not as well recognized previously.
In summary, it has become increasingly clear that MRI is the most sen
The Future of MS
While it is not yet a curable disease, there is growing evidence that MS prognosis has improved and will continue to improve. This is based on incremental decreases in acute MS exacerbations, progressive disability, and MRI lesion activity, as well as a combination of the three—no evidence of disease activity (NEDA).
Not only are drug therapies becoming more effective, but patients and physicians now have many more treatment options to carefully consider with regard to efficacy, side effect profiles, treatment frequency, route of administration, cost, and quality of life. Newer drugs with different mechanisms of action such as cladribine, now approved in Europe, fulfill most of these beneficial criteria (see Giovannoni et al, 2010). More promising MS treatments, including long-acting induction therapies, are still being evaluated. As with other complex diseases, multiple therapies are likely to be used as well.
In summary, compared with the time before 1993, MS will be much less likely to be a progressive disease, and quality of life will be much improved. In my opinion, patients will be less fearful about their prognosis than ever before, and with appropriate evaluations and treatments, we may realize that disabling MS will be far less common.

Suggested Reading
Brown JW, Pardini M, Brownlee WJ, et al. An abnormal periventricular magnetization transfer ratio gradient occurs early in multiple sclerosis. Brain. 2017;140(2):387-398.
Cadavid D, Wolansky LJ, Skurnick J, et al. Efficacy of treatment of MS with IFNbeta-1b or glatiramer acetate by monthly brain MRI in the BECOME study. Neurology. 2009;72(23):1976-1983.
Cook SD, Dhib-Jalbut S, Dowling P, et al. Use of magnetic resonance imaging as well as clinical disease activity in the clinical classification of multiple sclerosis and assessment of its course: a report from an international CMSC consensus conference, March 5-7, 2010. Int J MS Care. 2012;14(3):105-114.
Dowling PC, Bosch VV, Cook SD. Possible beneficial effect of high-dose intravenous steroid therapy in acute demyelinating disease and transverse myelitis. Neurology. 1980;30(7 Pt 2):33-36.
Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426.
Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364(9451):2106-2112.
Lincoln JA, Cadavid D, Pollard J, et al. We should use magnetic resonance imaging to classify and monitor the course of multiple sclerosis. Arch Neurol. 2009;66(3):412-414.
Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911.
Maranzano J, Rudko DA, Nakamura K, et al. MRI evidence of acute inflammation in leukocortical lesions of patients with early multiple sclerosis. Neurology. 2017;89(7):714-721.
McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127.
Miller HG, Gibbons JL. Acute disseminated encephalomyelitis and acute disseminated sclerosis; results of treatment with A.C.T.H. Br Med J. 1953;2(4850):1345-1348.
Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13(3):227-231.
Rose AS, Kuzma JW, Kurtzke JF, et al. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo--final report. Neurology. 1970;20(5):1-59.
Troiano R, Hafstein M, Ruderman M, et al. Effect of high-dose intravenous steroid administration on contrast-enhancing computed tomographic scan lesions in multiple sclerosis. Ann Neurol. 1984;15(3):257-263.
Troiano RA, Hafstein MP, Zito G, et al. The effect of oral corticosteroid dosage on CT enhancing multiple sclerosis plaques. J Neurol Sci. 1985;70(1):67-72.
Young IR, Hall AS, Pallis A, et al. Nuclear magnetic resonance imaging of the brain in multiple sclerosis. Lancet. 1981;2(8255):1063-1066.

