User login
Is an IUD a good contraceptive choice for a never sexually active teen?
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
Expert Commentary
Data demonstrate efficacy and safety of the IUD in adolescents. In addition, IUDs (particularly the levonorgestrel-containing IUD) have many noncontraceptive benefits. There is still reluctance, however, among clinicians to use IUDs in adolescents. In a sample of fellows of the American College of Obstetricians and Gynecologists, only 43% considered adolescents appropriate candidates for use of an IUD.1
Study details
In this retrospective chart review, Kebodeaux and Schwartz sought to compare successful IUD insertion rates on first attempt in 120 sexually active (SA) and 82 never sexually active (NSA) adolescents. The IUD type used for all women was the 52-mg levonorgestrel IUD (Mirena), except for 3 copper IUDs (Paragard) used in the SA group. The primary indications for IUD use were contraception (85.2%) in the SA group and abnormal uterine bleeding (43.9%) and menstrual suppression (24.4%) in the NSA group.
In the NSA group, 82.9% of adolescents had had some type of prior treatment affecting the menstrual cycle, compared with 60.9% in the SA group (P = .001).
Non–office insertion. Either a sedation unit or operating room was utilized in 5.5% of the IUD insertions in the SA group and 47.6% of the NSA group. Among the 39 adolescents in the NSA group undergoing non–office insertion, 19 (48.7%) had special needs (learning or intellectual disabilities, autism/autism spectrum, or physical disabilities, such as cerebral palsy). Only 1 adolescent with special needs in the NSA group had an office insertion compared with 5 out of 6 in the SA group.
The performance of another procedure other than the IUD insertion (including diagnostic laparoscopy and hymenectomy) was common among adolescents undergoing procedures in the sedation unit or operating room who did not have special needs. It is also important to note that adolescents with special needs were routinely offered insertion under anesthesia while SA adolescents were offered insertion under anesthesia only if they were undergoing another procedure as well.
Study strengths and weaknesses
The study’s strengths include IUD insertions performed at a children’s hospital by providers with experience working with adolescent populations. This likely accounts for the high rates of “tolerance of the procedure well” (93.8% in the SA group vs 81.7% in the NSA group; P = .006). The study also included a patient population—adolescents with special needs—that has not been studied relative to IUD use previously.
A significant weakness of the study, however, is that there are no long-term follow-up data, particularly related to continuation rates.
These study findings provides further support to combat the myth that adolescents, particularly if nulliparous or not sexually active, are not suitable candidates for IUD use. However, if they have never been sexually active or have special needs, IUD insertion under sedation or in an operating room may be necessary. It is also likely that selection of the IUD as an option by an adolescent and overall tolerance of the insertion procedure requires providers with experience in caring for adolescents as well as providers possessing good counseling skills.
—Ronald T. Burkman, MD
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.
1. Luchowski AT, Anderson BL, Power ML, Reglan GB, Espey E, Shulkin J. Obstetrician-gynecologists and contraception: practice and opinions about the use of IUDs in nulliparous women, adolescents and other patient populations. Contraception. 2014;89:572-577.
Are women seeking short-acting contraception satisfied with LARC after giving it a try?
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Because of women’s personal preference and aversion, for various reasons, to LARC methods, the current estimated use rate of 17% for LARC methods would increase only to 24% to 29% even if major barriers, such as cost and availability, were removed.1 To gain more insight into this issue, Hubacher and colleagues sought to determine if LARC methods would meet the contraceptive needs and be acceptable to a population of women who were not seeking these methods actively and who might have some reservation about using them.
Details of the study
The authors approached women actively seeking 1 of the 2 SARC methods but not a LARC method for contraception. They enrolled 524 women into a cohort study in which they received their desired SARC method. In addition, 392 women agreed to be enrolled in a randomized clinical trial comparing women beginning a LARC method for the first time with a group receiving 1 of the 2 SARC methods.
Importance of covered costs. Of note, the women in the randomized trial had the costs of the insertion or removal of the LARC method covered; those randomly assigned to the comparative SARC arm had the costs of their oral contraceptives (OCs) or depot medroxyprogesterone acetate (DMPA) covered for the first year of use. Underwriting the costs in the randomized study was likely important for study recruitment, since 47% of participants who were randomized to the LARC group cited cost as one of the reasons they did not try a LARC method previously.
Satisfaction with contraceptive method. In addition to the differences in continuation rates and pregnancy rates noted, it is interesting that, among women who tried a LARC method and who had some persistent negative feelings about the method, 65.9% would try the method again.
Satisfaction levels were estimated using 3 choices, with “happiness” being the highest level of satisfaction, followed by “neutral” and “unhappy.” At 24 months, the number of women indicating happiness was similar among the 3 study groups: 71.4% for the LARC randomized group, 75.0% for the randomized SARC group, and 77.6% for the preferred SARC cohort group.
Among women who discontinued their LARC method, occurrence of adverse effects was the reason given 74.2% of the time, while among SARC method users in both groups there was no dominant reason for discontinuation. Also, among women who discontinued their method, the percentage indicating happiness was 32.2% for the LARC randomized group compared with 69.9% and 68.2% for the randomized and preference cohort SARC groups, respectively.
Study strengths and weaknesses
This study had several strengths. The population from which the study groups were obtained was demographically diverse and was appropriate for determining if women with reservations about LARC methods could have satisfactory outcomes similar to women who self-select LARC methods. Further, the 24 months of observations indicate that, for the most part, satisfaction persisted.
One of the study’s shortcomings is the limited data on the subsets, that is, the specific method chosen, within each of the study groups.
-- Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
- Foster DG, Barar R, Gould H, et al. Projections and opinions from 100 experts in long-acting reversible contraception. Contraception. 2015;92:543-552.
Contraceptive considerations for women with headache and migraine
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6
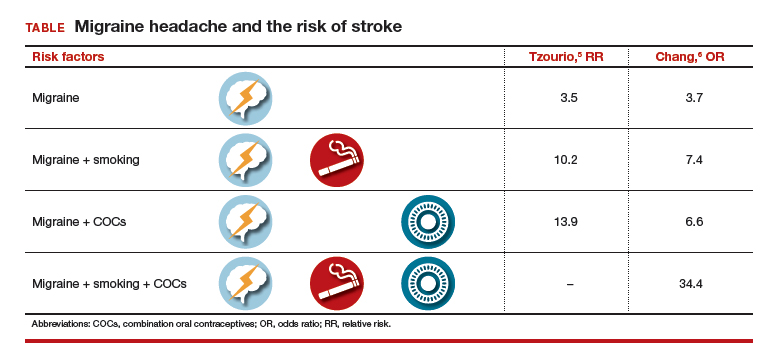
Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6

Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The use of hormonal contraception in women with headaches, especially migraine headaches, is an important topic. Approximately 43% of women in the United States report migraines.1 Roughly the same percentage of reproductive-aged women use hormonal contraception.2 Data suggest that all migraineurs have some increased risk of stroke. Therefore, can women with migraine headaches use combination hormonal contraception? And can women with severe headaches that are nonmigrainous use combination hormonal contraception? Let’s examine available data to help us answer these questions.
Risk factors for stroke
Migraine without aura is the most common subset, but migraine with aura is more problematic relative to the increased incidence of stroke.1
A migraine aura is visual 90% of the time.1 Symptoms can include flickering lights, spots, zigzag lines, a sense of pins and needles, or dysphasic speech. Aura precedes the headache and usually resolves within 1 hour after the aura begins.
In addition to migraine headaches, risk factors for stroke include increasing age, hypertension, the use of combination oral contraceptives (COCs), the contraceptive patch and ring, and smoking.1
Data indicate that the risk for ischemic stroke is increased in women with migraines even without the presence of other risk factors. In a meta-analysis of 14 observational studies, the risk of ischemic stroke among all migraineurs was about 2-fold (relative risk [RR], 2.2; 95% confidence interval [CI], 1.9–2.5) compared with the risk of ischemic stroke in women of the same age group who did not have migraine headaches. When there is migraine without aura, it was slightly less than 2-fold (RR, 1.8; 95% CI, 1.1–3.2). The risk of ischemic stroke among migraineurs with aura is increased more than 2 times compared with women without migraine (RR, 2.27; 95% CI, 1.61–3.19).3 However, the absolute risk of ischemic stroke among reproductive-aged women is 11 per 100,000 women years.4
Two observational studies show how additional risk factors increase that risk (TABLE).5,6 There are similar trends in terms of overall risk of stroke among women with all types of migraine. However, when you add smoking as an additional risk factor for women with migraine headaches, there is a substantial increase in the risk of stroke. When a woman who has migraines uses COCs, there is increased risk varying from 2-fold to almost 4-fold. When you combine migraine, smoking, and COCs, a very, very large risk factor (odds ratio [OR], 34.4; 95% CI, 3.27–3.61) was reported by Chang and colleagues.6

Although these risks are impressive, it is important to keep in mind that even with a 10-fold increase, we are only talking about 1 case per 1,000 migraineurs.4 Unfortunately, stroke often leads to major disability and even death, such that any reduction in risk is still important.
Preventing estrogen withdrawal or menstrual migraines
How should we treat a woman who uses hormonal contraception and reports estrogen withdrawal or menstrual migraines? Based on clinical evidence, there are 2 ways to reduce her symptoms:
- COCs. Reduce the hormone-free interval by having her take COCs for 3 to 4 days instead of 7 days, or eliminate the hormone-free interval altogether by continuous use of COCs, usually 3 months at a time.7
- NSAIDs. For those who do not want to alter how they take their hormonal product, use nonsteroidal anti-inflammatory drugs (NSAIDs) starting 7 days before the onset of menses and continuing for 13 days. In a clinical trial by Sances and colleagues, this plan reduced the frequency, duration, and severity of menstrual migraines.8
Probably altering how she takes the COC would make the most sense for most individuals instead of taking NSAIDs for 75% of each month.
Recommendations from the US MEC
The US Medical Eligibility Criteria (US MEC) from the Centers for Disease Control and Prevention (CDC) offers recommendations for contraceptive use9:
- For nonmigrainous headache, the CDC suggests that the benefits of using COCs outweigh the risks unless the headaches persist after 3 months of COC use.
- For migraine without aura, the benefits outweigh the risks in starting women who are younger than age 35 years on oral contraceptives. However, the risks of COCs outweigh the benefits in women who are age 35 years and older who develop migraine headache while on COCs, or who have risk factors for stroke.
- For migraine with aura, COCs are contraindicated.
- Progestin-only contraceptives. The CDC considers that the benefits of COC use outweigh any theoretical risk of stroke, even in women with risk factors or in women who have migraine with aura. Progestin-only contraceptives do not alter one’s risk of stroke, unlike contraceptives that contain estrogen.
My bottom line
Can women with migraine headaches begin the use of combination hormonal methods? Yes, if there is no aura in their migraines and they are not older than age 35.
Can women with severe headaches that are nonmigrainous use combination hormonal methods? Possibly, but you should discontinue COCs if headache severity persists or worsens, using a 3-month time period for evaluation.
How do you manage women with migraines during the hormone-free interval? Consider the continuous method or shorten the hormone-free interval.
Recommendations for complicated patients. Consulting the CDC’s US MEC database7 can provide assistance in your care of more complicated patients requesting contraception. I also recommend the book, “Contraception for the Medically Challenging Patient,” edited by Rebecca Allen and Carrie Cwiak.10 It links nicely with the CDC guidelines and presents more detail on each subject.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
- Stewart WF, Wood C, Reed MD, et al. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178.
- Finer LB, Frohwirth LF, Dauphinee LA, Singh S, Moore AM. Reasons U.S. women have abortions: quantitative and qualitative perspectives. Perspect Sex Reprod Health. 2005;37(3):110–118.
- Etminan M, Takkouche B, Isorna FC, Samii A. Risk of ischemic stroke in people with migraine: systematic review and meta-analysis of observational studies. BMJ. 2005;330(7482):63–66.
- Petitti DB, Sydney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stoke in users of low-dose oral contraceptives. N Engl J Med. 1996;335(1):8–15.
- Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischemic stroke in young women. BMJ. 1995;310:830–833.
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: case-control study. The World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ. 1999;318(7175):13–18.
- Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21(3):573–578.
- Sances G, Martignoni E, Fioroni L, Blandini F, Facchinetti F, Nappi G. Naproxen sodium in menstrual migraine prophylaxis: a double-blind placebo controlled study. Headache. 1990;30(11):705–709.
- US Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59(RR-4):1–86. https://www.cdc .gov/mmwr/pdf/rr/rr59e0528.pdf. Accessed October 4, 2016.
- Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
Webcast: Emergency contraception: How to choose the right one for your patient
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Contraceptive considerations for women with headache and migraine
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Contraceptive considerations for women with headache and migraine
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Contraceptive considerations for women with headache and migraine
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
Overcoming LARC complications: 7 case challenges
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.
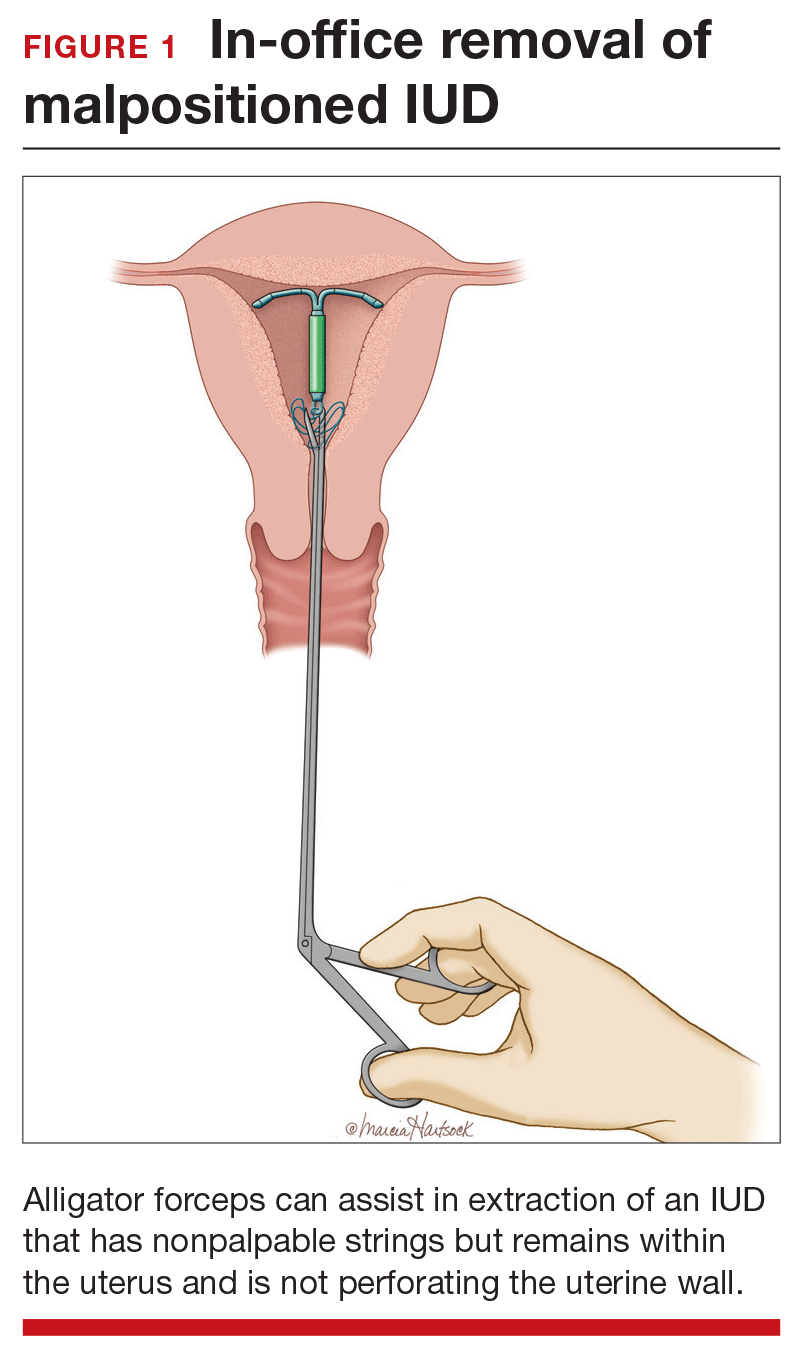
A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.
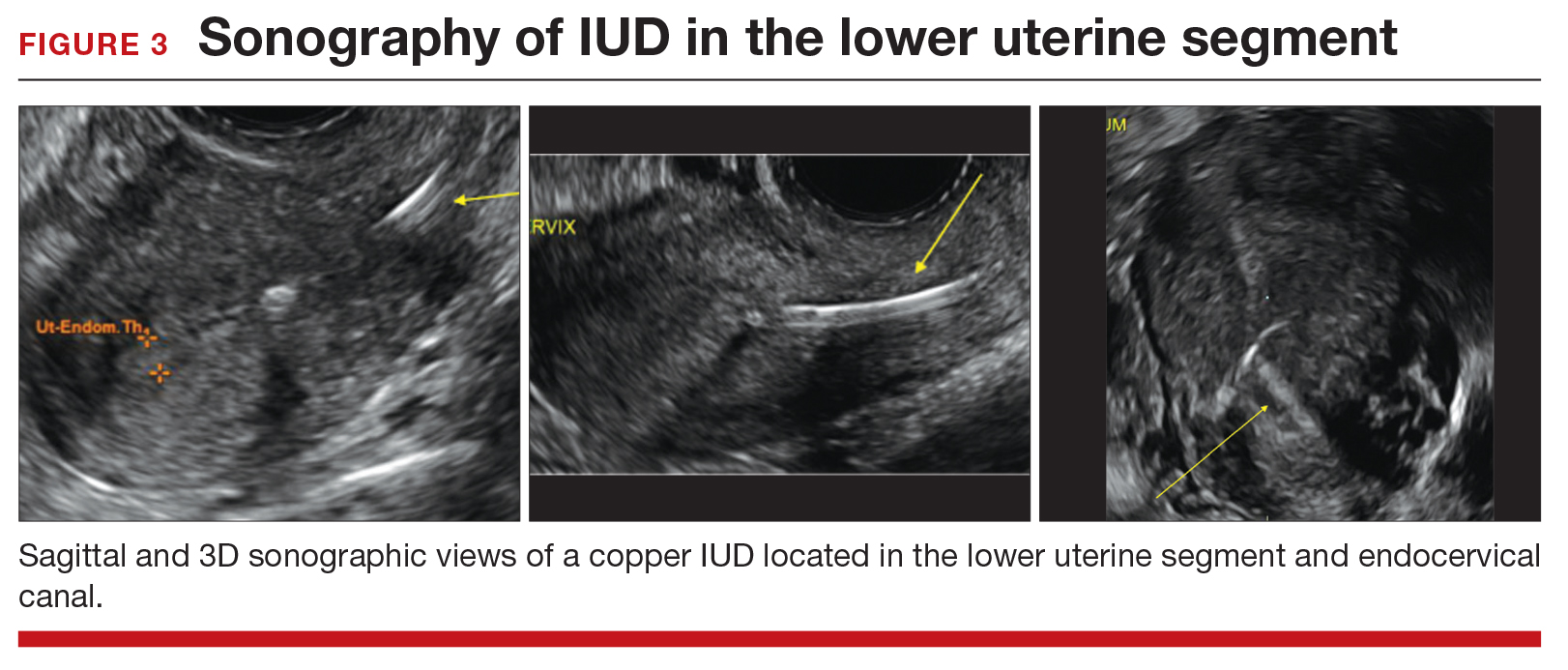
Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.

A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.

Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The use of long-acting reversible contraceptive (LARC) methods has shown a steady increase in the United States. The major factors for increasing acceptance include high efficacy, ease of use, and an acceptable adverse effect profile. Since these methods require placement under the skin (implantable device) or into the uterus (intrauterine devices [IUDs]), unique management issues arise during their usage. Recently, the American College of Obstetricians and Gynecologists (ACOG) released a committee opinion addressing several of these clinical challenges—among them: pain with insertion, what to do when the IUD strings are not visualized, and the plan of action for a nonpalpable IUD or contraceptive implant.1 In this article we present 7 cases, and successful management approaches, that reflect ACOG’s recent recommendations and our extensive clinical experience.
Read the first CHALLENGE: Pain with IUD insertion
CHALLENGE 1: Pain with IUD insertion
CASE First-time, nulliparous IUD user apprehensive about insertion pain
A 21-year-old woman (G0) presents for placement of a 52-mg levonorgestrel IUD for contraception and treatment of dysmenorrhea. Her medical and surgical histories are unremarkable. She has heard that IUD insertion “is more painful if you haven’t had a baby yet” and she asks what treatments are available to aid in pain relief.
What can you offer her?
A number of approaches have been used to reduce IUD insertion pain, including:
- placing lidocaine gel into or on the cervix
- lidocaine paracervical block
- preinsertion use of misoprostol or nonsteroidal anti-inflammatory drugs.
Authors of a recent Cochrane review2 indicated that none of these approaches were particularly effective at reducing insertion pain for nulliparous women. Naproxen sodium 550 mg or tramadol 50 mg taken 1 hour prior to IUD insertion have been found to decrease IUD insertion pain in multiparous patients.3 Misoprostol, apart from being ineffective in reducing insertion pain, also requires use for a number of hours before insertion and can cause painful uterine cramping, upset stomach, and diarrhea.2 Some studies do suggest that use of a paracervical block does reduce the pain associated with tenaculum placement but not the IUD insertion itself.
Related article:
Benefit of self-administered vaginal lidocaine gel in IUD placement
A reasonable pain management strategy for nulliparous patients. Given these data, there is not an evidence-based IUD insertion pain management strategy that can be used for the nulliparous case patient. A practical approach for nulliparous patients is to offer naproxen sodium or tramadol, which have been found to be beneficial in multiparous patients, to a nulliparous patient. Additionally, lidocaine gel applied to the cervix or tenaculum-site injection can be considered for tenaculum-associated pain, although it does not appear to help significantly with IUD insertion pain. Misoprostol should be avoided as it does not alleviate the pain of insertion and it can cause bothersome adverse effects.
Read CHALLENGE 2: IUD strings not visualized
CHALLENGE 2: IUD strings not visualized
CASE No strings palpated 6 weeks after postpartum IUD placement
A 26-year-old woman (G2P2) presents to your office for a postpartum visit 6 weeks after an uncomplicated cesarean delivery at term. She had requested that a 52-mg levonorgestrel IUD be placed at the time of delivery, and the delivery report describes an uneventful placement. The patient has not been able to feel the IUD strings using her fingers and you do not find them on examination. She does not remember the IUD falling out.
What are the next steps in her management?
Failure to palpate the IUD strings by the user or failure to visualize the strings is a fairly common occurrence. This is especially true when an IUD is placed immediatelypostpartum, as in this patient’s case.
When the strings cannot be palpated, it is important to exclude pregnancy and recommend a form of backup contraception, such as condoms and emergency contraception if appropriate, until evaluation can be completed.
Steps to locate a device. In the office setting, the strings often can be located by inserting a cytobrush into the endocervical canal to extract them. If that maneuver fails to locate them, an ultrasound should be completed to determine if the device is in the uterus. If the ultrasound does not detect the device in the uterus, obtain an anteroposterior (AP) x-ray encompassing the entire abdomen and pelvis. All IUDs used in the United States are radiopaque and will be observed on x-ray if present. If the IUD is identified, operative removal is indicated.
Related article:
How to identify and localize IUDs on ultrasound
Intraperitoneal location. If an IUD is found in this location, it is usually the result of a perforation that occurred at the time of insertion. In general, the device can be removed via laparoscopy. Occasionally, laparotomy is needed if there is significant pelvic infection, possible bowel perforation, or if there is an inability to locate the device at laparoscopy.4 The copper IUD is more inflammatory than the levonorgestrel IUDs.
Abdominal location. No matter the IUD type, operative removal of intra-abdominal IUDs should take place expeditiously after they are discovered.
In the case of expulsion. If the IUD is not seen on x-ray, expulsion is the likely cause. Expulsion tends to be more common among5:
- parous users
- those younger than age 20
- placements that immediately follow a delivery or second-trimester abortion.
Nulliparity and type of device are not associated with increased risk of expulsion.
Read CHALLENGE 3: Difficult IUD removal
CHALLENGE 3: Difficult IUD removal
CASE Strings not palpated in a patient with history of LEEP
A 37-year-old woman (G3P2) presents to your office for IUD removal. She underwent a loop electrosurgical excision procedure 2 years ago for cervical intraepithelial neoplasia (CIN) 2 and since then has not been able to feel the IUD strings. On pelvic examination, you do not palpate or visualize the IUD strings after speculum placement.
How can you achieve IUD removal for your patient?
When a patient requests that her IUD be removed, but the strings are not visible and the woman is not pregnant, employ ultrasonography to confirm the IUD remains intrauterine and to rule out expulsion or perforation.
Employ alligator forceps or an IUD hook. Once intrauterine position is confirmed, use an alligator forceps of suitable length and with a small diameter to extract the device (FIGURE 1). It is useful to utilize ultrasonography for guidance during the removal procedure. The alligator forceps will grasp both the IUD device itself and IUD strings well, so either can be targeted during removal.

A second useful tool for IUD removal is an IUD hook (FIGURE 2). In a similar way that a curette is used for endometrial sampling, IUD hooks can be used to drag the IUD from the uterus.

Anesthesia is not usually necessary for IUD removal with alligator forceps or an IUD hook, although it may be appropriate in select patients. Data are limited with regard to the utility of paracervical blocks in this situation.
Related article:
Surgical removal of malpositioned IUDs
Hysteroscopy is an option. If removal with an alligator forceps or IUD hook is unsuccessful, or if preferred by the clinician, hysteroscopic-guided removal is a management option. Hysteroscopic removal may be required if the IUD has become embedded in the uterine wall.
Read CHALLENGE 4: Nonfundal IUD location
CHALLENGE 4: Nonfundal IUD location
CASE Copper IUD found in lower uterine segment
A 31-year-old woman (G1P1) calls your office to report that she thinks her copper IUD strings are longer than before. Office examination confirms that the strings are noticeably longer than is typical. Pelvic ultrasonography shows the copper IUD in the lower uterine segment.
What is the appropriate course of action?
Occasionally, IUDs are noted to be located in the lower uterine segment (FIGURE 3) or cervix. With malposition, users may be experiencing cramping or abnormal bleeding.

Cervical malposition calls for removal. ACOG advises that, regardless of a patient’s presenting symptoms, clinicians should remove IUDs located in the cervix (ie, the stem below the internal os) due to an increased risk of pregnancy and address the woman’s contraceptive needs.
Related article:
STOP relying on 2D ultrasound for IUD localization
Lower-uterine-segment malposition man‑agement less clear. If the patient is symptomatic, remove the device and initiate some form of contraception. If the woman is asymptomatic, the woman should be given the option of having the device removed or left in place. The mechanisms of action of both the copper and levonorgestrel-releasing IUDs suggest that this lower location is unlikely to be associated with a significant decrease in efficacy.
Unfortunately, it is difficult to estimate the risk of pregnancy for a patient whose device is located in the lower uterine segment. Braaten and Goldberg discussed case-controlled data in their 2012 article that suggest malposition may be more important to the efficacy of copper IUDs than of levonorgestrel IUDs.6,7 As unintended pregnancy is an important risk to avoid, ultimately, it is the woman’s decision as to whether she wants removal or continued IUD use.
Read CHALLENGE 5: Pregnancy in an IUD user
CHALLENGE 5: Pregnancy in an IUD user
CASE 3-year copper IUD user with positive pregnancy test
A 25-year-old woman (G3P2) presents to your office because of missed menses and a positive home pregnancy test. Her last menstrual period was 6 weeks ago. She has had a copper IUD in place for 3 years and can feel the strings herself. She has experienced light cramping but no bleeding. Office examination is notable for the IUD stem present at the external cervical os. While the pregnancy is unplanned, the patient desires that it continue.
Should you remove the IUD?
The pregnancy rate among IUD users is less than 1%—a rate that is equivalent to that experienced by women undergoing tubal sterilization. Although there is an overall low risk of pregnancy, a higher proportion of pregnancies among IUD users compared with nonusers are ectopic. Therefore, subsequent management of pregnancy in an IUD user needs to be determined by, using ultrasound, both the location of the pregnancy and whether the IUD is in place.
If an ectopic pregnancy is found, it may be managed medically or surgically with the IUD left in place if desired. If you find an intrauterine pregnancy that is undesired, the IUD can be removed at the time of a surgical abortion or before the initiation of a medical abortion.
If you fail to locate the IUD either before or after the abortion procedure, use an AP x-ray of the entire abdomen and pelvis to determine whether the IUD is in the peritoneal cavity or whether it was likely expelled prior to the pregnancy.
Related article:
In which clinical situations can the use of the 52-mg levonorgestrel-releasing IUD (Mirena) and the TCu380A copper-IUD (ParaGard) be extended?
With a desired pregnancy, if the strings are visible, remove the IUD with gentle traction. If the IUD is left in place, the risk of spontaneous abortion is significantly increased. If the strings are not seen, but the device was noted to be in the cervix by ultrasound, remove the device if the stem is below the internal cervical os. For IUDs that are located above the cervix, removal should not be attempted; counsel the patient about the increased risk of spontaneous abortion, infection, and preterm delivery.
Read CHALLENGE 6: Pregnancy in an implant user
CHALLENGE 6: Pregnancy in an implant user
CASE 3-week implant user with positive pregnancy test
Your 21-year-old patient who received a contraceptive implant 3 weeks earlier now pre‑sents with nausea and abdominal cramping. Her last menstrual period was 6 weeks ago. She has regular cycles that are 28 days in length. Results of urine pregnancy testing are positive. Prior to using the implant, the patient inconsistently used condoms.
How should you counsel your patient?
The rate of pregnancy among implant users is very low; it is estimated at 5 pregnancies per 10,000 implant users per year.8 As in this case, apparent “failures” of the contraceptive implant actually may represent placements that occurred before a very early pregnancy was recognized. Similar to IUDs, the proportion of pregnancies that are ectopic among implant users compared to nonusers may be higher.
With a pregnancy that is ectopic or that is intrauterine and undesired, the device may be left in and use continued after the pregnancy has been terminated. Although the effectiveness of medication abortion with pre-existing contraceptive implant in situ is not well known, researchers have demonstrated that medication abortion initiated at the same time as contraceptive implant insertion does not influence success of the medication abortion.9
Related article:
2016 Update on contraception
For women with desired intrauterine pregnancies, remove the device as soon as feasible and counsel the woman that there is no known teratogenic risk associated with the contraceptive implant.
Read CHALLENGE 7: Nonpalpable contraceptive implant
CHALLENGE 7: Nonpalpable contraceptive implant
CASE Patient requests device removal to attempt conception
A 30-year-old woman (G2P2) presents for contraceptive implant removal because she would like to have another child. The device was placed 30 months ago in the patient’s left arm. The insertion note in the patient’s medical record is unremarkable, and standard insertion technique was used. On physical examination, you cannot palpate the device.
What is your next course of action?
Nonpalpable implants, particularly if removal is desired, present a significant clinical challenge. Do not attempt removing a nonpalpable implant before trying to locate the device through past medical records or radiography. Records that describe the original insertion, particularly the location and type of device, are helpful.
Related article:
2015 Update on contraception
Appropriate imaging assistance. Ultrasonography with a high frequency linear array transducer (10 MHz or greater) may allow an experienced radiologist to identify the implant—including earlier versions without barium (Implanon) and later ones with barium (Nexplanon). Magnetic resonance imaging (MRI), computed tomography scan, or plain x-ray also can be used to detect a barium-containing device; MRI can be used to locate a non−barium-containing implant.
Carry out removal using ultrasonographic guidance. If a deep insertion is felt to be close to a neurovascular bundle, device removal should be carried out in an operating room by a surgeon familiar with the anatomy of the upper arm.
When an implant cannot be located despite radiography. This is an infrequent occurrence. Merck, the manufacturer of the etonorgestrel implant, provides advice and support in this circumstance. (Visit https://www.merckconnect.com/nexplanon/over view.html.)
Recently, published case reports detail episodes of implants inserted into the venous system with migration to the heart or lungs.10 While this phenomenon is considered rare, the manufacturer has recommended that insertion of the contraceptive implant avoid the sulcus between the biceps and triceps muscles.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 672: clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128(3):e69−e77.
- Lopez LM, Bernholc A, Zeng Y, et al. Interventions for pain with intrauterine device insertion. Cochrane Database Syst Rev. 2015;(7):CD007373.
- Karabayirli S, Ayrim AA, Muslu B. Comparison of the analgesic effects of oral tramadol and naproxen sodium on pain relief during IUD insertion. J Minim Invasive Gynecol. 2012;19(5):581−584.
- Kho KA, Chamsy DJ. Perforated intraperitoneal intrauterine contraceptive devices: diagnosis, management, and clinical outcomes. J Minim Invasive Gynecol. 2014;21(4):596−601.
- Madden T, McNicholas C, Zhao Q, Secura GM, Eisenberg DL, Peipert JF. Association of age and parity with intrauterine device expulsion. Obstet Gynecol. 2014;124(4):718−726.
- Patil E, Bednarek PH. Immediate intrauterine device insertion following surgical abortion. Obstet Gynecol Clin North Am. 2015;42(4):583−546.
- Braaten and Goldberg. OBG Manag. Malpositioned IUDs: When you should intervene (and when you should not). OBG Manag. 2012;24(8):38−46.
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397−404.
- Raymond EG, Weaver MA, Tan YL, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2017;127(2):306−312.
- Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2016. In press.
Webcast: Contraceptive considerations for women with headache and migraine
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
Webcast: Hormonal contraception and risk of venous thromboembolism

Access Dr. Burkman's Webcasts on contraception:
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resource for your practice:

Access Dr. Burkman's Webcasts on contraception:
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resource for your practice:

Access Dr. Burkman's Webcasts on contraception:
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resource for your practice:
Webcast: Oral contraceptives and breast cancer: What’s the risk?
Access Dr. Burkman's Webcasts on contraception:
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resource for your practice:
Access Dr. Burkman's Webcasts on contraception:
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resource for your practice:
Access Dr. Burkman's Webcasts on contraception:
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resource for your practice:
Webcast: Providing LARC methods of contraception to adolescents
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Emergency contraception: How to choose the right one for your patient
- Contraceptive considerations for women with headache and migraine
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Emergency contraception: How to choose the right one for your patient
- Contraceptive considerations for women with headache and migraine
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Access Dr. Burkman's Webcasts on contraception:
- Emergency contraception: How to choose the right one for your patient
- Contraceptive considerations for women with headache and migraine
- Hormonal contraception and risk of venous thromboembolism
- Oral contraceptives and breast cancer: What’s the risk?
- Factors that contribute to overall contraceptive efficacy and risks
- Obesity and contraceptive efficacy and risks
- How to use the CDC's online tools to manage complex cases in contraception
Helpful resources for your practice:
- United States Medical Eligibility Criteria for Contraceptive Use, 2016
- United States Medical Eligibility Criteria (US MEC) for Contraceptive Use, 2010
- Summary Chart of US Medical Eligibility for Contraceptive Use
- Book recommendation: Allen RH, Cwiak CA, eds. Contraception for the medically challenging patient. New York, New York: Springer New York; 2014.
With no budge in more than 20 years, are US unintended pregnancy rates finally on the decline?
Expert Commentary The unintended pregnancy rate has hovered around 50% for at least 20 years despite vigorous efforts to educate both the public and health care providers on the importance of using effective contraceptive methods. During that time, new contraceptives were developed and older methods were improved to reduce risk and adverse effects. Despite these efforts, however, an estimated 48% of all unintended pregnancies in the United States occurred among contraceptive users.1 Results of the study by Finer and Zolna on the recent decline in unintended pregnancies suggest there may be some light at the end of the tunnel.
Details of the studyThe study authors used data from the National Survey of Family Growth (NSFG) and other sources to calculate rates of pregnancy in the United States for 2008 and 2011, including rates based on pregnancy intentions and outcome. About 45% of pregnancies in 2011 were unintended, compared with 51% in 2008. The rate in 2011 represents the lowest rate of unintended pregnancy in more than 3 decades.
Rates reduced in many population subgroupsThe percentage of unintended pregnancies ending in abortion remained stable at 40% in 2008 and 42% in 2011. The largest changes in rate of unintended pregnancy from 2008 to 2011 occurred in women aged 15 to 17 years (−44%), women cohabiting (−29%), those with incomes at 100% to 199% of the federal poverty level (−32%), women who were not high school graduates (−28%), and Hispanic women (−26%). Other population subgroups also showed improvement but to a lesser extent than those described here.
The study authors concede that some of the reduction in unintended pregnancies can be attributed to the economic recession that occurred during the study time frame, when many women intentionally reduced or delayed childbearing. The more likely explanation, they point out, is the increased use of long-acting reversible contraception (LARC), particularly the intrauterine device (IUD). Notably, among US women using contraception, the rates of IUD use increased from 4% in 2007 to 12% in 2012.2
Nevertheless, while the unintended pregnancy rate has shown improvement, the rate in the United States still lags considerably behind that of other industrialized nations. In Western Europe, for example, the unintended pregnancy rate was 34% in 2012.3
What this evidence means for practiceAs the study data suggest, use of contraceptive methods that do not rely on a frequent activity by the user, such as LARC methods, is associated with improved adherence. Consequently, all LARC methods, including the IUD, are associated with a pregnancy rate of about 1% or less; this rate is equal to or better than the rates seen with many forms of tubal sterilization, and it is superior to that seen with other methods, such as oral contraceptives, which have a contraceptive failure rate of about 9%.4
Finally, to correct disparities noted in this study that may be related particularly to access to contraceptive methods, the Affordable Care Act contains provisions that should lead to greater availability of contraceptive services in the United States.
—Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38(2):90–96.
- Use of highly effective contraceptives in the US continues to rise, with likely implications for declines in unintended pregnancy and abortion. New York: Guttmacher Institute, 2014. http://www.guttmacher.org/media/inthenews/2014/12/12/index.html. Published December 12, 2014. Accessed April 21, 2016.
- Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann. 2014;45(3):301–314.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154-161.
Expert Commentary The unintended pregnancy rate has hovered around 50% for at least 20 years despite vigorous efforts to educate both the public and health care providers on the importance of using effective contraceptive methods. During that time, new contraceptives were developed and older methods were improved to reduce risk and adverse effects. Despite these efforts, however, an estimated 48% of all unintended pregnancies in the United States occurred among contraceptive users.1 Results of the study by Finer and Zolna on the recent decline in unintended pregnancies suggest there may be some light at the end of the tunnel.
Details of the studyThe study authors used data from the National Survey of Family Growth (NSFG) and other sources to calculate rates of pregnancy in the United States for 2008 and 2011, including rates based on pregnancy intentions and outcome. About 45% of pregnancies in 2011 were unintended, compared with 51% in 2008. The rate in 2011 represents the lowest rate of unintended pregnancy in more than 3 decades.
Rates reduced in many population subgroupsThe percentage of unintended pregnancies ending in abortion remained stable at 40% in 2008 and 42% in 2011. The largest changes in rate of unintended pregnancy from 2008 to 2011 occurred in women aged 15 to 17 years (−44%), women cohabiting (−29%), those with incomes at 100% to 199% of the federal poverty level (−32%), women who were not high school graduates (−28%), and Hispanic women (−26%). Other population subgroups also showed improvement but to a lesser extent than those described here.
The study authors concede that some of the reduction in unintended pregnancies can be attributed to the economic recession that occurred during the study time frame, when many women intentionally reduced or delayed childbearing. The more likely explanation, they point out, is the increased use of long-acting reversible contraception (LARC), particularly the intrauterine device (IUD). Notably, among US women using contraception, the rates of IUD use increased from 4% in 2007 to 12% in 2012.2
Nevertheless, while the unintended pregnancy rate has shown improvement, the rate in the United States still lags considerably behind that of other industrialized nations. In Western Europe, for example, the unintended pregnancy rate was 34% in 2012.3
What this evidence means for practiceAs the study data suggest, use of contraceptive methods that do not rely on a frequent activity by the user, such as LARC methods, is associated with improved adherence. Consequently, all LARC methods, including the IUD, are associated with a pregnancy rate of about 1% or less; this rate is equal to or better than the rates seen with many forms of tubal sterilization, and it is superior to that seen with other methods, such as oral contraceptives, which have a contraceptive failure rate of about 9%.4
Finally, to correct disparities noted in this study that may be related particularly to access to contraceptive methods, the Affordable Care Act contains provisions that should lead to greater availability of contraceptive services in the United States.
—Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Expert Commentary The unintended pregnancy rate has hovered around 50% for at least 20 years despite vigorous efforts to educate both the public and health care providers on the importance of using effective contraceptive methods. During that time, new contraceptives were developed and older methods were improved to reduce risk and adverse effects. Despite these efforts, however, an estimated 48% of all unintended pregnancies in the United States occurred among contraceptive users.1 Results of the study by Finer and Zolna on the recent decline in unintended pregnancies suggest there may be some light at the end of the tunnel.
Details of the studyThe study authors used data from the National Survey of Family Growth (NSFG) and other sources to calculate rates of pregnancy in the United States for 2008 and 2011, including rates based on pregnancy intentions and outcome. About 45% of pregnancies in 2011 were unintended, compared with 51% in 2008. The rate in 2011 represents the lowest rate of unintended pregnancy in more than 3 decades.
Rates reduced in many population subgroupsThe percentage of unintended pregnancies ending in abortion remained stable at 40% in 2008 and 42% in 2011. The largest changes in rate of unintended pregnancy from 2008 to 2011 occurred in women aged 15 to 17 years (−44%), women cohabiting (−29%), those with incomes at 100% to 199% of the federal poverty level (−32%), women who were not high school graduates (−28%), and Hispanic women (−26%). Other population subgroups also showed improvement but to a lesser extent than those described here.
The study authors concede that some of the reduction in unintended pregnancies can be attributed to the economic recession that occurred during the study time frame, when many women intentionally reduced or delayed childbearing. The more likely explanation, they point out, is the increased use of long-acting reversible contraception (LARC), particularly the intrauterine device (IUD). Notably, among US women using contraception, the rates of IUD use increased from 4% in 2007 to 12% in 2012.2
Nevertheless, while the unintended pregnancy rate has shown improvement, the rate in the United States still lags considerably behind that of other industrialized nations. In Western Europe, for example, the unintended pregnancy rate was 34% in 2012.3
What this evidence means for practiceAs the study data suggest, use of contraceptive methods that do not rely on a frequent activity by the user, such as LARC methods, is associated with improved adherence. Consequently, all LARC methods, including the IUD, are associated with a pregnancy rate of about 1% or less; this rate is equal to or better than the rates seen with many forms of tubal sterilization, and it is superior to that seen with other methods, such as oral contraceptives, which have a contraceptive failure rate of about 9%.4
Finally, to correct disparities noted in this study that may be related particularly to access to contraceptive methods, the Affordable Care Act contains provisions that should lead to greater availability of contraceptive services in the United States.
—Ronald T. Burkman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38(2):90–96.
- Use of highly effective contraceptives in the US continues to rise, with likely implications for declines in unintended pregnancy and abortion. New York: Guttmacher Institute, 2014. http://www.guttmacher.org/media/inthenews/2014/12/12/index.html. Published December 12, 2014. Accessed April 21, 2016.
- Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann. 2014;45(3):301–314.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154-161.
- Finer LB, Henshaw SK. Disparities in rates of unintended pregnancy in the United States, 1994 and 2001. Perspect Sex Reprod Health. 2006;38(2):90–96.
- Use of highly effective contraceptives in the US continues to rise, with likely implications for declines in unintended pregnancy and abortion. New York: Guttmacher Institute, 2014. http://www.guttmacher.org/media/inthenews/2014/12/12/index.html. Published December 12, 2014. Accessed April 21, 2016.
- Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann. 2014;45(3):301–314.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154-161.