User login
New Developments in Adult Vaccination: Challenges and Opportunities to Protect Vulnerable Veterans From Pneumococcal Disease
Streptococcus pneumoniae (S pneumoniae), also known as pneumococcus, is a successful human pathogen with significant clinical impact that causes pneumonia and invasive infections, including bacteremia and meningitis.1 In the preantibiotic era, nearly 80% of patients with bacteremic pneumococcal pneumonia died.2 The introduction of sulfas and penicillin in the mid-20th century, subsequent refinements in antibiotic chemotherapy, and improvements in supportive care rendered pneumococcal disease readily treatable, notwithstanding the threat of antibiotic-resistant pneumococcus.3 Despite the availability of effective antibiotic therapy against S pneumoniae, pneumococcal disease remains a significant cause of morbidity and mortality among people with increased susceptibility, such as older adults and those living with chronic illness or immunosuppressive conditions. In developed countries like the U.S., where a growing portion of the population is vulnerable to S pneumoniae by virtue of their advanced age and underlying medical conditions, pneumococcal disease is still an important public health concern.4
Penumococcal Vaccines: A Long Time Coming
Vaccination against S pneumoniae has proven an efficacious strategy to reduce the morbidity and mortality associated with this pathogen.5 The original efforts to develop a pneumococcal vaccine culminated in 1945 with a vaccine containing pneumococcal capsular polysaccharides, which elicited a protective immune response among U.S. soldiers (Table 1).Subsequent investigations determined that the protective response was specific to pneumococcal disease caused by the 4 pneumococcal capsular serotypes included in the vaccine, that the carrier rate of pneumococcus with the vaccine serotypes decreased by about 50%, and that the incidence of pneumonia from the vaccine serotypes was reduced even in nonimmunized soldiers.6 These early observations remain relevant to our contemporary understanding of the impact of pneumococcal vaccination: Protection is limited to serotypes included in the vaccine; the vaccine reduces colonization; and the vaccine leads to herd immunity—the protection of unvaccinated subjects.
Despite this achievement, the use of vaccines as a strategy to combat pneumococcal disease was upstaged in the subsequent decades by the success of antibiotics. Renewed interest in pneumococcal vaccines resulted from the efforts of Robert Austrian, MD, who astutely observed that “highly effective antimicrobial drugs must be supplemented by other measures, both prophylactic and therapeutic, if the significant mortality resulting still from pneumococcal infection is to be reduced.”7
After initial studies carried out in South African gold miners, the FDA approved a pneumococcal polysaccharide vaccine (PPSV) against 23 of about 90 circulating serotypes.8 The CDC and the Advisory Committee on Immunization Practices (ACIP) initially recommended PPSV-23 for persons perceived to be at high risk for pneumococcal disease, including those with chronic diseases, immunocompromising conditions, and older adults.9
Three decades later, the analysis of a large body of available evidence demonstrated the protective effects of PPSV-23 against invasive pneumococcal disease caused by pneumococcal types included in the vaccine, especially bacteremic pneumonia (about 75% reduction, odds ratio [OR] 0.26, 95% confidence interval [CI] 0.14-0.45).10 A perceived shortcoming of PPSV-23, which some experts dispute, is the lack of definite protection against nonbacteremic pneumococcal pneumonia.11 Studies of nursing home residents demonstrated a 50% reduction in the incidence of both pneumococcal pneumonia and all-cause pneumonia, suggesting that PPSV-23 offers protection against noninvasive pneumococcal pneumonia in specific populations.12 Additional potential limitations of PPSV-23 include reduced benefit in patients aged > 65 years and waning of protection over time.13
Related: Identification and Management of Middle East Respiratory Syndrome
Protein-Conjugate Vaccines
Clearly, the most important limitation of PPSV-23, inherent to all capsular polysaccharide vaccines, is that it does not elicit an immune response in children aged < 2 years. The successful development of a vaccine against Haemophilus influenzae type b (Hib) gave rise to a new generation of pneumococcal vaccines.14 Specifically, the Hib vaccine covalently binds, or conjugates, the capsule polysaccharide to an antigenic protein, leading to effective T-cell–mediated antibody production in infants and toddlers.
In 2000, children received the first iteration of a protein-conjugate vaccine containing the 7 most relevant pneumococcal serotypes (PCV-7).15 The effects of PCV-7 on pneumococcal disease have been extraordinary, practically eliminating infections caused by the pneumococcal serotypes included in the vaccine. Immunizing children with PCV-7 also ushered in a fundamental public health benefit for adults aged > 65 years: a reduction of nearly 90% in the incidence of pneumococcal infections caused by serotypes included in the vaccine. By eliciting mucosal immunity, which leads to decreased nasal carriage of the covered pneumococcal strains among children, PVC-7 generates herd immunity, leading to reductions in transmission, colonization, and infection with vaccine serotypes among adults.16
In 2010, a conjugate vaccine containing 13 serotypes (PCV-13) replaced PCV-7 administration for children.17,18 The PCV-13 is expected to protect children and the herd from disease caused by 6 additional pneumococcal serotypes, including those that surged as replacement strains, filling the ecologic niche created by PCV-7, such as the epidemiologically relevant serotype 19A.19,20
Immune-Compromised Adults
One of the shortcomings of PPSV-23 is its lack of efficacy in patients with advanced HIV, a group with an exquisite vulnerability to pneumococcal disease, as demonstrated by a randomized controlled trial of PPSV-23 in African patients with advanced, untreated HIV infection.21 Similarly, there is concern that patients with lymphoma, leukemia, multiple myeloma, and Hodgkin disease, also at high risk of pneumococcal infection, do not mount sufficient immune responses to polysaccharide antigens or that these responses are adversely affected by chemotherapy or immune suppressing medications. In these populations, a conjugate vaccine (PCV-7 or PCV-13) may elicit a more robust and durable immune response than a polysaccharide vaccine (PPSV-23). A randomized placebo-controlled trial demonstrated the efficacy of PCV-7 in protecting patients with advanced HIV against pneumococcal disease in Africa.22 Based in part on this observation, the ACIP now recommends the use of PCV-13 in patients with HIV and other immunosuppressing conditions, including chronic renal failure.23 A direct comparison of the relative protection of PPSV-23 vs PCV-7 in this population has not been performed.
Pneumococcal Vaccines in the Elderly
Although PPSV-23 was widely adopted in the U.S. with the intention to protect adults aged > 65 years from pneumococcal infection, this vaccine did not achieve global appeal. In the Netherlands, for instance, PPSV-23 had very low penetration and was never endorsed by public health authorities. Concerns have been issued about the decreased immunogenicity of a polysaccharide vaccine in older adults, invoking the concept of immune senescence, a term that describes a diminished capacity to mount robust immune responses due to aging.
Protein-conjugated antigens, on the other hand, can elicit a better initial immune response than a polysaccharide vaccine can in an older adult. Whether the effect is sustained and translates to better clinical outcomes remains unknown. The adoption of PCV-13 and the continued role of PPSV-23 in adults aged > 65 years have been examined carefully by experts.24,25
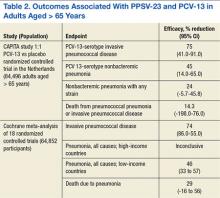
Dutch investigators organized the Community Acquired Pneumonia Immunization Trial in Adults (CAPITA), randomizing about 85,000 adults aged > 65 years toreceive eitherPCV-13 or a placebo.26 After 3 years of observation, the occurrence of invasive pneumococcal disease (caused by the vaccine serotypes) decreased by 85% in participants who received the vaccine compared with those who received a placebo. Additionally, the incidence of pneumococcal pneumonia (caused by the vaccine serotypes) decreased by 45% (Table 2). The findings of this trial largely inform the recommendation issued by APIC in October 2014 to administer PCV-13 vaccine to adults aged > 65 years.27
Polysaccharide Vaccine in Adults
A crucial limitation of the CAPITA study is that it does not provide a head-to-head comparison with PPSV-23. Similarly, the recommendation to use PCV-13 in patients with immune system compromising conditions did not arise from a direct comparison between PCV-13 and PPSV-23. In both groups of patients, ACIP continues to recommend PPSV-23 to extend protection to the 10 additional pneumococcal serotypes not included in PCV-13.28 Additionally, ACIP maintains its long-standing recommendation to administer PPSV-23 to adults aged < 65 years who have diabetes mellitus, or chronic liver, lung, or heart disease, or those who are tobacco smokers or misuse alcohol.
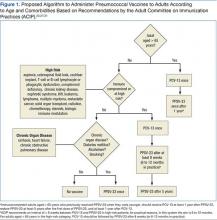
Notably, the order in which the vaccines are administered may influence their effectiveness. In adults aged 50 to 64 years, initial vaccination with PCV-13 followed by PPSV-23 lead to a better antipneumococcal immune response.14,29 Specifically, PCV-13 enhanced the response to subsequent administration of PPSV-23, whereas an initial PPSV-23 vaccine resulted in a decreased response to subsequent administration of PCV-13. After a 1-year interval between vaccines, immune titers to a second vaccination were not inferior.30 All the above considerations have shaped the ACIP recommendations for the administration of pneumococcal vaccines.27,31 Pneumococcal vaccination was a straightforward exercise when PPSV-23 was the only vaccine available for adults. The advent of PCV-13, however, renders pneumococcal immunization of adults more complicated (Figure 1).
Improving Vaccination Rates
The complex recommendations to vaccinate adults against pneumococcus may reveal obstacles to effective pneumococcal vaccination programs.32 From the perspective of health care institutions, the adoption of the new pneumococcal vaccine implies an added cost. A pneumococcal vaccination strategy that incorporates PCV-13 may be cost-effective, at least under certain parameters.33-35 There remains, however, the issue of affordability and the opportunity cost—the need to decrease funding for other health care programs to accommodate an increased budget for pneumococcal vaccination. Logistically, maintaining a reliable supply of vaccines to meet the demand of practitioners at various sites requires careful planning. Consequently, tracking vaccinated and unvaccinated patients and carefully coordinating among clinical providers, nurses, and pharmacists are essential.
From the perspective of clinical providers, an additional pneumococcal vaccine complicating the vaccination schedule for adults represents an increased burden. Providers will need information to reach their own conclusions regarding the rationale behind the development and use of pneumococcal conjugate vaccines, the existing and evolving recommendations from public health authorities, and the strengths and limitations of the evidence supporting the use of pneumococcal vaccines. Otherwise, providers may find it difficult to incorporate new data and guidelines supporting pneumococcal vaccination into their decision-making (Boxes 1-4). An additional and formidable challenge is to carve out time during an already busy clinical encounter to discuss pneumococcal vaccines and other immunizations.36
Related: The Importance of an Antimicrobial Stewardship Program
Similarly, older adults or patients with chronic health conditions may not recognize the important role that vaccines can play in their health maintenance and are likely to prioritize other issues during medical visits. It is not obvious for patients that multiple vaccines may be necessary to prevent pneumococcal disease. Furthermore, many patients, not unreasonably, may assume that their yearly influenza vaccine is sufficiently protective against pneumonia. Therefore, patients need to be educated about the rationale behind pneumococcal conjugate vaccination. Ultimately, access to immunization—the opportunity for patients to have an encounter with their providers and with the health care system that results in the administration of an appropriate vaccine—will determine whether goals for pneumococcal vaccination are achieved.
The evolving landscape for the implementation of pneumococcal vaccines creates the need to develop, implement, and refine organizational changes to adhere to the new guidelines for the use of PCV-13 and PPSV-23 vaccines. These interventions, if effective, may help improve pneumococcal vaccination coverage among adults (Table 3).
Harnessing the Power of the EMR
Interventions to improve the adherence to pneumococcal vaccination guidelines begin by identifying persons eligible for vaccination based on their age, their vaccination status (ie, persons previously unvaccinated or due for vaccination according to the recommended schedule), or the presence of medical conditions conferring high risk for pneumococcal disease. This, in turn, depends on adequate documentation of patients’ underlying medical diagnosis, as well as up-to-date records of vaccine administration to patients.
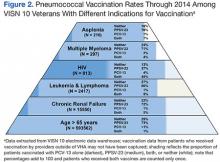
Health care systems possessing a mature and sophisticated electronic medical record (EMR), such as the VHA Computerized Patient Record System (CPRS), are in a good position to wield such information to plan, implement, and assure the quality of activities designed to improve pneumococcal vaccination rates. An analysis of the proportion of veterans in VISN 10 who received pneumococcal vaccination revealed that even with the advantages of a robust EMR and a highly developed infrastructure devoted to primary care, pneumococcal vaccine coverage remains below the 60% target goal, although well above national averages (Figure 2).37,38
Standing Orders
Standing orders make it possible for nurses and pharmacists to administer vaccines according to a preestablished protocol without a physician’s direct evaluation of each patient. Standing orders are a versatile intervention, with a record of effective implementation in both outpatient and inpatient facilities, in acute-care and long-term care facilities, and in most instances where patients interact with the health care system. Based on strong scientific evidence, ACIP recommends the adoption of standing-order programs to improve pneumococcal vaccination rates among adults.39 Indeed, standing-order programs may prove a very effective intervention to fulfill the recommendation to administer PCV-13 to adults aged > 65 years.
The Virtual Vaccination Clinic
Unfortunately, with 2 vaccines that have to be administered at different times to various groups of patients at risk, the current state of pneumococcal vaccination may be too complex to be readily reduced to a comprehensible set of standing orders. An innovative way to realize the benefit of standing orders is to target high-risk groups for pneumococcal disease who are eligible for vaccination by selecting them using the EMR and entering standing orders tailored to their specific vaccination needs. The selection of patients according to comorbidities and vaccination status and the determination of the appropriate pneumococcal vaccine takes place in the context of a “virtual” vaccination clinic.40
Enhancing Vaccinations
Further improvement in pneumococcal vaccination rates are likely to result from interventions that increase the demand for vaccines among patients and practitioners. Efforts to disseminate information and provide advice regarding pneumococcal vaccination are likely to result in patients seeking and clinicians offering the appropriate vaccine. Similarly, interventions to enhance the supply of vaccines at the point of care may reduce barriers that patients might encounter when attempting to receive vaccinations.
Another set of system-based interventions that can assist clinicians in making timely and appropriate vaccination decisions are EMR reminders, especially those targeting patients at high risk for pneumococcal disease because of underlying illnesses.41 Previous experience with pneumococcal vaccination in patients aged > 65 years indicates that EMR reminders facilitate improvements in vaccination. The involvement of a panel manager who coordinated with the primary care provider and contacted patients directly augmented the effect of the EMR reminder by 25%.42
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
Patient reminder and recall systems also demonstrated effectiveness in improving immunization rates.43 In certain groups, notification of patients has been achieved through electronic methods, such as short text messaging or e-mail.44 Determining which interventions within a bundle are essential may be impossible, because the various interventions reinforce one another, and the likelihood of patients benefitting from at least one of the activities increases when multiple interventions are administered together. Therefore, the Task Force on Community Preventive Services supports combining provider reminder systems with education and other measures that encourage use of vaccines in patients and providers.45
Box answer key: 1: A; 2: A; 3: B; 4: A.
Conclusion
The increasing role of vaccines in the health maintenance of adults represents a change in paradigm for primary care and specialty providers. Physicians must assess the value and limitations of vaccines and find time to discuss immunizations with their adult patients. Health care systems can increase opportunities for vaccination and facilitate encounters that result in vaccination by educating patients and health care personnel and through the innovative use of reminders and standing orders in the EMR. Undertaking these activities may limit the burden of pneumococcal disease, an important cause of morbidity and mortality in adults that is preventable through immunization.
Acknowledgements
This work is dedicated to the memory of John M. Rieger, Information Technology Specialist at the Cleveland VAMC and Chief Master Sergeant, Air Force Reserve.
Author Disclosure
This work was supported by a research grant from Pfizer and by the Louis Stokes Cleveland VAMC, the VISN 10 Geriatric Research Education and Clinical Center, and the Clinical and Translational Science Collaborative of Cleveland (award UL1TR000439 from the National Center for Advancing Translational Sciences of the National Institutes of Health NIH). The content is the responsibility of the authors and does not represent the official views of the NIH or the VA.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144-154.
2. Tilghman RC, Finland M. Clinical significance of bacteremia in pneumococcic pneumonia. Arch Intern Med (Chic). 1937;59(4):602-619.
3. Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2010;36(3):197-204.
4. Lexau CA, Lynfield R, Danila R, et al; Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043-2051.
5. Musher DM. How effective is vaccination in preventing pneumococcal disease? Infect Dis Clin North Am. 2013;27(1):229-241.
6. Macleod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82(6):445-465.
7. Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759-776.
8. Austrian R. The Jeremiah Metzger Lecture: Of gold and pneumococci: a history of pneumococcal vaccines in South Africa. Trans Am Clin Climatol Assoc. 1978;89:141-161.
9. Centers for Disease Control (CDC). Pneumococcal polysaccharide vaccine. MMWR Morb Mortal Wkly Rep. 1981;30(33):410-412, 417-419.
10. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422.
11. Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180(1):48-58.
12. Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004.
13. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Eng J Med. 1991;325(21):1453-1460.
14. Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis. 2011;52(5):633-640.
15. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
16. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Eng J Med. 2013;369(2):155-163.
17. Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
18. Centers for Disease Control and Prevention (CDC). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children--Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):258-261.
19. Iroh Tam PY, Madoff LC, Coombes B, Pelton SI. Invasive pneumococcal disease after implementation of 13-valent conjugate vaccine. Pediatrics. 2014;134(2):210-217.
20. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535-543.
21. French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106-2111.
22. French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Eng J Med. 2010;362(9):812-822.
23. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816-819.
24. Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis. 2012;55(2):259-264.
25. Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin Infect Dis. 2012;55(2):265-267.
26. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Eng J Med. 2015;372(12):1114-1125.
27. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822-825.
28. Grabenstein JD. Effectiveness and serotype coverage: key criteria for pneumococcal vaccines for adults. Clin Infect Dis. 2012;55(2):255-258.
29. Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594-3602.
30. Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32(20):2364-2374.
31. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-947.
32. Walton LR, Orenstein WA, Pickering LK. Lessons learned from making and implementing vaccine recommendations in the U.S. Am J Prev Med. 2015;pii:S0749-3797(15)00333-00335 [Epub ahead of print].
33. Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011-6021.
34. Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950-3956.
35. Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804-812.
36. Kyaw MH, Greene CM, Schaffner W, et al; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Adults with invasive pneumococcal disease: missed opportunities for vaccination. Am J Prev Med. 2006;31(4):286-292.
37. Williams WW, Lu PJ, O'Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(4):95-102.
38. Committee on Review of Priorities in the National Vaccine Plan, Institute of Medicine. Priorities for the National Vaccine Plan. Washington, DC: National Academies Press; 2010.
39. McKibben LJ, Stange PV, Sneller VP, Strikas RA, Rodewald LE; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49(RR-1):15-16.
40. Jump RLP, Banks R, Wilson B, et al. A virtual clinic improves pneumococcal vaccination for asplenic veterans at high-risk for pneumococcal disease. Open Forum Infect Dis. In press.
41. Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61(11):1505-1510.
42. Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552-1558.
43. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
44. Ghadieh AS, Hamadeh GN, Mahmassani DM, Lakkis NA. The effect of various types of patients' reminders on the uptake of pneumococcal vaccine in adults: a randomized controlled trial. Vaccine. 2015;33(43):5868-5872.
45. Ndiaye SM, Hopkins DP, Shefer AM, et al; Task Force on Community Preventive Services. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: a systematic review. Am J Prev Med. 2005;28(5)(suppl):248-279.
Streptococcus pneumoniae (S pneumoniae), also known as pneumococcus, is a successful human pathogen with significant clinical impact that causes pneumonia and invasive infections, including bacteremia and meningitis.1 In the preantibiotic era, nearly 80% of patients with bacteremic pneumococcal pneumonia died.2 The introduction of sulfas and penicillin in the mid-20th century, subsequent refinements in antibiotic chemotherapy, and improvements in supportive care rendered pneumococcal disease readily treatable, notwithstanding the threat of antibiotic-resistant pneumococcus.3 Despite the availability of effective antibiotic therapy against S pneumoniae, pneumococcal disease remains a significant cause of morbidity and mortality among people with increased susceptibility, such as older adults and those living with chronic illness or immunosuppressive conditions. In developed countries like the U.S., where a growing portion of the population is vulnerable to S pneumoniae by virtue of their advanced age and underlying medical conditions, pneumococcal disease is still an important public health concern.4
Penumococcal Vaccines: A Long Time Coming
Vaccination against S pneumoniae has proven an efficacious strategy to reduce the morbidity and mortality associated with this pathogen.5 The original efforts to develop a pneumococcal vaccine culminated in 1945 with a vaccine containing pneumococcal capsular polysaccharides, which elicited a protective immune response among U.S. soldiers (Table 1).Subsequent investigations determined that the protective response was specific to pneumococcal disease caused by the 4 pneumococcal capsular serotypes included in the vaccine, that the carrier rate of pneumococcus with the vaccine serotypes decreased by about 50%, and that the incidence of pneumonia from the vaccine serotypes was reduced even in nonimmunized soldiers.6 These early observations remain relevant to our contemporary understanding of the impact of pneumococcal vaccination: Protection is limited to serotypes included in the vaccine; the vaccine reduces colonization; and the vaccine leads to herd immunity—the protection of unvaccinated subjects.
Despite this achievement, the use of vaccines as a strategy to combat pneumococcal disease was upstaged in the subsequent decades by the success of antibiotics. Renewed interest in pneumococcal vaccines resulted from the efforts of Robert Austrian, MD, who astutely observed that “highly effective antimicrobial drugs must be supplemented by other measures, both prophylactic and therapeutic, if the significant mortality resulting still from pneumococcal infection is to be reduced.”7
After initial studies carried out in South African gold miners, the FDA approved a pneumococcal polysaccharide vaccine (PPSV) against 23 of about 90 circulating serotypes.8 The CDC and the Advisory Committee on Immunization Practices (ACIP) initially recommended PPSV-23 for persons perceived to be at high risk for pneumococcal disease, including those with chronic diseases, immunocompromising conditions, and older adults.9
Three decades later, the analysis of a large body of available evidence demonstrated the protective effects of PPSV-23 against invasive pneumococcal disease caused by pneumococcal types included in the vaccine, especially bacteremic pneumonia (about 75% reduction, odds ratio [OR] 0.26, 95% confidence interval [CI] 0.14-0.45).10 A perceived shortcoming of PPSV-23, which some experts dispute, is the lack of definite protection against nonbacteremic pneumococcal pneumonia.11 Studies of nursing home residents demonstrated a 50% reduction in the incidence of both pneumococcal pneumonia and all-cause pneumonia, suggesting that PPSV-23 offers protection against noninvasive pneumococcal pneumonia in specific populations.12 Additional potential limitations of PPSV-23 include reduced benefit in patients aged > 65 years and waning of protection over time.13
Related: Identification and Management of Middle East Respiratory Syndrome
Protein-Conjugate Vaccines
Clearly, the most important limitation of PPSV-23, inherent to all capsular polysaccharide vaccines, is that it does not elicit an immune response in children aged < 2 years. The successful development of a vaccine against Haemophilus influenzae type b (Hib) gave rise to a new generation of pneumococcal vaccines.14 Specifically, the Hib vaccine covalently binds, or conjugates, the capsule polysaccharide to an antigenic protein, leading to effective T-cell–mediated antibody production in infants and toddlers.
In 2000, children received the first iteration of a protein-conjugate vaccine containing the 7 most relevant pneumococcal serotypes (PCV-7).15 The effects of PCV-7 on pneumococcal disease have been extraordinary, practically eliminating infections caused by the pneumococcal serotypes included in the vaccine. Immunizing children with PCV-7 also ushered in a fundamental public health benefit for adults aged > 65 years: a reduction of nearly 90% in the incidence of pneumococcal infections caused by serotypes included in the vaccine. By eliciting mucosal immunity, which leads to decreased nasal carriage of the covered pneumococcal strains among children, PVC-7 generates herd immunity, leading to reductions in transmission, colonization, and infection with vaccine serotypes among adults.16
In 2010, a conjugate vaccine containing 13 serotypes (PCV-13) replaced PCV-7 administration for children.17,18 The PCV-13 is expected to protect children and the herd from disease caused by 6 additional pneumococcal serotypes, including those that surged as replacement strains, filling the ecologic niche created by PCV-7, such as the epidemiologically relevant serotype 19A.19,20
Immune-Compromised Adults
One of the shortcomings of PPSV-23 is its lack of efficacy in patients with advanced HIV, a group with an exquisite vulnerability to pneumococcal disease, as demonstrated by a randomized controlled trial of PPSV-23 in African patients with advanced, untreated HIV infection.21 Similarly, there is concern that patients with lymphoma, leukemia, multiple myeloma, and Hodgkin disease, also at high risk of pneumococcal infection, do not mount sufficient immune responses to polysaccharide antigens or that these responses are adversely affected by chemotherapy or immune suppressing medications. In these populations, a conjugate vaccine (PCV-7 or PCV-13) may elicit a more robust and durable immune response than a polysaccharide vaccine (PPSV-23). A randomized placebo-controlled trial demonstrated the efficacy of PCV-7 in protecting patients with advanced HIV against pneumococcal disease in Africa.22 Based in part on this observation, the ACIP now recommends the use of PCV-13 in patients with HIV and other immunosuppressing conditions, including chronic renal failure.23 A direct comparison of the relative protection of PPSV-23 vs PCV-7 in this population has not been performed.
Pneumococcal Vaccines in the Elderly
Although PPSV-23 was widely adopted in the U.S. with the intention to protect adults aged > 65 years from pneumococcal infection, this vaccine did not achieve global appeal. In the Netherlands, for instance, PPSV-23 had very low penetration and was never endorsed by public health authorities. Concerns have been issued about the decreased immunogenicity of a polysaccharide vaccine in older adults, invoking the concept of immune senescence, a term that describes a diminished capacity to mount robust immune responses due to aging.
Protein-conjugated antigens, on the other hand, can elicit a better initial immune response than a polysaccharide vaccine can in an older adult. Whether the effect is sustained and translates to better clinical outcomes remains unknown. The adoption of PCV-13 and the continued role of PPSV-23 in adults aged > 65 years have been examined carefully by experts.24,25
Dutch investigators organized the Community Acquired Pneumonia Immunization Trial in Adults (CAPITA), randomizing about 85,000 adults aged > 65 years toreceive eitherPCV-13 or a placebo.26 After 3 years of observation, the occurrence of invasive pneumococcal disease (caused by the vaccine serotypes) decreased by 85% in participants who received the vaccine compared with those who received a placebo. Additionally, the incidence of pneumococcal pneumonia (caused by the vaccine serotypes) decreased by 45% (Table 2). The findings of this trial largely inform the recommendation issued by APIC in October 2014 to administer PCV-13 vaccine to adults aged > 65 years.27
Polysaccharide Vaccine in Adults
A crucial limitation of the CAPITA study is that it does not provide a head-to-head comparison with PPSV-23. Similarly, the recommendation to use PCV-13 in patients with immune system compromising conditions did not arise from a direct comparison between PCV-13 and PPSV-23. In both groups of patients, ACIP continues to recommend PPSV-23 to extend protection to the 10 additional pneumococcal serotypes not included in PCV-13.28 Additionally, ACIP maintains its long-standing recommendation to administer PPSV-23 to adults aged < 65 years who have diabetes mellitus, or chronic liver, lung, or heart disease, or those who are tobacco smokers or misuse alcohol.
Notably, the order in which the vaccines are administered may influence their effectiveness. In adults aged 50 to 64 years, initial vaccination with PCV-13 followed by PPSV-23 lead to a better antipneumococcal immune response.14,29 Specifically, PCV-13 enhanced the response to subsequent administration of PPSV-23, whereas an initial PPSV-23 vaccine resulted in a decreased response to subsequent administration of PCV-13. After a 1-year interval between vaccines, immune titers to a second vaccination were not inferior.30 All the above considerations have shaped the ACIP recommendations for the administration of pneumococcal vaccines.27,31 Pneumococcal vaccination was a straightforward exercise when PPSV-23 was the only vaccine available for adults. The advent of PCV-13, however, renders pneumococcal immunization of adults more complicated (Figure 1).
Improving Vaccination Rates
The complex recommendations to vaccinate adults against pneumococcus may reveal obstacles to effective pneumococcal vaccination programs.32 From the perspective of health care institutions, the adoption of the new pneumococcal vaccine implies an added cost. A pneumococcal vaccination strategy that incorporates PCV-13 may be cost-effective, at least under certain parameters.33-35 There remains, however, the issue of affordability and the opportunity cost—the need to decrease funding for other health care programs to accommodate an increased budget for pneumococcal vaccination. Logistically, maintaining a reliable supply of vaccines to meet the demand of practitioners at various sites requires careful planning. Consequently, tracking vaccinated and unvaccinated patients and carefully coordinating among clinical providers, nurses, and pharmacists are essential.
From the perspective of clinical providers, an additional pneumococcal vaccine complicating the vaccination schedule for adults represents an increased burden. Providers will need information to reach their own conclusions regarding the rationale behind the development and use of pneumococcal conjugate vaccines, the existing and evolving recommendations from public health authorities, and the strengths and limitations of the evidence supporting the use of pneumococcal vaccines. Otherwise, providers may find it difficult to incorporate new data and guidelines supporting pneumococcal vaccination into their decision-making (Boxes 1-4). An additional and formidable challenge is to carve out time during an already busy clinical encounter to discuss pneumococcal vaccines and other immunizations.36
Related: The Importance of an Antimicrobial Stewardship Program
Similarly, older adults or patients with chronic health conditions may not recognize the important role that vaccines can play in their health maintenance and are likely to prioritize other issues during medical visits. It is not obvious for patients that multiple vaccines may be necessary to prevent pneumococcal disease. Furthermore, many patients, not unreasonably, may assume that their yearly influenza vaccine is sufficiently protective against pneumonia. Therefore, patients need to be educated about the rationale behind pneumococcal conjugate vaccination. Ultimately, access to immunization—the opportunity for patients to have an encounter with their providers and with the health care system that results in the administration of an appropriate vaccine—will determine whether goals for pneumococcal vaccination are achieved.
The evolving landscape for the implementation of pneumococcal vaccines creates the need to develop, implement, and refine organizational changes to adhere to the new guidelines for the use of PCV-13 and PPSV-23 vaccines. These interventions, if effective, may help improve pneumococcal vaccination coverage among adults (Table 3).
Harnessing the Power of the EMR
Interventions to improve the adherence to pneumococcal vaccination guidelines begin by identifying persons eligible for vaccination based on their age, their vaccination status (ie, persons previously unvaccinated or due for vaccination according to the recommended schedule), or the presence of medical conditions conferring high risk for pneumococcal disease. This, in turn, depends on adequate documentation of patients’ underlying medical diagnosis, as well as up-to-date records of vaccine administration to patients.
Health care systems possessing a mature and sophisticated electronic medical record (EMR), such as the VHA Computerized Patient Record System (CPRS), are in a good position to wield such information to plan, implement, and assure the quality of activities designed to improve pneumococcal vaccination rates. An analysis of the proportion of veterans in VISN 10 who received pneumococcal vaccination revealed that even with the advantages of a robust EMR and a highly developed infrastructure devoted to primary care, pneumococcal vaccine coverage remains below the 60% target goal, although well above national averages (Figure 2).37,38
Standing Orders
Standing orders make it possible for nurses and pharmacists to administer vaccines according to a preestablished protocol without a physician’s direct evaluation of each patient. Standing orders are a versatile intervention, with a record of effective implementation in both outpatient and inpatient facilities, in acute-care and long-term care facilities, and in most instances where patients interact with the health care system. Based on strong scientific evidence, ACIP recommends the adoption of standing-order programs to improve pneumococcal vaccination rates among adults.39 Indeed, standing-order programs may prove a very effective intervention to fulfill the recommendation to administer PCV-13 to adults aged > 65 years.
The Virtual Vaccination Clinic
Unfortunately, with 2 vaccines that have to be administered at different times to various groups of patients at risk, the current state of pneumococcal vaccination may be too complex to be readily reduced to a comprehensible set of standing orders. An innovative way to realize the benefit of standing orders is to target high-risk groups for pneumococcal disease who are eligible for vaccination by selecting them using the EMR and entering standing orders tailored to their specific vaccination needs. The selection of patients according to comorbidities and vaccination status and the determination of the appropriate pneumococcal vaccine takes place in the context of a “virtual” vaccination clinic.40
Enhancing Vaccinations
Further improvement in pneumococcal vaccination rates are likely to result from interventions that increase the demand for vaccines among patients and practitioners. Efforts to disseminate information and provide advice regarding pneumococcal vaccination are likely to result in patients seeking and clinicians offering the appropriate vaccine. Similarly, interventions to enhance the supply of vaccines at the point of care may reduce barriers that patients might encounter when attempting to receive vaccinations.
Another set of system-based interventions that can assist clinicians in making timely and appropriate vaccination decisions are EMR reminders, especially those targeting patients at high risk for pneumococcal disease because of underlying illnesses.41 Previous experience with pneumococcal vaccination in patients aged > 65 years indicates that EMR reminders facilitate improvements in vaccination. The involvement of a panel manager who coordinated with the primary care provider and contacted patients directly augmented the effect of the EMR reminder by 25%.42
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
Patient reminder and recall systems also demonstrated effectiveness in improving immunization rates.43 In certain groups, notification of patients has been achieved through electronic methods, such as short text messaging or e-mail.44 Determining which interventions within a bundle are essential may be impossible, because the various interventions reinforce one another, and the likelihood of patients benefitting from at least one of the activities increases when multiple interventions are administered together. Therefore, the Task Force on Community Preventive Services supports combining provider reminder systems with education and other measures that encourage use of vaccines in patients and providers.45
Box answer key: 1: A; 2: A; 3: B; 4: A.
Conclusion
The increasing role of vaccines in the health maintenance of adults represents a change in paradigm for primary care and specialty providers. Physicians must assess the value and limitations of vaccines and find time to discuss immunizations with their adult patients. Health care systems can increase opportunities for vaccination and facilitate encounters that result in vaccination by educating patients and health care personnel and through the innovative use of reminders and standing orders in the EMR. Undertaking these activities may limit the burden of pneumococcal disease, an important cause of morbidity and mortality in adults that is preventable through immunization.
Acknowledgements
This work is dedicated to the memory of John M. Rieger, Information Technology Specialist at the Cleveland VAMC and Chief Master Sergeant, Air Force Reserve.
Author Disclosure
This work was supported by a research grant from Pfizer and by the Louis Stokes Cleveland VAMC, the VISN 10 Geriatric Research Education and Clinical Center, and the Clinical and Translational Science Collaborative of Cleveland (award UL1TR000439 from the National Center for Advancing Translational Sciences of the National Institutes of Health NIH). The content is the responsibility of the authors and does not represent the official views of the NIH or the VA.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Streptococcus pneumoniae (S pneumoniae), also known as pneumococcus, is a successful human pathogen with significant clinical impact that causes pneumonia and invasive infections, including bacteremia and meningitis.1 In the preantibiotic era, nearly 80% of patients with bacteremic pneumococcal pneumonia died.2 The introduction of sulfas and penicillin in the mid-20th century, subsequent refinements in antibiotic chemotherapy, and improvements in supportive care rendered pneumococcal disease readily treatable, notwithstanding the threat of antibiotic-resistant pneumococcus.3 Despite the availability of effective antibiotic therapy against S pneumoniae, pneumococcal disease remains a significant cause of morbidity and mortality among people with increased susceptibility, such as older adults and those living with chronic illness or immunosuppressive conditions. In developed countries like the U.S., where a growing portion of the population is vulnerable to S pneumoniae by virtue of their advanced age and underlying medical conditions, pneumococcal disease is still an important public health concern.4
Penumococcal Vaccines: A Long Time Coming
Vaccination against S pneumoniae has proven an efficacious strategy to reduce the morbidity and mortality associated with this pathogen.5 The original efforts to develop a pneumococcal vaccine culminated in 1945 with a vaccine containing pneumococcal capsular polysaccharides, which elicited a protective immune response among U.S. soldiers (Table 1).Subsequent investigations determined that the protective response was specific to pneumococcal disease caused by the 4 pneumococcal capsular serotypes included in the vaccine, that the carrier rate of pneumococcus with the vaccine serotypes decreased by about 50%, and that the incidence of pneumonia from the vaccine serotypes was reduced even in nonimmunized soldiers.6 These early observations remain relevant to our contemporary understanding of the impact of pneumococcal vaccination: Protection is limited to serotypes included in the vaccine; the vaccine reduces colonization; and the vaccine leads to herd immunity—the protection of unvaccinated subjects.
Despite this achievement, the use of vaccines as a strategy to combat pneumococcal disease was upstaged in the subsequent decades by the success of antibiotics. Renewed interest in pneumococcal vaccines resulted from the efforts of Robert Austrian, MD, who astutely observed that “highly effective antimicrobial drugs must be supplemented by other measures, both prophylactic and therapeutic, if the significant mortality resulting still from pneumococcal infection is to be reduced.”7
After initial studies carried out in South African gold miners, the FDA approved a pneumococcal polysaccharide vaccine (PPSV) against 23 of about 90 circulating serotypes.8 The CDC and the Advisory Committee on Immunization Practices (ACIP) initially recommended PPSV-23 for persons perceived to be at high risk for pneumococcal disease, including those with chronic diseases, immunocompromising conditions, and older adults.9
Three decades later, the analysis of a large body of available evidence demonstrated the protective effects of PPSV-23 against invasive pneumococcal disease caused by pneumococcal types included in the vaccine, especially bacteremic pneumonia (about 75% reduction, odds ratio [OR] 0.26, 95% confidence interval [CI] 0.14-0.45).10 A perceived shortcoming of PPSV-23, which some experts dispute, is the lack of definite protection against nonbacteremic pneumococcal pneumonia.11 Studies of nursing home residents demonstrated a 50% reduction in the incidence of both pneumococcal pneumonia and all-cause pneumonia, suggesting that PPSV-23 offers protection against noninvasive pneumococcal pneumonia in specific populations.12 Additional potential limitations of PPSV-23 include reduced benefit in patients aged > 65 years and waning of protection over time.13
Related: Identification and Management of Middle East Respiratory Syndrome
Protein-Conjugate Vaccines
Clearly, the most important limitation of PPSV-23, inherent to all capsular polysaccharide vaccines, is that it does not elicit an immune response in children aged < 2 years. The successful development of a vaccine against Haemophilus influenzae type b (Hib) gave rise to a new generation of pneumococcal vaccines.14 Specifically, the Hib vaccine covalently binds, or conjugates, the capsule polysaccharide to an antigenic protein, leading to effective T-cell–mediated antibody production in infants and toddlers.
In 2000, children received the first iteration of a protein-conjugate vaccine containing the 7 most relevant pneumococcal serotypes (PCV-7).15 The effects of PCV-7 on pneumococcal disease have been extraordinary, practically eliminating infections caused by the pneumococcal serotypes included in the vaccine. Immunizing children with PCV-7 also ushered in a fundamental public health benefit for adults aged > 65 years: a reduction of nearly 90% in the incidence of pneumococcal infections caused by serotypes included in the vaccine. By eliciting mucosal immunity, which leads to decreased nasal carriage of the covered pneumococcal strains among children, PVC-7 generates herd immunity, leading to reductions in transmission, colonization, and infection with vaccine serotypes among adults.16
In 2010, a conjugate vaccine containing 13 serotypes (PCV-13) replaced PCV-7 administration for children.17,18 The PCV-13 is expected to protect children and the herd from disease caused by 6 additional pneumococcal serotypes, including those that surged as replacement strains, filling the ecologic niche created by PCV-7, such as the epidemiologically relevant serotype 19A.19,20
Immune-Compromised Adults
One of the shortcomings of PPSV-23 is its lack of efficacy in patients with advanced HIV, a group with an exquisite vulnerability to pneumococcal disease, as demonstrated by a randomized controlled trial of PPSV-23 in African patients with advanced, untreated HIV infection.21 Similarly, there is concern that patients with lymphoma, leukemia, multiple myeloma, and Hodgkin disease, also at high risk of pneumococcal infection, do not mount sufficient immune responses to polysaccharide antigens or that these responses are adversely affected by chemotherapy or immune suppressing medications. In these populations, a conjugate vaccine (PCV-7 or PCV-13) may elicit a more robust and durable immune response than a polysaccharide vaccine (PPSV-23). A randomized placebo-controlled trial demonstrated the efficacy of PCV-7 in protecting patients with advanced HIV against pneumococcal disease in Africa.22 Based in part on this observation, the ACIP now recommends the use of PCV-13 in patients with HIV and other immunosuppressing conditions, including chronic renal failure.23 A direct comparison of the relative protection of PPSV-23 vs PCV-7 in this population has not been performed.
Pneumococcal Vaccines in the Elderly
Although PPSV-23 was widely adopted in the U.S. with the intention to protect adults aged > 65 years from pneumococcal infection, this vaccine did not achieve global appeal. In the Netherlands, for instance, PPSV-23 had very low penetration and was never endorsed by public health authorities. Concerns have been issued about the decreased immunogenicity of a polysaccharide vaccine in older adults, invoking the concept of immune senescence, a term that describes a diminished capacity to mount robust immune responses due to aging.
Protein-conjugated antigens, on the other hand, can elicit a better initial immune response than a polysaccharide vaccine can in an older adult. Whether the effect is sustained and translates to better clinical outcomes remains unknown. The adoption of PCV-13 and the continued role of PPSV-23 in adults aged > 65 years have been examined carefully by experts.24,25
Dutch investigators organized the Community Acquired Pneumonia Immunization Trial in Adults (CAPITA), randomizing about 85,000 adults aged > 65 years toreceive eitherPCV-13 or a placebo.26 After 3 years of observation, the occurrence of invasive pneumococcal disease (caused by the vaccine serotypes) decreased by 85% in participants who received the vaccine compared with those who received a placebo. Additionally, the incidence of pneumococcal pneumonia (caused by the vaccine serotypes) decreased by 45% (Table 2). The findings of this trial largely inform the recommendation issued by APIC in October 2014 to administer PCV-13 vaccine to adults aged > 65 years.27
Polysaccharide Vaccine in Adults
A crucial limitation of the CAPITA study is that it does not provide a head-to-head comparison with PPSV-23. Similarly, the recommendation to use PCV-13 in patients with immune system compromising conditions did not arise from a direct comparison between PCV-13 and PPSV-23. In both groups of patients, ACIP continues to recommend PPSV-23 to extend protection to the 10 additional pneumococcal serotypes not included in PCV-13.28 Additionally, ACIP maintains its long-standing recommendation to administer PPSV-23 to adults aged < 65 years who have diabetes mellitus, or chronic liver, lung, or heart disease, or those who are tobacco smokers or misuse alcohol.
Notably, the order in which the vaccines are administered may influence their effectiveness. In adults aged 50 to 64 years, initial vaccination with PCV-13 followed by PPSV-23 lead to a better antipneumococcal immune response.14,29 Specifically, PCV-13 enhanced the response to subsequent administration of PPSV-23, whereas an initial PPSV-23 vaccine resulted in a decreased response to subsequent administration of PCV-13. After a 1-year interval between vaccines, immune titers to a second vaccination were not inferior.30 All the above considerations have shaped the ACIP recommendations for the administration of pneumococcal vaccines.27,31 Pneumococcal vaccination was a straightforward exercise when PPSV-23 was the only vaccine available for adults. The advent of PCV-13, however, renders pneumococcal immunization of adults more complicated (Figure 1).
Improving Vaccination Rates
The complex recommendations to vaccinate adults against pneumococcus may reveal obstacles to effective pneumococcal vaccination programs.32 From the perspective of health care institutions, the adoption of the new pneumococcal vaccine implies an added cost. A pneumococcal vaccination strategy that incorporates PCV-13 may be cost-effective, at least under certain parameters.33-35 There remains, however, the issue of affordability and the opportunity cost—the need to decrease funding for other health care programs to accommodate an increased budget for pneumococcal vaccination. Logistically, maintaining a reliable supply of vaccines to meet the demand of practitioners at various sites requires careful planning. Consequently, tracking vaccinated and unvaccinated patients and carefully coordinating among clinical providers, nurses, and pharmacists are essential.
From the perspective of clinical providers, an additional pneumococcal vaccine complicating the vaccination schedule for adults represents an increased burden. Providers will need information to reach their own conclusions regarding the rationale behind the development and use of pneumococcal conjugate vaccines, the existing and evolving recommendations from public health authorities, and the strengths and limitations of the evidence supporting the use of pneumococcal vaccines. Otherwise, providers may find it difficult to incorporate new data and guidelines supporting pneumococcal vaccination into their decision-making (Boxes 1-4). An additional and formidable challenge is to carve out time during an already busy clinical encounter to discuss pneumococcal vaccines and other immunizations.36
Related: The Importance of an Antimicrobial Stewardship Program
Similarly, older adults or patients with chronic health conditions may not recognize the important role that vaccines can play in their health maintenance and are likely to prioritize other issues during medical visits. It is not obvious for patients that multiple vaccines may be necessary to prevent pneumococcal disease. Furthermore, many patients, not unreasonably, may assume that their yearly influenza vaccine is sufficiently protective against pneumonia. Therefore, patients need to be educated about the rationale behind pneumococcal conjugate vaccination. Ultimately, access to immunization—the opportunity for patients to have an encounter with their providers and with the health care system that results in the administration of an appropriate vaccine—will determine whether goals for pneumococcal vaccination are achieved.
The evolving landscape for the implementation of pneumococcal vaccines creates the need to develop, implement, and refine organizational changes to adhere to the new guidelines for the use of PCV-13 and PPSV-23 vaccines. These interventions, if effective, may help improve pneumococcal vaccination coverage among adults (Table 3).
Harnessing the Power of the EMR
Interventions to improve the adherence to pneumococcal vaccination guidelines begin by identifying persons eligible for vaccination based on their age, their vaccination status (ie, persons previously unvaccinated or due for vaccination according to the recommended schedule), or the presence of medical conditions conferring high risk for pneumococcal disease. This, in turn, depends on adequate documentation of patients’ underlying medical diagnosis, as well as up-to-date records of vaccine administration to patients.
Health care systems possessing a mature and sophisticated electronic medical record (EMR), such as the VHA Computerized Patient Record System (CPRS), are in a good position to wield such information to plan, implement, and assure the quality of activities designed to improve pneumococcal vaccination rates. An analysis of the proportion of veterans in VISN 10 who received pneumococcal vaccination revealed that even with the advantages of a robust EMR and a highly developed infrastructure devoted to primary care, pneumococcal vaccine coverage remains below the 60% target goal, although well above national averages (Figure 2).37,38
Standing Orders
Standing orders make it possible for nurses and pharmacists to administer vaccines according to a preestablished protocol without a physician’s direct evaluation of each patient. Standing orders are a versatile intervention, with a record of effective implementation in both outpatient and inpatient facilities, in acute-care and long-term care facilities, and in most instances where patients interact with the health care system. Based on strong scientific evidence, ACIP recommends the adoption of standing-order programs to improve pneumococcal vaccination rates among adults.39 Indeed, standing-order programs may prove a very effective intervention to fulfill the recommendation to administer PCV-13 to adults aged > 65 years.
The Virtual Vaccination Clinic
Unfortunately, with 2 vaccines that have to be administered at different times to various groups of patients at risk, the current state of pneumococcal vaccination may be too complex to be readily reduced to a comprehensible set of standing orders. An innovative way to realize the benefit of standing orders is to target high-risk groups for pneumococcal disease who are eligible for vaccination by selecting them using the EMR and entering standing orders tailored to their specific vaccination needs. The selection of patients according to comorbidities and vaccination status and the determination of the appropriate pneumococcal vaccine takes place in the context of a “virtual” vaccination clinic.40
Enhancing Vaccinations
Further improvement in pneumococcal vaccination rates are likely to result from interventions that increase the demand for vaccines among patients and practitioners. Efforts to disseminate information and provide advice regarding pneumococcal vaccination are likely to result in patients seeking and clinicians offering the appropriate vaccine. Similarly, interventions to enhance the supply of vaccines at the point of care may reduce barriers that patients might encounter when attempting to receive vaccinations.
Another set of system-based interventions that can assist clinicians in making timely and appropriate vaccination decisions are EMR reminders, especially those targeting patients at high risk for pneumococcal disease because of underlying illnesses.41 Previous experience with pneumococcal vaccination in patients aged > 65 years indicates that EMR reminders facilitate improvements in vaccination. The involvement of a panel manager who coordinated with the primary care provider and contacted patients directly augmented the effect of the EMR reminder by 25%.42
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
Patient reminder and recall systems also demonstrated effectiveness in improving immunization rates.43 In certain groups, notification of patients has been achieved through electronic methods, such as short text messaging or e-mail.44 Determining which interventions within a bundle are essential may be impossible, because the various interventions reinforce one another, and the likelihood of patients benefitting from at least one of the activities increases when multiple interventions are administered together. Therefore, the Task Force on Community Preventive Services supports combining provider reminder systems with education and other measures that encourage use of vaccines in patients and providers.45
Box answer key: 1: A; 2: A; 3: B; 4: A.
Conclusion
The increasing role of vaccines in the health maintenance of adults represents a change in paradigm for primary care and specialty providers. Physicians must assess the value and limitations of vaccines and find time to discuss immunizations with their adult patients. Health care systems can increase opportunities for vaccination and facilitate encounters that result in vaccination by educating patients and health care personnel and through the innovative use of reminders and standing orders in the EMR. Undertaking these activities may limit the burden of pneumococcal disease, an important cause of morbidity and mortality in adults that is preventable through immunization.
Acknowledgements
This work is dedicated to the memory of John M. Rieger, Information Technology Specialist at the Cleveland VAMC and Chief Master Sergeant, Air Force Reserve.
Author Disclosure
This work was supported by a research grant from Pfizer and by the Louis Stokes Cleveland VAMC, the VISN 10 Geriatric Research Education and Clinical Center, and the Clinical and Translational Science Collaborative of Cleveland (award UL1TR000439 from the National Center for Advancing Translational Sciences of the National Institutes of Health NIH). The content is the responsibility of the authors and does not represent the official views of the NIH or the VA.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144-154.
2. Tilghman RC, Finland M. Clinical significance of bacteremia in pneumococcic pneumonia. Arch Intern Med (Chic). 1937;59(4):602-619.
3. Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2010;36(3):197-204.
4. Lexau CA, Lynfield R, Danila R, et al; Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043-2051.
5. Musher DM. How effective is vaccination in preventing pneumococcal disease? Infect Dis Clin North Am. 2013;27(1):229-241.
6. Macleod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82(6):445-465.
7. Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759-776.
8. Austrian R. The Jeremiah Metzger Lecture: Of gold and pneumococci: a history of pneumococcal vaccines in South Africa. Trans Am Clin Climatol Assoc. 1978;89:141-161.
9. Centers for Disease Control (CDC). Pneumococcal polysaccharide vaccine. MMWR Morb Mortal Wkly Rep. 1981;30(33):410-412, 417-419.
10. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422.
11. Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180(1):48-58.
12. Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004.
13. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Eng J Med. 1991;325(21):1453-1460.
14. Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis. 2011;52(5):633-640.
15. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
16. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Eng J Med. 2013;369(2):155-163.
17. Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
18. Centers for Disease Control and Prevention (CDC). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children--Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):258-261.
19. Iroh Tam PY, Madoff LC, Coombes B, Pelton SI. Invasive pneumococcal disease after implementation of 13-valent conjugate vaccine. Pediatrics. 2014;134(2):210-217.
20. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535-543.
21. French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106-2111.
22. French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Eng J Med. 2010;362(9):812-822.
23. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816-819.
24. Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis. 2012;55(2):259-264.
25. Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin Infect Dis. 2012;55(2):265-267.
26. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Eng J Med. 2015;372(12):1114-1125.
27. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822-825.
28. Grabenstein JD. Effectiveness and serotype coverage: key criteria for pneumococcal vaccines for adults. Clin Infect Dis. 2012;55(2):255-258.
29. Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594-3602.
30. Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32(20):2364-2374.
31. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-947.
32. Walton LR, Orenstein WA, Pickering LK. Lessons learned from making and implementing vaccine recommendations in the U.S. Am J Prev Med. 2015;pii:S0749-3797(15)00333-00335 [Epub ahead of print].
33. Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011-6021.
34. Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950-3956.
35. Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804-812.
36. Kyaw MH, Greene CM, Schaffner W, et al; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Adults with invasive pneumococcal disease: missed opportunities for vaccination. Am J Prev Med. 2006;31(4):286-292.
37. Williams WW, Lu PJ, O'Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(4):95-102.
38. Committee on Review of Priorities in the National Vaccine Plan, Institute of Medicine. Priorities for the National Vaccine Plan. Washington, DC: National Academies Press; 2010.
39. McKibben LJ, Stange PV, Sneller VP, Strikas RA, Rodewald LE; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49(RR-1):15-16.
40. Jump RLP, Banks R, Wilson B, et al. A virtual clinic improves pneumococcal vaccination for asplenic veterans at high-risk for pneumococcal disease. Open Forum Infect Dis. In press.
41. Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61(11):1505-1510.
42. Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552-1558.
43. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
44. Ghadieh AS, Hamadeh GN, Mahmassani DM, Lakkis NA. The effect of various types of patients' reminders on the uptake of pneumococcal vaccine in adults: a randomized controlled trial. Vaccine. 2015;33(43):5868-5872.
45. Ndiaye SM, Hopkins DP, Shefer AM, et al; Task Force on Community Preventive Services. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: a systematic review. Am J Prev Med. 2005;28(5)(suppl):248-279.
1. Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144-154.
2. Tilghman RC, Finland M. Clinical significance of bacteremia in pneumococcic pneumonia. Arch Intern Med (Chic). 1937;59(4):602-619.
3. Jones RN, Jacobs MR, Sader HS. Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia. Int J Antimicrob Agents. 2010;36(3):197-204.
4. Lexau CA, Lynfield R, Danila R, et al; Active Bacterial Core Surveillance Team. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043-2051.
5. Musher DM. How effective is vaccination in preventing pneumococcal disease? Infect Dis Clin North Am. 2013;27(1):229-241.
6. Macleod CM, Hodges RG, Heidelberger M, Bernhard WG. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82(6):445-465.
7. Austrian R, Gold J. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann Intern Med. 1964;60:759-776.
8. Austrian R. The Jeremiah Metzger Lecture: Of gold and pneumococci: a history of pneumococcal vaccines in South Africa. Trans Am Clin Climatol Assoc. 1978;89:141-161.
9. Centers for Disease Control (CDC). Pneumococcal polysaccharide vaccine. MMWR Morb Mortal Wkly Rep. 1981;30(33):410-412, 417-419.
10. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013;1:CD000422.
11. Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180(1):48-58.
12. Maruyama T, Taguchi O, Niederman MS, et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ. 2010;340:c1004.
13. Shapiro ED, Berg AT, Austrian R, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Eng J Med. 1991;325(21):1453-1460.
14. Musher DM, Sampath R, Rodriguez-Barradas MC. The potential role for protein-conjugate pneumococcal vaccine in adults: what is the supporting evidence? Clin Infect Dis. 2011;52(5):633-640.
15. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
16. Griffin MR, Zhu Y, Moore MR, Whitney CG, Grijalva CG. U.S. hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Eng J Med. 2013;369(2):155-163.
17. Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
18. Centers for Disease Control and Prevention (CDC). Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children--Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(9):258-261.
19. Iroh Tam PY, Madoff LC, Coombes B, Pelton SI. Invasive pneumococcal disease after implementation of 13-valent conjugate vaccine. Pediatrics. 2014;134(2):210-217.
20. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535-543.
21. French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355(9221):2106-2111.
22. French N, Gordon SB, Mwalukomo T, et al. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Eng J Med. 2010;362(9):812-822.
23. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012;61(40):816-819.
24. Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis. 2012;55(2):259-264.
25. Musher DM. Editorial commentary: should 13-valent protein-conjugate pneumococcal vaccine be used routinely in adults? Clin Infect Dis. 2012;55(2):265-267.
26. Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Eng J Med. 2015;372(12):1114-1125.
27. Tomczyk S, Bennett NM, Stoecker C, et al; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014;63(37):822-825.
28. Grabenstein JD. Effectiveness and serotype coverage: key criteria for pneumococcal vaccines for adults. Clin Infect Dis. 2012;55(2):255-258.
29. Jackson LA, Gurtman A, van Cleeff M, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31(35):3594-3602.
30. Greenberg RN, Gurtman A, Frenck RW, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32(20):2364-2374.
31. Kobayashi M, Bennett NM, Gierke R, et al. Intervals between PCV13 and PPSV23 vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2015;64(34):944-947.
32. Walton LR, Orenstein WA, Pickering LK. Lessons learned from making and implementing vaccine recommendations in the U.S. Am J Prev Med. 2015;pii:S0749-3797(15)00333-00335 [Epub ahead of print].
33. Cho BH, Stoecker C, Link-Gelles R, Moore MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. 2013;31(50):6011-6021.
34. Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31(37):3950-3956.
35. Smith KJ, Wateska AR, Nowalk MP, Raymund M, Nuorti JP, Zimmerman RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. 2012;307(8):804-812.
36. Kyaw MH, Greene CM, Schaffner W, et al; Active Bacterial Core Surveillance Program of the Emerging Infections Program Network. Adults with invasive pneumococcal disease: missed opportunities for vaccination. Am J Prev Med. 2006;31(4):286-292.
37. Williams WW, Lu PJ, O'Halloran A, et al; Centers for Disease Control and Prevention (CDC). Vaccination coverage among adults, excluding influenza vaccination - United States, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(4):95-102.
38. Committee on Review of Priorities in the National Vaccine Plan, Institute of Medicine. Priorities for the National Vaccine Plan. Washington, DC: National Academies Press; 2010.
39. McKibben LJ, Stange PV, Sneller VP, Strikas RA, Rodewald LE; Advisory Committee on Immunization Practices. Use of standing orders programs to increase adult vaccination rates. MMWR Recomm Rep. 2000;49(RR-1):15-16.
40. Jump RLP, Banks R, Wilson B, et al. A virtual clinic improves pneumococcal vaccination for asplenic veterans at high-risk for pneumococcal disease. Open Forum Infect Dis. In press.
41. Ledwich LJ, Harrington TM, Ayoub WT, Sartorius JA, Newman ED. Improved influenza and pneumococcal vaccination in rheumatology patients taking immunosuppressants using an electronic health record best practice alert. Arthritis Rheum. 2009;61(11):1505-1510.
42. Loo TS, Davis RB, Lipsitz LA, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med. 2011;171(17):1552-1558.
43. Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941.
44. Ghadieh AS, Hamadeh GN, Mahmassani DM, Lakkis NA. The effect of various types of patients' reminders on the uptake of pneumococcal vaccine in adults: a randomized controlled trial. Vaccine. 2015;33(43):5868-5872.
45. Ndiaye SM, Hopkins DP, Shefer AM, et al; Task Force on Community Preventive Services. Interventions to improve influenza, pneumococcal polysaccharide, and hepatitis B vaccination coverage among high-risk adults: a systematic review. Am J Prev Med. 2005;28(5)(suppl):248-279.
Carbapenem-resistant Enterobacteriaceae: A menace to our most vulnerable patients
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
KEY POINTS
- The utility of carbapenems is being undermined by the emergence of resistance in Enterobacteriaceae and other bacteria.
- The clinical impact of CRE falls on elderly patients exposed to these organisms in hospitals and long-term care facilities. In this vulnerable group, invasive infections with CRE exact a high death rate.
- Long-term care facilities play an important role in the transmission dynamics of CRE.
- Tigecycline and colistin are treatments of last resort against infections caused by CRE. Their use in combination with other agents, especially carbapenems, may improve outcomes and needs to be explored further.
- Early detection of CRE in the microbiology laboratory is key to guiding infection control and treatment decisions and supporting surveillance efforts.