User login
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
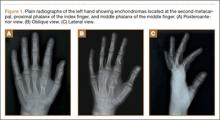
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
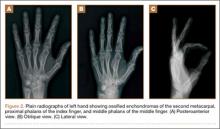
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
Ollier disease, or multiple enchondromatosis, is a rare nonfamilial condition characterized by multiple cartilaginous tumors often beginning in early childhood. There is significant variation in disease distribution, location, size, number of lesions, and behavior, but the tumors are often located unilaterally.1 Enchondromas are most commonly found in the metacarpals, metatarsals, and phalanges, and develop from metaphyseal bone in close proximity to the physis. They frequently present as painless masses or are incidentally noted during the evaluation of another musculoskeletal condition. Radiographically, enchondromas of the hands and feet appear as oval radiolucencies with thinned, sclerotic rims. The lesions have varying degrees of mineralization and endosteal scalloping, and may expand the bone.2 Enchondromas usually enlarge until skeletal maturity and have been observed to ossify spontaneously.1,3 The clinical course of Ollier disease is variable, and a number of cases of significant hand deformity and malignant transformation have been reported.4-6
In this case report, we present a mild form of Ollier disease isolated to the patient’s left hand, which we followed for 8 years, demonstrating part of the natural history of these lesions. We discuss the patient’s clinical features, radiologic findings, diagnosis, treatment, prognosis, and follow-up, as well as review the literature. The patient and the patient’s family provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old, right-handed girl was referred to our department for the evaluation of left-hand masses. At age 3 years, the patient underwent a chondroma excision from the middle phalanx of her middle finger on her left hand. No operative or pathology report was available from this surgery, and the patient tolerated the procedure well without any complications. At the time of presentation, the masses did not cause any pain, motor or sensory dysfunction, or any systemic symptoms. No history of recent or distant trauma was elicited. The patient’s medical and family history was unremarkable.
On physical examination, there was a firm, immobile, nontender palpable mass over the dorsal aspect of the distal second metacarpal bone of the left hand. The mass extended medially between the second and third metacarpals. A second small, firm, nontender left-hand mass was palpated over the volar aspect of her proximal phalanx on her index finger. She was neurovascularly intact with full active range of motion of the metacarpophalangeal and proximal and distal interphalangeal joints. There was no angular deformity of the digits. Plain radiographs taken at the time of initial presentation showed a 2.3×1.7-cm radiolucent lesion located in the metaphysis and diaphysis of the second metacarpal of the left hand (Figures 1A-1C). The lesion had varying degrees of mineralization with cortical thinning and expansion in the volar, dorsal, radial, and ulnar directions, consistent with a chondroid lesion. The second and third lesions were oval radiolucencies with sclerotic rims located at the metaphyseal-diaphyseal junction of the proximal phalanx of the index finger and middle phalanx of the middle finger, respectively. No fractures were identified in the radiographs, and the physes were open at this time. The patient was diagnosed with multiple enchondromatosis, or Ollier disease.
Our case showed 1 episode of pain and tenderness to palpation at the second proximal phalanx approximately 6 months after initial presentation. We attributed the pain and tenderness to a small pathologic fracture but did not see radiographic evidence of this. We elected to provide a trial of supportive measures, such as splinting and buddy taping, and to monitor the pain with a tentative plan of open biopsy with curettage and bone grafting if the pain persisted or evidence of fracture was seen on radiographs. The pain and tenderness to palpation resolved at a follow-up visit, and the surgery was deferred.
The patient was treated nonoperatively at initial presentation given the lack of significant cosmetic deformity or functional compromise and was advised close follow-up at 3 and 6 months. Given the absence of disease progression, annual checks (ie, clinical examination and radiographs) in a skeletally immature patient were decided on after consultation with the patient and parent. The family was educated about the possibility of pathologic fracture from minimal trauma to the hand versus the small risk of iatrogenic physeal injury with surgical curettage and bone grafting. No protective splinting was offered. A favorable prognosis and reassurance was provided to the patient and family, given the absence of symptoms, low suspicion and risk of malignant transformation, and stability of the lesion. Serial radiographs showed gradual increases in the lesions’ sizes but were consistent with the stable growth of the metacarpal and phalanges. With the patient nearing skeletal maturity, no pathologic fractures were identified on radiography during follow-up, and the risks of surgery lessened with growth; however, the continued absence of symptoms led to the mutual decision to continue observation.
Nearly 8 years after initial presentation, plain radiographs showed closed physes and partially ossified bone masses (Figures 2A-2C). The metacarpal lesion measured 3.2×1.5 cm, and the cortex appeared thickened and regular. The proximal phalanx lesion had a thickened cortex without periosteal reaction, and the middle phalanx lesion appeared to be completely healed. The patient has been asymptomatic for many years, and she has retained complete function of her left hand without any growth retardation, angular deformity, or pathologic fracture. A small but potential risk of malignant transformation was discussed with the patient and her family, as was the need for lifetime follow-up. We intend to follow the enchondromas clinically and radiographically every 2 years and obtain new radiographs if the mass presents with new clinical findings, such as enlargement or pain, for surveillance of tumor transformation. If the patient desired or symptoms developed, curettage and bone grafting would be offered, and the surgical tissue would be sent for pathologic analysis. A bone scan that was obtained at the request of the patient, when she was 21 years old, showed no other sites of disease besides the fingers.
Discussion
Multiple enchondromatosis was first described by Ollier at the turn of the 19th century and has been estimated to affect one in every 100,000 persons.1 The low prevalence and variable manifestations of Ollier disease lead clinicians to handle the disease and its complications, namely skeletal deformity and malignant transformation, on a case-by-case approach. Additionally, the prognosis of Ollier disease with malignant transformation is quite variable, with studies reporting the estimated incidence as 5% to 50%.7 Muramatsu and colleagues6 reported that the occurrence of malignant transformation of multiple enchondromas limited to the bones of the hand was extremely rare, with only 12 cases of malignant transformation. Enchondromas of the pelvis, scapula, and long bones of the extremities have increased risks and rates of secondary transformation to chondrosarcoma.8
A recent large European multicenter retrospective study investigating the clinical characteristics and behavior of enchondromas in 144 patients with Ollier disease has provided new information regarding this rare disease.7 Verdegaal and colleagues7 divided patients into 3 categories depending on their distribution of enchondromas. The development of chondrosarcoma was notably different between individuals with enchondromas limited to the small bones of the hands and feet (15%, group I) versus individuals with enchondromas limited to the long bones and flat bones (43%, group II) or individuals with enchondromas of the short, long, and flat bones (46%, group III).7 The only location found to be statistically significant for the development of chondrosarcoma was the pelvis.
The clinical findings associated with risk of malignant transformation of enchondromas are increasing size of the lesion and onset of pain and tenderness. Dahlin and Salvador9 reported that only 60% of patients with chondrosarcoma of the hand experience pain. The absence of pain may lead to a delay in patient presentation to the clinician.5,6 Radiographic findings of malignant transformation include the classic features of temporal increases in the lesion’s size after skeletal maturity and cortical destruction associated with soft-tissue invasion. However, both findings are nonspecific for differentiating enchondromas from grade 1 chondrosarcomas as described by Geirnaerdt and colleagues.10
Sassoon and colleagues11 reported on a series of hand enchondromas treated operatively. Subgroup analysis between pathologic fractures treated primarily or in delayed fashion showed similar outcomes for achieving full motion and similar number of complications; however, they noted that the delayed group required 7 more weeks of immobilization. Additionally, review of the whole series showed 1 episode of metacarpal shortening and 1 occurrence of angular malalignment. In our patient, we were concerned about introducing an iatrogenic cosmetic deformity, and we believed a pathologic fracture could be managed expectantly. Overall, patients without pathologic fracture treated surgically experienced a complication rate of 12%, whereas patients with a fracture had a complication rate of 20%.11 The majority of patients with multiple enchondromatosis treated with surgical curettage and grafting had successful outcomes, with 86% of patients regaining full motion, but the recurrence rate was 21%.11 Patients with expansile lesions regained less motion than patients with nonexpansile lesions. There was a single lesion believed preoperatively to be an enchondroma, but it underwent malignant transformation, as confirmed on intraoperative pathology. This patient had Maffucci syndrome and was treated with an amputation through the metacarpophalangeal joint.
There are 3 options for treating hand enchondromas: observation, curettage alone, or curettage with bone grafting. There is no consensus about conservative management, timing of intervention, or risk of pathologic fracture. Each patient is treated individually with attention to reason for presentation, number of lesions, associated pain, deformity, or pathologic fracture. Operative criteria include high risk of pathologic fracture based on location of enchondroma, cortical thinning, and previous pathologic fracture with resulting angular deformity. Nonoperative management may increase the risk of pathologic fracture, particularly in patients involved in aggressive contact sports, but the physician may offer protective splinting or counsel the patient on activity modification. Our case provides a study of the natural history of multiple enchondromatosis and shows mild increases in the lesions’ sizes during the 8-year follow-up. This was an expected finding given the patient’s immature skeleton. The lesions’ cortices continued to ossify after the physes closed and now provides an excellent comparison for the identification of future malignant changes.
Histologic analysis of biopsied or surgically treated lesions contributes to the differentiation between benign hand enchondromas and chondrosarcoma. Pathologic findings must be correlated with clinical and radiographic findings because hand enchondromas contain cytologic features of chondrosarcoma.12 In a series of 55 patients with chondrosarcoma, Liu and colleagues8 reported no cases from the hand. Verdegaal and colleagues7 reported a total of 13 chondrosarcomas in the metacarpals and hand phalanges in 97 group I and III patients. Five of these lesions were grade 1, 2 were grade 2, 1 was grade 3, and 5 lesions were unknown.
For patients with multiple enchondromatosis limited to the hands, prognosis is relatively good with respect to risk of secondary chondrosarcoma transformation, metastasis of secondary chondrosarcoma, and death. Verdegaal and colleagues7 reported the rate of secondary transformation in the hand to be 15%. Patil and colleagues13 reported no distant metastases in 23 patients with hand chondrosarcoma at mean follow-up of 8.5 years (range, 2-19 years), although none of their patients had Ollier disease. Verdegaal and colleagues7 reported 7 of the 8 deaths in their study were related to development of pulmonary metastases; however, none originated from chondrosarcomas in the hand. Additionally, there were no disease-related deaths in 29 group I patients. Herget and colleagues,14 in summarizing the literature, postulated that the overall survival rate of patients with secondary chondrosarcoma at 5 years is approximately 90%.
In our case, the patient, who had 3 enchondromas isolated to the left hand, can be categorized in group I. Thus, this case highlights the natural history of a patient with hand enchondromas and demonstrates that enchondromatosis of the short tubular bones of the hands can mature and ossify.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.
1. Silve C, Jüppner H. Ollier disease. Orphanet J Rare Dis. 2006;1:37-42.
2. Baert A. Encyclopedia of Diagnostic Imaging. Vol. 1. Berlin, Germany: Springer; 2008.
3. Takigawa K. Chondroma of the bones of the hand. A review of 110 cases. J Bone Joint Surg Am. 1971;53(8):1591-1600.
4. Mosher J. Multiple enchondromatosis of the hand. A case report. J Bone Joint Surg Am. 1976;58(5):717-719.
5. Goto T, Motoi T, Komiya K, et al. Chondrosarcoma of the hand secondary to multiple enchondromatosis; report of two cases. Arch Orthop Trauma Surg. 2003;123(1):42-47.
6. Muramatsu K, Kawakami Y, Tani Y, Taguchi T. Malignant transformation of multiple enchondromas in the hand: case report. J Hand Surg Am. 2011;36(2):304-307.
7. Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771-1779.
8. Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376-1385.
9. Dahlin D, Salvador AH. Chondrosarcomas of bones of the hands and feet—a study of 30 cases. Cancer. 1974;34(3):755-760.
10. Geirnaerdt MJ, Hermans J, Bloem JL, et al. Usefulness of radiography in differentiating enchondroma from central grade I chondrosarcoma. AJR Am J Roentgenol. 1997;169(4):1097-1104.
11. Sassoon AA, Fitz-Gibbon PD, Harmsen WS, Moran SL. Enchondromas of the hand: factors affecting recurrence, healing, motion, and malignant transformation. J Hand Surg Am. 2012;37(6):1229-1234.
12. Ogose A, Unni KK, Swee R, May GK, Rowland CM, Sim FH. Chondrosarcoma of small bones of the hands and feet. Cancer. 1997;80(1):50-59.
13. Patil S, de Silva MV, Crossan J, Reid R. Chondrosarcoma of small bones of the hand. J Hand Surg Br. 2003;28(6):602-608.
14. Herget GW, Strohm P, Rottenburger C, et al. Insights in Enchondroma, Enchondromatosis and the risk of secondary Chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma. 2014;61(4):365-378.