User login
How to minimize the pain of local anesthetic administration
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
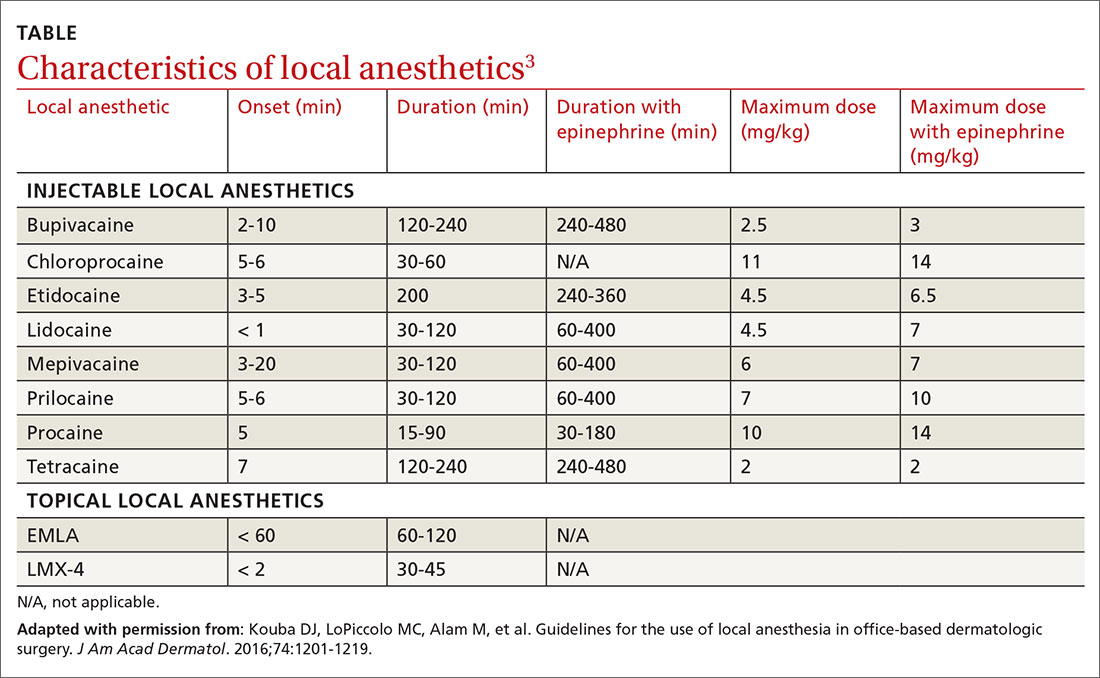
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.
Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
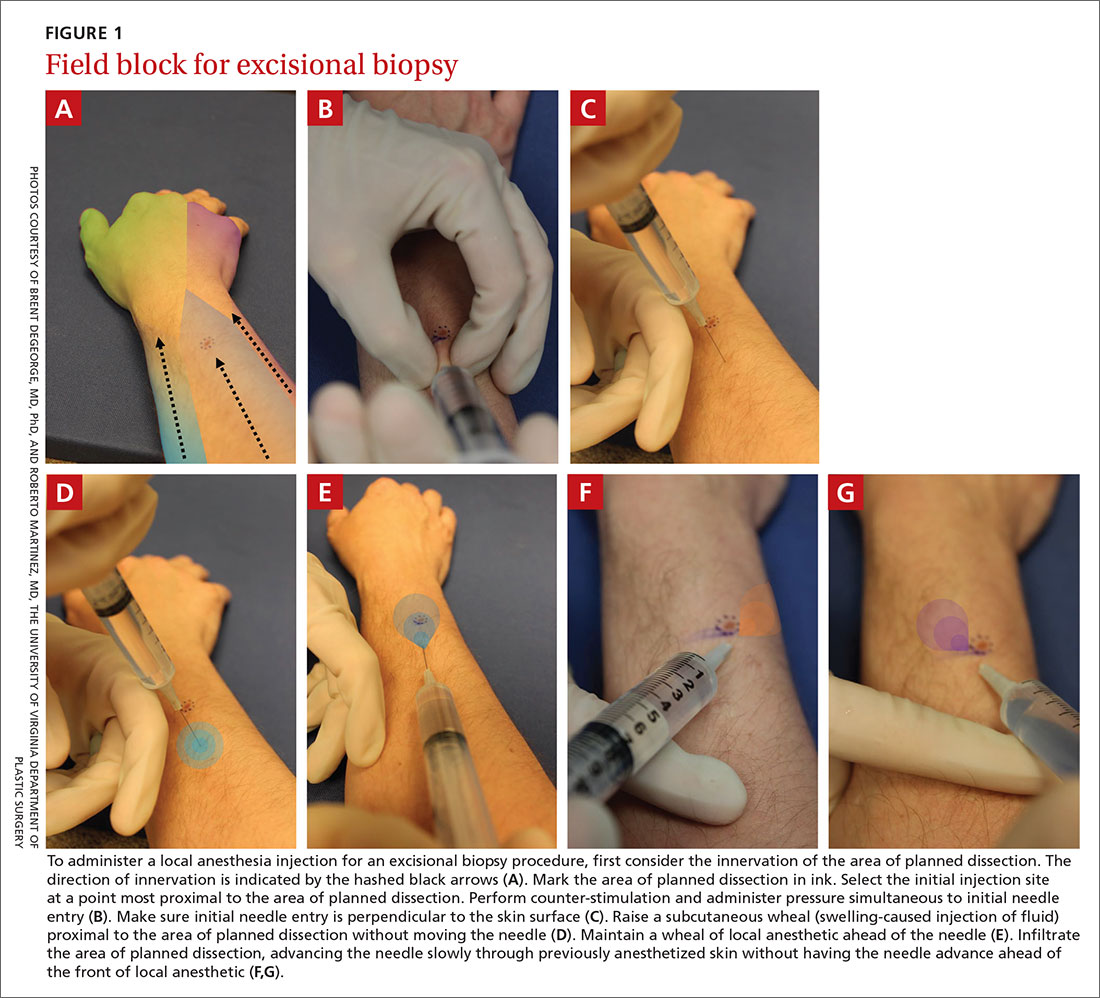
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42
Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
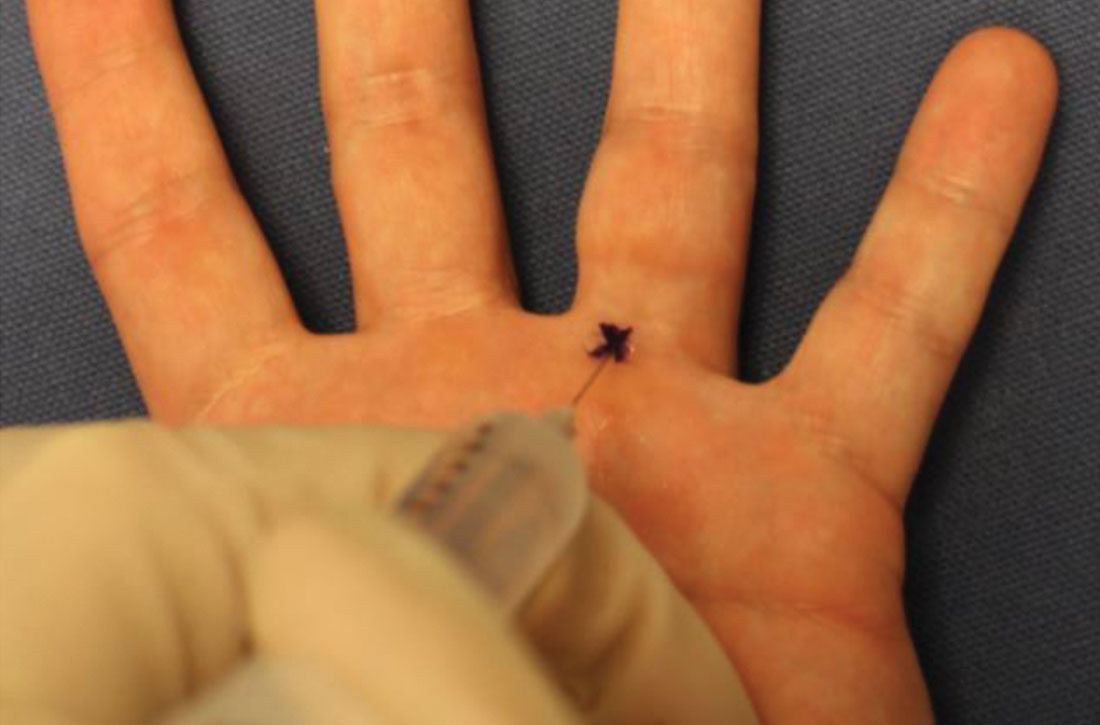
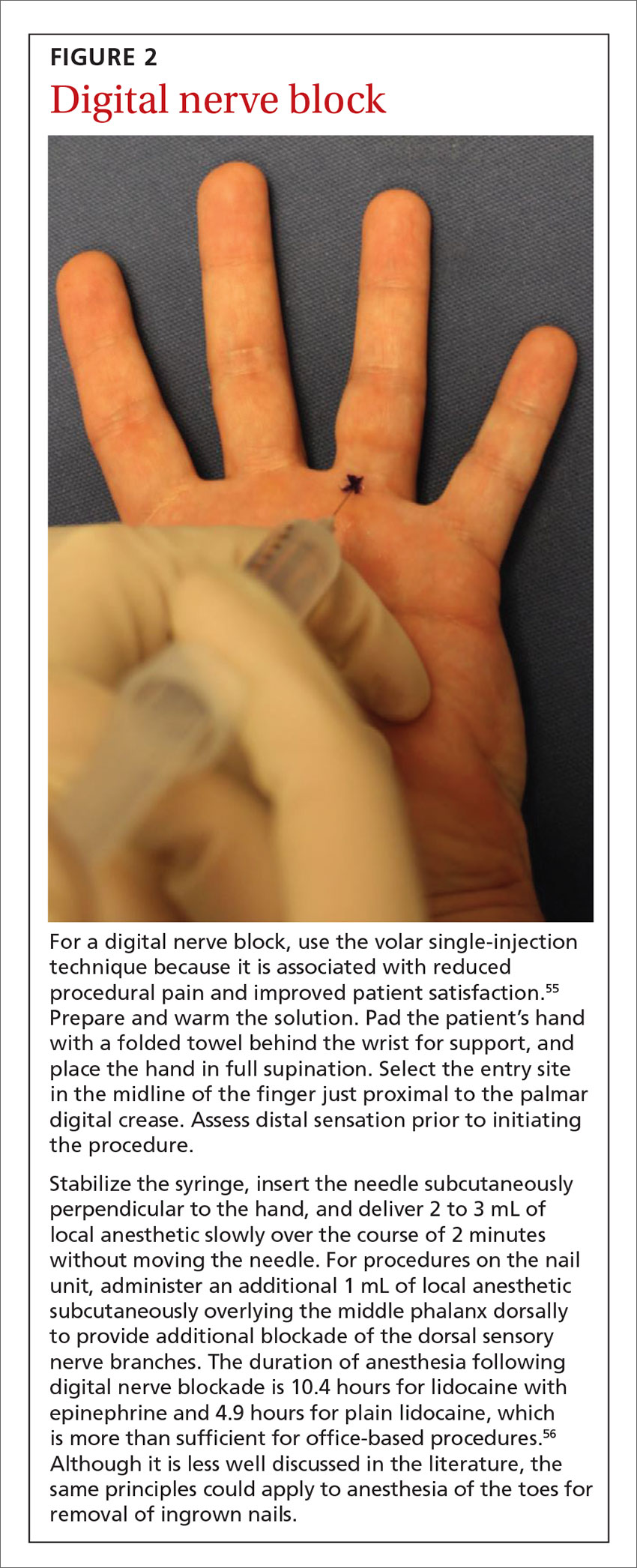
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.

Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42

Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.

CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
In-office procedures are increasingly emphasized as a way to reduce referrals, avoid treatment delay, and increase practice revenue. Local analgesia is administered before many in-office procedures such as biopsies, toenail removal, and laceration repair. Skin procedures are performed most commonly; nearly three-quarters (74%) of family physicians (FPs) provided these services in 2018.1 Administration of local anesthetic is often the most feared and uncomfortable step in the entire process.2
Knowledge of strategies to reduce pain associated with anesthetic administration can make a huge difference in the patient experience. This article explores evidence-based techniques for administering a local anesthetic with minimal patient discomfort.
4 factors influence the painof local anesthetic administration
Pain is perceived during the administration of local anesthetic because of the insertion of the needle and the increased pressure from the injection of fluid. The needle causes sharp, pricking “first pain” via large diameter, myelinated A-delta fibers, and the fluid induces unmyelinated C-fiber activation via tissue distention resulting in dull, diffuse “second pain.”
Four factors influence the experience of pain during administration of local anesthetic: the pharmacologic properties of the anesthetic itself, the equipment used, the environment, and the injection technique. Optimizing all 4 factors limits patient discomfort.
Pharmacologic agents: Lidocaine is often the agent of choice
Local anesthetics differ in maximal dosing, onset of action, and duration of effect (TABLE3). Given its ubiquity in clinics and hospitals, 1% lidocaine is often the agent of choice. Onset of effect occurs within minutes and lasts up to 2 hours. Alternative agents, such as bupivacaine or ropivacaine, may be considered to prolong the anesthetic effect; however, limited evidence exists to support their use in office-based procedures. Additionally, bupivacaine and ropivacaine may be associated with greater pain on injection and parasthesias lasting longer than the duration of pain control.4-6 In practice, maximal dosing is most important in the pediatric population, given the smaller size of the patients and their increased susceptibility to toxicity.

Calculating the maximum recommended dose. To calculate the maximum recommended dose of local anesthetic, you need to know the concentration of the anesthetic, the maximum allowable dose (mg/kg), and the weight of the patient.7,8 The concentration of the local anesthetic is converted from percentage to weight per unit volume (eg, 1% = 10 mg/mL; 0.5% = 5 mg/mL). Multiply the patient's weight (kg) by the maximum dose of local anesthetic (mg/kg) and divide by the concentration of the local anesthetic (mg/mL) to get the maximum recommended dose in milliliters. Walsh et al9 described a simplified formula to calculate the maximum allowable volume of local anesthetics in milliliters:
(maximum allowable dose in mg/kg) × (weight in kg) × (1 divided by the concentration of anesthetic).
For delivery of lidocaine with epinephrine in a 50-lb (22.7-kg) child, the calculation would be (7 mg/kg) × (22.7 kg) × (1 divided by 10 mg/mL) = 15.9 mL.
Continue to: The advantages (and misconceptions) of epinephrine
The advantages (and misconceptions) of epinephrine
The advantage of adding epinephrine is that it prolongs the effect of the anesthesia and it decreases bleeding. Epinephrine is commonly available as a premixed solution with lidocaine or bupivacaine at a concentration of 1:100,000 and is generally differentiated from “plain” local anesthetic by a red label and cap. Although maximum vasoconstriction may occur as long as 30 minutes after injection,10 adequate vasoconstriction is achieved in 7 to 10 minutes for excision of skin lesions.11
Traditional teaching recommends against using epinephrine in the “fingers, toes, penis, ears, or nose” because of potential arterial spasm, ischemia, and gangrene distal to the injection site.12 These concerns were based on experiences with procaine and cocaine mixed with epinephrine. Studies suffered from multiple confounders, including tourniquets and nonstandardized epinephrine concentrations.13-15
No association of distal ischemia with epinephrine use was identified in a recent Cochrane Review or in another multicenter prospective study.16,17 Phentolamine, a non-selective alpha-adrenergic receptor antagonist and vasodilator, can be administered to reverse vasoconstriction following inadvertent administration of high-dose epinephrine (1:1000) via anaphylaxis autoinjector kits.
Dosing of phentolamine is 1 mL of 1 mg/mL solution delivered subcutaneously to the affected area; reversal decreases the duration of vasoconstriction from 320 minutes to approximately 85 minutes.18 As always, when applying literature to clinical practice, one must keep in mind the risks and benefits of any intervention. As such, in patients with pre-existing vascular disease, vaso-occlusive or vasospastic disease, or compromised perfusion due to trauma, one must weigh the benefits of the hemostatic effect against potential ischemia of already susceptible tissues. In such instances, omitting epinephrine from the solution is reasonable.
The benefits of sodium bicarbonate
The acidity of the solution contributes to the level of pain associated with administration of local anesthesia. Previously opened containers become more acidic.19 Addition of 8.4% sodium bicarbonate, at a ratio of 1 mL per 10 mL of 1% lidocaine with 1:100,000 epinephrine, neutralizes the pH to 7.4.19 A Cochrane Review showed that correction of pH to physiologic levels results in a significant reduction in pain.20
Continue to: This solution can be...
This solution can be easily prepared, as standard syringes hold an additional milliliter (ie, 10-mL syringes hold 11 mL) and, thus, can accommodate the additional volume of bicarbonate.21
Warming the solution helps, too
Warming the solution to body temperature prior to injection decreases pain on injection.22 This may be done in a variety of ways depending on available in-office equipment. Water baths, incubators, fluid warmers, heating pads, or specific syringe warmers may be used. Multiple studies have shown improvement in patient satisfaction with warming.23 Moreover, warming and buffering solution provide a synergistic effect on pain reduction.23
Equipment: Size matters
Smaller diameter needles. Reducing the outer diameter of the needle used for injection improves pain by reducing activation of nociceptors.24-26 Reduced inner diameter restricts injection speed, which further reduces pain.25 We recommend 27- to 30-gauge needles for subcutaneous injection and 25- to 27-gauge needles for intra-articular or tendon sheath injections.
Appropriate syringe size. Filling a syringe to capacity results in maximal deployment of the plunger. This requires greater handspan, which can lead to fatigue and loss of control during injection.26,27 Using a syringe filled to approximately half its capacity results in improved dexterity. We recommend 10-mL syringes with 5 mL to 6 mL of local anesthetic for small procedures and 20-mL syringes filled with 10 mL to 12 mL for larger procedures.
Topical local anesthetics may be used either as an adjunct to decrease pain during injection or as the primary anesthetic.28 A variety of agents are available for clinical use, including eutectic mixture of local anesthetics (EMLA), lidocaine-epinephrine-tetracaine (LET), lidocaine, benzocaine, and tetracaine. FPs should be familiar with their different pharmacokinetic profiles.
Continue to: EMLA is a mixture of...
EMLA is a mixture of 25 mg/mL of lidocaine and 25 mg/mL of prilocaine. It is indicated for topical anesthesia on intact, nonmucosal, uninjured skin (maximal dose 20 g/200 cm2 of surface area). It is applied in a thick layer and covered with an occlusive dressing (eg, Tegaderm) to enhance dermal penetration. The depth of penetration increases with application time and may reach a maximum depth of 3 mm and 5 mm following 60-minute and 120-minute application times, respectively.28 Duration of effect is 60 to 120 minutes.
LET, which is a mixture of 4% lidocaine, 0.1% epinephrine, and 0.5% tetracaine, may be used on nonintact, nonmucosal surfaces. Typically, 1 mL to 5 mL of gel is applied directly to the target area and is followed by application of direct pressure for 15 to 30 minutes. LET is not effective on intact skin and is contraindicated in children < 2 years of age.28
Cooling sprays or ice. Topical skin refrigerants, or vapocoolants (eg, ethyl chloride spray), offer an option for short-term local anesthesia that is noninvasive and quick acting. Ethyl chloride is a gaseous substance that extracts heat as it evaporates from the skin, resulting in a transient local conduction block. Skin refrigerants are an option to consider for short procedures such as intra-articular injections, venipuncture, or skin tag excision, or as an adjunct prior to local anesthetic delivery.29-32 Research has shown that topical ethyl chloride spray also possesses antiseptic properties.29,33
Environment: Make a few simple changes
Direct observation of needle penetration is associated with increased pain; advising patients to avert their gaze will mitigate the perception of pain.34 Additionally, research has shown that creating a low-anxiety environment improves patient-reported outcomes in both children and adults.35 Music or audiovisual or multimedia aids, for example, decrease pain and anxiety, particularly among children, and can be readily accessed with smart devices.36-39
We also recommend avoiding terms such as “pinch,” “bee sting,” or “stick” in order to reduce patient anxiety. Instead, we use language such as, “This is the medicine that will numb the area so you will be comfortable during the procedure.”40
Continue to: Injection technique
Injection technique: Consider these helpful tips
Site of needle entry. Prior to injecting local anesthesia, assess the area where the procedure is planned (FIGURE 1). The initial injection site should be proximal along the path of innervation. If regional nerves are anesthetized proximally and infiltration of local anesthesia proceeds distally, the initial puncture will be painful; however, further injections will be through anesthetized skin. Additionally, consider and avoid regional vascular anatomy.41,42

Counter-stimulation. Applying firm pressure, massaging, or stroking the site prior to or during the injection decreases pain.43,44 This technique may be performed by firmly pinching the area of planned injection between the thumb and index fingers, inserting the needle into the pinched skin, and maintaining pressure on the area until the anesthetic effect is achieved.
Angle of needle insertion. Perpendicular entry of the needle into the skin appears to reduce injection site pain (FIGURE 1). Anecdotal reports are supported by a randomized, controlled crossover trial that demonstrated significantly reduced pain with perpendicular injection compared to delivery at 45°.45
Depth of injection. Subcutaneous needle placement is associated with significantly less pain than injection into superficial dermis.2,46 Dermal wheals cause distention of the dermis, increased intradermal pressure, and greater activation of pain afferents in comparison to injection in the subcutaneous space.46 One important exception is the shave biopsy in which dermal distention is, in fact, desirable to ensure adequate specimen collection.
Other methods of pain reduction should still be employed. In the setting of traumatic wounds when a laceration is present, injection into the subcutaneous fat through the wound is easy and associated with less pain than injection through intact skin.47
Continue to: Speed of injection
Speed of injection. Rapid injection of anesthesia is associated with worse injection site pain and decreased patient satisfaction.48-50 Slowing the rate of injection causes less rapid distention of the dermis and subcutaneous space, resulting in decreased pain afferent activation and increased time for nerve blockade. Its importance is underscored by a prospective, randomized trial that compared rate of administration with buffering of local anesthetics and demonstrated that slow administration impacted patient-perceived pain more than buffering solution.51
Needle stabilization. Following perpendicular entry of the needle into the area of planned infiltration, deliver 0.5 mL of local anesthetic into the subcutaneous space without movement of the needle tip.52 With a stabilized needle tip, pain associated with initial needle entry is no longer perceived within 15 to 30 seconds.
It is paramount to stabilize both the syringe and the area of infiltration to prevent patient movement from causing iatrogenic injury or the need for multiple needlesticks. This can be accomplished by maintaining the dominant hand in a position to inject (ie, thumb on the plunger).
Needle reinsertion. Once subcutaneous swelling of local anesthesia is obtained, the needle may be slowly advanced, maintaining a palpable subcutaneous wavefront of local anesthesia ahead of the needle tip as it moves proximally to distally.2,52 Any reinsertion of the needle should be through previously anesthetized skin; this blockade is assessed by the presence of palpable tumescence and blanching (from the epinephrine effect).53
An example of the application of these injection pearls is demonstrated in the administration of a digital nerve block in FIGURE 2.54,55 With the use of the techniques outlined here, the patient ideally experiences only the initial needle entry and is comfortable for the remainder of the procedure.

CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, 1215 Lee Street, Charlottesville, VA, 22903; [email protected].
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
1. American Academy of Family Physicians. Family Medicine Facts. 2018. www.aafp.org/about/the-aafp/family-medicine-specialty/facts/table-12(rev).html. Accessed April 27, 2020.
2. Strazar AR, Leynes PG, Lalonde DH. Minimizing the pain of local anesthesia injection. Plast Reconstr Surg. 2013;132:675-684.
3. Kouba DJ, LoPiccolo MC, Alam M, et al. Guidelines for the use of local anesthesia in office-based dermatologic surgery. J Am Acad Dermatol. 2016;74:1201-1219.
4. Vinycomb TI, Sahhar LJ. Comparison of local anesthetics for digital nerve blocks: a systematic review. J Hand Surg Am. 2014;39:744-751.e5.
5. Valvano MN, Leffler S. Comparison of bupivacaine and lidocaine/bupivacaine for local anesthesia/digital nerve block. Ann Emerg Med. 1996;27:490-492.
6. Spivey WH, McNamara RM, MacKenzie RS, et al. A clinical comparison of lidocaine and bupivacaine. Ann Emerg Med. 1987;16:752-757.
7. Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain Medicine. American Society of Regional Anesthesia and Pain Medicine checklist for managing local anesthetic systemic toxicity. Reg Anesth Pain Med. 2012;37:16-18.
8. Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152-161.
9. Walsh K, Arya R. A simple formula for quick and accurate calculation of maximum allowable volume of local anaesthetic agents. Br J Dermatol. 2015;172:825-826.
10. McKee DE, Lalonde DH, Thoma A, et al. Optimal time delay between epinephrine injection and incision to minimize bleeding. Plast Reconstr Surg. 2013;131:811-814.
11. Hult J, Sheikh R, Nguyen CD, et al. A waiting time of 7 min is sufficient to reduce bleeding in oculoplastic surgery following the administration of epinephrine together with local anaesthesia. Acta Ophthalmol. 2018;96:499-502.
12. McKee DE, Lalonde DH, Thoma A, et al. Achieving the optimal epinephrine effect in wide awake hand surgery using local anesthesia without a tourniquet. Hand (NY). 2015;10:613-615.
13. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
14. Thomson CJ, Lalonde DH, Denkler KA, et al. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119:260-266.
15. Lalonde DH, Lalonde JF. Discussion. Do not use epinephrine in digital blocks: myth or truth? Part II. A retrospective review of 1111 cases. Plast Reconstr Surg. 2010;126:2035-2036.
16. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
17. Lalonde D, Bell M, Benoit P, et al. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30:1061-1067.
18. Nodwell T, Lalonde D. How long does it take phentolamine to reverse adrenaline-induced vasoconstriction in the finger and hand? A prospective, randomized, blinded study: the Dalhousie Project experimental phase. Can J Plast Surg. 2003;11:187-190.
19. Frank SG, Lalonde DH. How acidic is the lidocaine we are injecting, and how much bicarbonate should we add? Can J Plast Surg. 2012;20:71-73.
20. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Cochrane Review: adjusting the pH of lidocaine for reducing pain on injection. Evidence-Based Child Heal. 2012;7:149-215.
21. Barros MFFH, da Rocha Luz Júnior A, Roncaglio B, et al. Evaluation of surgical treatment of carpal tunnel syndrome using local anesthesia. Rev Bras Ortop. 2016;51:36-39.
22. Hogan M-E, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58:86-98.e1.
23. Colaric KB, Overton DT, Moore K. Pain reduction in lidocaine administration through buffering and warming. Am J Emerg Med. 1998;16:353-356.
24. Arendt-Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37-43.
25. Edlich RF, Smith JF, Mayer NE, et al. Performance of disposable needle syringe systems for local anesthesia. J Emerg Med. 1987;5:83-90.
26. Reed KL, Malamed SF, Fonner AM. Local anesthesia Part 2: technical considerations. Anesth Prog. 2012;59:127-137.
27. Elliott TG. Tips for a better local anaesthetic. Australas J Dermatol. 1998;39:50-51.
28. Kumar M, Chawla R, Goyal M. Topical anesthesia. J Anaesthesiol Clin Pharmacol. 2015;31:450.
29. Polishchuk D, Gehrmann R, Tan V. Skin sterility after application of ethyl chloride spray. J Bone Joint Surg Am. 2012;94:118-120.
30. Franko OI, Stern PJ. Use and effectiveness of ethyl chloride for hand injections. J Hand Surg Am. 2017;42:175-181.e1.
31. Fossum K, Love SL, April MD. Topical ethyl chloride to reduce pain associated with venous catheterization: a randomized crossover trial. Am J Emerg Med. 2016;34:845-850.
32. Görgülü T, Torun M, Güler R, et al. Fast and painless skin tag excision with ethyl chloride. Aesthetic Plast Surg. 2015;39:644-645.
33. Azar FM, Lake JE, Grace SP, et al. Ethyl chloride improves antiseptic effect of betadine skin preparation for office procedures. J Surg Orthop Adv. 2012;21:84-87.
34. Oliveira NCAC, Santos JLF, Linhares MBM. Audiovisual distraction for pain relief in paediatric inpatients: a crossover study. Eur J Pain. 2017;21:178-187.
35. Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev. 2015;(12):CD006275.
36. Attar RH, Baghdadi ZD. Comparative efficacy of active and passive distraction during restorative treatment in children using an iPad versus audiovisual eyeglasses: a randomised controlled trial. Eur Arch Paediatr Dent. 2015;16:1-8.
37. Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013;(10):CD005179.
38. Ahmad Z, Chawla R, Jaffe W. A novel distraction technique to facilitate daycase paediatric surgery under local anaesthesia. J Plast Reconstr Aesthetic Surg. 2012;65:e21-e22.
39. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department. JAMA Pediatr. 2013;167:826.
40. Varelmann D, Pancaro C, Cappiello EC, et al. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg. 2010;110:868-870.
41. Nelson TW. Accidental intravascular injection of local anesthetic? Anesthesiology. 2008;109:1143-1144.
42. Taghavi Zenouz A, Ebrahimi H, Mahdipour M, et al. The incidence of intravascular needle entrance during inferior alveolar nerve block injection. J Dent Res Dent Clin Dent Prospects. 2008;2:38-41.
43. Taddio A, Ilersich AL, Ipp M, et al; HELPinKIDS Team. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi-randomized controlled trials. Clin Ther. 2009;31:S48-S76.
44. Aminabadi NA, Farahani RMZ, Balayi Gajan E. The efficacy of distraction and counterstimulation in the reduction of pain reaction to intraoral injection by pediatric patients. J Contemp Dent Pract. 2008;9:33-40.
45. Martires KJ, Malbasa CL, Bordeaux JS. A randomized controlled crossover trial: lidocaine injected at a 90-degree angle causes less pain than lidocaine injected at a 45-degree angle. J Am Acad Dermatol. 2011;65:1231-1233.
46. Zilinsky I, Bar-Meir E, Zaslansky R, et al. Ten commandments for minimal pain during administration of local anesthetics. J Drugs Dermatol. 2005;4:212-216.
47. Bartfield JM, Sokaris SJ, Raccio-Robak N. Local anesthesia for lacerations: pain of infiltration inside vs outside the wound. Acad Emerg Med. 1998;5:100-104.
48. Scarfone RJ, Jasani M, Gracely EJ. Pain of local anesthetics: rate of administration and buffering. Ann Emerg Med. 1998;31:36-40.
49. Kattan AE, Al-Shomer F, Al-Jerian A, et al. Pain on administration of non-alkalinised lidocaine for carpal tunnel decompression: a comparison between the Gale and the “advancing wheal” techniques. J Plast Surg Hand Surg. 2016;50:10-14.
50. Tangen LF, Lundbom JS, Skarsvåg TI, et al. The influence of injection speed on pain during injection of local anaesthetic. J Plast Surg Hand Surg. 2016;50:7-9.
51. McGlone R, Bodenham A. Reducing the pain of intradermal lignocaine injection by pH buffering. Arch Emerg Med. 1990;7:65-68.
52. Lalonde D, Wong A. Local anesthetics. Plast Reconstr Surg. 2014;134(4 Suppl 2):40S-49S.
53. Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16:248-263.
54. Williams JG, Lalonde DH. Randomized comparison of the single-injection volar subcutaneous block and the two-injection dorsal block for digital anesthesia. Plast Reconstr Surg. 2006;118:1195-1200.
55. Thomson CJ, Lalonde DH. Randomized double-blind comparison of duration of anesthesia among three commonly used agents in digital nerve block. Plast Reconstr Surg. 2006;118:429-432.
PRACTICE RECOMMENDATIONS
› Add epinephrine and sodium bicarbonate buffer to local anesthetic solution to reduce pain and procedural blood loss. A
› Use such techniques as counter-stimulation, a perpendicular angle of injection, a subcutaneous depth of injection, and a slow rate of injection to minimize patient discomfort. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Neonatal hyperbilirubinemia: An evidence-based approach
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.
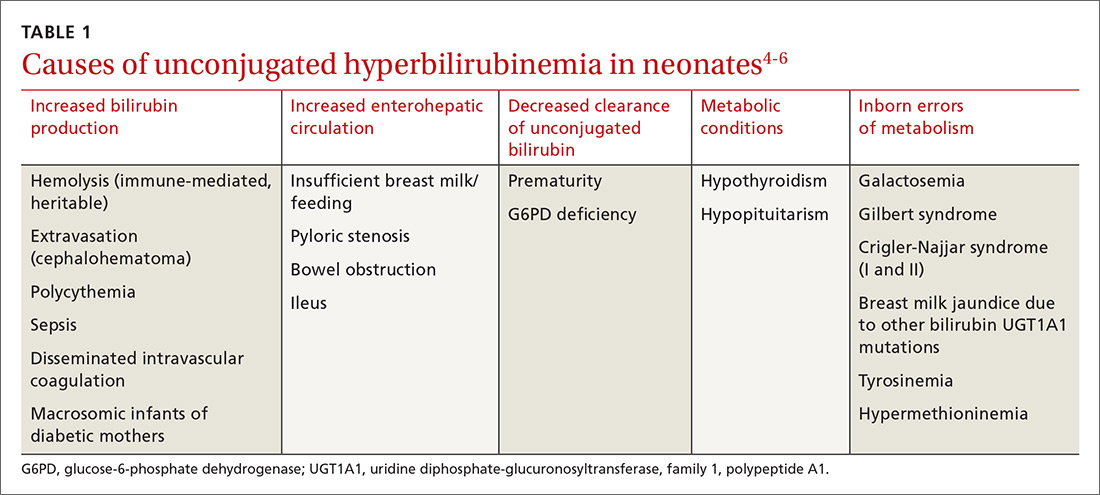
Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8
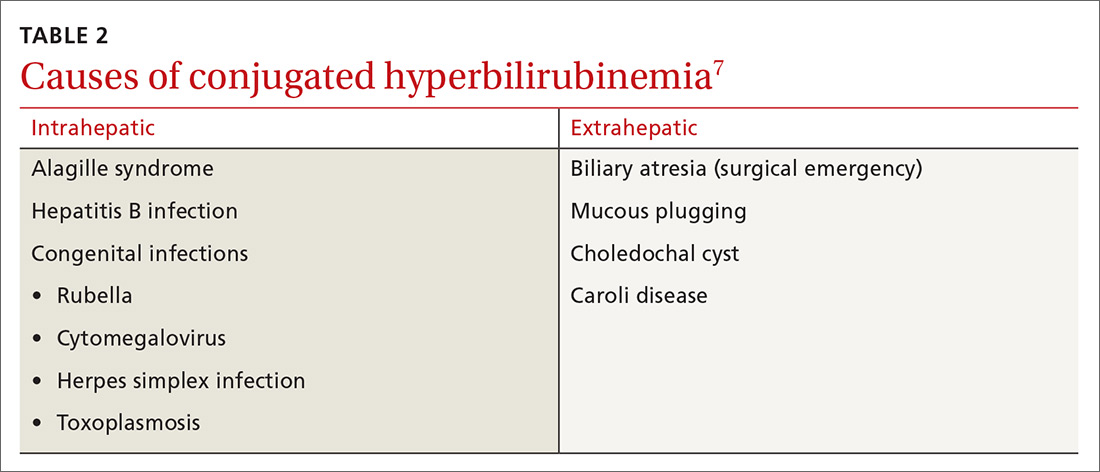
Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
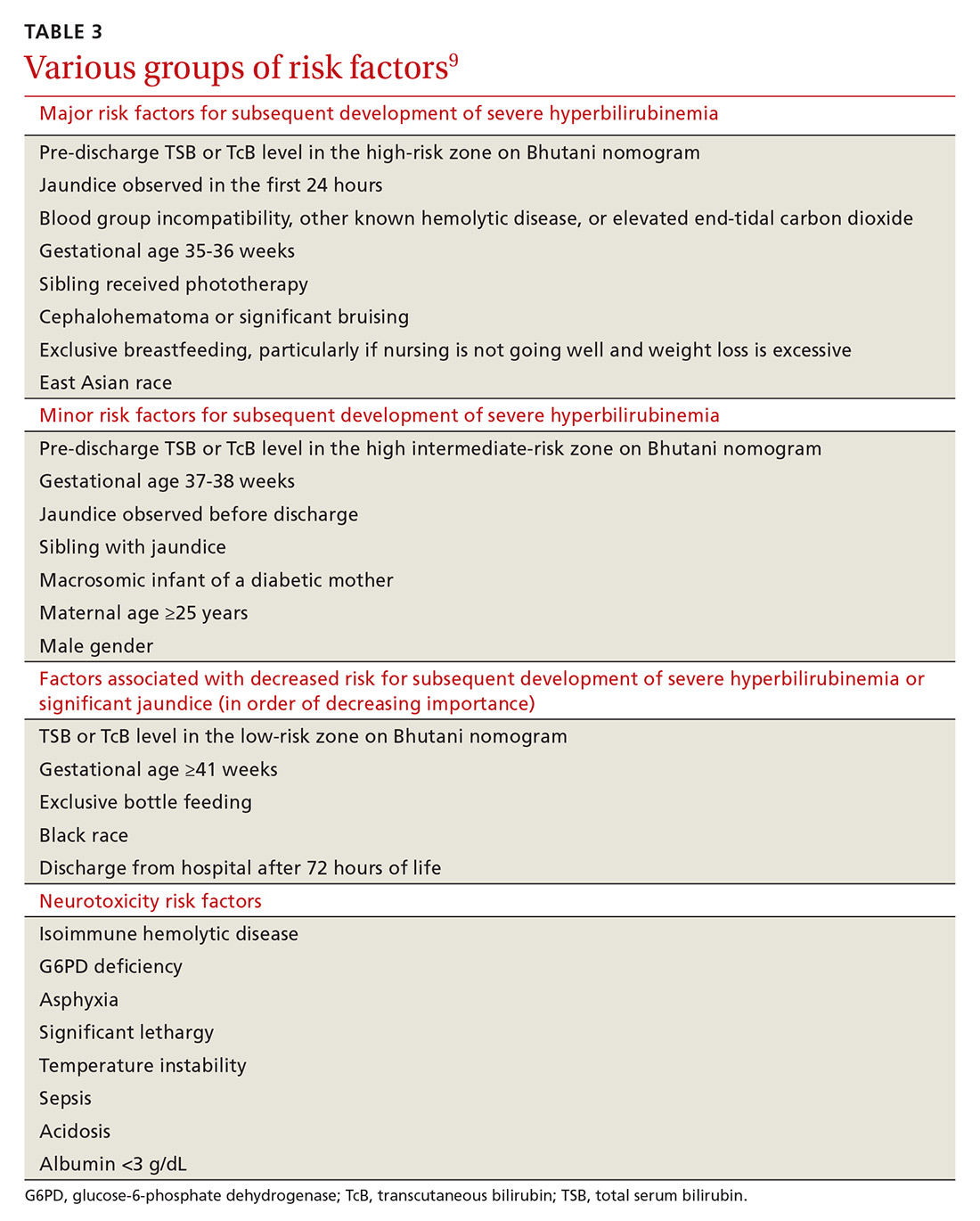
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9
Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; [email protected].
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.

Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8

Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9

Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; [email protected].
More than 60% of newborns appear clinically jaundiced in the first few weeks of life,1 most often due to physiologic jaundice. Mild hyperbilirubinemia peaks at Days 3 to 5 and returns to normal in the following weeks.1 However, approximately 10% of term and 25% of late preterm infants will undergo phototherapy for hyperbilirubinemia in an effort to prevent acute bilirubin encephalopathy (ABE) and kernicterus.2
Heightened vigilance to prevent these rare but devastating outcomes has made hyperbilirubinemia the most common cause of hospital readmission in infants in the United States3 and one with significant health care costs. This article summarizes the evidence and recommendations for the screening, evaluation, and management of hyperbilirubinemia in term infants.
But first, we begin with a quick look at the causes of hyperbilirubinemia.
Causes of conjugated vs unconjugated hyperbilirubinemia
Bilirubin is generated when red blood cells break down and release heme, which is metabolized into biliverdin and then to bilirubin. Unconjugated bilirubin binds to albumin in the blood and is transported to hepatocytes where conjugation occurs, allowing it to be excreted through the gastrointestinal tract. In neonates, most of the conjugated bilirubin that reaches the gut is then unconjugated, resulting in its recirculation. Additionally, neonates have an increased volume of red blood cells and a slow conjugating system. These factors all contribute to excess unconjugated bilirubin, which manifests as physiologic, nonpathologic jaundice.4TABLE 14-6 lists causes of unconjugated hyperbilirubinemia.

Elevated conjugated hyperbilirubinemia (conjugated bilirubin level ≥20% of total serum bilirubin [TSB]) is always pathologic and occurs due to intrahepatic or extrahepatic obstruction. TABLE 27 lists causes of conjugated hyperbilirubinemia. Infants found to have conjugated hyperbilirubinemia should undergo an additional work-up to determine the cause and identify potential complications of this disease.8

Given that the differential for conjugated hyperbilirubinemia is so broad and that it is often associated with severe disease requiring complicated and invasive treatments, infants with conjugated hyperbilirubinemia should be referred to a pediatric tertiary care facility with pediatric gastroenterologists, infectious disease specialists, and surgeons.7
What puts newborns at risk?
Major and minor risk factors for the development of severe hyperbilirubinemia in well newborns ≥35 weeks’ gestation are listed in TABLE 3.9 Those that carry the highest risk include gestational age <38 weeks, having a sibling who required phototherapy, visible jaundice by the time of discharge, and exclusive breastfeeding.9 Several more recent cohort studies, however, suggest that breastfeeding may not be a significant risk factor.10 The more risk factors present, the higher the risk. Infants who are formula fed, age ≥41 gestational weeks, or have no major or minor risk factors have a very low likelihood of developing severe hyperbilirubinemia.9

Continue to: The Bhutani curve...
The Bhutani curve11 is a widely used, validated nomogram based on pre-discharge hour-specific serum bilirubin measurements. (Go to http://pediatrics.aappublications.org/content/114/1/297 and see Figure 2.) It is the most reliable way to assess the risk for subsequent development of significant hyperbilirubinemia requiring phototherapy.9 As such, it is the basis for several online calculators and apps such as BiliTool (bilitool.org).
An alternative nomogram developed by Varvarigou et al12 is available for predicting significant hyperbilirubinemia based on transcutaneous bilirubin assessment. (Go to https://pdfs.semanticscholar.org/a9a6/5a988dba7a3442bcebc149ad5aea5e3c35d5.pdf and see Figure 3.) The American Academy of Pediatrics (AAP) supports the use of either bilirubin assessment for the screening and diagnosis of hyperbilirubinemia in infants ≥35 weeks’ gestation.9
Neurotoxicity risk factors. It is important to differentiate the major and minor risk factors for severe hyperbilirubinemia from neurotoxicity risk factors, also listed in TABLE 3.9 Neurotoxicity risk factors are indicative of conditions that may affect albumin binding of bilirubin and are thought to lower the threshold at which bilirubin may cross the blood-brain barrier and render the brain more susceptible to damage from bilirubin. These neurotoxicity risk factors should be applied to the AAP phototherapy nomogram (see Figure 3 at pediatrics.aappublications.org/content/114/1/297) to determine the threshold for initiation of phototherapy in infants with hyperbilirubinemia.9
Few investigators have attempted to define risk factors for the development of poor neurologic outcomes associated with hyperbilirubinemia. Evidence to date has not allowed the determination of a specific bilirubin level at which subsequent development of kernicterus occurs.9 The limited data available suggest a poor correlation between TSB level and bilirubin-induced neurologic dysfunction.13
(For more on kernicterus, as well as ABE and bilirubin-induced neurologic dysfunction [BIND], see “Why do we worry about hyperbilirubinemia?”9,14-16)
SIDEBAR
Why do we worry about hyperbilirubinemia?
When circulating bilirubin crosses the blood-brain barrier, neurologic dysfunction can occur that may become permanent. Bilirubin-induced neurologic dysfunction (BIND) occurs on a spectrum, beginning with acute bilirubin encephalopathy (ABE) and progressing to the irreversible condition—kernicterus.
The incidence of BIND has not been well documented; rates of kernicterus and ABE are thought to be about 1 in 133,000,16 but ABE and kernicterus are not reportable conditions in the United States, so exact prevalence is unknown.
Acute bilirubin encephalopathy may present subtly at first with lethargy, hypotonia, and a high-pitched cry. If not corrected, the condition can progress to hypertonicity (with arching of the neck and trunk), poor sucking, and irritability. It can lead to apnea, intractable seizures, respiratory failure, and even death.14
Kernicterus was originally a term used to describe the yellow bilirubin-staining of brainstem nuclei and the cerebellum seen on autopsy,9 but is now synonymous with chronic bilirubin encephalopathy. Kernicterus describes the irreversible manifestations of bilirubin neurotoxicity that often present as a classical tetrad of motor deficits (athetoid cerebral palsy), auditory processing deficits (with or without hearing loss), oculomotor deficits (especially impairments of upward vertical gaze), and enamel dysplasia of deciduous teeth.15 Abnormal magnetic resonance imaging of the globus pallidus and subthalamic nuclei is often seen in infants with kernicterus.14
Continue to: Diagnosis relies on TSB and/or TcB
Diagnosis relies on TSB and/or TcB
TSB measurement is the traditional and most widely used method for screening and diagnosing neonatal hyperbilirubinemia, but the blood draw is invasive and carries a risk (albeit low) of infection and anemia.17 Transcutaneous bilirubin (TcB) assessment is a noninvasive alternative that generally correlates well with TSB values ≤15 mg/dL,17-20 even in Hispanic, African, and multiethnic populations.18,21,22
Diagnosis of hyperbilirubinemia is made with TSB or TcB measured at >95th percentile for age in hours. TcB levels measured at >15 mg/dL should be confirmed with TSB measurement. Visual assessment of jaundice should not be used for diagnosis, as it may lead to errors.9-23
The total cost of testing is lower with TcB ($4-$15 per patient17) than with TSB when the cost of supplies and personnel are considered.24 Although more recent evidence suggests that TcB is an acceptable way to measure bilirubin in premature infants, no professional society currently recommends the use of TcB for the diagnosis of hyperbilirubinemia in infants25 <35 weeks’ gestation.
Screening recommendations lack consensus
There is a lack of consensus among professional societies on appropriate screening for neonatal hyperbilirubinemia, likely due to limited available data, necessitating expert-driven recommendations.
The AAP recommends universal screening of infants ≥35 weeks’ gestation prior to discharge with measurement of TSB/TcB and/or clinical assessment.9 The Canadian Pediatric Society recommends universal screening with TSB/TcB measurement in all infants in the first 72 hours of life.26
Continue to: The US Preventive Services Task Force...
The US Preventative Services Task Force, however, found insufficient evidence to recommend universal screening for infants ≥35 weeks’ gestation.27 The main rationale for their “I” recommendation was that although screening can identify infants at risk of developing severe hyperbilirubinemia, there is no clear evidence that identifying and treating elevated bilirubin levels results in the prevention of kernicterus.
The United Kingdom’s National Institute for Health and Care Excellence (NICE) guidelines do not support universal screening either.28 NICE recommends risk factor assessment and visual inspection for jaundice in all newborns and also additional physical examination for newborns with risk factors. NICE recommends against routine monitoring of bilirubin levels in infants who do not appear jaundiced.
All infants who appear jaundiced should be evaluated with either risk factor assessment or bilirubin measurement (TSB or TcB). Infants born to mothers who are Rh-negative or have type O blood should have cord blood tested for blood type, Rh status, and other antibodies with a direct Coombs test, as ABO and Rh incompatibility are major risk factors for development of hyperbilirubinemia because of hemolysis.8,9
A question of cost-efficacy? Data from a multicenter prospective clinical trial suggest a number needed to screen of 128,600 to prevent 1 case of kernicterus,29 making cost another important factor in the discussion about screening for neonatal hyperbilirubinemia. Universal screening is associated not only with the cost of TSB and TcB measurements, but also with the cost of phototherapy, rates of which are increased with universal screening.24,29,30 The cost of caring for 1 patient with kernicterus over a lifetime is estimated at $900,000, while the estimated cost to prevent 1 case of kernicterus with universal TSB/TcB screening is between $5.7 and $9.2 million.31
In Canada, universal screening was found to decrease emergency department visits for jaundice, but did not affect rates of readmission for hyperbilirubinemia, length of hospital stay, or rates of phototherapy after discharge.30
Continue to: Phototherapy: What kind of light, when to initiate
Phototherapy: What kind of light, when to initiate
The initial management of hyperbilirubinemia is phototherapy. Light directed at the skin converts bilirubin to lumirubin—a compound that unlike bilirubin does not require conjugation in the liver and can be directly excreted in the urine or bile.8
Light in the blue-green spectrum (460-490 nm) is most effective. Generally, phototherapy is more effective the closer the light is to the infant and the greater the surface area of skin the infant has exposed. There are many different types of lights used to provide phototherapy including fluorescent, halogen, light emitting diode (LED), and fiber optic lights, which are commonly used in home biliblankets.8 Fluorescent and halogen lights are the conventional methods, but newer LED systems are equally effective in terms of rate of decline of serum bilirubin levels, duration of phototherapy required, and need for exchange transfusion. Fiber optic lights work as well as other lights in preterm infants but are less effective in term infants. Using 2 fiber optic lights in term infants can increase efficacy to the level of a single conventional or LED source.32
Phototherapy thresholds. The AAP phototherapy curve (see Figure 3 at http://pediatrics.aappublications.org/content/114/1/297) is commonly used to determine phototherapy thresholds for infants with hyperbilirubinemia. This nomogram applies TSB level and age in hours to a “low,” “medium,” or “high” risk curve that is determined by the presence of neurotoxicity risk factors and gestational age. Infants on the “medium” and “high” risk curves have lower thresholds for initiation of phototherapy.9 The majority of infants born at gestational age ≥38 weeks being cared for in a newborn nursery will be assigned to a low risk curve on the AAP phototherapy nomogram, as many of the neurotoxicity risk factors that elevate risk would also be reasons for infants to be in an intensive care unit.
Online calculators and apps based on the AAP phototherapy nomogram, such as BiliTool (bilitool.org), offer recommendations for phototherapy thresholds and may suggest a time interval at which to repeat bilirubin testing if phototherapy is not indicated.
The additional work-up for infants requiring phototherapy often includes neonatal blood type, direct Coombs test, complete blood count and smear, and conjugated bilirubin level.9 Besser et al,33 however, found that 88% of infants requiring phototherapy had normal laboratory results. They also found that those infants with lab abnormalities often started phototherapy before 48 hours of age and did not have an appropriate decrease in bilirubin after initiation of phototherapy.
Continue to: Timing
Timing. Based on this data, it is reasonable to start phototherapy in term infants who develop jaundice at >48 to 72 hours of age without doing additional testing.
Bilirubin levels are expected to drop about 0.5 mg/dL per hour in the first 4 to 8 hours after starting phototherapy, but if the bilirubin measurement is not decreasing as expected or is increasing, additional work-up, with reticulocyte count, G6PD (glucose-6-phosphate dehydrogenase) concentration, end-tidal carbon dioxide determination (ETCO), and a bilirubin/albumin (B/A) ratio is warranted.8 Since unbound bilirubin can cross the blood-brain barrier, increased B/A ratio could theoretically be a predictor of bilirubin-induced neurologic dysfunction risk, but Iskander et al34 found that it was not superior to TSB levels in predicting neurotoxicity. ETCO may help identify children with ongoing hemolysis.8
The ideal time to stop phototherapy is not clear. Expert recommendations for phototherapy discontinuation thresholds range from 4-5 mg/dL to 13-14 mg/dL,8 while other clinicians stop phototherapy when bilirubin falls 1 to 2 mg/dL below the phototherapy initiation threshold. Phototherapy should be continued for any infant with signs of acute bilirubin encephalopathy, even if the bilirubin level is decreasing.9 Rebound hyperbilirubinemia is rare, and checking rebound bilirubin levels is not recommended.8
Safety. Phototherapy is generally considered safe, but both short- and long-term adverse effects are possible. Immediate adverse effects include intestinal hypermobility/diarrhea and temperature instability. Long-term issues include increased risks of the development of childhood asthma (odds ratio=1.4) and type 1 diabetes (odds ratio=3.79).35 Phototherapy can also be distressing for parents, as it requires frequent blood draws, physical separation, and possible disruption of breastfeeding.36 One study found a number needed to harm of 4 for cessation of breastfeeding at 1 month in jaundiced infants.37
Maintain breastfeeding. The AAP recommends breastfeeding be continued and promoted in infants who are jaundiced and receiving phototherapy.9 Maternal interaction with health care professionals who are encouraging of this practice was the best predictor of ongoing breastfeeding in a qualitative study of jaundiced infants and their families.38 Interrupting phototherapy for up to 30 minutes to allow for breastfeeding without eye covers has not been shown to decrease the efficacy of phototherapy.38
Continue to: Available evidence does not provide a clear answer...
Available evidence does not provide a clear answer regarding whether formula supplementation should be initiated in breastfed infants with hyperbilirubinemia. Cow’s milk formula supplementation decreases intestinal reabsorption of bilirubin, lowering serum bilirubin levels, but may interfere with successful breastfeeding.39 The Academy of Breastfeeding Medicine recommends an individual discussion about formula supplementation in place of, or prior to, phototherapy if an infant’s bilirubin is approaching (within 2-3 mg/dL) or above the threshold for phototherapy.39 Routine supplementation with intravenous fluids or other non–milk-based supplementation is not recommended for infants receiving phototherapy.9
Adjuvant therapies and exchange transfusion
Clofibrate, metalloporphyrins, and ursodiol have been studied in the management of unconjugated hyperbilirubinemia as augmentation to phototherapy. Honar et al40 found that ursodiol added at the time of phototherapy initiation demonstrated a significant reduction in peak bilirubin levels and duration of phototherapy in term infants with unconjugated hyperbilirubinemia without any adverse effects. Cochrane reviews of clofibrat5 and metalloporphyrins41 found that when added to phototherapy, these medications significantly decreased serum bilirubin levels and duration of phototherapy. However, there was insufficient evidence to recommend their use due to inadequate data on safety and long-term outcomes.
Exchange transfusion. Infants with bilirubin levels >25 mg/dL, those who are not responding to phototherapy, and those with evidence of acute bilirubin encephalopathy should be treated with exchange transfusion, with initiation based on an infant’s age in hours and neurotoxicity risk factors.9 Exchange transfusion involves taking small aliquots of blood from the infant and replacing them with donor red cells until the infant’s blood volume has been replaced twice to remove bilirubin and antibodies that may be causing hemolysis. It should be carried out in a neonatal intensive care unit due to significant risks.
Approximately 12% of infants have a complication from exchange transfusion including infection, electrolyte imbalances, thrombosis, thrombocytopenia, and necrotizing enterocolitis.8 The mortality rate in neonates without hemolysis who undergo exchange transfusion is 3 to 4 per 1000 treated.42
Post-discharge follow-up
Infants discharged before 72 hours of life should be seen within 2 days of discharge. Those infants with significant risk factors for development of severe hyperbilirubinemia should be seen within 1 day. Arrangements for follow-up should be made prior to discharge. Some infants discharged before 48 hours of life may require 2 follow-up visits. If follow-up cannot be ensured for an infant with risk factors for the development of severe hyperbilirubinemia, delay of discharge may be appropriate.9
CORRESPONDENCE
Katharine C. DeGeorge, MD, MS, Department of Family Medicine, University of Virginia, PO Box 800729, Charlottesville, VA, 22908-0729; [email protected].
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
1. Schwartz HP, Haberman BE, Ruddy RM. Hyperbilirubinemia: current guidelines and emerging therapies. Pediatr Emerg Care. 2011;27:884-889.
2. Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113:775-780.
3. Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101:995-998.
4. Maisels MJ. Neonatal jaundice. Pediatr Rev. 2006;27:443-454.
5. Gholitabar M, McGuire H, Rennie J, et al. Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia. Cochrane Database Syst Rev. 2012;12:CD009017.
6. Maruo Y, Morioka Y, Fujito H, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. 2014;165:36-41.e1.
7. Brumbaugh D, Mack C. Conjugated hyperbilirubinemia in children. Pediatr Rev. 2012;33:291-302.
8. Lauer BJ, Spector ND. Hyperbilirubinemia in the newborn. Pediatr Rev. 2011;32:341-349.
9. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297-316.
10. Bertini G, Dani C, Tronchin M, et al. Is breastfeeding really favoring early neonatal jaundice? Pediatrics. 2001;107:E41.
11. Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999;103:6-14.
12. Varvarigou A, Fouzas S, Skylogianni E, et al. Transcutaneous bilirubin nomogram for prediction of significant neonatal hyperbilirubinemia. Pediatrics. 2009;124:1052-1059.
13. Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128:e925-e931.
14. Wisnowski JL, Panigrahy A, Painter MJ, et al. Magnetic resonance imaging of bilirubin encephalopathy: current limitations and future promise. Semin Perinatol. 2014;38:422-428.
15. Shapiro SM. Chronic bilirubin encephalopathy: diagnosis and outcome. Semin Fetal Neonatal Med. 2010;15:157-163.
16. Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134:504-509.
17. Mahram M, Oveisi S, Jaberi N. Trans-cutaneous bilirubinometery versus serum bilirubin in neonatal jaundice. Acta Med Iran. 2015;53:764-769.
18. Campbell DM, Danayan KC, McGovern V, et al. Transcutaneous bilirubin measurement at the time of hospital discharge in a multiethnic newborn population. Paediatr Child Health. 2011;16:141-145.
19. Bhutani VK, Gourley GR, Adler S, et al. Noninvasive measurement of total serum bilirubin in a multiracial predischarge newborn population to assess the risk of severe hyperbilirubinemia. Pediatrics. 2000;106:E17.
20. Holland L, Blick K. Implementing and validating transcutaneous bilirubinometry for neonates. Am J Clin Pathol. 2009;132:555-561.
21. Kolman KB, Mathieson KM, Frias C. A comparison of transcutaneous and total serum bilirubin in newborn Hispanic infants at 35 or more weeks of gestation. J Am Board Fam Med. 2007;20:266-271.
22. Slusher TM, Angyo IA, Bode-Thomas F, et al. Transcutaneous bilirubin measurements and serum total bilirubin levels in indigenous African infants. Pediatrics. 2004;113:1636-1641.
23. Riskin A, Tamir A, Kugelman A, et al. Is visual assessment of jaundice reliable as a screening tool to detect significant neonatal hyperbilirubinemia? J Pediatr. 2008;152:782-787.
24. Bhutani VK, Vilms RJ, Hamerman-Johnson L. Universal bilirubin screening for severe neonatal hyperbilirubinemia. J Perinatol. 2010;30 Suppl:S6-S15.
25. Nagar G, Vandermeer B, Campbell S, et al. Reliability of transcutaneous bilirubin devices in preterm infants: a systematic review. Pediatrics. 2013;132:871-881.
26. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants (35 or more weeks’ gestation) - summary. Paediatr Child Health. 2007;12:401-418.
27. US Preventive Services Task Force. Screening of infants for hyperbilirubinemia to prevent chronic bilirubin encephalopathy: US Preventive Services Task Force recommendation statement. Pediatrics. 2009;124:1172-1177.
28. National Institute for Health and Care Excellence. Jaundice in newborn babies under 28 days. Clinical guideline [CG98].https://www.nice.org.uk/guidance/cg98. Updated October 2016. Accessed October 17, 2018.
29. Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125:e1143-e1148.
30. Darling EK, Ramsay T, Sprague AE, et al. Universal bilirubin screening and health care utilization. Pediatrics. 2014;134:e1017-e1024.
31. Suresh GK, Clark RE. Cost-effectiveness of strategies that are intended to prevent kernicterus in newborn infants. Pediatrics. 2004;114:917-924.
32. Kumar P, Chawla D, Deorari A. Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates. Cochrane Database Syst Rev. 2011;7:CD007969.
33. Besser I, Perry ZH, Mesner O, et al. Yield of recommended blood tests for neonates requiring phototherapy for hyperbilirubinemia. Isr Med Assoc J. 2010;12:220-224.
34. Iskander I, Gamaleldin R, El Houchi S, et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics. 2014;134:e1330-e1339.
35. Aspberg S, Dahlquist G, Kahan T, Källén B. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733-e739.
36. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
37. Kemper K, Forsyth B, McCarthy P. Jaundice, terminating breast-feeding, and the vulnerable child. Pediatrics. 1989;84:773-778.
38. Willis SK, Hannon PR, Scrimshaw SC. The impact of the maternal experience with a jaundiced newborn on the breastfeeding relationship. J Fam Pract. 2002;51:465.
39. The Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #22: guidelines for management of jaundice in the breastfeeding infant equal to or greater than 35 weeks’ gestation. Breastfeed Med. 2010;5:87-93.
40. Honar N, Ghashghaei Saadi E, Saki F, et al. Effect of ursodeoxycholic acid on indirect hyperbilirubinemia in neonates treated with phototherapy. J Pediatr Gastroenterol Nutr. 2016;62:97-100.
41. Suresh GK, Martin CL, Soll RF. Metalloporphyrins for treatment of unconjugated hyperbilirubinemia in neonates. Cochrane Database Syst Rev. 2003;2:CD004207.
42. Muchowski KE. Evaluation and treatment of neonatal hyperbilirubinemia. Am Fam Physician. 2014;89:873-878.
PRACTICE RECOMMENDATIONS
› Diagnose hyperbilirubinemia in infants with bilirubin measured at >95th percentile for age in hours. Do not use visual assessment of jaundice for diagnosis as it may lead to errors. C
› Determine the threshold for initiation of phototherapy by applying serum bilirubin and age in hours to the American Academy of Pediatrics phototherapy nomogram along a risk curve assigned based on gestational age and neurotoxicity risk factors (not major and minor risk factors for severe hyperbilirubinemia). C
› Make arrangements to ensure that all infants are seen by a health care provider within 2 days of discharge (within 1 day if significant risk factors for development of severe hyperbilirubinemia are present). C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series