User login
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
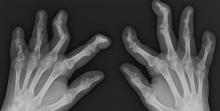
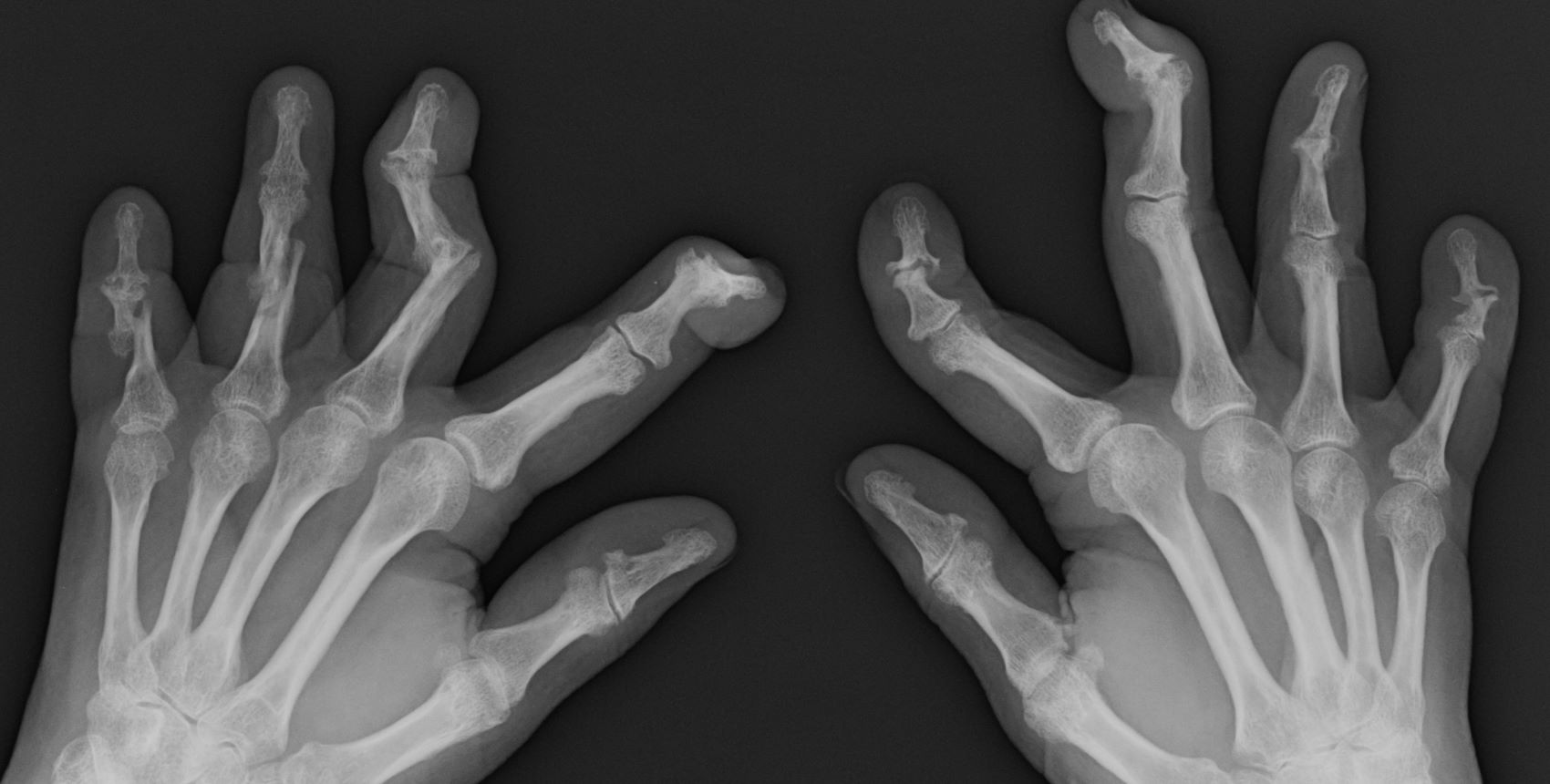
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Psoriatic arthritis (PsA) is an immune-mediated arthritis that is almost always associated with plaque psoriasis. PsA is diagnosed in about 20% of patients with plaque psoriasis, and, in most patients, a diagnosis of plaque psoriasis precedes PsA development. However, in this patient, PsA appears to have become symptomatic at about the same time as the skin symptoms behind his ears, which is consistent with studies showing that PsA co-occurs with plaque psoriasis in 15% of patients and precedes plaque psoriasis diagnosis in 17% of patients. Environmental risk factors for PsA in genetically susceptible patients with plaque psoriasis include joint trauma, streptococcal infection, and certain antibiotics. Smoking appears to have a protective effect in PsA. PsA is more highly associated with severe plaque psoriasis than with mild plaque psoriasis.
PsA has a heterogeneous presentation, which may challenge diagnosis. It is classified by the degree of joint involvement as oligoarticular (four or fewer joints) or polyarticular (five or more joints). Radiographically, there are five main types of PsA, one of which is predominant involvement of the distal interphalangeal joint (DIP) joints — the form seen in this patient. The other four types of PsA are symmetrical peripheral polyarthritis, asymmetrical mono- or oligoarthritis, axial spondyloarthropathy, and arthritis mutilans. DIP joint involvement with proliferative bone changes suggests a diagnosis of PsA over rheumatoid arthritis.
PsA is diagnosed using radiography, skin biopsy of affected skin areas, and complete blood and metabolic assessments. With advanced PsA, radiographs typically reveal bone destruction and disease-related bone formation (juxta-articular bone formation), resulting in erosions, joint destruction, and joint-space narrowing. Juxta-articular bone formation (presenting poorly defined ossification adjacent to the joint margin) is often the earliest radiographic clue before erosions may occur. Enthesitis in multiple entheses is typical of PsA vs osteoarthritis or mechanical injury. There are no specific tests to confirm PsA. Patients with PsA usually are rheumatoid factor negative. Inflammatory biomarkers, such as C-reactive protein and erythrocyte sedimentation rate, are elevated in about 40% of patients with PsA.
In addition to plaques, extra-articular manifestations of PsA may include nail changes and chronic bilateral ocular disease. PsA is an articular manifestation of psoriasis but is still a systemic disease with deleterious effects on cardiometabolic factors; increased mortality with myocardial infarction; and possible involvement of other immune-mediated diseases, such as inflammatory bowel disease, that share a common pathway of tumor necrosis factor (TNF) alpha overexpression.
Early treatment with disease-modifying drugs plus nonpharmacologic interventions is crucial to minimizing disability progression and optimizing patients' quality of life. Nonpharmacologic interventions include physical and occupational therapy and exercise. Symptomatic therapies (nonsteroidal anti-inflammatory drugs or steroids) can be used to relieve mild symptoms. Assessment for cardiometabolic, renal, and other systemic impacts of PsA is essential.
Biologic therapies are key to preventing disease progression. The most broadly targeted are the TNF-alpha inhibitors, which are commonly recommended as first-line therapy in moderate to severe PsA or in patients with radiographic damage. The interleukin (IL)-17 inhibitors ixekizumab and secukinumab and the IL-12/23 inhibitor ustekinumab have targets downstream of TNF-alpha. Treatment options also include oral small molecule inhibitors of phosphodiesterase 4 (apremilast) or Janus kinase (tofacitinib, upadacitinib). Recognition of the role of IL-23 as a key cytokine in PsA development through promotion of Th17 differentiation has led to availability of drugs specifically targeting IL-23(p19) (guselkumab, tildrakizumab, and risankizumab). All have been studied, but only guselkumab and risankizumab currently are approved for treatment of PsA. Each class of drugs has specific benefits and risks, which should be discussed with patients before treatment initiation. Patient comorbidities should also be considered in choosing treatment.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 58-year-old man presents with pain in fingers of several months' duration, which is moderately relieved with over-the-counter naproxen. He is concerned about "crooked" fingers and is worried that his work will be affected. He is overweight (BMI, 28.8), hypertensive, hypercholesterolemic, and a nonsmoker. The patient also reports a 6-month history of itchy "scalp" behind the ears, not relieved with dandruff shampoos. Physical exam reveals advanced deformity in index and middle fingers of both hands and no evident deformity in the wrists or metacarpophalangeal joints. Nails are pitted and discolored. Scalp behind ears shows well-demarcated, scaly patches. Lab work, radiography, and biopsy of the retroauricular area are ordered.