User login
The care of people with pain has been wrought with ineffective and unnecessary treatment, including the misuse of opioids, largely because we do not have an accurate conceptualization of pain. The absence of animal and human models of central nervous system (CNS) pain processing ensures that our understanding of pain will remain incomplete for the foreseeable future, but enough evidence exists to help family physicians develop an understanding of pain that goes beyond what we learned in medical school and that can help us more effectively treat patients with pain.
In this review, we will briefly discuss the established concepts of nociceptive and neuropathic pain. And then, with those concepts in mind, we will explore a third type of pain that for lack of a better term, we will call “pain for psychological reasons.” We hypothesize that this pain may be the consequence of changes in nervous system function that arise from developmental trauma, other traumatic experiences in a patient’s life, or mental health disorders. It is this third type of pain that may offer us insights into conditions such as fibromyalgia.
While we do not yet have validated diagnostic criteria for this third type of pain, we believe that there is enough information to present initial criteria so that one may distinguish it from nociceptive and neuropathic pain.
Nociceptive and neuropathic pain: The current paradigm
Nociceptive pain. The sensory pain experience, or nociceptive pain, is produced by noxious stimuli that either damage, or are capable of damaging, tissues (eg, burns, cuts, fractures, inflammation, and increased pressure in a hollow viscus). Noxious stimuli are detected at the molecular level by specific pain sensory receptors embedded in our tissues called nociceptors.
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps—transduction, transmission, modulation, and perception—which are described in “From periphery to brain: The process of nociceptive pain.”1-4
Neuropathic pain. While nociceptive pain can be easily traced from a peripheral nociceptive fiber to the brain and typically resolves when the nociceptive stimulus stops, neuropathic pain (NPP) results from changes to the function of the nervous system and is typically caused by injury to the nerves. Such changes, referred to as neuronal sensitization, may not quickly resolve, as is the case with postherpetic neuralgia. In fact, the changes can become permanent. NPP fundamentally differs from nociceptive pain because it results from changes in the central processing of pain that can lead a person to perceive pain sensations even in the absence of tissue pathology.
Common causes of NPP that persists even after tissue damage has healed include trauma (eg, amputation of a limb), ischemia (eg, pressure palsy), disease (eg, the metabolic injury of diabetes or the injury caused by a shingles infection), and drug treatment (eg, chemotherapy). The underlying mechanisms of NPP and the neuronal plasticity (the ability of the nervous system to rewire itself) that initiate and then maintain NPP are important areas of active research that may eventually lead to the development of more effective treatments.
Timing is critical. Neuroplastic changes in the nervous system following nerve injury are time-dependent. Synaptic plasticity can occur within seconds to minutes, while cellular plasticity occurs within hours to days. Synaptic and cellular plasticity happen relatively fast and may be reversible.
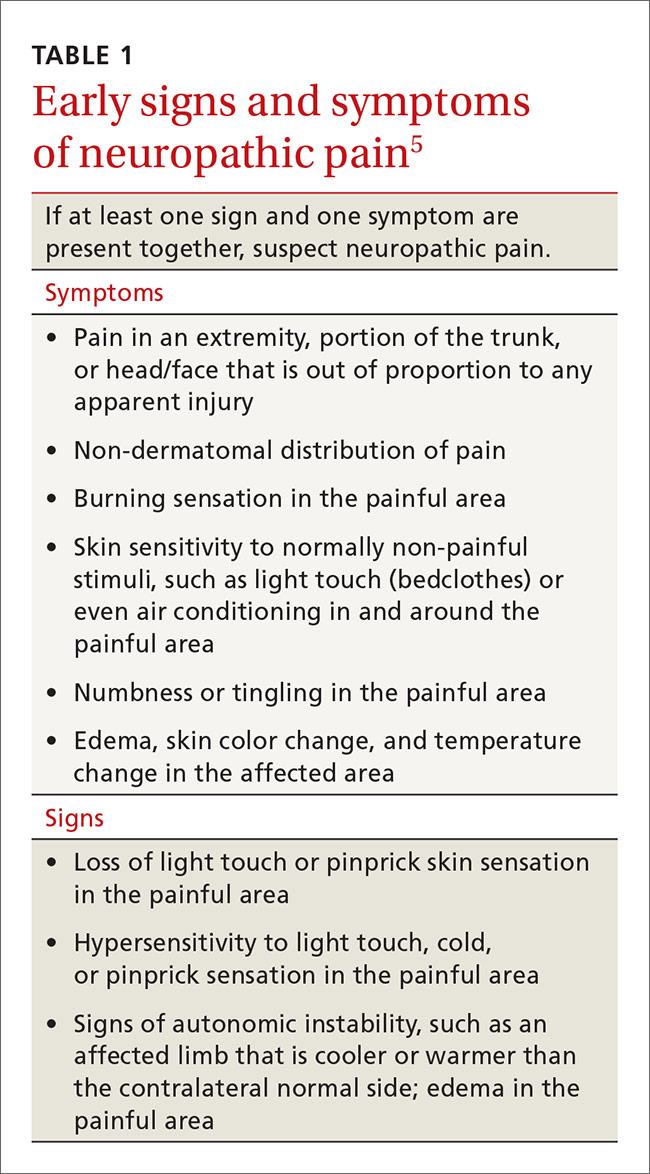
In contrast, systems plasticity (when new CNS neuronal connections are formed in response to nerve injury) takes place over the months and years following nerve injury and is often irreversible. When we recognize NPP and intervene before system neuroplastic changes occur, it may be possible to prevent pain from becoming chronic (TABLE 15). In cases of nerve injury, researchers have long suspected that early and aggressive pain treatment within the first few months that may include sympathetic and peripheral neural blockade reduces the likelihood that the patient will have chronic pain.6,7
It’s time to update our understanding of pain
The International Association for the Study of Pain (IASP)—a group of health care providers, scientists, and policymakers seeking to improve pain relief worldwide—notes in its definition of pain that the complaint, “I hurt” does not necessarily imply that there is a painful stimulus in the form of tissue injury.8 Yet most of us have been taught to think of pain solely as the result of tissue pathology, and we assume that emotional factors merely modify how the physical damage is perceived. This traditional concept of pain is incomplete. It leads clinicians to misdiagnose the cause of pain, initiate expensive and unnecessary treatment, engage in well-meaning but misguided prescribing behavior, and miss opportunities to help patients.
SIDEBAR
From periphery to brain: The process of nociceptive pain1-4
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps:
In transduction, nociceptors containing special molecular proteins respond to noxious modalities, such as thermal, mechanical, or chemical stimuli, and trigger nerve impulses in the nociceptive nerve fibers (nerves dedicated to pain sensation).
During transmission—the second stage of the process—information from the nociceptors in the periphery (skin, muscle, viscera) is relayed to the spinal cord mainly by 2 types of nociceptive neurons: C-fibers and A delta (Aδ) fibers. Both approach the spinal cord in a peripheral nerve and then enter the spinal cord in the dorsal root entry zone. Because Aδ fibers are thinly myelinated, they send impulses faster than unmyelinated C fibers. This is why when injury occurs, we first feel sharp, acute pain that then slowly diffuses into a duller ache.
Once the incoming signal is transmitted to the CNS at the spinal cord, primary afferent neurons synapse on second order neurons. From there, information travels on to the thalamus via multiple neurons that have the capacity to change their response patterns when activity of nociceptive fibers is sustained (as occurs in the setting of a tissue or nerve injury and perhaps in the setting of psychological trauma). This is known as modulation of the incoming nociceptive stimulus. During this step of the process, stimuli can be amplified, suppressed, or even transformed from one type to another (eg, a light touch can be modulated in such a way that it will be perceived as a burning sensation). Also, it is this step that is affected by many medications, by intrathecal drug infusions, and by spinal neurostimulators.
In perception, the thalamus then directs the pain sensation to multiple brain centers. At this step, the stimulus is finally consciously perceived as pain by the individual.
Cortical pain circuits can be activated without physical input (ie, no tissue damage, noxious stimuli, or nerve injury). This becomes important in understanding pain syndromes, such as fibromyalgia.
Pain in the absence of any pathophysiologic cause or injury
The clinician’s search for a pain diagnosis is typically predicated on the notion that there must be an underlying tissue injury of severity equal to the severity of the patient’s pain complaints. This approach to a pain evaluation rests on 2 assumptions that are not true for all patients:
- Pain is simply a sensory experience that is always caused by tissue damage of some type.
- The severity of the pain experienced by a patient should be tightly bound to the severity of the pain stimulus (ie, tissue damage).
These assumptions are true of acute nociceptive pain, they may or may not be true for NPP, but they do not apply to the third type of pain—pain for psychological reasons. While tissue pathology in humans and animals with nociceptive pain is usually visible, measurable, and correlates with observed pain behaviors, the damage to nerve tissue and the ensuing changes in nervous system function with NPP are not always visible or able to be imaged. These changes produce pain that can appear more severe than expected based on a brief exam. Some of the time, however, characteristic symptoms and physical signs of NPP will be present, and perhaps electrodiagnostic or other tests will be abnormal, thus providing some objective sense of changes in nervous system function.
In contrast, pain behavior due to the third type of pain usually appears very much out of proportion, and unbound to, tissue pathology. Furthermore, the patient’s pain behaviors often reflect heightened emotional pain processing (TABLE 29). The resulting emotionally charged presentation can be alarming and suggestive of extreme tissue injury, but there may be absolutely no evidence of tissue injury or pathology.

Functional change in the CNS
There is evidence from experimental studies that psychologic factors change nervous system function. In one review, the authors concluded, “Pain…can vary widely between people and even within an individual depending on…the psychological state of the person.”10 In a second review, the authors concluded that our emotional state has an enormous influence on pain; a negative emotional state increases pain, whereas a positive state lowers pain.11
But can psychological factors induce long-term changes in nervous system function analogous to the systems neuroplasticity responsible for irreversible changes in NPP? And can psychologically induced changes in nervous system sensory processing lead to pain without any tissue or nerve damage?
We theorize that a functional change in the CNS can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents). (More on this in a bit.) When such changes occur, mildly painful stimuli are amplified and processed through overly sensitized, dysregulated, ramped-up emotional and somatosensory pain circuits in the brain. This is analogous to the functional changes in the nervous system that occur with NPP; however, when the nervous system changes are due to psychological factors, there may be no tissue or nerve injury.
Childhood trauma influences adult pain. One of the more compelling narratives emerging in health care has to do with the influence that childhood developmental trauma can have on health, including pain. In his chapter on the impact of early life trauma on health and disease, Lanius states:12
“Women were 50% more likely than men to have experienced 5 or more categories of adverse childhood experiences. We believe that here is a key to what in mainstream epidemiology appears as women’s natural proneness to ill-defined health problems like fibromyalgia, chronic fatigue syndrome, obesity, irritable bowel syndrome, and chronic non-malignant pain syndromes. In light of our findings, we now see these as medical constructs, artifacts resulting from medical blindness to social realities and ignorance of the impact of gender.”
Lanius12 suggests that adverse childhood experiences13 (trauma such as abuse and sexual assault) can lead to long-term changes within the nervous system, including areas of pain processing. My coauthor and I describe these changes here in terms of nervous system sensitization or dysregulation, and we believe that these changes lead to a bias toward hyperactivation of emotional pain circuits, which leads to the emotionally laden pain behaviors that often seem out of proportion to tissue pathology.
1. Dubner R, Gold M. The neurobiology of pain. Proc Natl Acad Sci U S A. 1999;96:7627-7630.
2. Markenson JA. Mechanisms of chronic pain. Am J Med. 1996;101:S6-S18.
3. Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968-971.
4. Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78:415-418.
5. Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48:332-338.
6. Fassoulaki A, Triga A, Melemeni A, et al. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg. 2005;101:1427-1432.
7. Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362-379.
8. International Association for the Study of Pain Web site. IASP Taxonomy. Available at: http://www.iasp-pain.org/Taxonomy. Accessed January 10, 2016.
9. Waddell G, McCulloch JA, Kummel E, et al. Nonorganic physical signs in low-back pain. Spine. 1980;5:117-125.
10. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502-511.
11. Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195-199.
12. Felitti VJ, Anda RF. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders, and sexual behavior: implications for healthcare. In: Lanius R, Vermetten E, eds. The Hidden Epidemic: The Impact of Early Life Trauma on Health and Disease. Cambridge University Press; 2010. Available at: http://www.unnaturalcauses.org/assets/uploads/file/ACE%20Study-Lanius.pdf. Accessed January 11, 2016.
13. Centers for Disease Control and Prevention. Injury prevention and control: Division of violence prevention. Adverse childhood experiences. Available at: http://www.cdc.gov/violenceprevention/acestudy/. Accessed January 11, 2016.
14. Kross E, Berman MG, Mischel W, et al. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci. 2011;108:6270-6275.
15. Geuze E, Westenberg HGM, Jochims A, et al. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76-85.
16. Mickleborough MJ, Daniels JK, Coupland NJ. Effects of trauma-related cues on pain processing in posttraumatic stress disorder: an fMRI investigation. J Psychiatry Neurosci. 2011;36: 6-14.
17. Noll-Hussong M, Otti A, Laeer L, et al. Aftermath of sexual abuse history on adult patients suffering from chronic functional pain syndromes: an fMRI pilot study. J Psychoso Res. 2010; 68:483-487.
The care of people with pain has been wrought with ineffective and unnecessary treatment, including the misuse of opioids, largely because we do not have an accurate conceptualization of pain. The absence of animal and human models of central nervous system (CNS) pain processing ensures that our understanding of pain will remain incomplete for the foreseeable future, but enough evidence exists to help family physicians develop an understanding of pain that goes beyond what we learned in medical school and that can help us more effectively treat patients with pain.
In this review, we will briefly discuss the established concepts of nociceptive and neuropathic pain. And then, with those concepts in mind, we will explore a third type of pain that for lack of a better term, we will call “pain for psychological reasons.” We hypothesize that this pain may be the consequence of changes in nervous system function that arise from developmental trauma, other traumatic experiences in a patient’s life, or mental health disorders. It is this third type of pain that may offer us insights into conditions such as fibromyalgia.
While we do not yet have validated diagnostic criteria for this third type of pain, we believe that there is enough information to present initial criteria so that one may distinguish it from nociceptive and neuropathic pain.
Nociceptive and neuropathic pain: The current paradigm
Nociceptive pain. The sensory pain experience, or nociceptive pain, is produced by noxious stimuli that either damage, or are capable of damaging, tissues (eg, burns, cuts, fractures, inflammation, and increased pressure in a hollow viscus). Noxious stimuli are detected at the molecular level by specific pain sensory receptors embedded in our tissues called nociceptors.
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps—transduction, transmission, modulation, and perception—which are described in “From periphery to brain: The process of nociceptive pain.”1-4
Neuropathic pain. While nociceptive pain can be easily traced from a peripheral nociceptive fiber to the brain and typically resolves when the nociceptive stimulus stops, neuropathic pain (NPP) results from changes to the function of the nervous system and is typically caused by injury to the nerves. Such changes, referred to as neuronal sensitization, may not quickly resolve, as is the case with postherpetic neuralgia. In fact, the changes can become permanent. NPP fundamentally differs from nociceptive pain because it results from changes in the central processing of pain that can lead a person to perceive pain sensations even in the absence of tissue pathology.
Common causes of NPP that persists even after tissue damage has healed include trauma (eg, amputation of a limb), ischemia (eg, pressure palsy), disease (eg, the metabolic injury of diabetes or the injury caused by a shingles infection), and drug treatment (eg, chemotherapy). The underlying mechanisms of NPP and the neuronal plasticity (the ability of the nervous system to rewire itself) that initiate and then maintain NPP are important areas of active research that may eventually lead to the development of more effective treatments.
Timing is critical. Neuroplastic changes in the nervous system following nerve injury are time-dependent. Synaptic plasticity can occur within seconds to minutes, while cellular plasticity occurs within hours to days. Synaptic and cellular plasticity happen relatively fast and may be reversible.
In contrast, systems plasticity (when new CNS neuronal connections are formed in response to nerve injury) takes place over the months and years following nerve injury and is often irreversible. When we recognize NPP and intervene before system neuroplastic changes occur, it may be possible to prevent pain from becoming chronic (TABLE 15). In cases of nerve injury, researchers have long suspected that early and aggressive pain treatment within the first few months that may include sympathetic and peripheral neural blockade reduces the likelihood that the patient will have chronic pain.6,7

It’s time to update our understanding of pain
The International Association for the Study of Pain (IASP)—a group of health care providers, scientists, and policymakers seeking to improve pain relief worldwide—notes in its definition of pain that the complaint, “I hurt” does not necessarily imply that there is a painful stimulus in the form of tissue injury.8 Yet most of us have been taught to think of pain solely as the result of tissue pathology, and we assume that emotional factors merely modify how the physical damage is perceived. This traditional concept of pain is incomplete. It leads clinicians to misdiagnose the cause of pain, initiate expensive and unnecessary treatment, engage in well-meaning but misguided prescribing behavior, and miss opportunities to help patients.
SIDEBAR
From periphery to brain: The process of nociceptive pain1-4
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps:
In transduction, nociceptors containing special molecular proteins respond to noxious modalities, such as thermal, mechanical, or chemical stimuli, and trigger nerve impulses in the nociceptive nerve fibers (nerves dedicated to pain sensation).
During transmission—the second stage of the process—information from the nociceptors in the periphery (skin, muscle, viscera) is relayed to the spinal cord mainly by 2 types of nociceptive neurons: C-fibers and A delta (Aδ) fibers. Both approach the spinal cord in a peripheral nerve and then enter the spinal cord in the dorsal root entry zone. Because Aδ fibers are thinly myelinated, they send impulses faster than unmyelinated C fibers. This is why when injury occurs, we first feel sharp, acute pain that then slowly diffuses into a duller ache.
Once the incoming signal is transmitted to the CNS at the spinal cord, primary afferent neurons synapse on second order neurons. From there, information travels on to the thalamus via multiple neurons that have the capacity to change their response patterns when activity of nociceptive fibers is sustained (as occurs in the setting of a tissue or nerve injury and perhaps in the setting of psychological trauma). This is known as modulation of the incoming nociceptive stimulus. During this step of the process, stimuli can be amplified, suppressed, or even transformed from one type to another (eg, a light touch can be modulated in such a way that it will be perceived as a burning sensation). Also, it is this step that is affected by many medications, by intrathecal drug infusions, and by spinal neurostimulators.
In perception, the thalamus then directs the pain sensation to multiple brain centers. At this step, the stimulus is finally consciously perceived as pain by the individual.
Cortical pain circuits can be activated without physical input (ie, no tissue damage, noxious stimuli, or nerve injury). This becomes important in understanding pain syndromes, such as fibromyalgia.
Pain in the absence of any pathophysiologic cause or injury
The clinician’s search for a pain diagnosis is typically predicated on the notion that there must be an underlying tissue injury of severity equal to the severity of the patient’s pain complaints. This approach to a pain evaluation rests on 2 assumptions that are not true for all patients:
- Pain is simply a sensory experience that is always caused by tissue damage of some type.
- The severity of the pain experienced by a patient should be tightly bound to the severity of the pain stimulus (ie, tissue damage).
These assumptions are true of acute nociceptive pain, they may or may not be true for NPP, but they do not apply to the third type of pain—pain for psychological reasons. While tissue pathology in humans and animals with nociceptive pain is usually visible, measurable, and correlates with observed pain behaviors, the damage to nerve tissue and the ensuing changes in nervous system function with NPP are not always visible or able to be imaged. These changes produce pain that can appear more severe than expected based on a brief exam. Some of the time, however, characteristic symptoms and physical signs of NPP will be present, and perhaps electrodiagnostic or other tests will be abnormal, thus providing some objective sense of changes in nervous system function.
In contrast, pain behavior due to the third type of pain usually appears very much out of proportion, and unbound to, tissue pathology. Furthermore, the patient’s pain behaviors often reflect heightened emotional pain processing (TABLE 29). The resulting emotionally charged presentation can be alarming and suggestive of extreme tissue injury, but there may be absolutely no evidence of tissue injury or pathology.

Functional change in the CNS
There is evidence from experimental studies that psychologic factors change nervous system function. In one review, the authors concluded, “Pain…can vary widely between people and even within an individual depending on…the psychological state of the person.”10 In a second review, the authors concluded that our emotional state has an enormous influence on pain; a negative emotional state increases pain, whereas a positive state lowers pain.11
But can psychological factors induce long-term changes in nervous system function analogous to the systems neuroplasticity responsible for irreversible changes in NPP? And can psychologically induced changes in nervous system sensory processing lead to pain without any tissue or nerve damage?
We theorize that a functional change in the CNS can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents). (More on this in a bit.) When such changes occur, mildly painful stimuli are amplified and processed through overly sensitized, dysregulated, ramped-up emotional and somatosensory pain circuits in the brain. This is analogous to the functional changes in the nervous system that occur with NPP; however, when the nervous system changes are due to psychological factors, there may be no tissue or nerve injury.
Childhood trauma influences adult pain. One of the more compelling narratives emerging in health care has to do with the influence that childhood developmental trauma can have on health, including pain. In his chapter on the impact of early life trauma on health and disease, Lanius states:12
“Women were 50% more likely than men to have experienced 5 or more categories of adverse childhood experiences. We believe that here is a key to what in mainstream epidemiology appears as women’s natural proneness to ill-defined health problems like fibromyalgia, chronic fatigue syndrome, obesity, irritable bowel syndrome, and chronic non-malignant pain syndromes. In light of our findings, we now see these as medical constructs, artifacts resulting from medical blindness to social realities and ignorance of the impact of gender.”
Lanius12 suggests that adverse childhood experiences13 (trauma such as abuse and sexual assault) can lead to long-term changes within the nervous system, including areas of pain processing. My coauthor and I describe these changes here in terms of nervous system sensitization or dysregulation, and we believe that these changes lead to a bias toward hyperactivation of emotional pain circuits, which leads to the emotionally laden pain behaviors that often seem out of proportion to tissue pathology.
The care of people with pain has been wrought with ineffective and unnecessary treatment, including the misuse of opioids, largely because we do not have an accurate conceptualization of pain. The absence of animal and human models of central nervous system (CNS) pain processing ensures that our understanding of pain will remain incomplete for the foreseeable future, but enough evidence exists to help family physicians develop an understanding of pain that goes beyond what we learned in medical school and that can help us more effectively treat patients with pain.
In this review, we will briefly discuss the established concepts of nociceptive and neuropathic pain. And then, with those concepts in mind, we will explore a third type of pain that for lack of a better term, we will call “pain for psychological reasons.” We hypothesize that this pain may be the consequence of changes in nervous system function that arise from developmental trauma, other traumatic experiences in a patient’s life, or mental health disorders. It is this third type of pain that may offer us insights into conditions such as fibromyalgia.
While we do not yet have validated diagnostic criteria for this third type of pain, we believe that there is enough information to present initial criteria so that one may distinguish it from nociceptive and neuropathic pain.
Nociceptive and neuropathic pain: The current paradigm
Nociceptive pain. The sensory pain experience, or nociceptive pain, is produced by noxious stimuli that either damage, or are capable of damaging, tissues (eg, burns, cuts, fractures, inflammation, and increased pressure in a hollow viscus). Noxious stimuli are detected at the molecular level by specific pain sensory receptors embedded in our tissues called nociceptors.
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps—transduction, transmission, modulation, and perception—which are described in “From periphery to brain: The process of nociceptive pain.”1-4
Neuropathic pain. While nociceptive pain can be easily traced from a peripheral nociceptive fiber to the brain and typically resolves when the nociceptive stimulus stops, neuropathic pain (NPP) results from changes to the function of the nervous system and is typically caused by injury to the nerves. Such changes, referred to as neuronal sensitization, may not quickly resolve, as is the case with postherpetic neuralgia. In fact, the changes can become permanent. NPP fundamentally differs from nociceptive pain because it results from changes in the central processing of pain that can lead a person to perceive pain sensations even in the absence of tissue pathology.
Common causes of NPP that persists even after tissue damage has healed include trauma (eg, amputation of a limb), ischemia (eg, pressure palsy), disease (eg, the metabolic injury of diabetes or the injury caused by a shingles infection), and drug treatment (eg, chemotherapy). The underlying mechanisms of NPP and the neuronal plasticity (the ability of the nervous system to rewire itself) that initiate and then maintain NPP are important areas of active research that may eventually lead to the development of more effective treatments.
Timing is critical. Neuroplastic changes in the nervous system following nerve injury are time-dependent. Synaptic plasticity can occur within seconds to minutes, while cellular plasticity occurs within hours to days. Synaptic and cellular plasticity happen relatively fast and may be reversible.
In contrast, systems plasticity (when new CNS neuronal connections are formed in response to nerve injury) takes place over the months and years following nerve injury and is often irreversible. When we recognize NPP and intervene before system neuroplastic changes occur, it may be possible to prevent pain from becoming chronic (TABLE 15). In cases of nerve injury, researchers have long suspected that early and aggressive pain treatment within the first few months that may include sympathetic and peripheral neural blockade reduces the likelihood that the patient will have chronic pain.6,7

It’s time to update our understanding of pain
The International Association for the Study of Pain (IASP)—a group of health care providers, scientists, and policymakers seeking to improve pain relief worldwide—notes in its definition of pain that the complaint, “I hurt” does not necessarily imply that there is a painful stimulus in the form of tissue injury.8 Yet most of us have been taught to think of pain solely as the result of tissue pathology, and we assume that emotional factors merely modify how the physical damage is perceived. This traditional concept of pain is incomplete. It leads clinicians to misdiagnose the cause of pain, initiate expensive and unnecessary treatment, engage in well-meaning but misguided prescribing behavior, and miss opportunities to help patients.
SIDEBAR
From periphery to brain: The process of nociceptive pain1-4
The process by which noxious stimuli lead to the experience of sensory pain consists of 4 steps:
In transduction, nociceptors containing special molecular proteins respond to noxious modalities, such as thermal, mechanical, or chemical stimuli, and trigger nerve impulses in the nociceptive nerve fibers (nerves dedicated to pain sensation).
During transmission—the second stage of the process—information from the nociceptors in the periphery (skin, muscle, viscera) is relayed to the spinal cord mainly by 2 types of nociceptive neurons: C-fibers and A delta (Aδ) fibers. Both approach the spinal cord in a peripheral nerve and then enter the spinal cord in the dorsal root entry zone. Because Aδ fibers are thinly myelinated, they send impulses faster than unmyelinated C fibers. This is why when injury occurs, we first feel sharp, acute pain that then slowly diffuses into a duller ache.
Once the incoming signal is transmitted to the CNS at the spinal cord, primary afferent neurons synapse on second order neurons. From there, information travels on to the thalamus via multiple neurons that have the capacity to change their response patterns when activity of nociceptive fibers is sustained (as occurs in the setting of a tissue or nerve injury and perhaps in the setting of psychological trauma). This is known as modulation of the incoming nociceptive stimulus. During this step of the process, stimuli can be amplified, suppressed, or even transformed from one type to another (eg, a light touch can be modulated in such a way that it will be perceived as a burning sensation). Also, it is this step that is affected by many medications, by intrathecal drug infusions, and by spinal neurostimulators.
In perception, the thalamus then directs the pain sensation to multiple brain centers. At this step, the stimulus is finally consciously perceived as pain by the individual.
Cortical pain circuits can be activated without physical input (ie, no tissue damage, noxious stimuli, or nerve injury). This becomes important in understanding pain syndromes, such as fibromyalgia.
Pain in the absence of any pathophysiologic cause or injury
The clinician’s search for a pain diagnosis is typically predicated on the notion that there must be an underlying tissue injury of severity equal to the severity of the patient’s pain complaints. This approach to a pain evaluation rests on 2 assumptions that are not true for all patients:
- Pain is simply a sensory experience that is always caused by tissue damage of some type.
- The severity of the pain experienced by a patient should be tightly bound to the severity of the pain stimulus (ie, tissue damage).
These assumptions are true of acute nociceptive pain, they may or may not be true for NPP, but they do not apply to the third type of pain—pain for psychological reasons. While tissue pathology in humans and animals with nociceptive pain is usually visible, measurable, and correlates with observed pain behaviors, the damage to nerve tissue and the ensuing changes in nervous system function with NPP are not always visible or able to be imaged. These changes produce pain that can appear more severe than expected based on a brief exam. Some of the time, however, characteristic symptoms and physical signs of NPP will be present, and perhaps electrodiagnostic or other tests will be abnormal, thus providing some objective sense of changes in nervous system function.
In contrast, pain behavior due to the third type of pain usually appears very much out of proportion, and unbound to, tissue pathology. Furthermore, the patient’s pain behaviors often reflect heightened emotional pain processing (TABLE 29). The resulting emotionally charged presentation can be alarming and suggestive of extreme tissue injury, but there may be absolutely no evidence of tissue injury or pathology.

Functional change in the CNS
There is evidence from experimental studies that psychologic factors change nervous system function. In one review, the authors concluded, “Pain…can vary widely between people and even within an individual depending on…the psychological state of the person.”10 In a second review, the authors concluded that our emotional state has an enormous influence on pain; a negative emotional state increases pain, whereas a positive state lowers pain.11
But can psychological factors induce long-term changes in nervous system function analogous to the systems neuroplasticity responsible for irreversible changes in NPP? And can psychologically induced changes in nervous system sensory processing lead to pain without any tissue or nerve damage?
We theorize that a functional change in the CNS can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents). (More on this in a bit.) When such changes occur, mildly painful stimuli are amplified and processed through overly sensitized, dysregulated, ramped-up emotional and somatosensory pain circuits in the brain. This is analogous to the functional changes in the nervous system that occur with NPP; however, when the nervous system changes are due to psychological factors, there may be no tissue or nerve injury.
Childhood trauma influences adult pain. One of the more compelling narratives emerging in health care has to do with the influence that childhood developmental trauma can have on health, including pain. In his chapter on the impact of early life trauma on health and disease, Lanius states:12
“Women were 50% more likely than men to have experienced 5 or more categories of adverse childhood experiences. We believe that here is a key to what in mainstream epidemiology appears as women’s natural proneness to ill-defined health problems like fibromyalgia, chronic fatigue syndrome, obesity, irritable bowel syndrome, and chronic non-malignant pain syndromes. In light of our findings, we now see these as medical constructs, artifacts resulting from medical blindness to social realities and ignorance of the impact of gender.”
Lanius12 suggests that adverse childhood experiences13 (trauma such as abuse and sexual assault) can lead to long-term changes within the nervous system, including areas of pain processing. My coauthor and I describe these changes here in terms of nervous system sensitization or dysregulation, and we believe that these changes lead to a bias toward hyperactivation of emotional pain circuits, which leads to the emotionally laden pain behaviors that often seem out of proportion to tissue pathology.
1. Dubner R, Gold M. The neurobiology of pain. Proc Natl Acad Sci U S A. 1999;96:7627-7630.
2. Markenson JA. Mechanisms of chronic pain. Am J Med. 1996;101:S6-S18.
3. Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968-971.
4. Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78:415-418.
5. Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48:332-338.
6. Fassoulaki A, Triga A, Melemeni A, et al. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg. 2005;101:1427-1432.
7. Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362-379.
8. International Association for the Study of Pain Web site. IASP Taxonomy. Available at: http://www.iasp-pain.org/Taxonomy. Accessed January 10, 2016.
9. Waddell G, McCulloch JA, Kummel E, et al. Nonorganic physical signs in low-back pain. Spine. 1980;5:117-125.
10. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502-511.
11. Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195-199.
12. Felitti VJ, Anda RF. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders, and sexual behavior: implications for healthcare. In: Lanius R, Vermetten E, eds. The Hidden Epidemic: The Impact of Early Life Trauma on Health and Disease. Cambridge University Press; 2010. Available at: http://www.unnaturalcauses.org/assets/uploads/file/ACE%20Study-Lanius.pdf. Accessed January 11, 2016.
13. Centers for Disease Control and Prevention. Injury prevention and control: Division of violence prevention. Adverse childhood experiences. Available at: http://www.cdc.gov/violenceprevention/acestudy/. Accessed January 11, 2016.
14. Kross E, Berman MG, Mischel W, et al. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci. 2011;108:6270-6275.
15. Geuze E, Westenberg HGM, Jochims A, et al. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76-85.
16. Mickleborough MJ, Daniels JK, Coupland NJ. Effects of trauma-related cues on pain processing in posttraumatic stress disorder: an fMRI investigation. J Psychiatry Neurosci. 2011;36: 6-14.
17. Noll-Hussong M, Otti A, Laeer L, et al. Aftermath of sexual abuse history on adult patients suffering from chronic functional pain syndromes: an fMRI pilot study. J Psychoso Res. 2010; 68:483-487.
1. Dubner R, Gold M. The neurobiology of pain. Proc Natl Acad Sci U S A. 1999;96:7627-7630.
2. Markenson JA. Mechanisms of chronic pain. Am J Med. 1996;101:S6-S18.
3. Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968-971.
4. Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78:415-418.
5. Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48:332-338.
6. Fassoulaki A, Triga A, Melemeni A, et al. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg. 2005;101:1427-1432.
7. Woolf CJ, Chong MS. Preemptive analgesia—treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362-379.
8. International Association for the Study of Pain Web site. IASP Taxonomy. Available at: http://www.iasp-pain.org/Taxonomy. Accessed January 10, 2016.
9. Waddell G, McCulloch JA, Kummel E, et al. Nonorganic physical signs in low-back pain. Spine. 1980;5:117-125.
10. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502-511.
11. Villemure C, Bushnell MC. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195-199.
12. Felitti VJ, Anda RF. The relationship of adverse childhood experiences to adult medical disease, psychiatric disorders, and sexual behavior: implications for healthcare. In: Lanius R, Vermetten E, eds. The Hidden Epidemic: The Impact of Early Life Trauma on Health and Disease. Cambridge University Press; 2010. Available at: http://www.unnaturalcauses.org/assets/uploads/file/ACE%20Study-Lanius.pdf. Accessed January 11, 2016.
13. Centers for Disease Control and Prevention. Injury prevention and control: Division of violence prevention. Adverse childhood experiences. Available at: http://www.cdc.gov/violenceprevention/acestudy/. Accessed January 11, 2016.
14. Kross E, Berman MG, Mischel W, et al. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci. 2011;108:6270-6275.
15. Geuze E, Westenberg HGM, Jochims A, et al. Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry. 2007;64:76-85.
16. Mickleborough MJ, Daniels JK, Coupland NJ. Effects of trauma-related cues on pain processing in posttraumatic stress disorder: an fMRI investigation. J Psychiatry Neurosci. 2011;36: 6-14.
17. Noll-Hussong M, Otti A, Laeer L, et al. Aftermath of sexual abuse history on adult patients suffering from chronic functional pain syndromes: an fMRI pilot study. J Psychoso Res. 2010; 68:483-487.
From The Journal of Family Practice | 2016;65(9):598-600,602-605.