User login
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
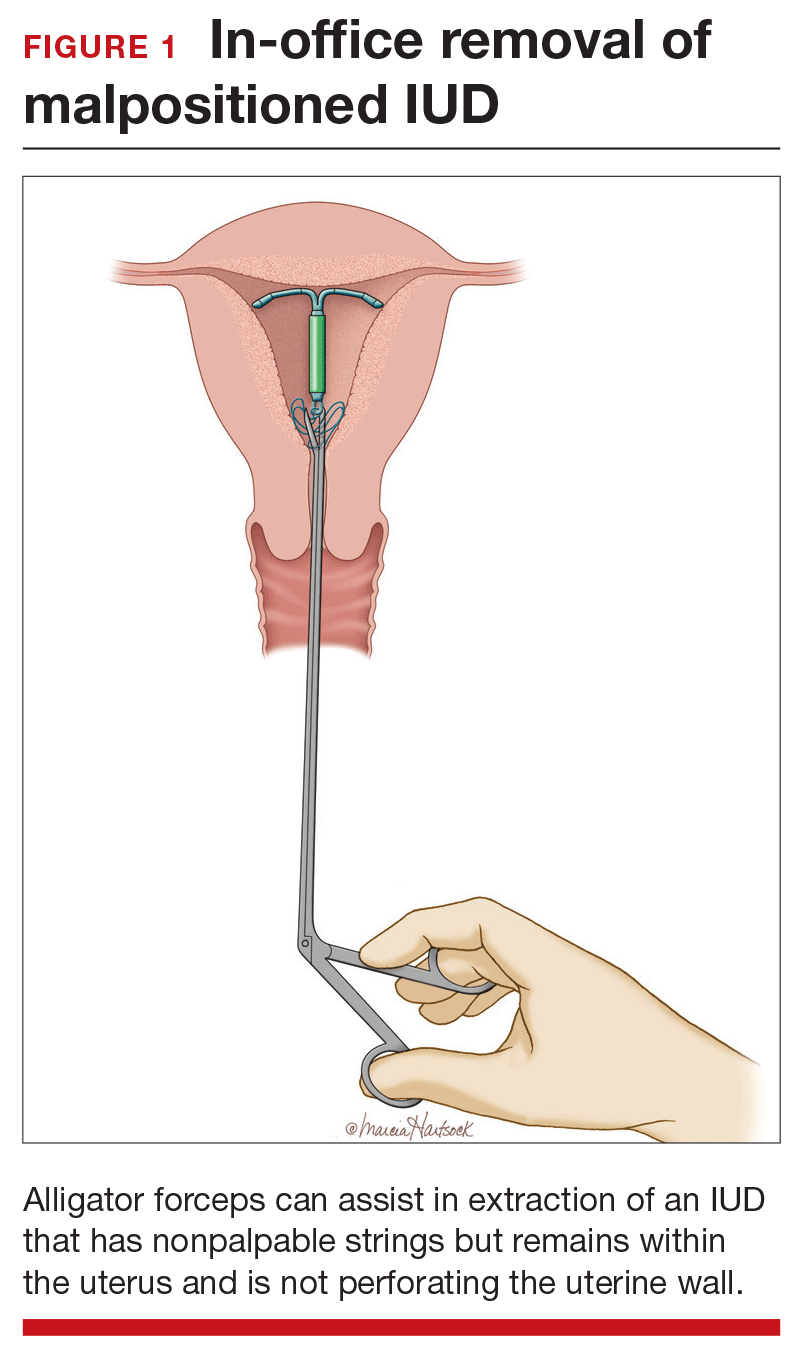
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.
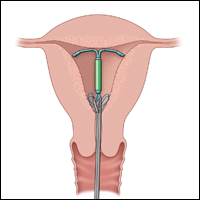
A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
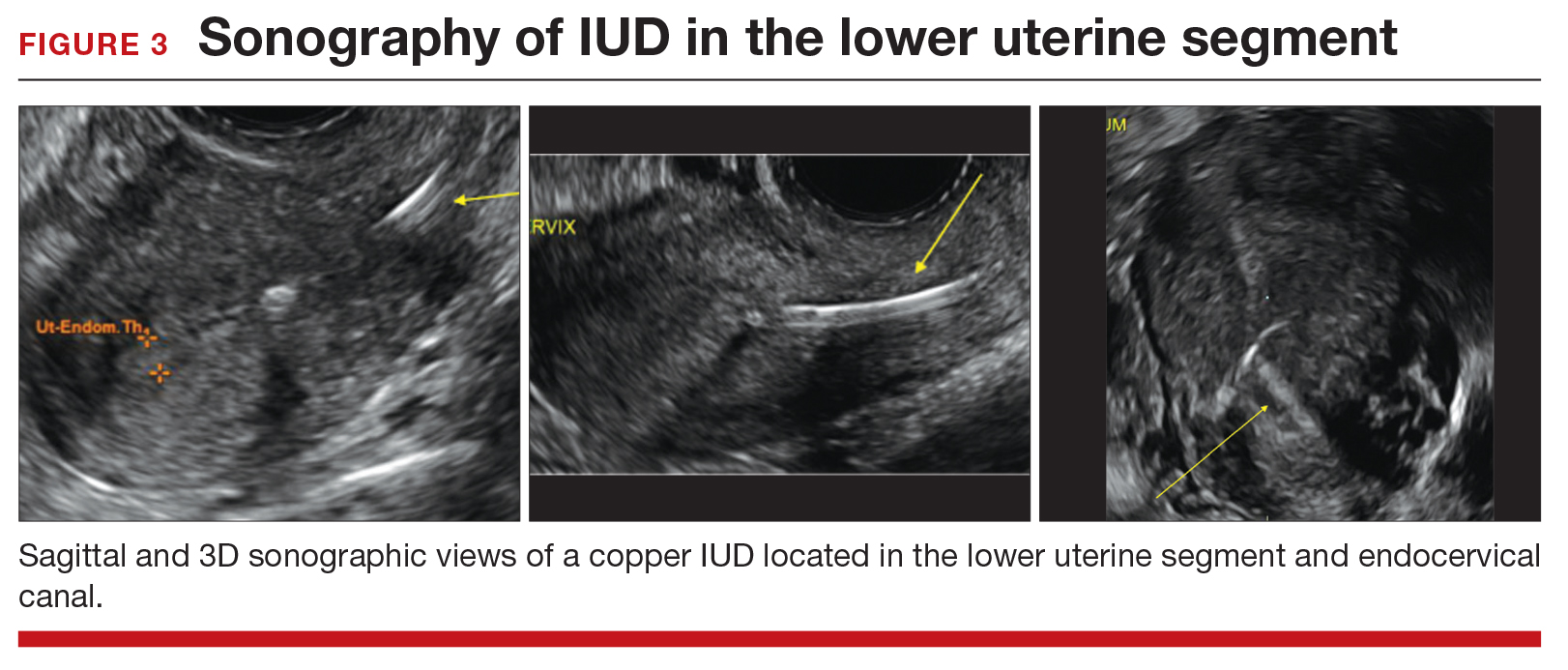
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.
Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.

A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.

Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.

A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.

Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.