User login
An enhanced understanding of platelet biology, as reviewed in the previous article in this supplement, has made it possible to identify a wide variety of platelet agonists. This knowledge has fostered the development of a host of pharmacologic strategies to block agonists such as cyclooxygenase, thromboxane, adenosine diphosphate (ADP), and thrombin, among others. This article will discuss the pharmacologic properties of novel antiplatelet agents, as well as alternative dosing of the established antiplatelet agent clopidogrel, and will review data from available comparative and placebo-controlled trials of these agents. The article concludes with comparative perspectives on the potential roles and relative advantages of these agents in the evolving management of patients with acute coronary syndromes (ACS).
CLOPIDOGREL AND THE CHALLENGE OF VARIABLE RESPONSE
Clopidogrel, a member of the thienopyridine class of ADP receptor inhibitors, is well established for use in patients with ACS at a loading dose of 300 mg followed by a maintenance dose of 75 mg/day. At this loading dose, inhibition of platelet aggregation to ADP is approximately 30%, and the time to peak effect is approximately 4 to 6 hours.1
As with most other drugs, the response to clopidogrel is variable. However, in contrast to the accepted measures of response to antihypertensive or lipid-lowering drugs, there are no routinely used tests for measuring response to antiplatelet therapies. As a result, a “one size fits all” strategy in the dosing of clopidogrel has prevailed.
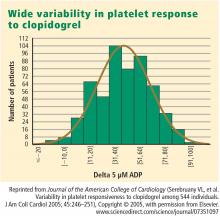
This variability in response is clinically relevant. In a study assessing clopidogrel responsiveness by ADP-induced platelet aggregation in 60 patients who experienced ST-segment-elevation myocardial infarction (MI), Matetzky et al found that the lowest levels of clopidogrel responsiveness were associated with a significantly elevated rate (P = .007) of recurrent cardiovascular events 6 months after the MI.3 Gurbel et al found a similar association between clopidogrel responsiveness and subacute stent thrombosis in a study of 120 patients using two different methods—light transmission aggregotomy to 5 μmol/L of ADP, and the ratio of vasodilator-stimulated phosphoprotein reactivity—to assess clopidogrel responsiveness.4
Increasing the loading dose raises response rates
One proposed method for boosting responsiveness to clopidogrel in suboptimal responders is the use of a higher dose. In a study of 190 patients undergoing coronary stenting, increasing the loading dose from 300 mg to 600 mg reduced the rate of clopidogrel resistance (defined as a < 10% absolute change in aggregation to 5 μM of ADP at 24 hours) from 28% to 8% (P < .001),5 a finding that supports the notion of enhanced response at doses up to 600 mg. Single loading doses in excess of 600 mg yield diminishing returns in terms of platelet inhibition, most likely as a result of clopidogrel pharmacokinetics.6
Compared with 300 mg of clopidogrel, the more potent platelet inhibitory effect of a 600-mg dose translated to a two-thirds reduction (P = .041) in the composite end point of death, MI, or target vessel revascularization at 30 days in a study of 255 patients with stable coronary artery disease undergoing percutaneous coronary intervention (PCI).7 The reduction in this composite end point with high-dose clopidogrel was driven by a reduction in the incidence of periprocedural MI.
In a separate study of 292 patients with non‑ST-segment-elevation ACS who were scheduled for PCI, the superior platelet response to 600 mg versus 300 mg of clopidogrel translated to a 60% reduction in adverse thrombotic events (P = .02), and this benefit extended beyond rates of periprocedural MI.8
Similar results with increased maintenance dose
Similarly, emerging data suggest that raising the maintenance dose of clopidogrel can also raise response rates. In a study of 60 patients, doubling the maintenance dose of clopidogrel after PCI from 75 mg/day to 150 mg/day resulted in improved platelet inhibition as assessed by rapid platelet function analysis.9 Likewise, a 150-mg/day maintenance dose of clopidogrel was associated with a superior antiplatelet effect compared with 75 mg/day in a study of 40 patients with type 2 diabetes.10
Large definitive trial is under way
In the wake of these smaller trials, a large randomized trial known as CURRENT is comparing a strategy of high-dose clopidogrel with standard-dose clopidogrel in patients with ACS for whom an early invasive management strategy is planned.11 The high-dose regimen involves a 600-mg loading dose followed by 150 mg/day for 1 week and then 75 mg/day for 3 weeks, whereas the standard-dose regimen involves a 300-mg loading dose followed by 75 mg/day for 4 weeks. Both groups are being further randomized to low-dose aspirin (75 to 100 mg/day) or high-dose aspirin (300 to 325 mg/day) for 30 days after PCI. With a target enrollment well beyond 10,000 patients, CURRENT should definitively clarify the relative efficacy and safety of high-dose clopidogrel in this setting.
Tailoring clopidogrel therapy
Investigators have explored tailoring the dosing of clopidogrel around the time of PCI based on the degree of platelet inhibition. In one study, administering additional loading doses of clopidogrel, up to a total of 2,400 mg, before PCI in patients with a suboptimal degree of platelet inhibition resulted in a lower rate of ischemic complications following PCI.12
PRASUGREL, A NOVEL THIENOPYRIDINE
Prasugrel is an investigational third-generation thienopyridine currently under US Food and Drug Administration (FDA) review for use in patients with ACS being managed with PCI. Like clopidogrel, prasugrel is a prodrug that requires conversion to an active metabolite prior to binding to the platelet P2Y12 receptor for ADP to confer antiplatelet activity. Prasugrel is metabolized more efficiently than clopidogrel, allowing for faster activation and superior bioavailability to produce a greater and more consistent antiplatelet effect.1,13
The active metabolites of clopidogrel and prasugrel are no different in their ability to inhibit platelet aggregation, but approximately 85% of clopidogrel is inactivated by esterases, with the remaining 15% being converted to the active metabolite using the cytochrome P450 pathway via two successive oxidative steps in the liver.14 In contrast, esterases facilitate the transformation of prasugrel to its active metabolite.14 This activation requires only one oxidative step that can occur in either the liver or the gut through cytochrome P450.
Both prasugrel and clopidogrel are irreversible P2Y12 receptor blockers. For this reason, one must wait approximately 5 days after the last dose of either medication for generation of a sufficient number of new platelets to allow restoration of normal platelet-mediated hemostasis.
Inhibition of platelet aggregation relative to clopidogrel
In a study among healthy volunteers, inhibition of platelet aggregation was significantly higher after a 60-mg loading dose of prasugrel compared with a 300-mg loading dose of clopidogrel.13 Further, suboptimal responders to clopidogrel who crossed over to prasugrel had levels of platelet inhibition as high as 80% following prasugrel administration. The time to peak effect of prasugrel was about 1 hour. Inhibition of platelet aggregation was more consistent following dosing of prasugrel compared with clopidogrel.13
In a study of 201 patients undergoing cardiac catheterization with planned PCI, Wiviott et al demonstrated better levels of inhibition of platelet aggregation at 6 hours after a 60-mg loading dose of prasugrel than after a 600-mg loading dose of clopidogrel (P < .0001).1
Clinical effects relative to clopidogrel: TRITON-TIMI 38
A large phase 3 clinical trial—the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38—was conducted to compare the effects of prasugrel and standard-dose clopidogrel on death and ischemic end points in 13,608 patients with ACS scheduled to undergo PCI.15 Patients randomized to clopidogrel were given the standard regimen of a 300-mg loading dose followed by a 75-mg daily maintenance dose; those randomized to prasugrel were given a 60-mg loading dose followed by a 10-mg daily maintenance dose. The study drug was typically given immediately before PCI, a time frame that may mimic real-life use but that favored the faster-onset prasugrel over the slower-onset clopidogrel. Both groups also received low-dose aspirin. Approximately half of the patients in each group were treated with a glycoprotein IIb/IIIa inhibitor. The median duration of therapy was approximately 15 months.
Efficacy. The primary end point—a composite of cardiovascular death, MI, or stroke—occurred in 9.9% of patients randomized to prasugrel compared with 12.1% of those randomized to clopidogrel, corresponding to a 19% relative risk reduction (P = .0004) with prasugrel. Based on these results, 46 patients would need to be treated with prasugrel rather than with clopidogrel to prevent 1 additional cardiovascular death, MI, or stroke.15
The reduction in the primary end point with prasugrel was driven primarily by a reduction in nonfatal MI; nonsignificant trends favored prasugrel over clopidogrel on rates of cardiovascular death and all-cause mortality, but there was no difference in stroke rates. Prasugrel’s effect was consistent across subgroups based on MI type, sex, age, the type of stent used, adjunctive antithrombotic therapy, and renal function.15
In the subgroup of patients with diabetes, the relative reduction in the primary end point with prasugrel compared with clopidogrel was 30% (P < .001), and the respective relative reduction among patients with diabetes who required insulin was 37%.16
Safety. Higher antiplatelet potency carries the trade-off of increased bleeding, and this trade-off was apparent with prasugrel in TRITON-TIMI 38.15 TIMI major bleeding (not counting bleeding related to coronary artery bypass graft surgery [CABG]) occurred significantly more often in prasugrel-treated subjects than in those receiving clopidogrel (2.4% vs 1.8%; P = .03), as did life-threatening bleeds (1.4% vs 0.9%; P = .01). Because absolute rates of major bleeding were low in each treatment group, based on these results, 167 patients would need to be treated with prasugrel rather than clopidogrel to result in 1 excess non-CABG-related major bleeding episode. Rates of intracranial hemorrhage were identical in the two treatment groups.15
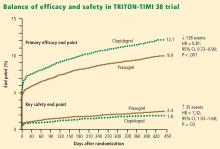
Net clinical outcome and therapeutic considerations. Overall analysis of the balance of efficacy and safety in TRITON-TIMI 38 revealed that 138 events were prevented with randomization to prasugrel instead of clopidogrel, at a cost of 35 additional TIMI major bleeds (Figure 2).15
In a post hoc analysis of net clinical outcome, in which major bleeding events were added to the primary composite efficacy end point, prasugrel was associated with a 13% relative risk reduction (P = .004).15 Twenty-three MIs were prevented per 1,000 treated patients with the use of prasugrel instead of clopidogrel, at a cost of 6 excess non-CABG-related major bleeds.15
Another post hoc assessment identified three subgroups who had a significantly increased risk of TIMI major bleeds with randomization to prasugrel15:
- Patients aged 75 years or older
- Patients with a body weight less than 60 kg
- Patients with a history of stroke or transient ischemic attack (TIA).
In these three subgroups, the net clinical effect either was neutral (for those aged ≥ 75years and for those weighing < 60 kg) or favored clopidogrel (for those with a history of stroke or TIA). The group with a history of stroke or TIA represented 4% of the entire cohort, and the TRITON-TIMI 38 investigators recommended avoiding prasugrel in patients with a history of these events. The other two subgroups with a significantly increased bleeding risk with prasugrel represented 16% of the entire cohort, and in these two groups the investigators suggested a pharmacokinetics-guided reduction in the maintenance dose of prasugrel, although a recommendation for such dosing is based on modeling and not actual outcomes data.15
Stent thrombosis. A subanalysis of TRITON-TIMI 38 examined the risk of stent thrombosis in the 12,844 patients enrolled in the trial who had stents implanted.17 Stent thrombosis was assessed using the Academic Research Consortium definitions of definite, probable, and possible stent thrombosis.18 The risk of definite or probable stent thrombosis was halved (hazard ratio = 0.48; P < .0001) with the use of prasugrel compared with clopidogrel, and the reduction was highly significant regardless of the type of stent implanted or the way stent thrombosis was defined. Significant reductions in both early (within the first 30 days) stent thrombosis (P < .0001) and late (beyond 30 days) stent thrombosis (P = .03) were observed in the prasugrel arm compared with the clopidogrel arm.17
AZD6140, A REVERSIBLE P2Y12 RECEPTOR ANTAGONIST
AZD6140, another investigational antiplatelet agent, is an orally active reversible P2Y12 receptor antagonist, in contrast to the thienopyridines, which are irreversible inhibitors. A member of the cyclo-pentyl-triazolo-pyrimidine (CPTP) class, AZD6140 has a rapid onset of action (≤ 2 hours) and does not require metabolic activation. Its plasma half-life is approximately 12 hours, which translates to twice-daily dosing.
Inhibition of platelet aggregation relative to clopidogrel
In a study of clopidogrel-naïve patients with ACS, inhibition of platelet aggregation 12 hours after administration of AZD6140 was approximately 75% with 90-mg, 180-mg, and 270-mg doses, significantly greater than the 30% inhibition achieved after administration of 300 mg of clopidogrel (P < .0002 for all doses of AZD6140 vs clopidogrel).19 Whereas steady state was achieved in approximately 4 to 6 hours with clopidogrel, it was achieved in approximately 2 hours or less with AZD6140.
Clinical safety and efficacy relative to clopidogrel
In a dose-ranging study of AZD6140, adjudicated bleeding rates were similar among two different doses of AZD6140 (90 mg twice daily and 180 mg twice daily) and clopidogrel 75 mg once daily, with no evidence of a dose effect for major bleeding with AZD6140.20 Although this study, conducted in 990 patients with ACS, was underpowered for efficacy end points, rates of adjudicated MI were numerically lower in each of the AZD6140 groups than in the clopidogrel group.
A more definitive evaluation of the relative effcicacy and safety of AZD6140 is expected from the ongoing PLATO trial, which is comparing 90 mg of AZD6140 twice daily with clopidogrel 75 mg/day among 18,000 patients randomized to one of the two treatments within 24 hours of an index ACS event.21
CANGRELOR, A RAPID PARENTERAL P2Y12 RECEPTOR ANTAGONIST
Cangrelor (formerly known as AR-C69931MX) is an intravenously (IV) administered P2Y12 receptor antagonist under investigation for treatment of ACS and use during PCI and other coronary procedures. The compound is an adenosine triphosphate analogue with a plasma half-life of 5 to 9 minutes. Cangrelor is highly reversible, as platelet function returns to normal within 20 minutes of dosing. Within 15 minutes of initiation, cangrelor produces profound platelet inhibition and rapidly achieves steady state; peak effect occurs within minutes.22 The response to cangrelor is highly consistent, with virtually all recipients achieving the same degree of platelet inhibition. Platelet response approaches baseline 15 minutes after termination.22
If approved by the FDA, cangrelor would be administered similar to the way that glycoprotein IIb/IIIa inhibitors are, as it would be used primarily in the catheterization laboratory and then discontinued after the procedure, at which point transition to a long-term oral therapy would be necessary.
Clinical effects relative to abciximab
Cangrelor has been compared with the glycoprotein IIb/IIIa inhibitor abciximab and placebo in 249 patients undergoing elective or urgent PCI.22 Rates of the combined end point of death, MI, or need for repeat revascularization at 30 days were similar with cangrelor and abciximab (5.7% vs 5.4%, respectively; P = NS), both of which were lower than the rate with placebo (10.0%). Major or minor bleeding through 7 days occurred in numerically fewer cangrelor recipients compared with abciximab recipients (7.0% vs 9.0%), although the small sample size precluded evaluation for statistical significance.
Clinical effects relative to clopidogrel—the CHAMPION trials
A phase 3 trial program consisting of two multinational studies of cangrelor—the Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) program—is currently under way.
CHAMPION-PCI is enrolling 9,000 patients presenting with ACS who are being randomized in a double-blind fashion at the start of PCI to a 600-mg loading dose of clopidogrel or to cangrelor given as an IV bolus of 30 μg/kg followed by an IV infusion of 4 μg/kg/min. The primary end point is a composite of all-cause mortality, MI, or ischemia-driven revascularization in the 48 hours following randomization. Secondary end points include rates of all-cause mortality and MI at 48 hours.23
CHAMPION-PLATFORM is enrolling 4,400 patients scheduled for PCI as a result of ACS who are being randomized in a double-blind, double-dummy manner to (1) cangrelor bolus and infusion plus oral placebo or (2) oral clopidogrel plus placebo bolus and infusion before their index procedures. Dosages of the two agents are the same as in CHAMPION-PCI. The primary end point is a composite of death, MI, or urgent target vessel revascularization at 48 hours. Secondary end points include 30-day and 1-year clinical outcomes.23
The rationale for the CHAMPION investigations stems from the need to initiate clopidogrel before a patient is taken to the catheterization laboratory, owing to the inability to achieve a high degree of platelet inhibition until 4 to 6 hours after clopidogrel administration. Although this strategy can be undertaken without complication for most patients, a subset of patients with three-vessel disease or left-main disease will require CABG, which then must be delayed several days until clopidogrel’s platelet-inhibiting effect diminishes. A rapid-acting IV inhibitor of the P2Y12 receptor such as cangrelor would obviate this concern.
THROMBIN INHIBITORS
Thrombin plays an important role in platelet activation, and thrombin receptor antagonists may represent a safer means of inhibiting platelet activation relative to traditional antiplatelet agents. This theoretical safety advantage stems from the notion that blocking the action of platelets at the thrombin receptor would preserve platelets’ function as mediators of primary hemostasis. Because thrombin’s activation of platelets should occur only during clot formation, blocking platelet activation at the thrombin receptor would interrupt thrombin’s ability to propagate platelet activation during formation of coronary artery clots.
One agent in this class that is being studied extensively is SCH 530348, an oral thrombin receptor antagonist with potent antiplatelet activity. Its peak antiplatelet potency is achieved within hours when a loading dose is given, and within days without a loading dose. Wearing-off of the action of SCH 530348 takes weeks.24
Inhibition of platelet aggregation with thrombin receptor antagonists is measured in response to the thrombin receptor antagonist peptide (TRAP), not ADP. The proportion of subjects treated with SCH 530348 who achieve greater than 80% inhibition of platelet aggregation to 15 μM of TRAP ranges from 91% (with 0.5 mg of SCH 530348) to 100% (with 1.0 mg and 2.5 mg) at both 30 days and 60 days.25
Clinical effects in placebo-controlled trials
SCH 530348 was studied in the Thrombin Receptor Antagonist (TRA)–PCI trial, a dose-ranging study in which patients were randomized to one of three oral loading doses of the study drug (10 mg, 20 mg, or 40 mg) on top of a clopidogrel loading dose before undergoing cardiac catheterization for planned PCI; patients were then randomized to one of three maintenance doses of SCH 530348 (0.5 mg, 1.0 mg, or 2.5 mg) or placebo (depending on loading therapy) for 60 days.25
Among the 573 patients undergoing PCI , the rate of TIMI major or minor bleeding was not significantly higher with any dose of SCH 530348 compared with placebo,25 supporting the hypothesis that thrombin receptor antagonism inhibits platelet aggregation without a significant increase in bleeding.
Although the TRA-PCI study was not powered to detect differences in clinical event rates, a reduction in the rate of major adverse cardiovascular events was observed in a dose-dependent manner with SCH 530348 compared with placebo in the PCI cohort.25
On the basis of the TRA-PCI trial, a pair of phase 3 trials of SCH 530348 have been launched—the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P-TIMI 50) study and the Thrombin Receptor Antagonist for Clinical Event Reduction in ACS (TRA-CER) study.
TRA 2°P-TIMI 50 is a multinational double-blind study enrolling 19,500 patients with prior MI or stroke or with existing peripheral arterial disease. Patients are being randomized to placebo plus standard medical care (including aspirin and clopidogrel) or to 2.5 mg of SCH 530348 once daily plus standard medical care. The primary end point is the composite of cardiovascular death, MI, urgent coronary revascularization, or stroke.26
TRA-CER is a multinational double-blind study with planned enrollment of 10,000 patients with non-ST-segment-elevation MI. Patients are being randomized to placebo plus standard medical care (including aspirin or clopidogrel) or to SCH 530348 (using the oral 40-mg loading dose and a maintenance dose of 2.5 mg once daily) plus standard medical care. The primary end point is the composite of cardiovascular death, MI, rehospitalization for ACS, urgent coronary revascularization, or stroke. The key secondary end point is the composite of cardiovascular death, MI, or stroke.27
COMPARATIVE CONSIDERATIONS
Inhibition of platelet aggregation
Clopidogrel achieves about 30% inhibition of platelet aggregation to ADP at its current FDA-approved loading dose of 300 mg and about 40% inhibition when its dose is doubled to 600 mg. These levels of inhibition are increased to 75% to 80% by clopidogrel’s fellow thienopyridine prasugrel, and this increase is attributable to prasugrel’s more efficient metabolism from prodrug to active metabolite. The reversible P2Y12 receptor antagonist AZD6140 achieves a comparable 75% to 80% inhibition of platelet aggregation. The parenterally administered P2Y12 receptor antagonist cangrelor achieves greater than 90% inhibition, as does the oral thrombin receptor antagonist SCH 530348, although the latter agent’s inhibition is to the agonist TRAP rather than ADP.
Time to peak effect
The time to peak effect with clopidogrel is approximately 4 hours regardless of the loading dose used (300 mg or 600 mg); this is substantially reduced with all of the investigational agents except SCH 530348. The novel agents’ reduced time to peak effect can offer advantages in speeding patients’ readiness to undergo catheterization procedures. This is particularly true for the IV agent cangrelor, which achieves its peak effect within minutes, although the 1-hour to 2-hour time frame with oral agents prasugrel and AZD6140 also would usually obviate any need to delay catheterization.
Consistency of platelet response
Standard-dose clopidogrel has the least consistency of platelet response among the therapies reviewed. Although increasing the clopidogrel dose yields somewhat greater consistency in response, it is still lower than the very high degrees of consistency observed with all of the novel compounds, each of which appears to achieve the same degree of inhibition of aggregation in virtually all patients.
Offset of effect
Both of the thienopyridines—clopidogrel and prasugrel—have an offset of effect of about 5 days, which requires delay of surgery, if possible, for several days in patients taking these agents. This is not an issue for the reversible oral agent AZD6140, whose offset of action takes just 1 to 2 days. While this rapid wearing-off of effect translates to a potential advantage for AZD6140, it also poses the potential drawback that a missed dose or two may leave the patient exposed to the risk of a thrombotic event. Cangrelor’s rapid offset of 20 minutes promotes its envisioned use as a catheterization lab–based medication like the glycoprotein IIb/IIIa inhibitors that can be started right before a PCI procedure and stopped immediately afterward. Because SCH 530348 has a very long half-life and thus a weeks-long washout period, the practicality of its use may depend on the hypothesis that thrombin receptor antagonists do not interfere with primary hemostasis, which is supported by data to date but remains to be definitively confirmed.
CONCLUSIONS
Clopidogrel achieves modest platelet inhibition with wide variability in response. Higher doses of clopidogrel achieve modestly greater degrees of inhibition than standard doses, and appear to result in a decreased rate of ischemic events. Although higher doses of clopidogrel have been embraced by some clinicians, we await definitive phase 3 trial evidence of net benefit before making high-dose clopidogrel the new standard of care.
Compared with clopidogrel, the investigational thienopyridine prasugrel is a more potent and consistent blocker of the ADP receptor. It results in a decreased rate of ischemic events relative to clopidogrel, including a 50% reduction in the rate of stent thrombosis, but is associated with an increased rate of bleeding. If prasugrel is approved for marketing, its use should be avoided in patients with a history of stroke or TIA, and avoidance or dose adjustment may be necessary in patients aged 75 years or older and in patients weighing less than 60 kg.
Other novel antiplatelet agents being evaluated for use in patients with ACS—the reversible oral ADP receptor blocker AZD6140, the rapid-acting IV ADP receptor blocker cangrelor, and oral thrombin receptor antagonists—offer potential advantages that need to be examined in the context of large-scale clinical trials.
- Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation–Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004; 109:3171–3175.
- Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol 2005; 46:1827–1832.
- Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol 2005; 45:1392–1396.
- von Beckerath N, Taubert D, Pogatsa-Murray G, Schömig E, Kastrati A, Schömig A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) Trial. Circulation 2005; 112:2946–2950.
- Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation 2005; 111:2099–2106.
- Cuisset T, Frere C, Quilici J, et al. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol 2006; 48:1339–1345.
- von Beckerath N, Kastrati A, Wieczorek A, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J 2007; 28:1814–1819.
- Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007; 115:708–716.
- Clopidogrel optimal loading dose usage to reduce recurrent events/optimal antiplatelet strategy for interventions (CURRENT/OASIS7). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00335452. Updated September 1, 2008. Accessed December 16, 2008.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolic formation. Am Heart J 2007; 153:66.e9–e16.
- Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med 2003; 3:113–122.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38. Circulation 2008; 118:1626–1636.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol 2007; 50:1852–1856.
- Cannon CP, Husted S, Harrington RA, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007; 50:1844–1851.
- A comparison of AZD6140 and clopidogrel in patients with acute coronary syndrome (PLATO). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00391872. Updated December 3, 2008. Accessed December 5, 2008.
- Greenbaum AB, Grines CL, Bittl JA, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J 2006; 151:689.e1–689.e10.
- A clinical trial to demonstrate the efficacy of cangrelor (PCI). Clinical Trials.gov Web site. http://www.clinicaltrials.gov/ct/show/nct00305162. Updated December 3, 2008. Accessed December 5, 2008.
- Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov 2003; 2:15–28.
- Moliterno DJ, Becker RC, Jennings LK, et al; TRA-PCI Study Investigators. Results of a multinational randomized, double-blind, placebo-controlled study of a novel thrombin receptor antagonist (SCH 530348) in percutaneous coronary intervention. Presented at: 56th Annual Scientific Session of the American College of Cardiology; March 24–27, 2007; New Orleans, LA.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with atherosclerosis (TRA 2°P-TIMI 50). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00526474. Updated November 13, 2008. Accessed December 16, 2008.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with acute coronary syndrome (TRA-CER). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00527943. Updated November 12, 2008. Accessed December 16, 2008.
An enhanced understanding of platelet biology, as reviewed in the previous article in this supplement, has made it possible to identify a wide variety of platelet agonists. This knowledge has fostered the development of a host of pharmacologic strategies to block agonists such as cyclooxygenase, thromboxane, adenosine diphosphate (ADP), and thrombin, among others. This article will discuss the pharmacologic properties of novel antiplatelet agents, as well as alternative dosing of the established antiplatelet agent clopidogrel, and will review data from available comparative and placebo-controlled trials of these agents. The article concludes with comparative perspectives on the potential roles and relative advantages of these agents in the evolving management of patients with acute coronary syndromes (ACS).
CLOPIDOGREL AND THE CHALLENGE OF VARIABLE RESPONSE
Clopidogrel, a member of the thienopyridine class of ADP receptor inhibitors, is well established for use in patients with ACS at a loading dose of 300 mg followed by a maintenance dose of 75 mg/day. At this loading dose, inhibition of platelet aggregation to ADP is approximately 30%, and the time to peak effect is approximately 4 to 6 hours.1
As with most other drugs, the response to clopidogrel is variable. However, in contrast to the accepted measures of response to antihypertensive or lipid-lowering drugs, there are no routinely used tests for measuring response to antiplatelet therapies. As a result, a “one size fits all” strategy in the dosing of clopidogrel has prevailed.
This variability in response is clinically relevant. In a study assessing clopidogrel responsiveness by ADP-induced platelet aggregation in 60 patients who experienced ST-segment-elevation myocardial infarction (MI), Matetzky et al found that the lowest levels of clopidogrel responsiveness were associated with a significantly elevated rate (P = .007) of recurrent cardiovascular events 6 months after the MI.3 Gurbel et al found a similar association between clopidogrel responsiveness and subacute stent thrombosis in a study of 120 patients using two different methods—light transmission aggregotomy to 5 μmol/L of ADP, and the ratio of vasodilator-stimulated phosphoprotein reactivity—to assess clopidogrel responsiveness.4
Increasing the loading dose raises response rates
One proposed method for boosting responsiveness to clopidogrel in suboptimal responders is the use of a higher dose. In a study of 190 patients undergoing coronary stenting, increasing the loading dose from 300 mg to 600 mg reduced the rate of clopidogrel resistance (defined as a < 10% absolute change in aggregation to 5 μM of ADP at 24 hours) from 28% to 8% (P < .001),5 a finding that supports the notion of enhanced response at doses up to 600 mg. Single loading doses in excess of 600 mg yield diminishing returns in terms of platelet inhibition, most likely as a result of clopidogrel pharmacokinetics.6
Compared with 300 mg of clopidogrel, the more potent platelet inhibitory effect of a 600-mg dose translated to a two-thirds reduction (P = .041) in the composite end point of death, MI, or target vessel revascularization at 30 days in a study of 255 patients with stable coronary artery disease undergoing percutaneous coronary intervention (PCI).7 The reduction in this composite end point with high-dose clopidogrel was driven by a reduction in the incidence of periprocedural MI.
In a separate study of 292 patients with non‑ST-segment-elevation ACS who were scheduled for PCI, the superior platelet response to 600 mg versus 300 mg of clopidogrel translated to a 60% reduction in adverse thrombotic events (P = .02), and this benefit extended beyond rates of periprocedural MI.8
Similar results with increased maintenance dose
Similarly, emerging data suggest that raising the maintenance dose of clopidogrel can also raise response rates. In a study of 60 patients, doubling the maintenance dose of clopidogrel after PCI from 75 mg/day to 150 mg/day resulted in improved platelet inhibition as assessed by rapid platelet function analysis.9 Likewise, a 150-mg/day maintenance dose of clopidogrel was associated with a superior antiplatelet effect compared with 75 mg/day in a study of 40 patients with type 2 diabetes.10
Large definitive trial is under way
In the wake of these smaller trials, a large randomized trial known as CURRENT is comparing a strategy of high-dose clopidogrel with standard-dose clopidogrel in patients with ACS for whom an early invasive management strategy is planned.11 The high-dose regimen involves a 600-mg loading dose followed by 150 mg/day for 1 week and then 75 mg/day for 3 weeks, whereas the standard-dose regimen involves a 300-mg loading dose followed by 75 mg/day for 4 weeks. Both groups are being further randomized to low-dose aspirin (75 to 100 mg/day) or high-dose aspirin (300 to 325 mg/day) for 30 days after PCI. With a target enrollment well beyond 10,000 patients, CURRENT should definitively clarify the relative efficacy and safety of high-dose clopidogrel in this setting.
Tailoring clopidogrel therapy
Investigators have explored tailoring the dosing of clopidogrel around the time of PCI based on the degree of platelet inhibition. In one study, administering additional loading doses of clopidogrel, up to a total of 2,400 mg, before PCI in patients with a suboptimal degree of platelet inhibition resulted in a lower rate of ischemic complications following PCI.12
PRASUGREL, A NOVEL THIENOPYRIDINE
Prasugrel is an investigational third-generation thienopyridine currently under US Food and Drug Administration (FDA) review for use in patients with ACS being managed with PCI. Like clopidogrel, prasugrel is a prodrug that requires conversion to an active metabolite prior to binding to the platelet P2Y12 receptor for ADP to confer antiplatelet activity. Prasugrel is metabolized more efficiently than clopidogrel, allowing for faster activation and superior bioavailability to produce a greater and more consistent antiplatelet effect.1,13
The active metabolites of clopidogrel and prasugrel are no different in their ability to inhibit platelet aggregation, but approximately 85% of clopidogrel is inactivated by esterases, with the remaining 15% being converted to the active metabolite using the cytochrome P450 pathway via two successive oxidative steps in the liver.14 In contrast, esterases facilitate the transformation of prasugrel to its active metabolite.14 This activation requires only one oxidative step that can occur in either the liver or the gut through cytochrome P450.
Both prasugrel and clopidogrel are irreversible P2Y12 receptor blockers. For this reason, one must wait approximately 5 days after the last dose of either medication for generation of a sufficient number of new platelets to allow restoration of normal platelet-mediated hemostasis.
Inhibition of platelet aggregation relative to clopidogrel
In a study among healthy volunteers, inhibition of platelet aggregation was significantly higher after a 60-mg loading dose of prasugrel compared with a 300-mg loading dose of clopidogrel.13 Further, suboptimal responders to clopidogrel who crossed over to prasugrel had levels of platelet inhibition as high as 80% following prasugrel administration. The time to peak effect of prasugrel was about 1 hour. Inhibition of platelet aggregation was more consistent following dosing of prasugrel compared with clopidogrel.13
In a study of 201 patients undergoing cardiac catheterization with planned PCI, Wiviott et al demonstrated better levels of inhibition of platelet aggregation at 6 hours after a 60-mg loading dose of prasugrel than after a 600-mg loading dose of clopidogrel (P < .0001).1
Clinical effects relative to clopidogrel: TRITON-TIMI 38
A large phase 3 clinical trial—the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38—was conducted to compare the effects of prasugrel and standard-dose clopidogrel on death and ischemic end points in 13,608 patients with ACS scheduled to undergo PCI.15 Patients randomized to clopidogrel were given the standard regimen of a 300-mg loading dose followed by a 75-mg daily maintenance dose; those randomized to prasugrel were given a 60-mg loading dose followed by a 10-mg daily maintenance dose. The study drug was typically given immediately before PCI, a time frame that may mimic real-life use but that favored the faster-onset prasugrel over the slower-onset clopidogrel. Both groups also received low-dose aspirin. Approximately half of the patients in each group were treated with a glycoprotein IIb/IIIa inhibitor. The median duration of therapy was approximately 15 months.
Efficacy. The primary end point—a composite of cardiovascular death, MI, or stroke—occurred in 9.9% of patients randomized to prasugrel compared with 12.1% of those randomized to clopidogrel, corresponding to a 19% relative risk reduction (P = .0004) with prasugrel. Based on these results, 46 patients would need to be treated with prasugrel rather than with clopidogrel to prevent 1 additional cardiovascular death, MI, or stroke.15
The reduction in the primary end point with prasugrel was driven primarily by a reduction in nonfatal MI; nonsignificant trends favored prasugrel over clopidogrel on rates of cardiovascular death and all-cause mortality, but there was no difference in stroke rates. Prasugrel’s effect was consistent across subgroups based on MI type, sex, age, the type of stent used, adjunctive antithrombotic therapy, and renal function.15
In the subgroup of patients with diabetes, the relative reduction in the primary end point with prasugrel compared with clopidogrel was 30% (P < .001), and the respective relative reduction among patients with diabetes who required insulin was 37%.16
Safety. Higher antiplatelet potency carries the trade-off of increased bleeding, and this trade-off was apparent with prasugrel in TRITON-TIMI 38.15 TIMI major bleeding (not counting bleeding related to coronary artery bypass graft surgery [CABG]) occurred significantly more often in prasugrel-treated subjects than in those receiving clopidogrel (2.4% vs 1.8%; P = .03), as did life-threatening bleeds (1.4% vs 0.9%; P = .01). Because absolute rates of major bleeding were low in each treatment group, based on these results, 167 patients would need to be treated with prasugrel rather than clopidogrel to result in 1 excess non-CABG-related major bleeding episode. Rates of intracranial hemorrhage were identical in the two treatment groups.15
Net clinical outcome and therapeutic considerations. Overall analysis of the balance of efficacy and safety in TRITON-TIMI 38 revealed that 138 events were prevented with randomization to prasugrel instead of clopidogrel, at a cost of 35 additional TIMI major bleeds (Figure 2).15
In a post hoc analysis of net clinical outcome, in which major bleeding events were added to the primary composite efficacy end point, prasugrel was associated with a 13% relative risk reduction (P = .004).15 Twenty-three MIs were prevented per 1,000 treated patients with the use of prasugrel instead of clopidogrel, at a cost of 6 excess non-CABG-related major bleeds.15
Another post hoc assessment identified three subgroups who had a significantly increased risk of TIMI major bleeds with randomization to prasugrel15:
- Patients aged 75 years or older
- Patients with a body weight less than 60 kg
- Patients with a history of stroke or transient ischemic attack (TIA).
In these three subgroups, the net clinical effect either was neutral (for those aged ≥ 75years and for those weighing < 60 kg) or favored clopidogrel (for those with a history of stroke or TIA). The group with a history of stroke or TIA represented 4% of the entire cohort, and the TRITON-TIMI 38 investigators recommended avoiding prasugrel in patients with a history of these events. The other two subgroups with a significantly increased bleeding risk with prasugrel represented 16% of the entire cohort, and in these two groups the investigators suggested a pharmacokinetics-guided reduction in the maintenance dose of prasugrel, although a recommendation for such dosing is based on modeling and not actual outcomes data.15
Stent thrombosis. A subanalysis of TRITON-TIMI 38 examined the risk of stent thrombosis in the 12,844 patients enrolled in the trial who had stents implanted.17 Stent thrombosis was assessed using the Academic Research Consortium definitions of definite, probable, and possible stent thrombosis.18 The risk of definite or probable stent thrombosis was halved (hazard ratio = 0.48; P < .0001) with the use of prasugrel compared with clopidogrel, and the reduction was highly significant regardless of the type of stent implanted or the way stent thrombosis was defined. Significant reductions in both early (within the first 30 days) stent thrombosis (P < .0001) and late (beyond 30 days) stent thrombosis (P = .03) were observed in the prasugrel arm compared with the clopidogrel arm.17
AZD6140, A REVERSIBLE P2Y12 RECEPTOR ANTAGONIST
AZD6140, another investigational antiplatelet agent, is an orally active reversible P2Y12 receptor antagonist, in contrast to the thienopyridines, which are irreversible inhibitors. A member of the cyclo-pentyl-triazolo-pyrimidine (CPTP) class, AZD6140 has a rapid onset of action (≤ 2 hours) and does not require metabolic activation. Its plasma half-life is approximately 12 hours, which translates to twice-daily dosing.
Inhibition of platelet aggregation relative to clopidogrel
In a study of clopidogrel-naïve patients with ACS, inhibition of platelet aggregation 12 hours after administration of AZD6140 was approximately 75% with 90-mg, 180-mg, and 270-mg doses, significantly greater than the 30% inhibition achieved after administration of 300 mg of clopidogrel (P < .0002 for all doses of AZD6140 vs clopidogrel).19 Whereas steady state was achieved in approximately 4 to 6 hours with clopidogrel, it was achieved in approximately 2 hours or less with AZD6140.
Clinical safety and efficacy relative to clopidogrel
In a dose-ranging study of AZD6140, adjudicated bleeding rates were similar among two different doses of AZD6140 (90 mg twice daily and 180 mg twice daily) and clopidogrel 75 mg once daily, with no evidence of a dose effect for major bleeding with AZD6140.20 Although this study, conducted in 990 patients with ACS, was underpowered for efficacy end points, rates of adjudicated MI were numerically lower in each of the AZD6140 groups than in the clopidogrel group.
A more definitive evaluation of the relative effcicacy and safety of AZD6140 is expected from the ongoing PLATO trial, which is comparing 90 mg of AZD6140 twice daily with clopidogrel 75 mg/day among 18,000 patients randomized to one of the two treatments within 24 hours of an index ACS event.21
CANGRELOR, A RAPID PARENTERAL P2Y12 RECEPTOR ANTAGONIST
Cangrelor (formerly known as AR-C69931MX) is an intravenously (IV) administered P2Y12 receptor antagonist under investigation for treatment of ACS and use during PCI and other coronary procedures. The compound is an adenosine triphosphate analogue with a plasma half-life of 5 to 9 minutes. Cangrelor is highly reversible, as platelet function returns to normal within 20 minutes of dosing. Within 15 minutes of initiation, cangrelor produces profound platelet inhibition and rapidly achieves steady state; peak effect occurs within minutes.22 The response to cangrelor is highly consistent, with virtually all recipients achieving the same degree of platelet inhibition. Platelet response approaches baseline 15 minutes after termination.22
If approved by the FDA, cangrelor would be administered similar to the way that glycoprotein IIb/IIIa inhibitors are, as it would be used primarily in the catheterization laboratory and then discontinued after the procedure, at which point transition to a long-term oral therapy would be necessary.
Clinical effects relative to abciximab
Cangrelor has been compared with the glycoprotein IIb/IIIa inhibitor abciximab and placebo in 249 patients undergoing elective or urgent PCI.22 Rates of the combined end point of death, MI, or need for repeat revascularization at 30 days were similar with cangrelor and abciximab (5.7% vs 5.4%, respectively; P = NS), both of which were lower than the rate with placebo (10.0%). Major or minor bleeding through 7 days occurred in numerically fewer cangrelor recipients compared with abciximab recipients (7.0% vs 9.0%), although the small sample size precluded evaluation for statistical significance.
Clinical effects relative to clopidogrel—the CHAMPION trials
A phase 3 trial program consisting of two multinational studies of cangrelor—the Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) program—is currently under way.
CHAMPION-PCI is enrolling 9,000 patients presenting with ACS who are being randomized in a double-blind fashion at the start of PCI to a 600-mg loading dose of clopidogrel or to cangrelor given as an IV bolus of 30 μg/kg followed by an IV infusion of 4 μg/kg/min. The primary end point is a composite of all-cause mortality, MI, or ischemia-driven revascularization in the 48 hours following randomization. Secondary end points include rates of all-cause mortality and MI at 48 hours.23
CHAMPION-PLATFORM is enrolling 4,400 patients scheduled for PCI as a result of ACS who are being randomized in a double-blind, double-dummy manner to (1) cangrelor bolus and infusion plus oral placebo or (2) oral clopidogrel plus placebo bolus and infusion before their index procedures. Dosages of the two agents are the same as in CHAMPION-PCI. The primary end point is a composite of death, MI, or urgent target vessel revascularization at 48 hours. Secondary end points include 30-day and 1-year clinical outcomes.23
The rationale for the CHAMPION investigations stems from the need to initiate clopidogrel before a patient is taken to the catheterization laboratory, owing to the inability to achieve a high degree of platelet inhibition until 4 to 6 hours after clopidogrel administration. Although this strategy can be undertaken without complication for most patients, a subset of patients with three-vessel disease or left-main disease will require CABG, which then must be delayed several days until clopidogrel’s platelet-inhibiting effect diminishes. A rapid-acting IV inhibitor of the P2Y12 receptor such as cangrelor would obviate this concern.
THROMBIN INHIBITORS
Thrombin plays an important role in platelet activation, and thrombin receptor antagonists may represent a safer means of inhibiting platelet activation relative to traditional antiplatelet agents. This theoretical safety advantage stems from the notion that blocking the action of platelets at the thrombin receptor would preserve platelets’ function as mediators of primary hemostasis. Because thrombin’s activation of platelets should occur only during clot formation, blocking platelet activation at the thrombin receptor would interrupt thrombin’s ability to propagate platelet activation during formation of coronary artery clots.
One agent in this class that is being studied extensively is SCH 530348, an oral thrombin receptor antagonist with potent antiplatelet activity. Its peak antiplatelet potency is achieved within hours when a loading dose is given, and within days without a loading dose. Wearing-off of the action of SCH 530348 takes weeks.24
Inhibition of platelet aggregation with thrombin receptor antagonists is measured in response to the thrombin receptor antagonist peptide (TRAP), not ADP. The proportion of subjects treated with SCH 530348 who achieve greater than 80% inhibition of platelet aggregation to 15 μM of TRAP ranges from 91% (with 0.5 mg of SCH 530348) to 100% (with 1.0 mg and 2.5 mg) at both 30 days and 60 days.25
Clinical effects in placebo-controlled trials
SCH 530348 was studied in the Thrombin Receptor Antagonist (TRA)–PCI trial, a dose-ranging study in which patients were randomized to one of three oral loading doses of the study drug (10 mg, 20 mg, or 40 mg) on top of a clopidogrel loading dose before undergoing cardiac catheterization for planned PCI; patients were then randomized to one of three maintenance doses of SCH 530348 (0.5 mg, 1.0 mg, or 2.5 mg) or placebo (depending on loading therapy) for 60 days.25
Among the 573 patients undergoing PCI , the rate of TIMI major or minor bleeding was not significantly higher with any dose of SCH 530348 compared with placebo,25 supporting the hypothesis that thrombin receptor antagonism inhibits platelet aggregation without a significant increase in bleeding.
Although the TRA-PCI study was not powered to detect differences in clinical event rates, a reduction in the rate of major adverse cardiovascular events was observed in a dose-dependent manner with SCH 530348 compared with placebo in the PCI cohort.25
On the basis of the TRA-PCI trial, a pair of phase 3 trials of SCH 530348 have been launched—the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P-TIMI 50) study and the Thrombin Receptor Antagonist for Clinical Event Reduction in ACS (TRA-CER) study.
TRA 2°P-TIMI 50 is a multinational double-blind study enrolling 19,500 patients with prior MI or stroke or with existing peripheral arterial disease. Patients are being randomized to placebo plus standard medical care (including aspirin and clopidogrel) or to 2.5 mg of SCH 530348 once daily plus standard medical care. The primary end point is the composite of cardiovascular death, MI, urgent coronary revascularization, or stroke.26
TRA-CER is a multinational double-blind study with planned enrollment of 10,000 patients with non-ST-segment-elevation MI. Patients are being randomized to placebo plus standard medical care (including aspirin or clopidogrel) or to SCH 530348 (using the oral 40-mg loading dose and a maintenance dose of 2.5 mg once daily) plus standard medical care. The primary end point is the composite of cardiovascular death, MI, rehospitalization for ACS, urgent coronary revascularization, or stroke. The key secondary end point is the composite of cardiovascular death, MI, or stroke.27
COMPARATIVE CONSIDERATIONS
Inhibition of platelet aggregation
Clopidogrel achieves about 30% inhibition of platelet aggregation to ADP at its current FDA-approved loading dose of 300 mg and about 40% inhibition when its dose is doubled to 600 mg. These levels of inhibition are increased to 75% to 80% by clopidogrel’s fellow thienopyridine prasugrel, and this increase is attributable to prasugrel’s more efficient metabolism from prodrug to active metabolite. The reversible P2Y12 receptor antagonist AZD6140 achieves a comparable 75% to 80% inhibition of platelet aggregation. The parenterally administered P2Y12 receptor antagonist cangrelor achieves greater than 90% inhibition, as does the oral thrombin receptor antagonist SCH 530348, although the latter agent’s inhibition is to the agonist TRAP rather than ADP.
Time to peak effect
The time to peak effect with clopidogrel is approximately 4 hours regardless of the loading dose used (300 mg or 600 mg); this is substantially reduced with all of the investigational agents except SCH 530348. The novel agents’ reduced time to peak effect can offer advantages in speeding patients’ readiness to undergo catheterization procedures. This is particularly true for the IV agent cangrelor, which achieves its peak effect within minutes, although the 1-hour to 2-hour time frame with oral agents prasugrel and AZD6140 also would usually obviate any need to delay catheterization.
Consistency of platelet response
Standard-dose clopidogrel has the least consistency of platelet response among the therapies reviewed. Although increasing the clopidogrel dose yields somewhat greater consistency in response, it is still lower than the very high degrees of consistency observed with all of the novel compounds, each of which appears to achieve the same degree of inhibition of aggregation in virtually all patients.
Offset of effect
Both of the thienopyridines—clopidogrel and prasugrel—have an offset of effect of about 5 days, which requires delay of surgery, if possible, for several days in patients taking these agents. This is not an issue for the reversible oral agent AZD6140, whose offset of action takes just 1 to 2 days. While this rapid wearing-off of effect translates to a potential advantage for AZD6140, it also poses the potential drawback that a missed dose or two may leave the patient exposed to the risk of a thrombotic event. Cangrelor’s rapid offset of 20 minutes promotes its envisioned use as a catheterization lab–based medication like the glycoprotein IIb/IIIa inhibitors that can be started right before a PCI procedure and stopped immediately afterward. Because SCH 530348 has a very long half-life and thus a weeks-long washout period, the practicality of its use may depend on the hypothesis that thrombin receptor antagonists do not interfere with primary hemostasis, which is supported by data to date but remains to be definitively confirmed.
CONCLUSIONS
Clopidogrel achieves modest platelet inhibition with wide variability in response. Higher doses of clopidogrel achieve modestly greater degrees of inhibition than standard doses, and appear to result in a decreased rate of ischemic events. Although higher doses of clopidogrel have been embraced by some clinicians, we await definitive phase 3 trial evidence of net benefit before making high-dose clopidogrel the new standard of care.
Compared with clopidogrel, the investigational thienopyridine prasugrel is a more potent and consistent blocker of the ADP receptor. It results in a decreased rate of ischemic events relative to clopidogrel, including a 50% reduction in the rate of stent thrombosis, but is associated with an increased rate of bleeding. If prasugrel is approved for marketing, its use should be avoided in patients with a history of stroke or TIA, and avoidance or dose adjustment may be necessary in patients aged 75 years or older and in patients weighing less than 60 kg.
Other novel antiplatelet agents being evaluated for use in patients with ACS—the reversible oral ADP receptor blocker AZD6140, the rapid-acting IV ADP receptor blocker cangrelor, and oral thrombin receptor antagonists—offer potential advantages that need to be examined in the context of large-scale clinical trials.
An enhanced understanding of platelet biology, as reviewed in the previous article in this supplement, has made it possible to identify a wide variety of platelet agonists. This knowledge has fostered the development of a host of pharmacologic strategies to block agonists such as cyclooxygenase, thromboxane, adenosine diphosphate (ADP), and thrombin, among others. This article will discuss the pharmacologic properties of novel antiplatelet agents, as well as alternative dosing of the established antiplatelet agent clopidogrel, and will review data from available comparative and placebo-controlled trials of these agents. The article concludes with comparative perspectives on the potential roles and relative advantages of these agents in the evolving management of patients with acute coronary syndromes (ACS).
CLOPIDOGREL AND THE CHALLENGE OF VARIABLE RESPONSE
Clopidogrel, a member of the thienopyridine class of ADP receptor inhibitors, is well established for use in patients with ACS at a loading dose of 300 mg followed by a maintenance dose of 75 mg/day. At this loading dose, inhibition of platelet aggregation to ADP is approximately 30%, and the time to peak effect is approximately 4 to 6 hours.1
As with most other drugs, the response to clopidogrel is variable. However, in contrast to the accepted measures of response to antihypertensive or lipid-lowering drugs, there are no routinely used tests for measuring response to antiplatelet therapies. As a result, a “one size fits all” strategy in the dosing of clopidogrel has prevailed.
This variability in response is clinically relevant. In a study assessing clopidogrel responsiveness by ADP-induced platelet aggregation in 60 patients who experienced ST-segment-elevation myocardial infarction (MI), Matetzky et al found that the lowest levels of clopidogrel responsiveness were associated with a significantly elevated rate (P = .007) of recurrent cardiovascular events 6 months after the MI.3 Gurbel et al found a similar association between clopidogrel responsiveness and subacute stent thrombosis in a study of 120 patients using two different methods—light transmission aggregotomy to 5 μmol/L of ADP, and the ratio of vasodilator-stimulated phosphoprotein reactivity—to assess clopidogrel responsiveness.4
Increasing the loading dose raises response rates
One proposed method for boosting responsiveness to clopidogrel in suboptimal responders is the use of a higher dose. In a study of 190 patients undergoing coronary stenting, increasing the loading dose from 300 mg to 600 mg reduced the rate of clopidogrel resistance (defined as a < 10% absolute change in aggregation to 5 μM of ADP at 24 hours) from 28% to 8% (P < .001),5 a finding that supports the notion of enhanced response at doses up to 600 mg. Single loading doses in excess of 600 mg yield diminishing returns in terms of platelet inhibition, most likely as a result of clopidogrel pharmacokinetics.6
Compared with 300 mg of clopidogrel, the more potent platelet inhibitory effect of a 600-mg dose translated to a two-thirds reduction (P = .041) in the composite end point of death, MI, or target vessel revascularization at 30 days in a study of 255 patients with stable coronary artery disease undergoing percutaneous coronary intervention (PCI).7 The reduction in this composite end point with high-dose clopidogrel was driven by a reduction in the incidence of periprocedural MI.
In a separate study of 292 patients with non‑ST-segment-elevation ACS who were scheduled for PCI, the superior platelet response to 600 mg versus 300 mg of clopidogrel translated to a 60% reduction in adverse thrombotic events (P = .02), and this benefit extended beyond rates of periprocedural MI.8
Similar results with increased maintenance dose
Similarly, emerging data suggest that raising the maintenance dose of clopidogrel can also raise response rates. In a study of 60 patients, doubling the maintenance dose of clopidogrel after PCI from 75 mg/day to 150 mg/day resulted in improved platelet inhibition as assessed by rapid platelet function analysis.9 Likewise, a 150-mg/day maintenance dose of clopidogrel was associated with a superior antiplatelet effect compared with 75 mg/day in a study of 40 patients with type 2 diabetes.10
Large definitive trial is under way
In the wake of these smaller trials, a large randomized trial known as CURRENT is comparing a strategy of high-dose clopidogrel with standard-dose clopidogrel in patients with ACS for whom an early invasive management strategy is planned.11 The high-dose regimen involves a 600-mg loading dose followed by 150 mg/day for 1 week and then 75 mg/day for 3 weeks, whereas the standard-dose regimen involves a 300-mg loading dose followed by 75 mg/day for 4 weeks. Both groups are being further randomized to low-dose aspirin (75 to 100 mg/day) or high-dose aspirin (300 to 325 mg/day) for 30 days after PCI. With a target enrollment well beyond 10,000 patients, CURRENT should definitively clarify the relative efficacy and safety of high-dose clopidogrel in this setting.
Tailoring clopidogrel therapy
Investigators have explored tailoring the dosing of clopidogrel around the time of PCI based on the degree of platelet inhibition. In one study, administering additional loading doses of clopidogrel, up to a total of 2,400 mg, before PCI in patients with a suboptimal degree of platelet inhibition resulted in a lower rate of ischemic complications following PCI.12
PRASUGREL, A NOVEL THIENOPYRIDINE
Prasugrel is an investigational third-generation thienopyridine currently under US Food and Drug Administration (FDA) review for use in patients with ACS being managed with PCI. Like clopidogrel, prasugrel is a prodrug that requires conversion to an active metabolite prior to binding to the platelet P2Y12 receptor for ADP to confer antiplatelet activity. Prasugrel is metabolized more efficiently than clopidogrel, allowing for faster activation and superior bioavailability to produce a greater and more consistent antiplatelet effect.1,13
The active metabolites of clopidogrel and prasugrel are no different in their ability to inhibit platelet aggregation, but approximately 85% of clopidogrel is inactivated by esterases, with the remaining 15% being converted to the active metabolite using the cytochrome P450 pathway via two successive oxidative steps in the liver.14 In contrast, esterases facilitate the transformation of prasugrel to its active metabolite.14 This activation requires only one oxidative step that can occur in either the liver or the gut through cytochrome P450.
Both prasugrel and clopidogrel are irreversible P2Y12 receptor blockers. For this reason, one must wait approximately 5 days after the last dose of either medication for generation of a sufficient number of new platelets to allow restoration of normal platelet-mediated hemostasis.
Inhibition of platelet aggregation relative to clopidogrel
In a study among healthy volunteers, inhibition of platelet aggregation was significantly higher after a 60-mg loading dose of prasugrel compared with a 300-mg loading dose of clopidogrel.13 Further, suboptimal responders to clopidogrel who crossed over to prasugrel had levels of platelet inhibition as high as 80% following prasugrel administration. The time to peak effect of prasugrel was about 1 hour. Inhibition of platelet aggregation was more consistent following dosing of prasugrel compared with clopidogrel.13
In a study of 201 patients undergoing cardiac catheterization with planned PCI, Wiviott et al demonstrated better levels of inhibition of platelet aggregation at 6 hours after a 60-mg loading dose of prasugrel than after a 600-mg loading dose of clopidogrel (P < .0001).1
Clinical effects relative to clopidogrel: TRITON-TIMI 38
A large phase 3 clinical trial—the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON-TIMI) 38—was conducted to compare the effects of prasugrel and standard-dose clopidogrel on death and ischemic end points in 13,608 patients with ACS scheduled to undergo PCI.15 Patients randomized to clopidogrel were given the standard regimen of a 300-mg loading dose followed by a 75-mg daily maintenance dose; those randomized to prasugrel were given a 60-mg loading dose followed by a 10-mg daily maintenance dose. The study drug was typically given immediately before PCI, a time frame that may mimic real-life use but that favored the faster-onset prasugrel over the slower-onset clopidogrel. Both groups also received low-dose aspirin. Approximately half of the patients in each group were treated with a glycoprotein IIb/IIIa inhibitor. The median duration of therapy was approximately 15 months.
Efficacy. The primary end point—a composite of cardiovascular death, MI, or stroke—occurred in 9.9% of patients randomized to prasugrel compared with 12.1% of those randomized to clopidogrel, corresponding to a 19% relative risk reduction (P = .0004) with prasugrel. Based on these results, 46 patients would need to be treated with prasugrel rather than with clopidogrel to prevent 1 additional cardiovascular death, MI, or stroke.15
The reduction in the primary end point with prasugrel was driven primarily by a reduction in nonfatal MI; nonsignificant trends favored prasugrel over clopidogrel on rates of cardiovascular death and all-cause mortality, but there was no difference in stroke rates. Prasugrel’s effect was consistent across subgroups based on MI type, sex, age, the type of stent used, adjunctive antithrombotic therapy, and renal function.15
In the subgroup of patients with diabetes, the relative reduction in the primary end point with prasugrel compared with clopidogrel was 30% (P < .001), and the respective relative reduction among patients with diabetes who required insulin was 37%.16
Safety. Higher antiplatelet potency carries the trade-off of increased bleeding, and this trade-off was apparent with prasugrel in TRITON-TIMI 38.15 TIMI major bleeding (not counting bleeding related to coronary artery bypass graft surgery [CABG]) occurred significantly more often in prasugrel-treated subjects than in those receiving clopidogrel (2.4% vs 1.8%; P = .03), as did life-threatening bleeds (1.4% vs 0.9%; P = .01). Because absolute rates of major bleeding were low in each treatment group, based on these results, 167 patients would need to be treated with prasugrel rather than clopidogrel to result in 1 excess non-CABG-related major bleeding episode. Rates of intracranial hemorrhage were identical in the two treatment groups.15
Net clinical outcome and therapeutic considerations. Overall analysis of the balance of efficacy and safety in TRITON-TIMI 38 revealed that 138 events were prevented with randomization to prasugrel instead of clopidogrel, at a cost of 35 additional TIMI major bleeds (Figure 2).15
In a post hoc analysis of net clinical outcome, in which major bleeding events were added to the primary composite efficacy end point, prasugrel was associated with a 13% relative risk reduction (P = .004).15 Twenty-three MIs were prevented per 1,000 treated patients with the use of prasugrel instead of clopidogrel, at a cost of 6 excess non-CABG-related major bleeds.15
Another post hoc assessment identified three subgroups who had a significantly increased risk of TIMI major bleeds with randomization to prasugrel15:
- Patients aged 75 years or older
- Patients with a body weight less than 60 kg
- Patients with a history of stroke or transient ischemic attack (TIA).
In these three subgroups, the net clinical effect either was neutral (for those aged ≥ 75years and for those weighing < 60 kg) or favored clopidogrel (for those with a history of stroke or TIA). The group with a history of stroke or TIA represented 4% of the entire cohort, and the TRITON-TIMI 38 investigators recommended avoiding prasugrel in patients with a history of these events. The other two subgroups with a significantly increased bleeding risk with prasugrel represented 16% of the entire cohort, and in these two groups the investigators suggested a pharmacokinetics-guided reduction in the maintenance dose of prasugrel, although a recommendation for such dosing is based on modeling and not actual outcomes data.15
Stent thrombosis. A subanalysis of TRITON-TIMI 38 examined the risk of stent thrombosis in the 12,844 patients enrolled in the trial who had stents implanted.17 Stent thrombosis was assessed using the Academic Research Consortium definitions of definite, probable, and possible stent thrombosis.18 The risk of definite or probable stent thrombosis was halved (hazard ratio = 0.48; P < .0001) with the use of prasugrel compared with clopidogrel, and the reduction was highly significant regardless of the type of stent implanted or the way stent thrombosis was defined. Significant reductions in both early (within the first 30 days) stent thrombosis (P < .0001) and late (beyond 30 days) stent thrombosis (P = .03) were observed in the prasugrel arm compared with the clopidogrel arm.17
AZD6140, A REVERSIBLE P2Y12 RECEPTOR ANTAGONIST
AZD6140, another investigational antiplatelet agent, is an orally active reversible P2Y12 receptor antagonist, in contrast to the thienopyridines, which are irreversible inhibitors. A member of the cyclo-pentyl-triazolo-pyrimidine (CPTP) class, AZD6140 has a rapid onset of action (≤ 2 hours) and does not require metabolic activation. Its plasma half-life is approximately 12 hours, which translates to twice-daily dosing.
Inhibition of platelet aggregation relative to clopidogrel
In a study of clopidogrel-naïve patients with ACS, inhibition of platelet aggregation 12 hours after administration of AZD6140 was approximately 75% with 90-mg, 180-mg, and 270-mg doses, significantly greater than the 30% inhibition achieved after administration of 300 mg of clopidogrel (P < .0002 for all doses of AZD6140 vs clopidogrel).19 Whereas steady state was achieved in approximately 4 to 6 hours with clopidogrel, it was achieved in approximately 2 hours or less with AZD6140.
Clinical safety and efficacy relative to clopidogrel
In a dose-ranging study of AZD6140, adjudicated bleeding rates were similar among two different doses of AZD6140 (90 mg twice daily and 180 mg twice daily) and clopidogrel 75 mg once daily, with no evidence of a dose effect for major bleeding with AZD6140.20 Although this study, conducted in 990 patients with ACS, was underpowered for efficacy end points, rates of adjudicated MI were numerically lower in each of the AZD6140 groups than in the clopidogrel group.
A more definitive evaluation of the relative effcicacy and safety of AZD6140 is expected from the ongoing PLATO trial, which is comparing 90 mg of AZD6140 twice daily with clopidogrel 75 mg/day among 18,000 patients randomized to one of the two treatments within 24 hours of an index ACS event.21
CANGRELOR, A RAPID PARENTERAL P2Y12 RECEPTOR ANTAGONIST
Cangrelor (formerly known as AR-C69931MX) is an intravenously (IV) administered P2Y12 receptor antagonist under investigation for treatment of ACS and use during PCI and other coronary procedures. The compound is an adenosine triphosphate analogue with a plasma half-life of 5 to 9 minutes. Cangrelor is highly reversible, as platelet function returns to normal within 20 minutes of dosing. Within 15 minutes of initiation, cangrelor produces profound platelet inhibition and rapidly achieves steady state; peak effect occurs within minutes.22 The response to cangrelor is highly consistent, with virtually all recipients achieving the same degree of platelet inhibition. Platelet response approaches baseline 15 minutes after termination.22
If approved by the FDA, cangrelor would be administered similar to the way that glycoprotein IIb/IIIa inhibitors are, as it would be used primarily in the catheterization laboratory and then discontinued after the procedure, at which point transition to a long-term oral therapy would be necessary.
Clinical effects relative to abciximab
Cangrelor has been compared with the glycoprotein IIb/IIIa inhibitor abciximab and placebo in 249 patients undergoing elective or urgent PCI.22 Rates of the combined end point of death, MI, or need for repeat revascularization at 30 days were similar with cangrelor and abciximab (5.7% vs 5.4%, respectively; P = NS), both of which were lower than the rate with placebo (10.0%). Major or minor bleeding through 7 days occurred in numerically fewer cangrelor recipients compared with abciximab recipients (7.0% vs 9.0%), although the small sample size precluded evaluation for statistical significance.
Clinical effects relative to clopidogrel—the CHAMPION trials
A phase 3 trial program consisting of two multinational studies of cangrelor—the Cangrelor Versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition (CHAMPION) program—is currently under way.
CHAMPION-PCI is enrolling 9,000 patients presenting with ACS who are being randomized in a double-blind fashion at the start of PCI to a 600-mg loading dose of clopidogrel or to cangrelor given as an IV bolus of 30 μg/kg followed by an IV infusion of 4 μg/kg/min. The primary end point is a composite of all-cause mortality, MI, or ischemia-driven revascularization in the 48 hours following randomization. Secondary end points include rates of all-cause mortality and MI at 48 hours.23
CHAMPION-PLATFORM is enrolling 4,400 patients scheduled for PCI as a result of ACS who are being randomized in a double-blind, double-dummy manner to (1) cangrelor bolus and infusion plus oral placebo or (2) oral clopidogrel plus placebo bolus and infusion before their index procedures. Dosages of the two agents are the same as in CHAMPION-PCI. The primary end point is a composite of death, MI, or urgent target vessel revascularization at 48 hours. Secondary end points include 30-day and 1-year clinical outcomes.23
The rationale for the CHAMPION investigations stems from the need to initiate clopidogrel before a patient is taken to the catheterization laboratory, owing to the inability to achieve a high degree of platelet inhibition until 4 to 6 hours after clopidogrel administration. Although this strategy can be undertaken without complication for most patients, a subset of patients with three-vessel disease or left-main disease will require CABG, which then must be delayed several days until clopidogrel’s platelet-inhibiting effect diminishes. A rapid-acting IV inhibitor of the P2Y12 receptor such as cangrelor would obviate this concern.
THROMBIN INHIBITORS
Thrombin plays an important role in platelet activation, and thrombin receptor antagonists may represent a safer means of inhibiting platelet activation relative to traditional antiplatelet agents. This theoretical safety advantage stems from the notion that blocking the action of platelets at the thrombin receptor would preserve platelets’ function as mediators of primary hemostasis. Because thrombin’s activation of platelets should occur only during clot formation, blocking platelet activation at the thrombin receptor would interrupt thrombin’s ability to propagate platelet activation during formation of coronary artery clots.
One agent in this class that is being studied extensively is SCH 530348, an oral thrombin receptor antagonist with potent antiplatelet activity. Its peak antiplatelet potency is achieved within hours when a loading dose is given, and within days without a loading dose. Wearing-off of the action of SCH 530348 takes weeks.24
Inhibition of platelet aggregation with thrombin receptor antagonists is measured in response to the thrombin receptor antagonist peptide (TRAP), not ADP. The proportion of subjects treated with SCH 530348 who achieve greater than 80% inhibition of platelet aggregation to 15 μM of TRAP ranges from 91% (with 0.5 mg of SCH 530348) to 100% (with 1.0 mg and 2.5 mg) at both 30 days and 60 days.25
Clinical effects in placebo-controlled trials
SCH 530348 was studied in the Thrombin Receptor Antagonist (TRA)–PCI trial, a dose-ranging study in which patients were randomized to one of three oral loading doses of the study drug (10 mg, 20 mg, or 40 mg) on top of a clopidogrel loading dose before undergoing cardiac catheterization for planned PCI; patients were then randomized to one of three maintenance doses of SCH 530348 (0.5 mg, 1.0 mg, or 2.5 mg) or placebo (depending on loading therapy) for 60 days.25
Among the 573 patients undergoing PCI , the rate of TIMI major or minor bleeding was not significantly higher with any dose of SCH 530348 compared with placebo,25 supporting the hypothesis that thrombin receptor antagonism inhibits platelet aggregation without a significant increase in bleeding.
Although the TRA-PCI study was not powered to detect differences in clinical event rates, a reduction in the rate of major adverse cardiovascular events was observed in a dose-dependent manner with SCH 530348 compared with placebo in the PCI cohort.25
On the basis of the TRA-PCI trial, a pair of phase 3 trials of SCH 530348 have been launched—the Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P-TIMI 50) study and the Thrombin Receptor Antagonist for Clinical Event Reduction in ACS (TRA-CER) study.
TRA 2°P-TIMI 50 is a multinational double-blind study enrolling 19,500 patients with prior MI or stroke or with existing peripheral arterial disease. Patients are being randomized to placebo plus standard medical care (including aspirin and clopidogrel) or to 2.5 mg of SCH 530348 once daily plus standard medical care. The primary end point is the composite of cardiovascular death, MI, urgent coronary revascularization, or stroke.26
TRA-CER is a multinational double-blind study with planned enrollment of 10,000 patients with non-ST-segment-elevation MI. Patients are being randomized to placebo plus standard medical care (including aspirin or clopidogrel) or to SCH 530348 (using the oral 40-mg loading dose and a maintenance dose of 2.5 mg once daily) plus standard medical care. The primary end point is the composite of cardiovascular death, MI, rehospitalization for ACS, urgent coronary revascularization, or stroke. The key secondary end point is the composite of cardiovascular death, MI, or stroke.27
COMPARATIVE CONSIDERATIONS
Inhibition of platelet aggregation
Clopidogrel achieves about 30% inhibition of platelet aggregation to ADP at its current FDA-approved loading dose of 300 mg and about 40% inhibition when its dose is doubled to 600 mg. These levels of inhibition are increased to 75% to 80% by clopidogrel’s fellow thienopyridine prasugrel, and this increase is attributable to prasugrel’s more efficient metabolism from prodrug to active metabolite. The reversible P2Y12 receptor antagonist AZD6140 achieves a comparable 75% to 80% inhibition of platelet aggregation. The parenterally administered P2Y12 receptor antagonist cangrelor achieves greater than 90% inhibition, as does the oral thrombin receptor antagonist SCH 530348, although the latter agent’s inhibition is to the agonist TRAP rather than ADP.
Time to peak effect
The time to peak effect with clopidogrel is approximately 4 hours regardless of the loading dose used (300 mg or 600 mg); this is substantially reduced with all of the investigational agents except SCH 530348. The novel agents’ reduced time to peak effect can offer advantages in speeding patients’ readiness to undergo catheterization procedures. This is particularly true for the IV agent cangrelor, which achieves its peak effect within minutes, although the 1-hour to 2-hour time frame with oral agents prasugrel and AZD6140 also would usually obviate any need to delay catheterization.
Consistency of platelet response
Standard-dose clopidogrel has the least consistency of platelet response among the therapies reviewed. Although increasing the clopidogrel dose yields somewhat greater consistency in response, it is still lower than the very high degrees of consistency observed with all of the novel compounds, each of which appears to achieve the same degree of inhibition of aggregation in virtually all patients.
Offset of effect
Both of the thienopyridines—clopidogrel and prasugrel—have an offset of effect of about 5 days, which requires delay of surgery, if possible, for several days in patients taking these agents. This is not an issue for the reversible oral agent AZD6140, whose offset of action takes just 1 to 2 days. While this rapid wearing-off of effect translates to a potential advantage for AZD6140, it also poses the potential drawback that a missed dose or two may leave the patient exposed to the risk of a thrombotic event. Cangrelor’s rapid offset of 20 minutes promotes its envisioned use as a catheterization lab–based medication like the glycoprotein IIb/IIIa inhibitors that can be started right before a PCI procedure and stopped immediately afterward. Because SCH 530348 has a very long half-life and thus a weeks-long washout period, the practicality of its use may depend on the hypothesis that thrombin receptor antagonists do not interfere with primary hemostasis, which is supported by data to date but remains to be definitively confirmed.
CONCLUSIONS
Clopidogrel achieves modest platelet inhibition with wide variability in response. Higher doses of clopidogrel achieve modestly greater degrees of inhibition than standard doses, and appear to result in a decreased rate of ischemic events. Although higher doses of clopidogrel have been embraced by some clinicians, we await definitive phase 3 trial evidence of net benefit before making high-dose clopidogrel the new standard of care.
Compared with clopidogrel, the investigational thienopyridine prasugrel is a more potent and consistent blocker of the ADP receptor. It results in a decreased rate of ischemic events relative to clopidogrel, including a 50% reduction in the rate of stent thrombosis, but is associated with an increased rate of bleeding. If prasugrel is approved for marketing, its use should be avoided in patients with a history of stroke or TIA, and avoidance or dose adjustment may be necessary in patients aged 75 years or older and in patients weighing less than 60 kg.
Other novel antiplatelet agents being evaluated for use in patients with ACS—the reversible oral ADP receptor blocker AZD6140, the rapid-acting IV ADP receptor blocker cangrelor, and oral thrombin receptor antagonists—offer potential advantages that need to be examined in the context of large-scale clinical trials.
- Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation–Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004; 109:3171–3175.
- Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol 2005; 46:1827–1832.
- Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol 2005; 45:1392–1396.
- von Beckerath N, Taubert D, Pogatsa-Murray G, Schömig E, Kastrati A, Schömig A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) Trial. Circulation 2005; 112:2946–2950.
- Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation 2005; 111:2099–2106.
- Cuisset T, Frere C, Quilici J, et al. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol 2006; 48:1339–1345.
- von Beckerath N, Kastrati A, Wieczorek A, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J 2007; 28:1814–1819.
- Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007; 115:708–716.
- Clopidogrel optimal loading dose usage to reduce recurrent events/optimal antiplatelet strategy for interventions (CURRENT/OASIS7). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00335452. Updated September 1, 2008. Accessed December 16, 2008.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolic formation. Am Heart J 2007; 153:66.e9–e16.
- Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med 2003; 3:113–122.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38. Circulation 2008; 118:1626–1636.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol 2007; 50:1852–1856.
- Cannon CP, Husted S, Harrington RA, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007; 50:1844–1851.
- A comparison of AZD6140 and clopidogrel in patients with acute coronary syndrome (PLATO). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00391872. Updated December 3, 2008. Accessed December 5, 2008.
- Greenbaum AB, Grines CL, Bittl JA, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J 2006; 151:689.e1–689.e10.
- A clinical trial to demonstrate the efficacy of cangrelor (PCI). Clinical Trials.gov Web site. http://www.clinicaltrials.gov/ct/show/nct00305162. Updated December 3, 2008. Accessed December 5, 2008.
- Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov 2003; 2:15–28.
- Moliterno DJ, Becker RC, Jennings LK, et al; TRA-PCI Study Investigators. Results of a multinational randomized, double-blind, placebo-controlled study of a novel thrombin receptor antagonist (SCH 530348) in percutaneous coronary intervention. Presented at: 56th Annual Scientific Session of the American College of Cardiology; March 24–27, 2007; New Orleans, LA.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with atherosclerosis (TRA 2°P-TIMI 50). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00526474. Updated November 13, 2008. Accessed December 16, 2008.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with acute coronary syndrome (TRA-CER). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00527943. Updated November 12, 2008. Accessed December 16, 2008.
- Wiviott SD, Trenk D, Frelinger AL, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation–Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007; 116:2923–2932.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol 2005; 45:246–251.
- Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation 2004; 109:3171–3175.
- Gurbel PA, Bliden KP, Samara W, et al. Clopidogrel effect on platelet reactivity in patients with stent thrombosis: results of the CREST Study. J Am Coll Cardiol 2005; 46:1827–1832.
- Gurbel PA, Bliden KP, Hayes KM, Yoho JA, Herzog WR, Tantry US. The relation of dosing to clopidogrel responsiveness and the incidence of high post-treatment platelet aggregation in patients undergoing coronary stenting. J Am Coll Cardiol 2005; 45:1392–1396.
- von Beckerath N, Taubert D, Pogatsa-Murray G, Schömig E, Kastrati A, Schömig A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (Intracoronary Stenting and Antithrombotic Regimen: Choose Between 3 High Oral Doses for Immediate Clopidogrel Effect) Trial. Circulation 2005; 112:2946–2950.
- Patti G, Colonna G, Pasceri V, Pepe LL, Montinaro A, Di Sciascio G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: results from the ARMYDA-2 (Antiplatelet therapy for Reduction of MYocardial Damage during Angioplasty) study. Circulation 2005; 111:2099–2106.
- Cuisset T, Frere C, Quilici J, et al. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol 2006; 48:1339–1345.
- von Beckerath N, Kastrati A, Wieczorek A, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J 2007; 28:1814–1819.
- Angiolillo DJ, Shoemaker SB, Desai B, et al. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the Optimizing Antiplatelet Therapy in Diabetes Mellitus (OPTIMUS) study. Circulation 2007; 115:708–716.
- Clopidogrel optimal loading dose usage to reduce recurrent events/optimal antiplatelet strategy for interventions (CURRENT/OASIS7). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00335452. Updated September 1, 2008. Accessed December 16, 2008.
- Bonello L, Camoin-Jau L, Arques S, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol 2008; 51:1404–1411.
- Brandt JT, Payne CD, Wiviott SD, et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolic formation. Am Heart J 2007; 153:66.e9–e16.
- Herbert JM, Savi P. P2Y12, a new platelet ADP receptor, target of clopidogrel. Semin Vasc Med 2003; 3:113–122.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007; 357:2001–2015.
- Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction 38. Circulation 2008; 118:1626–1636.
- Wiviott SD, Braunwald E, McCabe CH, et al. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008; 371:1353–1363.
- Mauri L, Hsieh WH, Massaro JM, Ho KK, D’Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug-eluting stents. N Engl J Med 2007; 356:1020–1029.
- Storey RF, Husted S, Harrington RA, et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol 2007; 50:1852–1856.
- Cannon CP, Husted S, Harrington RA, et al. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007; 50:1844–1851.
- A comparison of AZD6140 and clopidogrel in patients with acute coronary syndrome (PLATO). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00391872. Updated December 3, 2008. Accessed December 5, 2008.
- Greenbaum AB, Grines CL, Bittl JA, et al. Initial experience with an intravenous P2Y12 platelet receptor antagonist in patients undergoing percutaneous coronary intervention: results from a 2-part, phase II, multicenter, randomized, placebo- and active-controlled trial. Am Heart J 2006; 151:689.e1–689.e10.
- A clinical trial to demonstrate the efficacy of cangrelor (PCI). Clinical Trials.gov Web site. http://www.clinicaltrials.gov/ct/show/nct00305162. Updated December 3, 2008. Accessed December 5, 2008.
- Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov 2003; 2:15–28.
- Moliterno DJ, Becker RC, Jennings LK, et al; TRA-PCI Study Investigators. Results of a multinational randomized, double-blind, placebo-controlled study of a novel thrombin receptor antagonist (SCH 530348) in percutaneous coronary intervention. Presented at: 56th Annual Scientific Session of the American College of Cardiology; March 24–27, 2007; New Orleans, LA.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with atherosclerosis (TRA 2°P-TIMI 50). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00526474. Updated November 13, 2008. Accessed December 16, 2008.
- Trial to assess the effects of SCH 530348 in preventing heart attack and stroke in patients with acute coronary syndrome (TRA-CER). Clinical Trials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00527943. Updated November 12, 2008. Accessed December 16, 2008.
KEY POINTS
- There is substantial interpatient variability in the response to clopidogrel.
- In the large TRITON-TIMI 38 trial, the composite rate of death, myocardial infarction, or stroke was reduced by 19% and the rate of stent thrombosis was halved in patients receiving prasugrel compared with standard-dose clopidogrel.
- The risk of major bleeding with prasugrel is highest in patients aged 75 or older, those weighing less than 60 kg, and those with a history of stroke or transient ischemic attack.
- Thrombin receptor antagonists are being studied to see if their use can reduce ischemic events without increasing bleeding.