User login
Streptococcus pneumoniae (S pneumoniae), also known as pneumococcus, is a successful human pathogen with significant clinical impact that causes pneumonia and invasive infections, including bacteremia and meningitis.1 In the preantibiotic era, nearly 80% of patients with bacteremic pneumococcal pneumonia died.2 The introduction of sulfas and penicillin in the mid-20th century, subsequent refinements in antibiotic chemotherapy, and improvements in supportive care rendered pneumococcal disease readily treatable, notwithstanding the threat of antibiotic-resistant pneumococcus.3 Despite the availability of effective antibiotic therapy against S pneumoniae, pneumococcal disease remains a significant cause of morbidity and mortality among people with increased susceptibility, such as older adults and those living with chronic illness or immunosuppressive conditions. In developed countries like the U.S., where a growing portion of the population is vulnerable to S pneumoniae by virtue of their advanced age and underlying medical conditions, pneumococcal disease is still an important public health concern.4
Penumococcal Vaccines: A Long Time Coming
Vaccination against S pneumoniae has proven an efficacious strategy to reduce the morbidity and mortality associated with this pathogen.5 The original efforts to develop a pneumococcal vaccine culminated in 1945 with a vaccine containing pneumococcal capsular polysaccharides, which elicited a protective immune response among U.S. soldiers (Table 1).Subsequent investigations determined that the protective response was specific to pneumococcal disease caused by the 4 pneumococcal capsular serotypes included in the vaccine, that the carrier rate of pneumococcus with the vaccine serotypes decreased by about 50%, and that the incidence of pneumonia from the vaccine serotypes was reduced even in nonimmunized soldiers.6 These early observations remain relevant to our contemporary understanding of the impact of pneumococcal vaccination: Protection is limited to serotypes included in the vaccine; the vaccine reduces colonization; and the vaccine leads to herd immunity—the protection of unvaccinated subjects.
Despite this achievement, the use of vaccines as a strategy to combat pneumococcal disease was upstaged in the subsequent decades by the success of antibiotics. Renewed interest in pneumococcal vaccines resulted from the efforts of Robert Austrian, MD, who astutely observed that “highly effective antimicrobial drugs must be supplemented by other measures, both prophylactic and therapeutic, if the significant mortality resulting still from pneumococcal infection is to be reduced.”7
After initial studies carried out in South African gold miners, the FDA approved a pneumococcal polysaccharide vaccine (PPSV) against 23 of about 90 circulating serotypes.8 The CDC and the Advisory Committee on Immunization Practices (ACIP) initially recommended PPSV-23 for persons perceived to be at high risk for pneumococcal disease, including those with chronic diseases, immunocompromising conditions, and older adults.9
Three decades later, the analysis of a large body of available evidence demonstrated the protective effects of PPSV-23 against invasive pneumococcal disease caused by pneumococcal types included in the vaccine, especially bacteremic pneumonia (about 75% reduction, odds ratio [OR] 0.26, 95% confidence interval [CI] 0.14-0.45).10 A perceived shortcoming of PPSV-23, which some experts dispute, is the lack of definite protection against nonbacteremic pneumococcal pneumonia.11 Studies of nursing home residents demonstrated a 50% reduction in the incidence of both pneumococcal pneumonia and all-cause pneumonia, suggesting that PPSV-23 offers protection against noninvasive pneumococcal pneumonia in specific populations.12 Additional potential limitations of PPSV-23 include reduced benefit in patients aged > 65 years and waning of protection over time.13
Related: Identification and Management of Middle East Respiratory Syndrome
Protein-Conjugate Vaccines
Clearly, the most important limitation of PPSV-23, inherent to all capsular polysaccharide vaccines, is that it does not elicit an immune response in children aged < 2 years. The successful development of a vaccine against Haemophilus influenzae type b (Hib) gave rise to a new generation of pneumococcal vaccines.14 Specifically, the Hib vaccine covalently binds, or conjugates, the capsule polysaccharide to an antigenic protein, leading to effective T-cell–mediated antibody production in infants and toddlers.
In 2000, children received the first iteration of a protein-conjugate vaccine containing the 7 most relevant pneumococcal serotypes (PCV-7).15 The effects of PCV-7 on pneumococcal disease have been extraordinary, practically eliminating infections caused by the pneumococcal serotypes included in the vaccine. Immunizing children with PCV-7 also ushered in a fundamental public health benefit for adults aged > 65 years: a reduction of nearly 90% in the incidence of pneumococcal infections caused by serotypes included in the vaccine. By eliciting mucosal immunity, which leads to decreased nasal carriage of the covered pneumococcal strains among children, PVC-7 generates herd immunity, leading to reductions in transmission, colonization, and infection with vaccine serotypes among adults.16
In 2010, a conjugate vaccine containing 13 serotypes (PCV-13) replaced PCV-7 administration for children.17,18 The PCV-13 is expected to protect children and the herd from disease caused by 6 additional pneumococcal serotypes, including those that surged as replacement strains, filling the ecologic niche created by PCV-7, such as the epidemiologically relevant serotype 19A.19,20
Immune-Compromised Adults
One of the shortcomings of PPSV-23 is its lack of efficacy in patients with advanced HIV, a group with an exquisite vulnerability to pneumococcal disease, as demonstrated by a randomized controlled trial of PPSV-23 in African patients with advanced, untreated HIV infection.21 Similarly, there is concern that patients with lymphoma, leukemia, multiple myeloma, and Hodgkin disease, also at high risk of pneumococcal infection, do not mount sufficient immune responses to polysaccharide antigens or that these responses are adversely affected by chemotherapy or immune suppressing medications. In these populations, a conjugate vaccine (PCV-7 or PCV-13) may elicit a more robust and durable immune response than a polysaccharide vaccine (PPSV-23). A randomized placebo-controlled trial demonstrated the efficacy of PCV-7 in protecting patients with advanced HIV against pneumococcal disease in Africa.22 Based in part on this observation, the ACIP now recommends the use of PCV-13 in patients with HIV and other immunosuppressing conditions, including chronic renal failure.23 A direct comparison of the relative protection of PPSV-23 vs PCV-7 in this population has not been performed.
Pneumococcal Vaccines in the Elderly
Although PPSV-23 was widely adopted in the U.S. with the intention to protect adults aged > 65 years from pneumococcal infection, this vaccine did not achieve global appeal. In the Netherlands, for instance, PPSV-23 had very low penetration and was never endorsed by public health authorities. Concerns have been issued about the decreased immunogenicity of a polysaccharide vaccine in older adults, invoking the concept of immune senescence, a term that describes a diminished capacity to mount robust immune responses due to aging.
Protein-conjugated antigens, on the other hand, can elicit a better initial immune response than a polysaccharide vaccine can in an older adult. Whether the effect is sustained and translates to better clinical outcomes remains unknown. The adoption of PCV-13 and the continued role of PPSV-23 in adults aged > 65 years have been examined carefully by experts.24,25
Dutch investigators organized the Community Acquired Pneumonia Immunization Trial in Adults (CAPITA), randomizing about 85,000 adults aged > 65 years toreceive eitherPCV-13 or a placebo.26 After 3 years of observation, the occurrence of invasive pneumococcal disease (caused by the vaccine serotypes) decreased by 85% in participants who received the vaccine compared with those who received a placebo. Additionally, the incidence of pneumococcal pneumonia (caused by the vaccine serotypes) decreased by 45% (Table 2). The findings of this trial largely inform the recommendation issued by APIC in October 2014 to administer PCV-13 vaccine to adults aged > 65 years.27
Polysaccharide Vaccine in Adults
A crucial limitation of the CAPITA study is that it does not provide a head-to-head comparison with PPSV-23. Similarly, the recommendation to use PCV-13 in patients with immune system compromising conditions did not arise from a direct comparison between PCV-13 and PPSV-23. In both groups of patients, ACIP continues to recommend PPSV-23 to extend protection to the 10 additional pneumococcal serotypes not included in PCV-13.28 Additionally, ACIP maintains its long-standing recommendation to administer PPSV-23 to adults aged < 65 years who have diabetes mellitus, or chronic liver, lung, or heart disease, or those who are tobacco smokers or misuse alcohol.
Notably, the order in which the vaccines are administered may influence their effectiveness. In adults aged 50 to 64 years, initial vaccination with PCV-13 followed by PPSV-23 lead to a better antipneumococcal immune response.14,29 Specifically, PCV-13 enhanced the response to subsequent administration of PPSV-23, whereas an initial PPSV-23 vaccine resulted in a decreased response to subsequent administration of PCV-13. After a 1-year interval between vaccines, immune titers to a second vaccination were not inferior.30 All the above considerations have shaped the ACIP recommendations for the administration of pneumococcal vaccines.27,31 Pneumococcal vaccination was a straightforward exercise when PPSV-23 was the only vaccine available for adults. The advent of PCV-13, however, renders pneumococcal immunization of adults more complicated (Figure 1).
Improving Vaccination Rates
The complex recommendations to vaccinate adults against pneumococcus may reveal obstacles to effective pneumococcal vaccination programs.32 From the perspective of health care institutions, the adoption of the new pneumococcal vaccine implies an added cost. A pneumococcal vaccination strategy that incorporates PCV-13 may be cost-effective, at least under certain parameters.33-35 There remains, however, the issue of affordability and the opportunity cost—the need to decrease funding for other health care programs to accommodate an increased budget for pneumococcal vaccination. Logistically, maintaining a reliable supply of vaccines to meet the demand of practitioners at various sites requires careful planning. Consequently, tracking vaccinated and unvaccinated patients and carefully coordinating among clinical providers, nurses, and pharmacists are essential.
From the perspective of clinical providers, an additional pneumococcal vaccine complicating the vaccination schedule for adults represents an increased burden. Providers will need information to reach their own conclusions regarding the rationale behind the development and use of pneumococcal conjugate vaccines, the existing and evolving recommendations from public health authorities, and the strengths and limitations of the evidence supporting the use of pneumococcal vaccines. Otherwise, providers may find it difficult to incorporate new data and guidelines supporting pneumococcal vaccination into their decision-making (Boxes 1-4). An additional and formidable challenge is to carve out time during an already busy clinical encounter to discuss pneumococcal vaccines and other immunizations.36
Related: The Importance of an Antimicrobial Stewardship Program
Similarly, older adults or patients with chronic health conditions may not recognize the important role that vaccines can play in their health maintenance and are likely to prioritize other issues during medical visits. It is not obvious for patients that multiple vaccines may be necessary to prevent pneumococcal disease. Furthermore, many patients, not unreasonably, may assume that their yearly influenza vaccine is sufficiently protective against pneumonia. Therefore, patients need to be educated about the rationale behind pneumococcal conjugate vaccination. Ultimately, access to immunization—the opportunity for patients to have an encounter with their providers and with the health care system that results in the administration of an appropriate vaccine—will determine whether goals for pneumococcal vaccination are achieved.
The evolving landscape for the implementation of pneumococcal vaccines creates the need to develop, implement, and refine organizational changes to adhere to the new guidelines for the use of PCV-13 and PPSV-23 vaccines. These interventions, if effective, may help improve pneumococcal vaccination coverage among adults (Table 3).
Harnessing the Power of the EMR
Interventions to improve the adherence to pneumococcal vaccination guidelines begin by identifying persons eligible for vaccination based on their age, their vaccination status (ie, persons previously unvaccinated or due for vaccination according to the recommended schedule), or the presence of medical conditions conferring high risk for pneumococcal disease. This, in turn, depends on adequate documentation of patients’ underlying medical diagnosis, as well as up-to-date records of vaccine administration to patients.
Health care systems possessing a mature and sophisticated electronic medical record (EMR), such as the VHA Computerized Patient Record System (CPRS), are in a good position to wield such information to plan, implement, and assure the quality of activities designed to improve pneumococcal vaccination rates. An analysis of the proportion of veterans in VISN 10 who received pneumococcal vaccination revealed that even with the advantages of a robust EMR and a highly developed infrastructure devoted to primary care, pneumococcal vaccine coverage remains below the 60% target goal, although well above national averages (Figure 2).37,38
Standing Orders
Standing orders make it possible for nurses and pharmacists to administer vaccines according to a preestablished protocol without a physician’s direct evaluation of each patient. Standing orders are a versatile intervention, with a record of effective implementation in both outpatient and inpatient facilities, in acute-care and long-term care facilities, and in most instances where patients interact with the health care system. Based on strong scientific evidence, ACIP recommends the adoption of standing-order programs to improve pneumococcal vaccination rates among adults.39 Indeed, standing-order programs may prove a very effective intervention to fulfill the recommendation to administer PCV-13 to adults aged > 65 years.
The Virtual Vaccination Clinic
Unfortunately, with 2 vaccines that have to be administered at different times to various groups of patients at risk, the current state of pneumococcal vaccination may be too complex to be readily reduced to a comprehensible set of standing orders. An innovative way to realize the benefit of standing orders is to target high-risk groups for pneumococcal disease who are eligible for vaccination by selecting them using the EMR and entering standing orders tailored to their specific vaccination needs. The selection of patients according to comorbidities and vaccination status and the determination of the appropriate pneumococcal vaccine takes place in the context of a “virtual” vaccination clinic.40
Enhancing Vaccinations
Further improvement in pneumococcal vaccination rates are likely to result from interventions that increase the demand for vaccines among patients and practitioners. Efforts to disseminate information and provide advice regarding pneumococcal vaccination are likely to result in patients seeking and clinicians offering the appropriate vaccine. Similarly, interventions to enhance the supply of vaccines at the point of care may reduce barriers that patients might encounter when attempting to receive vaccinations.
Another set of system-based interventions that can assist clinicians in making timely and appropriate vaccination decisions are EMR reminders, especially those targeting patients at high risk for pneumococcal disease because of underlying illnesses.41 Previous experience with pneumococcal vaccination in patients aged > 65 years indicates that EMR reminders facilitate improvements in vaccination. The involvement of a panel manager who coordinated with the primary care provider and contacted patients directly augmented the effect of the EMR reminder by 25%.42
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
Patient reminder and recall systems also demonstrated effectiveness in improving immunization rates.43 In certain groups, notification of patients has been achieved through electronic methods, such as short text messaging or e-mail.44 Determining which interventions within a bundle are essential may be impossible, because the various interventions reinforce one another, and the likelihood of patients benefitting from at least one of the activities increases when multiple interventions are administered together. Therefore, the Task Force on Community Preventive Services supports combining provider reminder systems with education and other measures that encourage use of vaccines in patients and providers.45
Box answer key: 1: A; 2: A; 3: B; 4: A.
Conclusion
The increasing role of vaccines in the health maintenance of adults represents a change in paradigm for primary care and specialty providers. Physicians must assess the value and limitations of vaccines and find time to discuss immunizations with their adult patients. Health care systems can increase opportunities for vaccination and facilitate encounters that result in vaccination by educating patients and health care personnel and through the innovative use of reminders and standing orders in the EMR. Undertaking these activities may limit the burden of pneumococcal disease, an important cause of morbidity and mortality in adults that is preventable through immunization.
Acknowledgements
This work is dedicated to the memory of John M. Rieger, Information Technology Specialist at the Cleveland VAMC and Chief Master Sergeant, Air Force Reserve.
Author Disclosure
This work was supported by a research grant from Pfizer and by the Louis Stokes Cleveland VAMC, the VISN 10 Geriatric Research Education and Clinical Center, and the Clinical and Translational Science Collaborative of Cleveland (award UL1TR000439 from the National Center for Advancing Translational Sciences of the National Institutes of Health NIH). The content is the responsibility of the authors and does not represent the official views of the NIH or the VA.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144-154.
2. Tilghman RC, Finland M. Clinical significance of bacteremia in pneumococcic pneumonia. Arch Intern Med (Chic). 1937;59(4):602-619.
3. Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2010;36(3):197-204.
4. Lexau CA, Lynfield R, Danila R, et al; Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043-2051.
5. Musher DM. How effective is vaccination in preventing pneumococcal disease? Infect Dis Clin North Am. 2013;27(1):229-241.
6. Macleod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82(6):445-465.
7. Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759-776.
8. Austrian R. The Jeremiah Metzger Lecture: Of gold and pneumococci: a history of pneumococcal vaccines in South Africa. Trans Am Clin Climatol Assoc. 1978;89:141-161.
9. Centers for Disease Control (CDC). Pneumococcal polysaccharide vaccine. MMWR Morb Mortal Wkly Rep. 1981;30(33):410-412, 417-419.
10. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422.
11. Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180(1):48-58.
12. Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004.
13. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Eng J Med. 1991;325(21):1453-1460.
14. Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis. 2011;52(5):633-640.
15. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
16. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Eng J Med. 2013;369(2):155-163.
17. Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
18. Centers for Disease Control and Prevention (CDC). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children--Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):258-261.
19. Iroh Tam PY, Madoff LC, Coombes B, Pelton SI. Invasive pneumococcal disease after implementation of 13-valent conjugate vaccine. Pediatrics. 2014;134(2):210-217.
20. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535-543.
21. French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106-2111.
22. French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Eng J Med. 2010;362(9):812-822.
23. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816-819.
24. Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis. 2012;55(2):259-264.
25. Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin Infect Dis. 2012;55(2):265-267.
26. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Eng J Med. 2015;372(12):1114-1125.
27. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822-825.
28. Grabenstein JD. Effectiveness and serotype coverage: key criteria for pneumococcal vaccines for adults. Clin Infect Dis. 2012;55(2):255-258.
29. Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594-3602.
30. Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32(20):2364-2374.
31. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-947.
32. Walton LR, Orenstein WA, Pickering LK. Lessons learned from making and implementing vaccine recommendations in the U.S. Am J Prev Med. 2015;pii:S0749-3797(15)00333-00335 [Epub ahead of print].
33. Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011-6021.
34. Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950-3956.
35. Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804-812.
36. Kyaw MH, Greene CM, Schaffner W, et al; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Adults with invasive pneumococcal disease: missed opportunities for vaccination. Am J Prev Med. 2006;31(4):286-292.
37. Williams WW, Lu PJ, O'Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(4):95-102.
38. Committee on Review of Priorities in the National Vaccine Plan, Institute of Medicine. Priorities for the National Vaccine Plan. Washington, DC: National Academies Press; 2010.
39. McKibben LJ, Stange PV, Sneller VP, Strikas RA, Rodewald LE; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49(RR-1):15-16.
40. Jump RLP, Banks R, Wilson B, et al. A virtual clinic improves pneumococcal vaccination for asplenic veterans at high-risk for pneumococcal disease. Open Forum Infect Dis. In press.
41. Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61(11):1505-1510.
42. Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552-1558.
43. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
44. Ghadieh AS, Hamadeh GN, Mahmassani DM, Lakkis NA. The effect of various types of patients' reminders on the uptake of pneumococcal vaccine in adults: a randomized controlled trial. Vaccine. 2015;33(43):5868-5872.
45. Ndiaye SM, Hopkins DP, Shefer AM, et al; Task Force on Community Preventive Services. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: a systematic review. Am J Prev Med. 2005;28(5)(suppl):248-279.
Streptococcus pneumoniae (S pneumoniae), also known as pneumococcus, is a successful human pathogen with significant clinical impact that causes pneumonia and invasive infections, including bacteremia and meningitis.1 In the preantibiotic era, nearly 80% of patients with bacteremic pneumococcal pneumonia died.2 The introduction of sulfas and penicillin in the mid-20th century, subsequent refinements in antibiotic chemotherapy, and improvements in supportive care rendered pneumococcal disease readily treatable, notwithstanding the threat of antibiotic-resistant pneumococcus.3 Despite the availability of effective antibiotic therapy against S pneumoniae, pneumococcal disease remains a significant cause of morbidity and mortality among people with increased susceptibility, such as older adults and those living with chronic illness or immunosuppressive conditions. In developed countries like the U.S., where a growing portion of the population is vulnerable to S pneumoniae by virtue of their advanced age and underlying medical conditions, pneumococcal disease is still an important public health concern.4
Penumococcal Vaccines: A Long Time Coming
Vaccination against S pneumoniae has proven an efficacious strategy to reduce the morbidity and mortality associated with this pathogen.5 The original efforts to develop a pneumococcal vaccine culminated in 1945 with a vaccine containing pneumococcal capsular polysaccharides, which elicited a protective immune response among U.S. soldiers (Table 1).Subsequent investigations determined that the protective response was specific to pneumococcal disease caused by the 4 pneumococcal capsular serotypes included in the vaccine, that the carrier rate of pneumococcus with the vaccine serotypes decreased by about 50%, and that the incidence of pneumonia from the vaccine serotypes was reduced even in nonimmunized soldiers.6 These early observations remain relevant to our contemporary understanding of the impact of pneumococcal vaccination: Protection is limited to serotypes included in the vaccine; the vaccine reduces colonization; and the vaccine leads to herd immunity—the protection of unvaccinated subjects.
Despite this achievement, the use of vaccines as a strategy to combat pneumococcal disease was upstaged in the subsequent decades by the success of antibiotics. Renewed interest in pneumococcal vaccines resulted from the efforts of Robert Austrian, MD, who astutely observed that “highly effective antimicrobial drugs must be supplemented by other measures, both prophylactic and therapeutic, if the significant mortality resulting still from pneumococcal infection is to be reduced.”7
After initial studies carried out in South African gold miners, the FDA approved a pneumococcal polysaccharide vaccine (PPSV) against 23 of about 90 circulating serotypes.8 The CDC and the Advisory Committee on Immunization Practices (ACIP) initially recommended PPSV-23 for persons perceived to be at high risk for pneumococcal disease, including those with chronic diseases, immunocompromising conditions, and older adults.9
Three decades later, the analysis of a large body of available evidence demonstrated the protective effects of PPSV-23 against invasive pneumococcal disease caused by pneumococcal types included in the vaccine, especially bacteremic pneumonia (about 75% reduction, odds ratio [OR] 0.26, 95% confidence interval [CI] 0.14-0.45).10 A perceived shortcoming of PPSV-23, which some experts dispute, is the lack of definite protection against nonbacteremic pneumococcal pneumonia.11 Studies of nursing home residents demonstrated a 50% reduction in the incidence of both pneumococcal pneumonia and all-cause pneumonia, suggesting that PPSV-23 offers protection against noninvasive pneumococcal pneumonia in specific populations.12 Additional potential limitations of PPSV-23 include reduced benefit in patients aged > 65 years and waning of protection over time.13
Related: Identification and Management of Middle East Respiratory Syndrome
Protein-Conjugate Vaccines
Clearly, the most important limitation of PPSV-23, inherent to all capsular polysaccharide vaccines, is that it does not elicit an immune response in children aged < 2 years. The successful development of a vaccine against Haemophilus influenzae type b (Hib) gave rise to a new generation of pneumococcal vaccines.14 Specifically, the Hib vaccine covalently binds, or conjugates, the capsule polysaccharide to an antigenic protein, leading to effective T-cell–mediated antibody production in infants and toddlers.
In 2000, children received the first iteration of a protein-conjugate vaccine containing the 7 most relevant pneumococcal serotypes (PCV-7).15 The effects of PCV-7 on pneumococcal disease have been extraordinary, practically eliminating infections caused by the pneumococcal serotypes included in the vaccine. Immunizing children with PCV-7 also ushered in a fundamental public health benefit for adults aged > 65 years: a reduction of nearly 90% in the incidence of pneumococcal infections caused by serotypes included in the vaccine. By eliciting mucosal immunity, which leads to decreased nasal carriage of the covered pneumococcal strains among children, PVC-7 generates herd immunity, leading to reductions in transmission, colonization, and infection with vaccine serotypes among adults.16
In 2010, a conjugate vaccine containing 13 serotypes (PCV-13) replaced PCV-7 administration for children.17,18 The PCV-13 is expected to protect children and the herd from disease caused by 6 additional pneumococcal serotypes, including those that surged as replacement strains, filling the ecologic niche created by PCV-7, such as the epidemiologically relevant serotype 19A.19,20
Immune-Compromised Adults
One of the shortcomings of PPSV-23 is its lack of efficacy in patients with advanced HIV, a group with an exquisite vulnerability to pneumococcal disease, as demonstrated by a randomized controlled trial of PPSV-23 in African patients with advanced, untreated HIV infection.21 Similarly, there is concern that patients with lymphoma, leukemia, multiple myeloma, and Hodgkin disease, also at high risk of pneumococcal infection, do not mount sufficient immune responses to polysaccharide antigens or that these responses are adversely affected by chemotherapy or immune suppressing medications. In these populations, a conjugate vaccine (PCV-7 or PCV-13) may elicit a more robust and durable immune response than a polysaccharide vaccine (PPSV-23). A randomized placebo-controlled trial demonstrated the efficacy of PCV-7 in protecting patients with advanced HIV against pneumococcal disease in Africa.22 Based in part on this observation, the ACIP now recommends the use of PCV-13 in patients with HIV and other immunosuppressing conditions, including chronic renal failure.23 A direct comparison of the relative protection of PPSV-23 vs PCV-7 in this population has not been performed.
Pneumococcal Vaccines in the Elderly
Although PPSV-23 was widely adopted in the U.S. with the intention to protect adults aged > 65 years from pneumococcal infection, this vaccine did not achieve global appeal. In the Netherlands, for instance, PPSV-23 had very low penetration and was never endorsed by public health authorities. Concerns have been issued about the decreased immunogenicity of a polysaccharide vaccine in older adults, invoking the concept of immune senescence, a term that describes a diminished capacity to mount robust immune responses due to aging.
Protein-conjugated antigens, on the other hand, can elicit a better initial immune response than a polysaccharide vaccine can in an older adult. Whether the effect is sustained and translates to better clinical outcomes remains unknown. The adoption of PCV-13 and the continued role of PPSV-23 in adults aged > 65 years have been examined carefully by experts.24,25
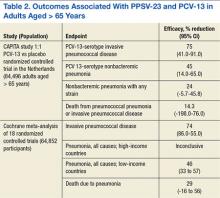
Dutch investigators organized the Community Acquired Pneumonia Immunization Trial in Adults (CAPITA), randomizing about 85,000 adults aged > 65 years toreceive eitherPCV-13 or a placebo.26 After 3 years of observation, the occurrence of invasive pneumococcal disease (caused by the vaccine serotypes) decreased by 85% in participants who received the vaccine compared with those who received a placebo. Additionally, the incidence of pneumococcal pneumonia (caused by the vaccine serotypes) decreased by 45% (Table 2). The findings of this trial largely inform the recommendation issued by APIC in October 2014 to administer PCV-13 vaccine to adults aged > 65 years.27
Polysaccharide Vaccine in Adults
A crucial limitation of the CAPITA study is that it does not provide a head-to-head comparison with PPSV-23. Similarly, the recommendation to use PCV-13 in patients with immune system compromising conditions did not arise from a direct comparison between PCV-13 and PPSV-23. In both groups of patients, ACIP continues to recommend PPSV-23 to extend protection to the 10 additional pneumococcal serotypes not included in PCV-13.28 Additionally, ACIP maintains its long-standing recommendation to administer PPSV-23 to adults aged < 65 years who have diabetes mellitus, or chronic liver, lung, or heart disease, or those who are tobacco smokers or misuse alcohol.
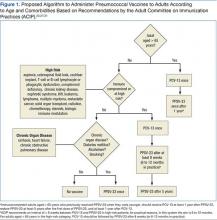
Notably, the order in which the vaccines are administered may influence their effectiveness. In adults aged 50 to 64 years, initial vaccination with PCV-13 followed by PPSV-23 lead to a better antipneumococcal immune response.14,29 Specifically, PCV-13 enhanced the response to subsequent administration of PPSV-23, whereas an initial PPSV-23 vaccine resulted in a decreased response to subsequent administration of PCV-13. After a 1-year interval between vaccines, immune titers to a second vaccination were not inferior.30 All the above considerations have shaped the ACIP recommendations for the administration of pneumococcal vaccines.27,31 Pneumococcal vaccination was a straightforward exercise when PPSV-23 was the only vaccine available for adults. The advent of PCV-13, however, renders pneumococcal immunization of adults more complicated (Figure 1).
Improving Vaccination Rates
The complex recommendations to vaccinate adults against pneumococcus may reveal obstacles to effective pneumococcal vaccination programs.32 From the perspective of health care institutions, the adoption of the new pneumococcal vaccine implies an added cost. A pneumococcal vaccination strategy that incorporates PCV-13 may be cost-effective, at least under certain parameters.33-35 There remains, however, the issue of affordability and the opportunity cost—the need to decrease funding for other health care programs to accommodate an increased budget for pneumococcal vaccination. Logistically, maintaining a reliable supply of vaccines to meet the demand of practitioners at various sites requires careful planning. Consequently, tracking vaccinated and unvaccinated patients and carefully coordinating among clinical providers, nurses, and pharmacists are essential.
From the perspective of clinical providers, an additional pneumococcal vaccine complicating the vaccination schedule for adults represents an increased burden. Providers will need information to reach their own conclusions regarding the rationale behind the development and use of pneumococcal conjugate vaccines, the existing and evolving recommendations from public health authorities, and the strengths and limitations of the evidence supporting the use of pneumococcal vaccines. Otherwise, providers may find it difficult to incorporate new data and guidelines supporting pneumococcal vaccination into their decision-making (Boxes 1-4). An additional and formidable challenge is to carve out time during an already busy clinical encounter to discuss pneumococcal vaccines and other immunizations.36
Related: The Importance of an Antimicrobial Stewardship Program
Similarly, older adults or patients with chronic health conditions may not recognize the important role that vaccines can play in their health maintenance and are likely to prioritize other issues during medical visits. It is not obvious for patients that multiple vaccines may be necessary to prevent pneumococcal disease. Furthermore, many patients, not unreasonably, may assume that their yearly influenza vaccine is sufficiently protective against pneumonia. Therefore, patients need to be educated about the rationale behind pneumococcal conjugate vaccination. Ultimately, access to immunization—the opportunity for patients to have an encounter with their providers and with the health care system that results in the administration of an appropriate vaccine—will determine whether goals for pneumococcal vaccination are achieved.
The evolving landscape for the implementation of pneumococcal vaccines creates the need to develop, implement, and refine organizational changes to adhere to the new guidelines for the use of PCV-13 and PPSV-23 vaccines. These interventions, if effective, may help improve pneumococcal vaccination coverage among adults (Table 3).
Harnessing the Power of the EMR
Interventions to improve the adherence to pneumococcal vaccination guidelines begin by identifying persons eligible for vaccination based on their age, their vaccination status (ie, persons previously unvaccinated or due for vaccination according to the recommended schedule), or the presence of medical conditions conferring high risk for pneumococcal disease. This, in turn, depends on adequate documentation of patients’ underlying medical diagnosis, as well as up-to-date records of vaccine administration to patients.
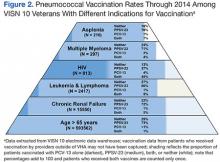
Health care systems possessing a mature and sophisticated electronic medical record (EMR), such as the VHA Computerized Patient Record System (CPRS), are in a good position to wield such information to plan, implement, and assure the quality of activities designed to improve pneumococcal vaccination rates. An analysis of the proportion of veterans in VISN 10 who received pneumococcal vaccination revealed that even with the advantages of a robust EMR and a highly developed infrastructure devoted to primary care, pneumococcal vaccine coverage remains below the 60% target goal, although well above national averages (Figure 2).37,38
Standing Orders
Standing orders make it possible for nurses and pharmacists to administer vaccines according to a preestablished protocol without a physician’s direct evaluation of each patient. Standing orders are a versatile intervention, with a record of effective implementation in both outpatient and inpatient facilities, in acute-care and long-term care facilities, and in most instances where patients interact with the health care system. Based on strong scientific evidence, ACIP recommends the adoption of standing-order programs to improve pneumococcal vaccination rates among adults.39 Indeed, standing-order programs may prove a very effective intervention to fulfill the recommendation to administer PCV-13 to adults aged > 65 years.
The Virtual Vaccination Clinic
Unfortunately, with 2 vaccines that have to be administered at different times to various groups of patients at risk, the current state of pneumococcal vaccination may be too complex to be readily reduced to a comprehensible set of standing orders. An innovative way to realize the benefit of standing orders is to target high-risk groups for pneumococcal disease who are eligible for vaccination by selecting them using the EMR and entering standing orders tailored to their specific vaccination needs. The selection of patients according to comorbidities and vaccination status and the determination of the appropriate pneumococcal vaccine takes place in the context of a “virtual” vaccination clinic.40
Enhancing Vaccinations
Further improvement in pneumococcal vaccination rates are likely to result from interventions that increase the demand for vaccines among patients and practitioners. Efforts to disseminate information and provide advice regarding pneumococcal vaccination are likely to result in patients seeking and clinicians offering the appropriate vaccine. Similarly, interventions to enhance the supply of vaccines at the point of care may reduce barriers that patients might encounter when attempting to receive vaccinations.
Another set of system-based interventions that can assist clinicians in making timely and appropriate vaccination decisions are EMR reminders, especially those targeting patients at high risk for pneumococcal disease because of underlying illnesses.41 Previous experience with pneumococcal vaccination in patients aged > 65 years indicates that EMR reminders facilitate improvements in vaccination. The involvement of a panel manager who coordinated with the primary care provider and contacted patients directly augmented the effect of the EMR reminder by 25%.42
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
Patient reminder and recall systems also demonstrated effectiveness in improving immunization rates.43 In certain groups, notification of patients has been achieved through electronic methods, such as short text messaging or e-mail.44 Determining which interventions within a bundle are essential may be impossible, because the various interventions reinforce one another, and the likelihood of patients benefitting from at least one of the activities increases when multiple interventions are administered together. Therefore, the Task Force on Community Preventive Services supports combining provider reminder systems with education and other measures that encourage use of vaccines in patients and providers.45
Box answer key: 1: A; 2: A; 3: B; 4: A.
Conclusion
The increasing role of vaccines in the health maintenance of adults represents a change in paradigm for primary care and specialty providers. Physicians must assess the value and limitations of vaccines and find time to discuss immunizations with their adult patients. Health care systems can increase opportunities for vaccination and facilitate encounters that result in vaccination by educating patients and health care personnel and through the innovative use of reminders and standing orders in the EMR. Undertaking these activities may limit the burden of pneumococcal disease, an important cause of morbidity and mortality in adults that is preventable through immunization.
Acknowledgements
This work is dedicated to the memory of John M. Rieger, Information Technology Specialist at the Cleveland VAMC and Chief Master Sergeant, Air Force Reserve.
Author Disclosure
This work was supported by a research grant from Pfizer and by the Louis Stokes Cleveland VAMC, the VISN 10 Geriatric Research Education and Clinical Center, and the Clinical and Translational Science Collaborative of Cleveland (award UL1TR000439 from the National Center for Advancing Translational Sciences of the National Institutes of Health NIH). The content is the responsibility of the authors and does not represent the official views of the NIH or the VA.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Streptococcus pneumoniae (S pneumoniae), also known as pneumococcus, is a successful human pathogen with significant clinical impact that causes pneumonia and invasive infections, including bacteremia and meningitis.1 In the preantibiotic era, nearly 80% of patients with bacteremic pneumococcal pneumonia died.2 The introduction of sulfas and penicillin in the mid-20th century, subsequent refinements in antibiotic chemotherapy, and improvements in supportive care rendered pneumococcal disease readily treatable, notwithstanding the threat of antibiotic-resistant pneumococcus.3 Despite the availability of effective antibiotic therapy against S pneumoniae, pneumococcal disease remains a significant cause of morbidity and mortality among people with increased susceptibility, such as older adults and those living with chronic illness or immunosuppressive conditions. In developed countries like the U.S., where a growing portion of the population is vulnerable to S pneumoniae by virtue of their advanced age and underlying medical conditions, pneumococcal disease is still an important public health concern.4
Penumococcal Vaccines: A Long Time Coming
Vaccination against S pneumoniae has proven an efficacious strategy to reduce the morbidity and mortality associated with this pathogen.5 The original efforts to develop a pneumococcal vaccine culminated in 1945 with a vaccine containing pneumococcal capsular polysaccharides, which elicited a protective immune response among U.S. soldiers (Table 1).Subsequent investigations determined that the protective response was specific to pneumococcal disease caused by the 4 pneumococcal capsular serotypes included in the vaccine, that the carrier rate of pneumococcus with the vaccine serotypes decreased by about 50%, and that the incidence of pneumonia from the vaccine serotypes was reduced even in nonimmunized soldiers.6 These early observations remain relevant to our contemporary understanding of the impact of pneumococcal vaccination: Protection is limited to serotypes included in the vaccine; the vaccine reduces colonization; and the vaccine leads to herd immunity—the protection of unvaccinated subjects.
Despite this achievement, the use of vaccines as a strategy to combat pneumococcal disease was upstaged in the subsequent decades by the success of antibiotics. Renewed interest in pneumococcal vaccines resulted from the efforts of Robert Austrian, MD, who astutely observed that “highly effective antimicrobial drugs must be supplemented by other measures, both prophylactic and therapeutic, if the significant mortality resulting still from pneumococcal infection is to be reduced.”7
After initial studies carried out in South African gold miners, the FDA approved a pneumococcal polysaccharide vaccine (PPSV) against 23 of about 90 circulating serotypes.8 The CDC and the Advisory Committee on Immunization Practices (ACIP) initially recommended PPSV-23 for persons perceived to be at high risk for pneumococcal disease, including those with chronic diseases, immunocompromising conditions, and older adults.9
Three decades later, the analysis of a large body of available evidence demonstrated the protective effects of PPSV-23 against invasive pneumococcal disease caused by pneumococcal types included in the vaccine, especially bacteremic pneumonia (about 75% reduction, odds ratio [OR] 0.26, 95% confidence interval [CI] 0.14-0.45).10 A perceived shortcoming of PPSV-23, which some experts dispute, is the lack of definite protection against nonbacteremic pneumococcal pneumonia.11 Studies of nursing home residents demonstrated a 50% reduction in the incidence of both pneumococcal pneumonia and all-cause pneumonia, suggesting that PPSV-23 offers protection against noninvasive pneumococcal pneumonia in specific populations.12 Additional potential limitations of PPSV-23 include reduced benefit in patients aged > 65 years and waning of protection over time.13
Related: Identification and Management of Middle East Respiratory Syndrome
Protein-Conjugate Vaccines
Clearly, the most important limitation of PPSV-23, inherent to all capsular polysaccharide vaccines, is that it does not elicit an immune response in children aged < 2 years. The successful development of a vaccine against Haemophilus influenzae type b (Hib) gave rise to a new generation of pneumococcal vaccines.14 Specifically, the Hib vaccine covalently binds, or conjugates, the capsule polysaccharide to an antigenic protein, leading to effective T-cell–mediated antibody production in infants and toddlers.
In 2000, children received the first iteration of a protein-conjugate vaccine containing the 7 most relevant pneumococcal serotypes (PCV-7).15 The effects of PCV-7 on pneumococcal disease have been extraordinary, practically eliminating infections caused by the pneumococcal serotypes included in the vaccine. Immunizing children with PCV-7 also ushered in a fundamental public health benefit for adults aged > 65 years: a reduction of nearly 90% in the incidence of pneumococcal infections caused by serotypes included in the vaccine. By eliciting mucosal immunity, which leads to decreased nasal carriage of the covered pneumococcal strains among children, PVC-7 generates herd immunity, leading to reductions in transmission, colonization, and infection with vaccine serotypes among adults.16
In 2010, a conjugate vaccine containing 13 serotypes (PCV-13) replaced PCV-7 administration for children.17,18 The PCV-13 is expected to protect children and the herd from disease caused by 6 additional pneumococcal serotypes, including those that surged as replacement strains, filling the ecologic niche created by PCV-7, such as the epidemiologically relevant serotype 19A.19,20
Immune-Compromised Adults
One of the shortcomings of PPSV-23 is its lack of efficacy in patients with advanced HIV, a group with an exquisite vulnerability to pneumococcal disease, as demonstrated by a randomized controlled trial of PPSV-23 in African patients with advanced, untreated HIV infection.21 Similarly, there is concern that patients with lymphoma, leukemia, multiple myeloma, and Hodgkin disease, also at high risk of pneumococcal infection, do not mount sufficient immune responses to polysaccharide antigens or that these responses are adversely affected by chemotherapy or immune suppressing medications. In these populations, a conjugate vaccine (PCV-7 or PCV-13) may elicit a more robust and durable immune response than a polysaccharide vaccine (PPSV-23). A randomized placebo-controlled trial demonstrated the efficacy of PCV-7 in protecting patients with advanced HIV against pneumococcal disease in Africa.22 Based in part on this observation, the ACIP now recommends the use of PCV-13 in patients with HIV and other immunosuppressing conditions, including chronic renal failure.23 A direct comparison of the relative protection of PPSV-23 vs PCV-7 in this population has not been performed.
Pneumococcal Vaccines in the Elderly
Although PPSV-23 was widely adopted in the U.S. with the intention to protect adults aged > 65 years from pneumococcal infection, this vaccine did not achieve global appeal. In the Netherlands, for instance, PPSV-23 had very low penetration and was never endorsed by public health authorities. Concerns have been issued about the decreased immunogenicity of a polysaccharide vaccine in older adults, invoking the concept of immune senescence, a term that describes a diminished capacity to mount robust immune responses due to aging.
Protein-conjugated antigens, on the other hand, can elicit a better initial immune response than a polysaccharide vaccine can in an older adult. Whether the effect is sustained and translates to better clinical outcomes remains unknown. The adoption of PCV-13 and the continued role of PPSV-23 in adults aged > 65 years have been examined carefully by experts.24,25
Dutch investigators organized the Community Acquired Pneumonia Immunization Trial in Adults (CAPITA), randomizing about 85,000 adults aged > 65 years toreceive eitherPCV-13 or a placebo.26 After 3 years of observation, the occurrence of invasive pneumococcal disease (caused by the vaccine serotypes) decreased by 85% in participants who received the vaccine compared with those who received a placebo. Additionally, the incidence of pneumococcal pneumonia (caused by the vaccine serotypes) decreased by 45% (Table 2). The findings of this trial largely inform the recommendation issued by APIC in October 2014 to administer PCV-13 vaccine to adults aged > 65 years.27
Polysaccharide Vaccine in Adults
A crucial limitation of the CAPITA study is that it does not provide a head-to-head comparison with PPSV-23. Similarly, the recommendation to use PCV-13 in patients with immune system compromising conditions did not arise from a direct comparison between PCV-13 and PPSV-23. In both groups of patients, ACIP continues to recommend PPSV-23 to extend protection to the 10 additional pneumococcal serotypes not included in PCV-13.28 Additionally, ACIP maintains its long-standing recommendation to administer PPSV-23 to adults aged < 65 years who have diabetes mellitus, or chronic liver, lung, or heart disease, or those who are tobacco smokers or misuse alcohol.
Notably, the order in which the vaccines are administered may influence their effectiveness. In adults aged 50 to 64 years, initial vaccination with PCV-13 followed by PPSV-23 lead to a better antipneumococcal immune response.14,29 Specifically, PCV-13 enhanced the response to subsequent administration of PPSV-23, whereas an initial PPSV-23 vaccine resulted in a decreased response to subsequent administration of PCV-13. After a 1-year interval between vaccines, immune titers to a second vaccination were not inferior.30 All the above considerations have shaped the ACIP recommendations for the administration of pneumococcal vaccines.27,31 Pneumococcal vaccination was a straightforward exercise when PPSV-23 was the only vaccine available for adults. The advent of PCV-13, however, renders pneumococcal immunization of adults more complicated (Figure 1).
Improving Vaccination Rates
The complex recommendations to vaccinate adults against pneumococcus may reveal obstacles to effective pneumococcal vaccination programs.32 From the perspective of health care institutions, the adoption of the new pneumococcal vaccine implies an added cost. A pneumococcal vaccination strategy that incorporates PCV-13 may be cost-effective, at least under certain parameters.33-35 There remains, however, the issue of affordability and the opportunity cost—the need to decrease funding for other health care programs to accommodate an increased budget for pneumococcal vaccination. Logistically, maintaining a reliable supply of vaccines to meet the demand of practitioners at various sites requires careful planning. Consequently, tracking vaccinated and unvaccinated patients and carefully coordinating among clinical providers, nurses, and pharmacists are essential.
From the perspective of clinical providers, an additional pneumococcal vaccine complicating the vaccination schedule for adults represents an increased burden. Providers will need information to reach their own conclusions regarding the rationale behind the development and use of pneumococcal conjugate vaccines, the existing and evolving recommendations from public health authorities, and the strengths and limitations of the evidence supporting the use of pneumococcal vaccines. Otherwise, providers may find it difficult to incorporate new data and guidelines supporting pneumococcal vaccination into their decision-making (Boxes 1-4). An additional and formidable challenge is to carve out time during an already busy clinical encounter to discuss pneumococcal vaccines and other immunizations.36
Related: The Importance of an Antimicrobial Stewardship Program
Similarly, older adults or patients with chronic health conditions may not recognize the important role that vaccines can play in their health maintenance and are likely to prioritize other issues during medical visits. It is not obvious for patients that multiple vaccines may be necessary to prevent pneumococcal disease. Furthermore, many patients, not unreasonably, may assume that their yearly influenza vaccine is sufficiently protective against pneumonia. Therefore, patients need to be educated about the rationale behind pneumococcal conjugate vaccination. Ultimately, access to immunization—the opportunity for patients to have an encounter with their providers and with the health care system that results in the administration of an appropriate vaccine—will determine whether goals for pneumococcal vaccination are achieved.
The evolving landscape for the implementation of pneumococcal vaccines creates the need to develop, implement, and refine organizational changes to adhere to the new guidelines for the use of PCV-13 and PPSV-23 vaccines. These interventions, if effective, may help improve pneumococcal vaccination coverage among adults (Table 3).
Harnessing the Power of the EMR
Interventions to improve the adherence to pneumococcal vaccination guidelines begin by identifying persons eligible for vaccination based on their age, their vaccination status (ie, persons previously unvaccinated or due for vaccination according to the recommended schedule), or the presence of medical conditions conferring high risk for pneumococcal disease. This, in turn, depends on adequate documentation of patients’ underlying medical diagnosis, as well as up-to-date records of vaccine administration to patients.
Health care systems possessing a mature and sophisticated electronic medical record (EMR), such as the VHA Computerized Patient Record System (CPRS), are in a good position to wield such information to plan, implement, and assure the quality of activities designed to improve pneumococcal vaccination rates. An analysis of the proportion of veterans in VISN 10 who received pneumococcal vaccination revealed that even with the advantages of a robust EMR and a highly developed infrastructure devoted to primary care, pneumococcal vaccine coverage remains below the 60% target goal, although well above national averages (Figure 2).37,38
Standing Orders
Standing orders make it possible for nurses and pharmacists to administer vaccines according to a preestablished protocol without a physician’s direct evaluation of each patient. Standing orders are a versatile intervention, with a record of effective implementation in both outpatient and inpatient facilities, in acute-care and long-term care facilities, and in most instances where patients interact with the health care system. Based on strong scientific evidence, ACIP recommends the adoption of standing-order programs to improve pneumococcal vaccination rates among adults.39 Indeed, standing-order programs may prove a very effective intervention to fulfill the recommendation to administer PCV-13 to adults aged > 65 years.
The Virtual Vaccination Clinic
Unfortunately, with 2 vaccines that have to be administered at different times to various groups of patients at risk, the current state of pneumococcal vaccination may be too complex to be readily reduced to a comprehensible set of standing orders. An innovative way to realize the benefit of standing orders is to target high-risk groups for pneumococcal disease who are eligible for vaccination by selecting them using the EMR and entering standing orders tailored to their specific vaccination needs. The selection of patients according to comorbidities and vaccination status and the determination of the appropriate pneumococcal vaccine takes place in the context of a “virtual” vaccination clinic.40
Enhancing Vaccinations
Further improvement in pneumococcal vaccination rates are likely to result from interventions that increase the demand for vaccines among patients and practitioners. Efforts to disseminate information and provide advice regarding pneumococcal vaccination are likely to result in patients seeking and clinicians offering the appropriate vaccine. Similarly, interventions to enhance the supply of vaccines at the point of care may reduce barriers that patients might encounter when attempting to receive vaccinations.
Another set of system-based interventions that can assist clinicians in making timely and appropriate vaccination decisions are EMR reminders, especially those targeting patients at high risk for pneumococcal disease because of underlying illnesses.41 Previous experience with pneumococcal vaccination in patients aged > 65 years indicates that EMR reminders facilitate improvements in vaccination. The involvement of a panel manager who coordinated with the primary care provider and contacted patients directly augmented the effect of the EMR reminder by 25%.42
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
Patient reminder and recall systems also demonstrated effectiveness in improving immunization rates.43 In certain groups, notification of patients has been achieved through electronic methods, such as short text messaging or e-mail.44 Determining which interventions within a bundle are essential may be impossible, because the various interventions reinforce one another, and the likelihood of patients benefitting from at least one of the activities increases when multiple interventions are administered together. Therefore, the Task Force on Community Preventive Services supports combining provider reminder systems with education and other measures that encourage use of vaccines in patients and providers.45
Box answer key: 1: A; 2: A; 3: B; 4: A.
Conclusion
The increasing role of vaccines in the health maintenance of adults represents a change in paradigm for primary care and specialty providers. Physicians must assess the value and limitations of vaccines and find time to discuss immunizations with their adult patients. Health care systems can increase opportunities for vaccination and facilitate encounters that result in vaccination by educating patients and health care personnel and through the innovative use of reminders and standing orders in the EMR. Undertaking these activities may limit the burden of pneumococcal disease, an important cause of morbidity and mortality in adults that is preventable through immunization.
Acknowledgements
This work is dedicated to the memory of John M. Rieger, Information Technology Specialist at the Cleveland VAMC and Chief Master Sergeant, Air Force Reserve.
Author Disclosure
This work was supported by a research grant from Pfizer and by the Louis Stokes Cleveland VAMC, the VISN 10 Geriatric Research Education and Clinical Center, and the Clinical and Translational Science Collaborative of Cleveland (award UL1TR000439 from the National Center for Advancing Translational Sciences of the National Institutes of Health NIH). The content is the responsibility of the authors and does not represent the official views of the NIH or the VA.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144-154.
2. Tilghman RC, Finland M. Clinical significance of bacteremia in pneumococcic pneumonia. Arch Intern Med (Chic). 1937;59(4):602-619.
3. Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2010;36(3):197-204.
4. Lexau CA, Lynfield R, Danila R, et al; Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043-2051.
5. Musher DM. How effective is vaccination in preventing pneumococcal disease? Infect Dis Clin North Am. 2013;27(1):229-241.
6. Macleod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82(6):445-465.
7. Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759-776.
8. Austrian R. The Jeremiah Metzger Lecture: Of gold and pneumococci: a history of pneumococcal vaccines in South Africa. Trans Am Clin Climatol Assoc. 1978;89:141-161.
9. Centers for Disease Control (CDC). Pneumococcal polysaccharide vaccine. MMWR Morb Mortal Wkly Rep. 1981;30(33):410-412, 417-419.
10. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422.
11. Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180(1):48-58.
12. Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004.
13. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Eng J Med. 1991;325(21):1453-1460.
14. Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis. 2011;52(5):633-640.
15. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
16. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Eng J Med. 2013;369(2):155-163.
17. Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
18. Centers for Disease Control and Prevention (CDC). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children--Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):258-261.
19. Iroh Tam PY, Madoff LC, Coombes B, Pelton SI. Invasive pneumococcal disease after implementation of 13-valent conjugate vaccine. Pediatrics. 2014;134(2):210-217.
20. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535-543.
21. French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106-2111.
22. French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Eng J Med. 2010;362(9):812-822.
23. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816-819.
24. Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis. 2012;55(2):259-264.
25. Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin Infect Dis. 2012;55(2):265-267.
26. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Eng J Med. 2015;372(12):1114-1125.
27. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822-825.
28. Grabenstein JD. Effectiveness and serotype coverage: key criteria for pneumococcal vaccines for adults. Clin Infect Dis. 2012;55(2):255-258.
29. Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594-3602.
30. Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32(20):2364-2374.
31. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-947.
32. Walton LR, Orenstein WA, Pickering LK. Lessons learned from making and implementing vaccine recommendations in the U.S. Am J Prev Med. 2015;pii:S0749-3797(15)00333-00335 [Epub ahead of print].
33. Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011-6021.
34. Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950-3956.
35. Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804-812.
36. Kyaw MH, Greene CM, Schaffner W, et al; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Adults with invasive pneumococcal disease: missed opportunities for vaccination. Am J Prev Med. 2006;31(4):286-292.
37. Williams WW, Lu PJ, O'Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(4):95-102.
38. Committee on Review of Priorities in the National Vaccine Plan, Institute of Medicine. Priorities for the National Vaccine Plan. Washington, DC: National Academies Press; 2010.
39. McKibben LJ, Stange PV, Sneller VP, Strikas RA, Rodewald LE; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49(RR-1):15-16.
40. Jump RLP, Banks R, Wilson B, et al. A virtual clinic improves pneumococcal vaccination for asplenic veterans at high-risk for pneumococcal disease. Open Forum Infect Dis. In press.
41. Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61(11):1505-1510.
42. Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552-1558.
43. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
44. Ghadieh AS, Hamadeh GN, Mahmassani DM, Lakkis NA. The effect of various types of patients' reminders on the uptake of pneumococcal vaccine in adults: a randomized controlled trial. Vaccine. 2015;33(43):5868-5872.
45. Ndiaye SM, Hopkins DP, Shefer AM, et al; Task Force on Community Preventive Services. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: a systematic review. Am J Prev Med. 2005;28(5)(suppl):248-279.
1. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144-154.
2. Tilghman RC, Finland M. Clinical significance of bacteremia in pneumococcic pneumonia. Arch Intern Med (Chic). 1937;59(4):602-619.
3. Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2010;36(3):197-204.
4. Lexau CA, Lynfield R, Danila R, et al; Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043-2051.
5. Musher DM. How effective is vaccination in preventing pneumococcal disease? Infect Dis Clin North Am. 2013;27(1):229-241.
6. Macleod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82(6):445-465.
7. Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759-776.
8. Austrian R. The Jeremiah Metzger Lecture: Of gold and pneumococci: a history of pneumococcal vaccines in South Africa. Trans Am Clin Climatol Assoc. 1978;89:141-161.
9. Centers for Disease Control (CDC). Pneumococcal polysaccharide vaccine. MMWR Morb Mortal Wkly Rep. 1981;30(33):410-412, 417-419.
10. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422.
11. Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180(1):48-58.
12. Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004.
13. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Eng J Med. 1991;325(21):1453-1460.
14. Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis. 2011;52(5):633-640.
15. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
16. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Eng J Med. 2013;369(2):155-163.
17. Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
18. Centers for Disease Control and Prevention (CDC). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children--Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):258-261.
19. Iroh Tam PY, Madoff LC, Coombes B, Pelton SI. Invasive pneumococcal disease after implementation of 13-valent conjugate vaccine. Pediatrics. 2014;134(2):210-217.
20. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535-543.
21. French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106-2111.
22. French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Eng J Med. 2010;362(9):812-822.
23. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816-819.
24. Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis. 2012;55(2):259-264.
25. Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin Infect Dis. 2012;55(2):265-267.
26. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Eng J Med. 2015;372(12):1114-1125.
27. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822-825.
28. Grabenstein JD. Effectiveness and serotype coverage: key criteria for pneumococcal vaccines for adults. Clin Infect Dis. 2012;55(2):255-258.
29. Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594-3602.
30. Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32(20):2364-2374.
31. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-947.
32. Walton LR, Orenstein WA, Pickering LK. Lessons learned from making and implementing vaccine recommendations in the U.S. Am J Prev Med. 2015;pii:S0749-3797(15)00333-00335 [Epub ahead of print].
33. Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011-6021.
34. Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950-3956.
35. Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804-812.
36. Kyaw MH, Greene CM, Schaffner W, et al; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Adults with invasive pneumococcal disease: missed opportunities for vaccination. Am J Prev Med. 2006;31(4):286-292.
37. Williams WW, Lu PJ, O'Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(4):95-102.
38. Committee on Review of Priorities in the National Vaccine Plan, Institute of Medicine. Priorities for the National Vaccine Plan. Washington, DC: National Academies Press; 2010.
39. McKibben LJ, Stange PV, Sneller VP, Strikas RA, Rodewald LE; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49(RR-1):15-16.
40. Jump RLP, Banks R, Wilson B, et al. A virtual clinic improves pneumococcal vaccination for asplenic veterans at high-risk for pneumococcal disease. Open Forum Infect Dis. In press.
41. Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61(11):1505-1510.
42. Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552-1558.
43. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
44. Ghadieh AS, Hamadeh GN, Mahmassani DM, Lakkis NA. The effect of various types of patients' reminders on the uptake of pneumococcal vaccine in adults: a randomized controlled trial. Vaccine. 2015;33(43):5868-5872.
45. Ndiaye SM, Hopkins DP, Shefer AM, et al; Task Force on Community Preventive Services. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: a systematic review. Am J Prev Med. 2005;28(5)(suppl):248-279.