User login
MONTRÉAL—Results from a phase 3 trial suggest a mesenchymal stem cell (MSC) product can treat steroid-refractory, acute graft-versus-host disease (GVHD) in children.
The product, remestemcel-L (MSC-100-IV), produced an overall response rate of 69% at day 28, with complete resolution of GVHD in 29% of patients.
Adverse events (AEs) in this trial were consistent with the known safety profile of remestemcel-L.
Joanne Kurtzberg, MD, of Duke University Medical Center in Durham, North Carolina, presented these results at ISCT 2018.
The trial was sponsored by Mesoblast International Sàrl, the company developing remestemcel-L.
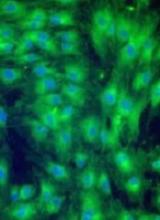
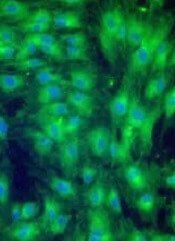
Remestemcel-L consists of human MSCs derived from donor bone marrow and expanded in culture.
Patients
The trial enrolled 55 patients who had acute GVHD and had failed to respond to steroid treatment. This was defined as progression within 3 days or no improvement within 7 days of consecutive treatment with at least 2 mg/kg/day of methylprednisolone or an equivalent product.
The patients had a median age of 7.6 years (range, 0.6 years to 17.9 years) at baseline, and 64% were male. Underlying diseases include acute myeloid leukemia (32.7%), acute lymphoblastic leukemia (21.8%), anemia (9.1%), chronic myeloid leukemia (7.3%), sickle cell disease (5.5%), juvenile myelomonocytic leukemia (3.6%), myelodysplastic syndromes (3.6%), and “other” disease (16.4%).
Most patients (87%) had received myeloablative conditioning, most (76%) had an unrelated donor, and roughly half (51%) received an HLA-mismatched transplant.
Fifty-five percent of patients received a bone marrow transplant, 25% received peripheral blood stem cells, and 20% received cord blood.
Forty-seven percent of patients had grade D GVHD at baseline, 42% had grade C, and 11% had grade B. Thirty-six percent of patients had multi-organ involvement (all with lower gastrointestinal), 38% had lower gastrointestinal involvement only, and 26% had skin involvement only.
Results
Fifty-four patients were treated with remestemcel-L. They received 8 injections over 4 weeks (twice weekly), consisting of 2 million cells per kg per injection.
The overall response rate at day 28 was 69%. Twenty-nine percent of patients achieved a complete response, defined as resolution of acute GVHD in all involved organs.
Forty percent of patients achieved a partial response, defined as organ-level improvement of at least one stage without worsening of any other organ.
All patients reported at least one treatment-emergent AE, and 61% had serious treatment-emergent AEs. The most common of these were infection (33%) and respiratory events (20%).
Four patients withdrew from the trial before day 100. One patient couldn’t receive treatment, 1 withdrew due to an AE (somnolence), 1 had parental consent withdrawn, and 1 was taken off study by the principal investigator.
There were 11 on-study deaths, but none were considered related to remestemcel-L. Eight deaths were due to infection, 1 due to GVHD progression, and 2 due to primary cancer relapse.
The day-100 survival analysis is pending.
MONTRÉAL—Results from a phase 3 trial suggest a mesenchymal stem cell (MSC) product can treat steroid-refractory, acute graft-versus-host disease (GVHD) in children.
The product, remestemcel-L (MSC-100-IV), produced an overall response rate of 69% at day 28, with complete resolution of GVHD in 29% of patients.
Adverse events (AEs) in this trial were consistent with the known safety profile of remestemcel-L.
Joanne Kurtzberg, MD, of Duke University Medical Center in Durham, North Carolina, presented these results at ISCT 2018.
The trial was sponsored by Mesoblast International Sàrl, the company developing remestemcel-L.
Remestemcel-L consists of human MSCs derived from donor bone marrow and expanded in culture.
Patients
The trial enrolled 55 patients who had acute GVHD and had failed to respond to steroid treatment. This was defined as progression within 3 days or no improvement within 7 days of consecutive treatment with at least 2 mg/kg/day of methylprednisolone or an equivalent product.
The patients had a median age of 7.6 years (range, 0.6 years to 17.9 years) at baseline, and 64% were male. Underlying diseases include acute myeloid leukemia (32.7%), acute lymphoblastic leukemia (21.8%), anemia (9.1%), chronic myeloid leukemia (7.3%), sickle cell disease (5.5%), juvenile myelomonocytic leukemia (3.6%), myelodysplastic syndromes (3.6%), and “other” disease (16.4%).
Most patients (87%) had received myeloablative conditioning, most (76%) had an unrelated donor, and roughly half (51%) received an HLA-mismatched transplant.
Fifty-five percent of patients received a bone marrow transplant, 25% received peripheral blood stem cells, and 20% received cord blood.
Forty-seven percent of patients had grade D GVHD at baseline, 42% had grade C, and 11% had grade B. Thirty-six percent of patients had multi-organ involvement (all with lower gastrointestinal), 38% had lower gastrointestinal involvement only, and 26% had skin involvement only.
Results
Fifty-four patients were treated with remestemcel-L. They received 8 injections over 4 weeks (twice weekly), consisting of 2 million cells per kg per injection.
The overall response rate at day 28 was 69%. Twenty-nine percent of patients achieved a complete response, defined as resolution of acute GVHD in all involved organs.
Forty percent of patients achieved a partial response, defined as organ-level improvement of at least one stage without worsening of any other organ.
All patients reported at least one treatment-emergent AE, and 61% had serious treatment-emergent AEs. The most common of these were infection (33%) and respiratory events (20%).
Four patients withdrew from the trial before day 100. One patient couldn’t receive treatment, 1 withdrew due to an AE (somnolence), 1 had parental consent withdrawn, and 1 was taken off study by the principal investigator.
There were 11 on-study deaths, but none were considered related to remestemcel-L. Eight deaths were due to infection, 1 due to GVHD progression, and 2 due to primary cancer relapse.
The day-100 survival analysis is pending.
MONTRÉAL—Results from a phase 3 trial suggest a mesenchymal stem cell (MSC) product can treat steroid-refractory, acute graft-versus-host disease (GVHD) in children.
The product, remestemcel-L (MSC-100-IV), produced an overall response rate of 69% at day 28, with complete resolution of GVHD in 29% of patients.
Adverse events (AEs) in this trial were consistent with the known safety profile of remestemcel-L.
Joanne Kurtzberg, MD, of Duke University Medical Center in Durham, North Carolina, presented these results at ISCT 2018.
The trial was sponsored by Mesoblast International Sàrl, the company developing remestemcel-L.
Remestemcel-L consists of human MSCs derived from donor bone marrow and expanded in culture.
Patients
The trial enrolled 55 patients who had acute GVHD and had failed to respond to steroid treatment. This was defined as progression within 3 days or no improvement within 7 days of consecutive treatment with at least 2 mg/kg/day of methylprednisolone or an equivalent product.
The patients had a median age of 7.6 years (range, 0.6 years to 17.9 years) at baseline, and 64% were male. Underlying diseases include acute myeloid leukemia (32.7%), acute lymphoblastic leukemia (21.8%), anemia (9.1%), chronic myeloid leukemia (7.3%), sickle cell disease (5.5%), juvenile myelomonocytic leukemia (3.6%), myelodysplastic syndromes (3.6%), and “other” disease (16.4%).
Most patients (87%) had received myeloablative conditioning, most (76%) had an unrelated donor, and roughly half (51%) received an HLA-mismatched transplant.
Fifty-five percent of patients received a bone marrow transplant, 25% received peripheral blood stem cells, and 20% received cord blood.
Forty-seven percent of patients had grade D GVHD at baseline, 42% had grade C, and 11% had grade B. Thirty-six percent of patients had multi-organ involvement (all with lower gastrointestinal), 38% had lower gastrointestinal involvement only, and 26% had skin involvement only.
Results
Fifty-four patients were treated with remestemcel-L. They received 8 injections over 4 weeks (twice weekly), consisting of 2 million cells per kg per injection.
The overall response rate at day 28 was 69%. Twenty-nine percent of patients achieved a complete response, defined as resolution of acute GVHD in all involved organs.
Forty percent of patients achieved a partial response, defined as organ-level improvement of at least one stage without worsening of any other organ.
All patients reported at least one treatment-emergent AE, and 61% had serious treatment-emergent AEs. The most common of these were infection (33%) and respiratory events (20%).
Four patients withdrew from the trial before day 100. One patient couldn’t receive treatment, 1 withdrew due to an AE (somnolence), 1 had parental consent withdrawn, and 1 was taken off study by the principal investigator.
There were 11 on-study deaths, but none were considered related to remestemcel-L. Eight deaths were due to infection, 1 due to GVHD progression, and 2 due to primary cancer relapse.
The day-100 survival analysis is pending.