User login
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
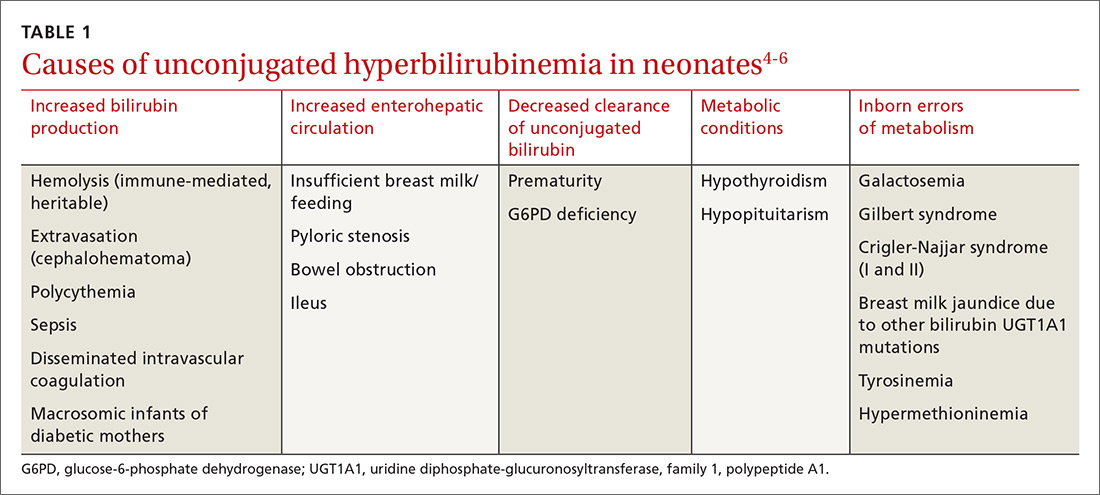
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.
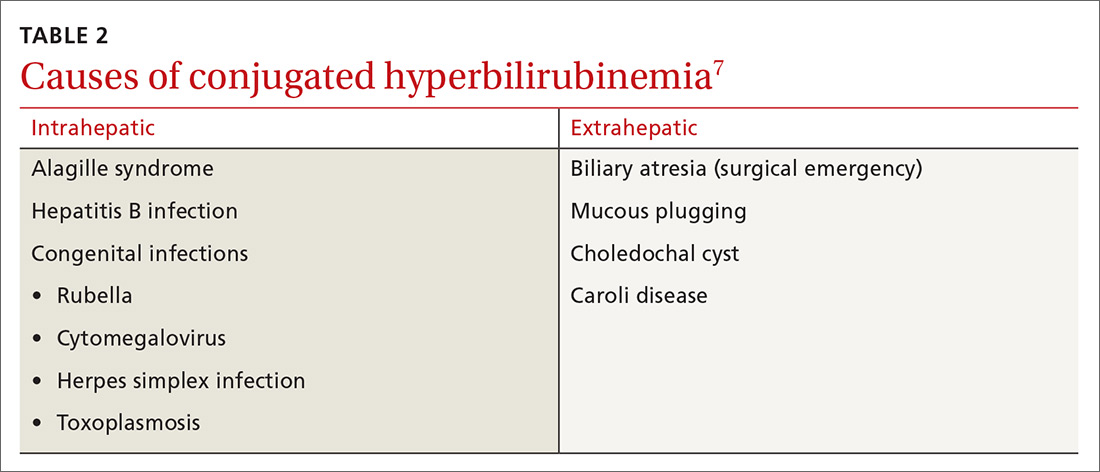
Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8
Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
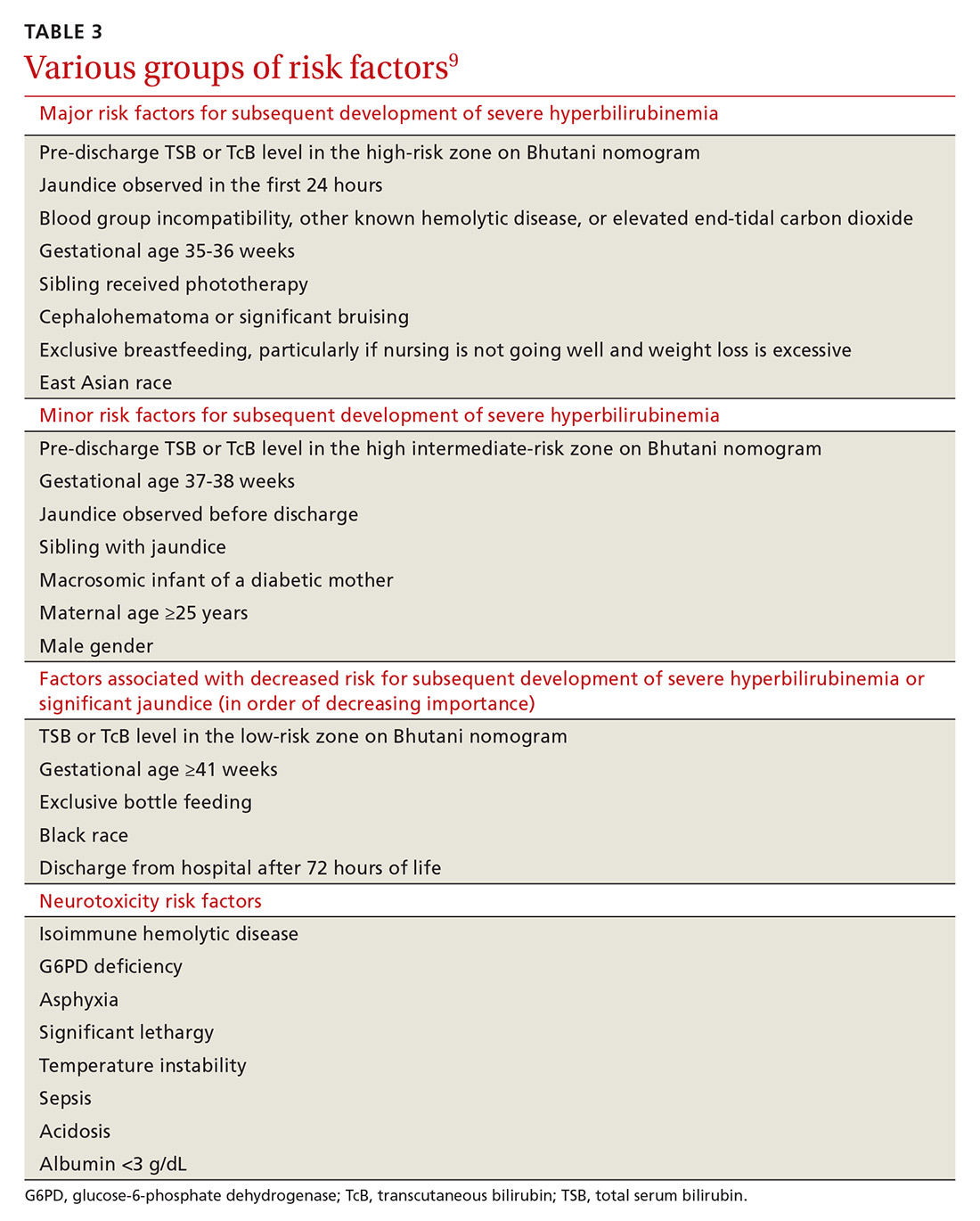
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9
Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; [email protected].
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.

Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8

Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9

Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; [email protected].
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.

Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8

Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9

Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; [email protected].
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
PRACTICE RECOMMENDATIONS
› Diagnose hyperbilirubinemia in infants with bilirubin measured at >95th percentile for age in hours. Do not use visual assessment of jaundice for diagnosis as it may lead to errors. C
› Determine the threshold for initiation of phototherapy by applying serum bilirubin and age in hours to the American Academy of Pediatrics phototherapy nomogram along a risk curve assigned based on gestational age and neurotoxicity risk factors (not major and minor risk factors for severe hyperbilirubinemia). C
› Make arrangements to ensure that all infants are seen by a health care provider within 2 days of discharge (within 1 day if significant risk factors for development of severe hyperbilirubinemia are present). C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
