User login
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
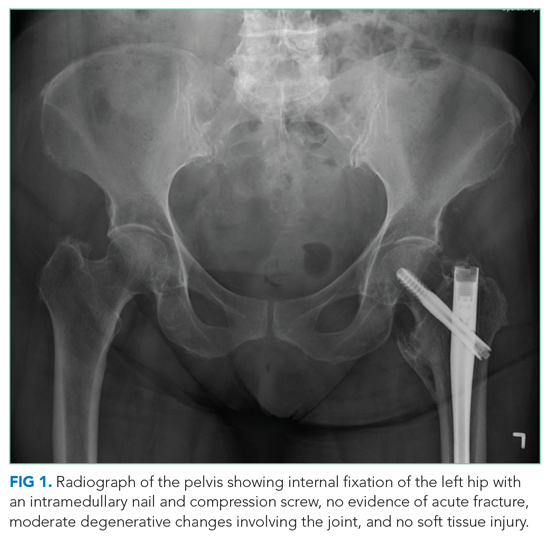
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.
The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
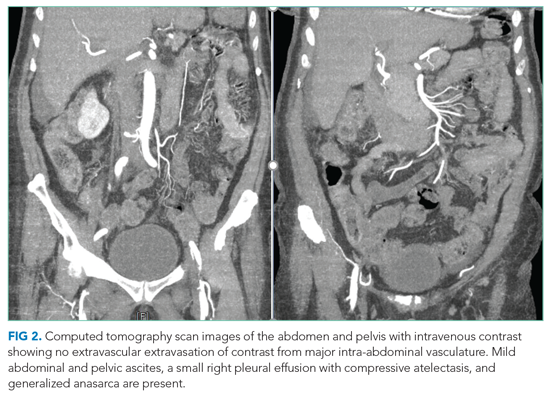
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170
The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
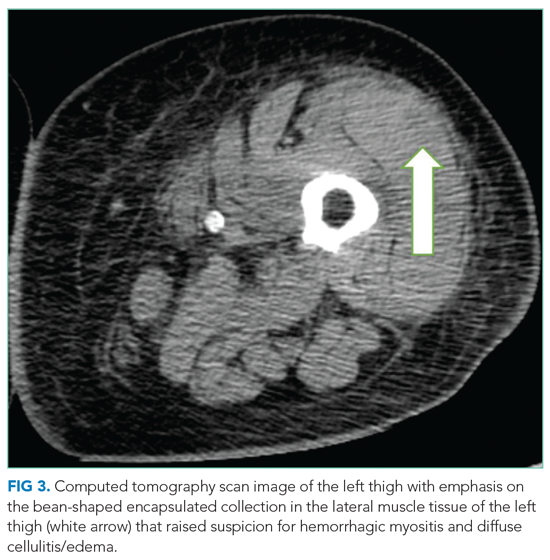
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.
Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
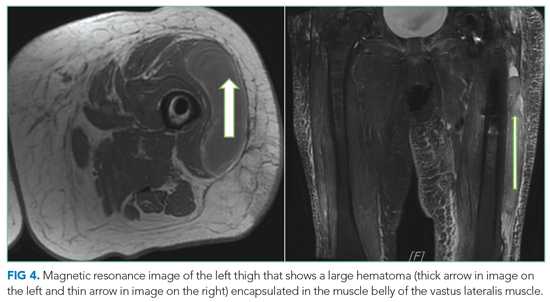
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.
DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.

The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170

The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.

Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.

DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
This icon represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
An 81-year-old woman with a remote history of left proximal femoral fracture (status post–open reduction and internal fixation) acutely developed severe pain in her left lateral thigh while at her home. A few days prior to her left thigh pain, the patient had routine blood work done. Her lab results (prior to the onset of her symptoms) revealed that her hemoglobin decreased from 10 g/dL, noted 9 months earlier, to 6.6 g/dL. Her primary care physician, who was planning to see the patient for her next regularly scheduled follow-up, was made aware of the patient’s decline in hemoglobin prior to the planned visit. The primary care physician called the patient to inform her about her concerning lab findings and coincidentally became aware of the acute, new-onset left thigh pain. The primary care physician requested that the patient be taken by her daughter to the emergency department (ED) for further evaluation.
The acute decrease in hemoglobin carries a broad differential and may or may not be related to the subsequent development of thigh pain. The presentation of an acute onset of pain in the thigh within the context of this patient’s age and gender suggests a femur fracture; this can be osteoporosis-related or a pathologic fracture associated with malignancy. Several malignancies are plausible, including multiple myeloma (given the anemia) or breast cancer. The proximal part of long bones is the most common site of pathologic fractures, and the femur accounts for half of these cases.
In the ED, she denied any recent trauma, hemoptysis, recent dark or bloody stools, vaginal bleeding, abdominal pain, or history of gastric ulcers. She had not experienced any similar episodes of thigh pain in the past. She had a history of atrial fibrillation, hypertension, diabetes mellitus type 2 with diabetic retinopathy and peripheral neuropathy, osteoporosis, nonalcoholic fatty liver disease (NAFLD), and internal hemorrhoids. Her medications included apixaban, metoprolol succinate, metformin, losartan, sitagliptin, calcium, vitamin D, alendronate, and fish oil. She had mild tenderness to palpation of her thigh, but her exam was otherwise normal. Radiography of the left hip and pelvis showed no acute fracture (Figure 1). An upper and lower endoscopy 3 years prior to her presentation revealed internal hemorrhoids.

The patient is taking apixaban, a direct factor Xa inhibitor. The absence of other obvious sources of bleeding suggests that the cause of anemia and pain is most likely bleeding into the anterior thigh compartment, exacerbated by the underlying anticoagulation. Since there was no trauma preceding this episode, the differential diagnosis must be expanded to include other, less common sources of bleeding, including a vascular anomaly such as a pseudoaneurysm or arteriovenous malformation. While the radiographs were normal, a CT scan or MRI may allow for identification of a fracture, other bone lesion, and/or hematoma.
A complete blood count revealed a hemoglobin of 6.6 g/dL (normal, 11.5-14.1 g/dL) with a mean corpuscular volume of 62 fL (normal, 79-96 fL). A CT scan of the abdomen and pelvis with intravenous contrast (Figure 2) was obtained to evaluate for intra-abdominal hemorrhage and retroperitoneal hematoma; it showed mild abdominal and pelvic ascites, a small right pleural effusion with compressive atelectasis, and generalized anasarca, but no evidence of bleeding. She was administered 2 units of packed red blood cells. Apixaban was held and 40 mg intravenous pantoprazole twice daily was started. Her iron level was 12 µg/dL (normal, 50-170

The studies reveal microcytic anemia associated with iron deficiency, as demonstrated by an elevated TIBC and very low ferritin. She also has a low-normal vitamin B12 level, which can contribute to poor red blood cell production; assessing methylmalonic acid levels would help to confirm whether true vitamin B12 deficiency is present. Anasarca can be secondary to severe hypoalbuminemia due to either protein-losing processes (eg, nephrotic syndrome, protein-losing enteropathy) or cirrhosis with poor synthetic function (given her history of NAFLD); it can also be secondary to severe heart failure or end-stage renal disease. The CT scan with contrast ruled out inferior vena cava thrombosis as a cause of ascites and did not reveal an obvious intra-abdominal malignancy as the cause of her anemia. Intestinal edema associated with anasarca can contribute to malabsorption (eg, iron, vitamin B12). The lack of abnormalities with respect to the liver and kidneys makes anasarca secondary to hepatic and renal dysfunction less likely.
The iron deficiency anemia prompted further evaluation for a gastrointestinal source of bleeding. Esophagogastroduodenoscopy showed a single, clean, 3-cm healing ulcer in the antrum, mild gastritis, and a superficial erosion in the duodenal bulb, all of which were biopsied. Because of inadequate bowel preparation, most of the colon was not optimally visualized and evaluation revealed only internal and external hemorrhoids in the rectum. On hospital day 4, the patient’s hemoglobin decreased from 9.6 g/dL to 7.3 g/dL. She had dark stools and also complained of left hip pain and swelling of the left knee and thigh. Another unit of packed red blood cells was given. A push enteroscopy and repeat colonoscopy showed no bleeding from the antral ulcer or from the internal and external hemorrhoids.
The patient has an antral ulcer, which most likely was a source of chronic blood loss and the underlying iron deficiency. However, the presence of healing and lack of signs of bleeding as demonstrated by negative repeat endoscopic studies suggests that the ulcer has little active contribution to the current anemia episode. A capsule enteroscopy could be performed, but most likely would be low yield. The presence of left thigh and knee swelling associated with worsening thigh pain raises the suspicion of a hemorrhagic process within the anterior thigh compartment, perhaps associated with an occult femoral fracture. A CT scan of the thigh would be valuable to identify a fracture or bone lesion as well as the presence of a hematoma. There are no widely available tests to evaluate apixaban anticoagulant activity; the anticoagulant effect would be expected to dissipate completely 36 to 48 hours after discontinuation in the context of normal renal function.
On hospital day 5, the patient’s left leg pain worsened. A physical exam showed edema of her entire left lower extremity with ecchymoses in several areas, including the left knee and lower thigh. A duplex ultrasound was negative for deep venous thrombosis, and X-ray of her left knee was normal. Her repeat hemoglobin was 8.8 g/dL. A repeat CT scan of the abdomen and pelvis again revealed no retroperitoneal bleeding. Orthopedic surgery was consulted on hospital day 7 and had low suspicion for compartment syndrome. Physical exam at that time showed mild swelling of the left thigh, moderate swelling of the left knee joint and pretibial area, two areas of ecchymosis on the left thigh, and diffuse ecchymosis of the left knee; all compartments were soft, and motor and nervous system functions were normal. A CT scan of the left lower extremity (Figure 3) revealed findings suspicious for hemorrhagic myositis with diffuse left thigh swelling with skin thickening and edema. There was no evidence of abscess, gas collection, foreign body, acute osteomyelitis, fracture, or dislocation. The patient’s hemoglobin remained stable.

Myopathies can be hereditary or acquired. Hereditary myopathies include congenital myopathies, muscular dystrophies, channelopathies, primary metabolic myopathies, and mitochondrial myopathies. Acquired myopathies include infectious myopathies, inflammatory myopathies, endocrine myopathies, secondary metabolic myopathies, and drug-induced and toxic myopathies. The findings of hemorrhagic myositis and skin edema are very intriguing, especially given their localized features. An overt femur fracture was previously ruled out, and an anterior thigh compartment syndrome was considered less likely after orthopedic surgery consultation. There is no description of the patient taking medications that could cause myopathy (such as statins), and there are also no clinical features suggestive of primary inflammatory myopathy, such as dermatomyositis. Increased suspicion of a focal inflammatory process such as localized scleroderma with regional inflammatory myopathy or another focal myopathy must be considered. The next diagnostic steps would include measuring the creatine kinase level, as well as obtaining an MRI of the leg to assess the nature and extent of the myopathy.
Multidisciplinary involvement, including hematology, rheumatology, and surgery, aided in narrowing the differential diagnosis. On hospital day 10, an MRI of the left thigh was performed for suspicion of diabetic myonecrosis (Figure 4). The MRI revealed a 10 cm × 3.6 cm × 22 cm intramuscular hematoma in the belly of the vastus lateralis muscle with associated soft tissue swelling, overlying subcutaneous edema, and skin thickening that was suggestive of hemorrhagic diabetic myonecrosis with some atypical features. A rheumatology consult was requested to evaluate for possible vasculitis in the left lower extremity, and vasculitis was not considered likely. The diagnosis of diabetic myonecrosis with associated intramuscular hemorrhage secondary to apixaban was made after careful reconsideration of the clinical presentation, imaging and laboratory data, and overall picture.

DISCUSSION
A clear schema for approaching the patient with acute, nontraumatic myopathies is important in avoiding diagnostic error. One effective schema is to divide myopathy into infectious and noninfectious categories. Causes of infectious myopathy include bacterial infections (eg, pyomyositis), inflammatory damage to muscles associated with viruses (eg, influenza), as well as rarer causes. Bacterial processes tend to be relatively focal and affect a specific muscle group or anatomic compartment, while viral causes are often more diffuse and occur in the context of a systemic viral syndrome. Bacterial causes range in severity, and life-threatening conditions, such as necrotizing soft tissue infection, must be considered. In this case, bacterial causes were less likely given the patient’s lack of fever, leukocytosis, and systemic signs of infection.1,2 However, these findings are not uniformly sensitive, and clinicians should not exclude potentially life- or limb-threatening infections without thorough evaluation. For example, pyomyositis may present without fever in the subacute stage, without leukocytosis if the patient is immunocompromised, and without overt pus if the infection is not in the suppurative stage.3 Viral causes were made less likely in this patient given the lack of a current or recent systemic viral syndrome.
Once infectious etiologies are deemed unlikely, noninfectious etiologies for nontraumatic myopathies should be considered. Some causes of noninfectious myopathy present with the muscle symptoms as a predominant feature, while others present in the context of another illness such as cancer, metabolic disorders, or other systemic disorders. Many noninfectious causes of myopathy associated with systemic illnesses have diffuse or relatively diffuse symptoms, with pain and/or weakness in multiple muscle groups, often in a bilateral distribution. Such examples include dermatomyositis and polymyositis as well as myositis associated with other rheumatologic conditions. Nontraumatic rhabdomyolysis is diffuse and can occur in association with medications and/or genetic conditions.
Angervall and Stener4 first described diabetic myonecrosis in 1965 as
The mainstay of the diagnosis of diabetic myonecrosis is a thorough history and physical examination and imaging. Routine laboratory evaluation is relatively unhelpful in diagnosing diabetic myonecrosis, but appropriate imaging can provide valuable supportive information. A CT scan and MRI are both helpful in excluding other etiologies as well as identifying features consistent with diabetic myonecrosis. A CT scan can help exclude a localized abscess, tumor, or bone destruction and, in affected patients, may show increased subcutaneous attenuation and increased muscle size with decreased attenuation secondary to edema.2 However, a CT scan may not give optimal assessment of muscle tissue, and therefore MRI may need to be considered. MRI T2 images have a sensitivity nearing 90% for detecting myonecrosis.1 The diagnostic value of MRI often obviates the need for muscle biopsy.
Spontaneous infarction with hemorrhagic features seen on imaging can be explained by a combination of damage from atherosclerotic or microvascular disease, an activated coagulation cascade, and an impaired fibrinolytic pathway.8 Hemorrhagic conversion in diabetic myonecrosis appears to be uncommon.9 In our case, we suspect that it developed because of the combination of bleeding risk from apixaban and the underlying mechanisms of diabetic myonecrosis.
The treatment of diabetic myonecrosis is mainly supportive, with an emphasis on rest, nonsteroidal anti-inflammatory agents, antiplatelet agents, and strict glycemic control.10 There is conflicting information about the value of limb immobilization versus active physical therapy as appropriate treatment modalities.11 Patients who present with clinical concern for sepsis or compartment syndrome require consultation for consideration of acute surgical intervention.10 The short-term prognosis is promising with supportive therapy, but the condition may recur.12 The recurrence rate may be as high as 40%, with a 2-year mortality of 10%.13 Ultimately, patients need to be followed closely in the outpatient setting to reduce the risk of recurrence.
In this patient, the simultaneous occurrence of focal pain and acute blood loss anemia led to a diagnosis of diabetic myonecrosis that was complicated by hemorrhagic conversion, a truly painful coincidence. The patient underwent a thorough evaluation for acute blood loss before the diagnosis was ultimately made. Clinicians should consider diabetic myonecrosis in patients with diabetes who present with acute muscle pain but no evidence of infection.
Key Teaching Points
- Diabetic myonecrosis is an underrecognized entity and should be included in the differential diagnosis for patients with diabetes who present with acute muscle pain and no history of trauma.
- Imaging with CT and/or MRI of the affected region is the mainstay of diagnosis; treatment is predicated on severity and risk factors and can range from conservative therapy to operative intervention.
- Although the prognosis is good in these patients, careful outpatient follow-up is necessary to oversee their recovery to help reduce the risk of recurrence.
Acknowledgment
The authors thank Dr Vijay Singh for his radiology input on image selection for this manuscript.
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
1. Ivanov M, Asif B, Jaffe R. Don’t move a muscle: a case of diabetic myonecrosis. Am J Med. 2018;131(11):e445-e448. https://doi.org/10.1016/j.amjmed.2018.07.002
2. Morcuende JA, Dobbs MB, Crawford H, Buckwalter JA. Diabetic muscle infarction. Iowa Orthop J. 2000;20:65-74.
3. Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21(3):473-494. https://doi.org/10.1128/CMR.00001-08
4. Angervall L, Stener B. Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1(1):39-42. https://doi.org/10.1007/BF01338714
5. Lawrence L, Tovar-Camargo O, Lansang MC, Makin V. Diabetic myonecrosis: a diagnostic and treatment challenge in longstanding diabetes. Case Rep Endocrinol. 2018;2018:1723695. https://doi.org/10.1155/2018/1723695
6. Horton WB, Taylor JS, Ragland TJ, Subauste AR. Diabetic muscle infarction: a systematic review. BMJ Open Diabetes Res Care. 2015;3(1):e000082. https://doi.org/10.1136/bmjdrc-2015-000082
7. Bhasin R, Ghobrial I. Diabetic myonecrosis: a diagnostic challenge in patients with long-standing diabetes. J Community Hosp Intern Med Perspect. 2013;3(1). https://doi.org/10.3402/jchimp.v3i1.20494
8. Bjornskov EK, Carry MR, Katz FH, Lefkowitz J, Ringel SP. Diabetic muscle infarction: a new perspective on pathogenesis and management. Neuromuscul Disord. 1995;5(1):39-45.
9. Cunningham J, Sharma R, Kirzner A, et al. Acute myonecrosis on MRI: etiologies in an oncological cohort and assessment of interobserver variability. Skeletal Radiol. 2016;45(8):1069-1078. https://doi.org/10.1007/s00256-016-2389-4
10. Khanna HK, Stevens AC. Diabetic myonecrosis: a rare complication of diabetes mellitus mimicking deep vein thrombosis. Am J Case Rep. 2017;18:38-41. https://doi.org/10.12659/ajcr.900903
11. Bunch TJ, Birskovich LM, Eiken PW. Diabetic myonecrosis in a previously healthy woman and review of a 25-year Mayo Clinic experience. Endocr Pract. 2002;8(5):343-346. https://doi.org/10.4158/EP.8.5.343
12. Mukherjee S, Aggarwal A, Rastogi A, et al. Spontaneous diabetic myonecrosis: report of four cases from a tertiary care institute. Endocrinol Diabetes Metab Case Rep. 2015;2015:150003. https://doi.org/10.1530/EDM-15-0003
13. Kapur S, McKendry RJ. Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11(1):8-12. https://doi.org/10.1097/01.rhu.0000152142.33358.f1
© 2021 Society of Hospital Medicine