User login
Endometriosis affects approximately 11% of women; the disease can be categorized as pelvic endometriosis and extrapelvic endometriosis, based on anatomic presentation. It is estimated that about 12% of extrapelvic disease involves the diaphragm or thoracic cavity.
While diaphragmatic endometriosis often is asymptomatic, patients who are symptomatic can experience progressive and incapacitating pain. because of a traditional focus on the lower pelvic region. Some cases are misdiagnosed as other conditions involving the gastrointestinal tract or of cardiothoracic origin, because of the propensity of diaphragmatic disease to occur posteriorly and hide behind the liver. The variable appearance of endometriotic lesions and the lack of reliable diagnostic or imaging tests also can contribute to delayed diagnosis.
Symptoms usually occur cyclically with the onset of menses, but sometimes are unrelated to menses. Most diaphragmatic lesions occur on the abdominal side and right hemidiaphragm, which may offer evidence for the theory that retrograde menstruation drives the development of endometriosis because of the clockwise flow of peritoneal fluid. However, lesions have been found on all parts of the diaphragm, including the left side only, the thoracic and visceral sides of the diaphragm, and the phrenic nerve. There is no correlation between the size/number of lesions and either pneumothorax or hemothorax, nor pain.
The best diagnostic method is thorough surveillance intraoperatively. In our practice, we routinely inspect the diaphragm for endometriosis at the time of video laparoscopy.
In women who have symptoms, it is important to ensure the best exposure of the diaphragm by properly considering the patient’s positioning and port placement, and by using an atraumatic liver retractor or grasping forceps to gently push the liver down and away from the visual/operative field. Posterior diaphragm viewing can also be enhanced by utilizing a 30-degree laparoscope angled toward the back. At times, it is helpful to cut the falciform ligament near the liver to expose the right side of the diaphragm completely while the patient is in steep reverse Trendelenburg position.
Most lesions in symptomatic patients can be successfully removed with hydrodissection and vaporization or excision. For asymptomatic patients with an incidental finding of diaphragmatic endometriosis, the suggestion is not to treat lesions in order to avoid the potential risk of injury to the diaphragm, phrenic nerve, lungs, or heart – especially when an adequate multidisciplinary team is not available.
Pathophysiology
In addition to retrograde menstruation, there are two other common theories regarding the pathophysiology of thoracic endometriosis. First, high prostaglandin F2-alpha at ovulation may result in vasospasm and ischemia of the lungs (resulting, in turn, in alveolar rupture and subsequent pneumothorax). Second, the loss of a mucus plug during menses may result in communication between the environment and peritoneal cavity.
What is clear is that patients who have symptoms consistent with pelvic endometriosis and chest complaints should be evaluated for both diaphragmatic and pelvic endometriosis. It’s also increasing clear that a multidisciplinary approach utilizing combined laparoscopy and thoracoscopy is a safe and effective method for addressing pelvic, diaphragmatic, and other thoracic endometriosis when other treatments have failed.
A multidisciplinary approach
Since the introduction of video laparoscopy and ease of evaluation of the upper abdomen, more extrapelvic endometriosis – including disease in the upper abdomen and diaphragm – is being diagnosed. The thoracic and visceral diaphragm are the most commonly described sites of thoracic endometriosis, and disease is often right sided, with parenchymal involvement less commonly reported.
Abdominopelvic and visceral diaphragmatic endometriosis are treated endoscopically with hydrodissection followed by excision or ablation. Superficial lesions away from the central diaphragm can be coagulated using bipolar current.
Thoracoscopic treatment varies, involving ablation or excision of smaller diaphragmatic lesions, pulmonary wedge resection of deep parenchymal nodules (using a stapling device), diaphragm resection of deep diaphragmatic lesions using a stapling device, or by excision and manual suturing.
Endoscopic diagnosis and treatment begins by introducing a 10-mm port at the umbilicus and placing three additional ports in the upper quadrant (right or left, depending on implant location). The arrangement (similar to that of a laparoscopic cholecystectomy or splenectomy) allows for examination of the posterior portion of the right hemidiaphragm and almost the entire left hemidiaphragm in addition to routine abdominopelvic exploration.
For better laparoscopic visualization, the patient is repositioned in steep reverse Trendelenburg, and the liver is gently pushed caudally to view the adjacent diaphragm. The upper abdominal walls and the liver also may be evaluated while in this position.
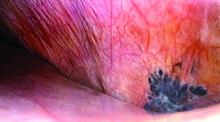
Bluish pigmented lesions are the most commonly reported form of diaphragmatic endometriosis, followed by lesions with a reddish-purple appearance. However, lesions can present with various colors and morphologic appearances, such as fibrotic white lesions or adhesions to the liver.
In our practice, we recommend using the CO2 laser (set at 20-25 watts) with hydrodissection for superficial lesions. The CO2 laser is much more precise and has a smaller depth of penetration and less thermal spread, compared with electrocautery. The CO2 laser beam also reaches otherwise hard-to-access areas behind the liver and has proven to be safe for vaporizing and/or excising many types of diaphragmatic lesions. We have successfully treated diaphragmatic endometriosis in the vicinity of the phrenic nerve and directly in line with the left ventricle.
Watch a video from Dr. Ceana Nezhat demonstrating a step wise vaporization and excision of diaphragmatic endometriosis utilizing different techniques.
(Courtesy Dr. Ceana Nezhat)
Plasma jet energy and ultrasonic energy are good alternatives when a CO2 laser is not available and are preferable to the use of cold scissors because of subsequent bleeding, which requires bipolar hemostasis.
Monopolar electrocautery is not as good a choice for treating diaphragmatic endometriosis because of higher depth of penetration, which may cause tissue necrosis and subsequent delayed diaphragmatic fenestrations. It also may cause unpredictable diaphragmatic muscular contractions and electrical conduction transmitted to the heart, inducing arrhythmia.
For patients treated via combined VALS and VATS procedures, endometriotic lesions involving the entire thickness of the diaphragm should be completely resected, and the defect can be repaired with either sutures or staples.
In all cases, special anesthesia considerations must be made given the inability to completely ventilate the lung. In our practice, we use a double-lumen endotracheal tube for single lung ventilation, if needed. A bronchial blocker is used to isolate the lung when the double-lumen endotracheal tube cannot be inserted.
It is important to note that we do not recommend VATS with VALS in all suspicious cases. We reserve VATS only for patients with catamenial pneumothorax, catamenial hemothorax, hemoptysis, and pulmonary nodules, defined as Thoracic Endometriosis Syndrome. We usually start with medical management first, then proceed to VALS, and finally, VATS, with the intention to treat if the patient fails nonsurgical treatments. It is better to avoid VATS, if possible, because it is associated with longer recovery and more pain; it should be done if all else fails.
If the patient has completed childbearing or passed reproductive age, bilateral salpingectomy, or hysterectomy with or without bilateral salpingo-oophorectomy, may be considered as the first step prior to more aggressive excisional procedures. This is especially true for widespread lesions, as branches of the phrenic nerve are difficult to see and injury could result in paralysis of the diaphragm. It’s important to appreciate that if estrogen stimulation to the diaphragmatic lesions is to cease for the long term, hormonal suppression or surgical treatment including bilateral oophorectomy should be utilized.
My colleagues and I have reported on our experience with a multidisciplinary approach in the treatment of diaphragmatic endometriosis in 25 patients. All had both pelvic and thoracic symptoms, and the majority had endometrial implants on both the thoracic and visceral sides of the diaphragm.
There were two postoperative complications: a diaphragmatic hernia and a vaginal cuff hematoma. Over a follow-up period of 3-18 months, all 25 patients had significant improvement or resolution of their chest complaints, and most remained asymptomatic for more than 6 months (JSLS. 2014 Jul-Sep;18[3]. pii: e2014.00312. doi: 10.4293/JSLS.2014.00312).
Dr. Ceana Nezhat is the fellowship director of Nezhat Medical Center, the medical director of training and education at Northside Hospital, and an adjunct clinical professor of gynecology and obstetrics at Emory University, all in Atlanta. He is president of SRS (Society of Reproductive Surgeons) and past president of AAGL (American Association of Gynecologic Laparoscopists). Dr. Nezhat is a consultant for Novuson Surgical, Karl Storz Endoscopy, Lumenis, and AbbVie; a medical advisor for Plasma Surgical, and a member of the scientific advisory board for SurgiQuest.
Suggested readings
1. Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operative Gynecologic Laparoscopy with Hysteroscopy. Fourth Edition. Cambridge University Press. 2013.
2. Am J Med. 1996 Feb;100(2):164-70.
3. Fertil Steril. 1998 Jun;69(6):1048-55.
4. Clin Obstet Gynecol. 1999 Sep;42(3):699-711.
5. JSLS. 2012 Jan-Mar; 16(1):140-2.
Endometriosis affects approximately 11% of women; the disease can be categorized as pelvic endometriosis and extrapelvic endometriosis, based on anatomic presentation. It is estimated that about 12% of extrapelvic disease involves the diaphragm or thoracic cavity.
While diaphragmatic endometriosis often is asymptomatic, patients who are symptomatic can experience progressive and incapacitating pain. because of a traditional focus on the lower pelvic region. Some cases are misdiagnosed as other conditions involving the gastrointestinal tract or of cardiothoracic origin, because of the propensity of diaphragmatic disease to occur posteriorly and hide behind the liver. The variable appearance of endometriotic lesions and the lack of reliable diagnostic or imaging tests also can contribute to delayed diagnosis.
Symptoms usually occur cyclically with the onset of menses, but sometimes are unrelated to menses. Most diaphragmatic lesions occur on the abdominal side and right hemidiaphragm, which may offer evidence for the theory that retrograde menstruation drives the development of endometriosis because of the clockwise flow of peritoneal fluid. However, lesions have been found on all parts of the diaphragm, including the left side only, the thoracic and visceral sides of the diaphragm, and the phrenic nerve. There is no correlation between the size/number of lesions and either pneumothorax or hemothorax, nor pain.
The best diagnostic method is thorough surveillance intraoperatively. In our practice, we routinely inspect the diaphragm for endometriosis at the time of video laparoscopy.
In women who have symptoms, it is important to ensure the best exposure of the diaphragm by properly considering the patient’s positioning and port placement, and by using an atraumatic liver retractor or grasping forceps to gently push the liver down and away from the visual/operative field. Posterior diaphragm viewing can also be enhanced by utilizing a 30-degree laparoscope angled toward the back. At times, it is helpful to cut the falciform ligament near the liver to expose the right side of the diaphragm completely while the patient is in steep reverse Trendelenburg position.
Most lesions in symptomatic patients can be successfully removed with hydrodissection and vaporization or excision. For asymptomatic patients with an incidental finding of diaphragmatic endometriosis, the suggestion is not to treat lesions in order to avoid the potential risk of injury to the diaphragm, phrenic nerve, lungs, or heart – especially when an adequate multidisciplinary team is not available.
Pathophysiology
In addition to retrograde menstruation, there are two other common theories regarding the pathophysiology of thoracic endometriosis. First, high prostaglandin F2-alpha at ovulation may result in vasospasm and ischemia of the lungs (resulting, in turn, in alveolar rupture and subsequent pneumothorax). Second, the loss of a mucus plug during menses may result in communication between the environment and peritoneal cavity.
What is clear is that patients who have symptoms consistent with pelvic endometriosis and chest complaints should be evaluated for both diaphragmatic and pelvic endometriosis. It’s also increasing clear that a multidisciplinary approach utilizing combined laparoscopy and thoracoscopy is a safe and effective method for addressing pelvic, diaphragmatic, and other thoracic endometriosis when other treatments have failed.
A multidisciplinary approach
Since the introduction of video laparoscopy and ease of evaluation of the upper abdomen, more extrapelvic endometriosis – including disease in the upper abdomen and diaphragm – is being diagnosed. The thoracic and visceral diaphragm are the most commonly described sites of thoracic endometriosis, and disease is often right sided, with parenchymal involvement less commonly reported.
Abdominopelvic and visceral diaphragmatic endometriosis are treated endoscopically with hydrodissection followed by excision or ablation. Superficial lesions away from the central diaphragm can be coagulated using bipolar current.
Thoracoscopic treatment varies, involving ablation or excision of smaller diaphragmatic lesions, pulmonary wedge resection of deep parenchymal nodules (using a stapling device), diaphragm resection of deep diaphragmatic lesions using a stapling device, or by excision and manual suturing.
Endoscopic diagnosis and treatment begins by introducing a 10-mm port at the umbilicus and placing three additional ports in the upper quadrant (right or left, depending on implant location). The arrangement (similar to that of a laparoscopic cholecystectomy or splenectomy) allows for examination of the posterior portion of the right hemidiaphragm and almost the entire left hemidiaphragm in addition to routine abdominopelvic exploration.
For better laparoscopic visualization, the patient is repositioned in steep reverse Trendelenburg, and the liver is gently pushed caudally to view the adjacent diaphragm. The upper abdominal walls and the liver also may be evaluated while in this position.
Bluish pigmented lesions are the most commonly reported form of diaphragmatic endometriosis, followed by lesions with a reddish-purple appearance. However, lesions can present with various colors and morphologic appearances, such as fibrotic white lesions or adhesions to the liver.
In our practice, we recommend using the CO2 laser (set at 20-25 watts) with hydrodissection for superficial lesions. The CO2 laser is much more precise and has a smaller depth of penetration and less thermal spread, compared with electrocautery. The CO2 laser beam also reaches otherwise hard-to-access areas behind the liver and has proven to be safe for vaporizing and/or excising many types of diaphragmatic lesions. We have successfully treated diaphragmatic endometriosis in the vicinity of the phrenic nerve and directly in line with the left ventricle.
Watch a video from Dr. Ceana Nezhat demonstrating a step wise vaporization and excision of diaphragmatic endometriosis utilizing different techniques.
(Courtesy Dr. Ceana Nezhat)
Plasma jet energy and ultrasonic energy are good alternatives when a CO2 laser is not available and are preferable to the use of cold scissors because of subsequent bleeding, which requires bipolar hemostasis.
Monopolar electrocautery is not as good a choice for treating diaphragmatic endometriosis because of higher depth of penetration, which may cause tissue necrosis and subsequent delayed diaphragmatic fenestrations. It also may cause unpredictable diaphragmatic muscular contractions and electrical conduction transmitted to the heart, inducing arrhythmia.
For patients treated via combined VALS and VATS procedures, endometriotic lesions involving the entire thickness of the diaphragm should be completely resected, and the defect can be repaired with either sutures or staples.
In all cases, special anesthesia considerations must be made given the inability to completely ventilate the lung. In our practice, we use a double-lumen endotracheal tube for single lung ventilation, if needed. A bronchial blocker is used to isolate the lung when the double-lumen endotracheal tube cannot be inserted.
It is important to note that we do not recommend VATS with VALS in all suspicious cases. We reserve VATS only for patients with catamenial pneumothorax, catamenial hemothorax, hemoptysis, and pulmonary nodules, defined as Thoracic Endometriosis Syndrome. We usually start with medical management first, then proceed to VALS, and finally, VATS, with the intention to treat if the patient fails nonsurgical treatments. It is better to avoid VATS, if possible, because it is associated with longer recovery and more pain; it should be done if all else fails.
If the patient has completed childbearing or passed reproductive age, bilateral salpingectomy, or hysterectomy with or without bilateral salpingo-oophorectomy, may be considered as the first step prior to more aggressive excisional procedures. This is especially true for widespread lesions, as branches of the phrenic nerve are difficult to see and injury could result in paralysis of the diaphragm. It’s important to appreciate that if estrogen stimulation to the diaphragmatic lesions is to cease for the long term, hormonal suppression or surgical treatment including bilateral oophorectomy should be utilized.
My colleagues and I have reported on our experience with a multidisciplinary approach in the treatment of diaphragmatic endometriosis in 25 patients. All had both pelvic and thoracic symptoms, and the majority had endometrial implants on both the thoracic and visceral sides of the diaphragm.
There were two postoperative complications: a diaphragmatic hernia and a vaginal cuff hematoma. Over a follow-up period of 3-18 months, all 25 patients had significant improvement or resolution of their chest complaints, and most remained asymptomatic for more than 6 months (JSLS. 2014 Jul-Sep;18[3]. pii: e2014.00312. doi: 10.4293/JSLS.2014.00312).
Dr. Ceana Nezhat is the fellowship director of Nezhat Medical Center, the medical director of training and education at Northside Hospital, and an adjunct clinical professor of gynecology and obstetrics at Emory University, all in Atlanta. He is president of SRS (Society of Reproductive Surgeons) and past president of AAGL (American Association of Gynecologic Laparoscopists). Dr. Nezhat is a consultant for Novuson Surgical, Karl Storz Endoscopy, Lumenis, and AbbVie; a medical advisor for Plasma Surgical, and a member of the scientific advisory board for SurgiQuest.
Suggested readings
1. Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operative Gynecologic Laparoscopy with Hysteroscopy. Fourth Edition. Cambridge University Press. 2013.
2. Am J Med. 1996 Feb;100(2):164-70.
3. Fertil Steril. 1998 Jun;69(6):1048-55.
4. Clin Obstet Gynecol. 1999 Sep;42(3):699-711.
5. JSLS. 2012 Jan-Mar; 16(1):140-2.
Endometriosis affects approximately 11% of women; the disease can be categorized as pelvic endometriosis and extrapelvic endometriosis, based on anatomic presentation. It is estimated that about 12% of extrapelvic disease involves the diaphragm or thoracic cavity.
While diaphragmatic endometriosis often is asymptomatic, patients who are symptomatic can experience progressive and incapacitating pain. because of a traditional focus on the lower pelvic region. Some cases are misdiagnosed as other conditions involving the gastrointestinal tract or of cardiothoracic origin, because of the propensity of diaphragmatic disease to occur posteriorly and hide behind the liver. The variable appearance of endometriotic lesions and the lack of reliable diagnostic or imaging tests also can contribute to delayed diagnosis.
Symptoms usually occur cyclically with the onset of menses, but sometimes are unrelated to menses. Most diaphragmatic lesions occur on the abdominal side and right hemidiaphragm, which may offer evidence for the theory that retrograde menstruation drives the development of endometriosis because of the clockwise flow of peritoneal fluid. However, lesions have been found on all parts of the diaphragm, including the left side only, the thoracic and visceral sides of the diaphragm, and the phrenic nerve. There is no correlation between the size/number of lesions and either pneumothorax or hemothorax, nor pain.
The best diagnostic method is thorough surveillance intraoperatively. In our practice, we routinely inspect the diaphragm for endometriosis at the time of video laparoscopy.
In women who have symptoms, it is important to ensure the best exposure of the diaphragm by properly considering the patient’s positioning and port placement, and by using an atraumatic liver retractor or grasping forceps to gently push the liver down and away from the visual/operative field. Posterior diaphragm viewing can also be enhanced by utilizing a 30-degree laparoscope angled toward the back. At times, it is helpful to cut the falciform ligament near the liver to expose the right side of the diaphragm completely while the patient is in steep reverse Trendelenburg position.
Most lesions in symptomatic patients can be successfully removed with hydrodissection and vaporization or excision. For asymptomatic patients with an incidental finding of diaphragmatic endometriosis, the suggestion is not to treat lesions in order to avoid the potential risk of injury to the diaphragm, phrenic nerve, lungs, or heart – especially when an adequate multidisciplinary team is not available.
Pathophysiology
In addition to retrograde menstruation, there are two other common theories regarding the pathophysiology of thoracic endometriosis. First, high prostaglandin F2-alpha at ovulation may result in vasospasm and ischemia of the lungs (resulting, in turn, in alveolar rupture and subsequent pneumothorax). Second, the loss of a mucus plug during menses may result in communication between the environment and peritoneal cavity.
What is clear is that patients who have symptoms consistent with pelvic endometriosis and chest complaints should be evaluated for both diaphragmatic and pelvic endometriosis. It’s also increasing clear that a multidisciplinary approach utilizing combined laparoscopy and thoracoscopy is a safe and effective method for addressing pelvic, diaphragmatic, and other thoracic endometriosis when other treatments have failed.
A multidisciplinary approach
Since the introduction of video laparoscopy and ease of evaluation of the upper abdomen, more extrapelvic endometriosis – including disease in the upper abdomen and diaphragm – is being diagnosed. The thoracic and visceral diaphragm are the most commonly described sites of thoracic endometriosis, and disease is often right sided, with parenchymal involvement less commonly reported.
Abdominopelvic and visceral diaphragmatic endometriosis are treated endoscopically with hydrodissection followed by excision or ablation. Superficial lesions away from the central diaphragm can be coagulated using bipolar current.
Thoracoscopic treatment varies, involving ablation or excision of smaller diaphragmatic lesions, pulmonary wedge resection of deep parenchymal nodules (using a stapling device), diaphragm resection of deep diaphragmatic lesions using a stapling device, or by excision and manual suturing.
Endoscopic diagnosis and treatment begins by introducing a 10-mm port at the umbilicus and placing three additional ports in the upper quadrant (right or left, depending on implant location). The arrangement (similar to that of a laparoscopic cholecystectomy or splenectomy) allows for examination of the posterior portion of the right hemidiaphragm and almost the entire left hemidiaphragm in addition to routine abdominopelvic exploration.
For better laparoscopic visualization, the patient is repositioned in steep reverse Trendelenburg, and the liver is gently pushed caudally to view the adjacent diaphragm. The upper abdominal walls and the liver also may be evaluated while in this position.
Bluish pigmented lesions are the most commonly reported form of diaphragmatic endometriosis, followed by lesions with a reddish-purple appearance. However, lesions can present with various colors and morphologic appearances, such as fibrotic white lesions or adhesions to the liver.
In our practice, we recommend using the CO2 laser (set at 20-25 watts) with hydrodissection for superficial lesions. The CO2 laser is much more precise and has a smaller depth of penetration and less thermal spread, compared with electrocautery. The CO2 laser beam also reaches otherwise hard-to-access areas behind the liver and has proven to be safe for vaporizing and/or excising many types of diaphragmatic lesions. We have successfully treated diaphragmatic endometriosis in the vicinity of the phrenic nerve and directly in line with the left ventricle.
Watch a video from Dr. Ceana Nezhat demonstrating a step wise vaporization and excision of diaphragmatic endometriosis utilizing different techniques.
(Courtesy Dr. Ceana Nezhat)
Plasma jet energy and ultrasonic energy are good alternatives when a CO2 laser is not available and are preferable to the use of cold scissors because of subsequent bleeding, which requires bipolar hemostasis.
Monopolar electrocautery is not as good a choice for treating diaphragmatic endometriosis because of higher depth of penetration, which may cause tissue necrosis and subsequent delayed diaphragmatic fenestrations. It also may cause unpredictable diaphragmatic muscular contractions and electrical conduction transmitted to the heart, inducing arrhythmia.
For patients treated via combined VALS and VATS procedures, endometriotic lesions involving the entire thickness of the diaphragm should be completely resected, and the defect can be repaired with either sutures or staples.
In all cases, special anesthesia considerations must be made given the inability to completely ventilate the lung. In our practice, we use a double-lumen endotracheal tube for single lung ventilation, if needed. A bronchial blocker is used to isolate the lung when the double-lumen endotracheal tube cannot be inserted.
It is important to note that we do not recommend VATS with VALS in all suspicious cases. We reserve VATS only for patients with catamenial pneumothorax, catamenial hemothorax, hemoptysis, and pulmonary nodules, defined as Thoracic Endometriosis Syndrome. We usually start with medical management first, then proceed to VALS, and finally, VATS, with the intention to treat if the patient fails nonsurgical treatments. It is better to avoid VATS, if possible, because it is associated with longer recovery and more pain; it should be done if all else fails.
If the patient has completed childbearing or passed reproductive age, bilateral salpingectomy, or hysterectomy with or without bilateral salpingo-oophorectomy, may be considered as the first step prior to more aggressive excisional procedures. This is especially true for widespread lesions, as branches of the phrenic nerve are difficult to see and injury could result in paralysis of the diaphragm. It’s important to appreciate that if estrogen stimulation to the diaphragmatic lesions is to cease for the long term, hormonal suppression or surgical treatment including bilateral oophorectomy should be utilized.
My colleagues and I have reported on our experience with a multidisciplinary approach in the treatment of diaphragmatic endometriosis in 25 patients. All had both pelvic and thoracic symptoms, and the majority had endometrial implants on both the thoracic and visceral sides of the diaphragm.
There were two postoperative complications: a diaphragmatic hernia and a vaginal cuff hematoma. Over a follow-up period of 3-18 months, all 25 patients had significant improvement or resolution of their chest complaints, and most remained asymptomatic for more than 6 months (JSLS. 2014 Jul-Sep;18[3]. pii: e2014.00312. doi: 10.4293/JSLS.2014.00312).
Dr. Ceana Nezhat is the fellowship director of Nezhat Medical Center, the medical director of training and education at Northside Hospital, and an adjunct clinical professor of gynecology and obstetrics at Emory University, all in Atlanta. He is president of SRS (Society of Reproductive Surgeons) and past president of AAGL (American Association of Gynecologic Laparoscopists). Dr. Nezhat is a consultant for Novuson Surgical, Karl Storz Endoscopy, Lumenis, and AbbVie; a medical advisor for Plasma Surgical, and a member of the scientific advisory board for SurgiQuest.
Suggested readings
1. Nezhat C, Nezhat F, Nezhat C. Nezhat’s Operative Gynecologic Laparoscopy with Hysteroscopy. Fourth Edition. Cambridge University Press. 2013.
2. Am J Med. 1996 Feb;100(2):164-70.
3. Fertil Steril. 1998 Jun;69(6):1048-55.
4. Clin Obstet Gynecol. 1999 Sep;42(3):699-711.
5. JSLS. 2012 Jan-Mar; 16(1):140-2.