User login
This is the second installment of a five-part monthly series that will discuss the pathologic, genomic, and clinical factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians1, a journal of the American Cancer Society, will also review exciting and innovative interventions to close this survival gap. This month’s column reviews tumor biology and genomics—the first element in the perfect storm.
Hormone receptor status and human epidermal growth factor receptor 2 (HER2)/neu
Breast cancer is not a single disease, and breast cancer subtype classifications are used in the clinical setting to determine prognosis and guide management. These different molecular subtypes are based on tumor markers, which include the presence or absence of three proteins: estrogen receptor (ER), progesterone receptor (PR), and HER2/neu. Hormone receptor status is a main factor in planning breast cancer treatment. Hormone receptor–positive breast tumors benefit from hormone therapies, such as selective ER modulators (for example, tamoxifen) and aromatase inhibitors (for example, anastrozole). Thus, these tumors have a more favorable disease-specific survival than do hormone receptor–negative tumors.2
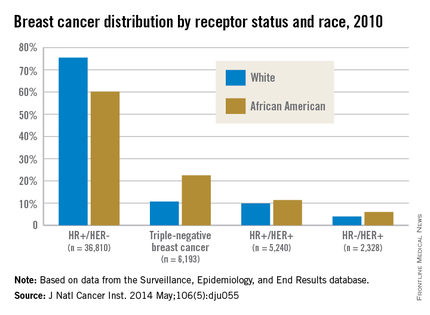
African American women are more likely to present with hormone receptor-negative tumors. In an analysis of the California Cancer Registry, which has collected patient ER and PR status since 1990, whites had a higher proportion of tumors that were ER positive or PR positive (or both) and HER2 negative (72% vs. 53%).3 DeSantis et al.4 reported similar results for this tumor type, with 76% of non-Hispanic whites having hormone receptor–positive, HER2-negative tumors vs. 62% of non-Hispanic blacks. Even with stratification by tumor stage, African Americans continue to have a significantly higher proportion of hormone receptor–negative tumors than do whites for localized and advanced disease.5
Although hormone receptor status varies significantly by race, HER2 status does not show the same divergence. HER2 overexpression is present in approximately 20% of invasive breast cancers. HER2-positive, hormone receptor–negative tumors demonstrate more-aggressive features and worse breast cancer–specific survival than do hormone receptor–positive and HER2-negative tumors,2 although survival has vastly improved with new HER2-targeted therapies such as trastuzumab and pertuzumab. Unlike hormone receptor status, there was no association between race and HER2-positive/ER-negative tumors in the Carolina Breast Cancer Study.2
Triple-negative breast cancer (TNBC)
TNBC is the subtype of breast cancer with the worst prognosis. TNBC gets its name because its tumor cells lack the markers for ER, PR, and HER2 overexpression. Thus, TNBC tumors are estrogen receptor negative (ER), progesterone receptor negative (PR), and HER2/neu negative (HER2). While other subtypes of breast cancer have benefited from drug development regarding hormonal therapies and HER2-targeted treatments, TNBC has not experienced the same pharmacologic breakthroughs.
As such, even after analyses control for the stage at diagnosis, women with this subtype have poorer survival than those with other breast cancers.6 African American women have a higher incidence of TNBC than white women.7 DeSantis et al.4 reported that 22% of breast cancers were triple negative in non-Hispanic black patients vs. only 11% in non-Hispanic white patients. The Carolina Breast Cancer Study found that 26% of African American women had TNBC, whereas 16% of non-African American women did.2 This subtype was most common among younger, premenopausal African American women (39% of diagnosed cancer subtypes). When TNBC patients were excluded from analysis in the Carolina Breast Cancer Study, breast cancer–specific survival remained significantly worse among premenopausal African American women, suggesting that although tumor biology in part explains the poor outcomes, the survival disparity story is more complex.
Germline mutations: BRCA1 and BRCA2 Mutations
In addition to tumor biology, cancer genomics has become increasingly important in determining cancer prognosis and guiding treatment. Approximately 5%-10% of breast cancer cases present in individuals with inherited mutations in autosomal dominant, highly penetrant breast cancer susceptibility genes.8 Accounting for 80%-90% of families containing multiple cases of breast and ovarian cancer, BRCA1 and BRCA2 germline mutations are the most common of the breast cancer susceptibility genes.9 These patients often are younger and have a higher-grade tumor that is hormone receptor negative, which also often matches the profile of the African American breast cancer patient.10
Despite similarities between BRCA1-associated breast cancers and breast cancer in African Americans, genetic abnormalities in African American breast cancer patients remain underresearched. Nanda et al.11 found that BRCA1 and BRCA2 mutations occur with appreciable frequency in high-risk families of African ancestry, with 28% testing positive for a deleterious mutation in one of these genes. This frequency was at a lower rate than that found in non-Hispanic, non-Jewish whites, who had a rate of 46%, because African Americans had a higher rate of polymorphisms or variants of unknown significance (44% vs. 12%). This large percentage of variants of unknown significance indicates that more analysis is needed to understand the clinical implications of these genetic variations. In another study from the Northern California site of the Breast Cancer Family Registry, the BRCA1 mutation prevalence was 16.7% in African American cases diagnosed under the age of 35 years vs. 7.2% in non-Hispanic, non-Ashkenazi Jewish whites in the same age category.12 High frequencies of mutations in BRCA1 and BRCA2 have also been reported in breast cancer patients of African ancestry from Nigeria and the Bahamas.13, 14
These results in African American patients highlight the need for further study of breast cancer genomics in minority populations. However, Armstrong et al.15 illuminated the existence of racial/ethnic disparities in patterns of referral to cancer risk clinics. In their study, African American women with a family history of breast or ovarian cancer were significantly less likely to undergo genetic counseling for BRCA1/2 testing than were white women with this family history. The results of this study were noteworthy for the magnitude of the disparity, with white women having almost five times greater odds of undergoing this clinically important evaluation. More than two decades after BRCA1 and BRCA2 genes were identified, larger studies are still needed in diverse populations to derive true estimates of the burden of mutations in both genes in underserved and understudied populations.
Although these differences in tumor biology and genomics tell part of the mortality disparity story, there is more to be told. In a study of African American and white patients in South Carolina, Adams et al.16 determined survival rates by ethnicity that were adjusted for disease stage and other prognostic characteristics. After they controlled for age, stage, ER, and HER2 expression as well as insurance status, African American women still had a twofold excess risk of death from breast cancer. Thus, in addition to differences in the innate characteristics of the breast tumors, racial differences in patterns of care for women with breast cancer must be considered in unraveling the observed disparity in mortality. The third installment of this series will discuss the second element of the perfect storm – patterns of care.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221-238.
2. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492-502.
3. Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12(6):R99.
4. DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2015 Oct 29. doi: 10.3322/caac.21320. [Epub ahead of print]
5. Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169(10):1251-9.
6. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721-8.
7. Ray M, Polite BN. Triple-negative breast cancers: a view from 10,000 feet. Cancer J. 2010;16(1):17-22.
8. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77(11):2318-24.
9. Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993;52(4):678-701.
10. Polite BN, Olopade OI. Breast cancer and race: a rising tide does not lift all boats equally. Perspect Biol Med. 2005;48(1 Suppl):S166-75.
11. Nanda R, Schumm LP, Cummings S, et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA. 2005;294(15):1925-33.
12. John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298(24):2869-76.
13. Fackenthal JD, Zhang J, Zhang B, et al. High prevalence of BRCA1 and BRCA2 mutations in unselected Nigerian breast cancer patients. Int J Cancer. 2012;131(5):1114-23.
14. Donenberg T, Lunn J, Curling D, et al. A high prevalence of BRCA1 mutations among breast cancer patients from the Bahamas. Breast Cancer Res Treat. 2011;125(2):591-6.
15. Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729-36.
16. Adams SA, Butler WM, Fulton J, et al. Racial disparities in breast cancer mortality in a multi-ethnic cohort in the Southeast. Cancer. 2012;118(10):2693-9.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
This is the second installment of a five-part monthly series that will discuss the pathologic, genomic, and clinical factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians1, a journal of the American Cancer Society, will also review exciting and innovative interventions to close this survival gap. This month’s column reviews tumor biology and genomics—the first element in the perfect storm.
Hormone receptor status and human epidermal growth factor receptor 2 (HER2)/neu
Breast cancer is not a single disease, and breast cancer subtype classifications are used in the clinical setting to determine prognosis and guide management. These different molecular subtypes are based on tumor markers, which include the presence or absence of three proteins: estrogen receptor (ER), progesterone receptor (PR), and HER2/neu. Hormone receptor status is a main factor in planning breast cancer treatment. Hormone receptor–positive breast tumors benefit from hormone therapies, such as selective ER modulators (for example, tamoxifen) and aromatase inhibitors (for example, anastrozole). Thus, these tumors have a more favorable disease-specific survival than do hormone receptor–negative tumors.2

African American women are more likely to present with hormone receptor-negative tumors. In an analysis of the California Cancer Registry, which has collected patient ER and PR status since 1990, whites had a higher proportion of tumors that were ER positive or PR positive (or both) and HER2 negative (72% vs. 53%).3 DeSantis et al.4 reported similar results for this tumor type, with 76% of non-Hispanic whites having hormone receptor–positive, HER2-negative tumors vs. 62% of non-Hispanic blacks. Even with stratification by tumor stage, African Americans continue to have a significantly higher proportion of hormone receptor–negative tumors than do whites for localized and advanced disease.5
Although hormone receptor status varies significantly by race, HER2 status does not show the same divergence. HER2 overexpression is present in approximately 20% of invasive breast cancers. HER2-positive, hormone receptor–negative tumors demonstrate more-aggressive features and worse breast cancer–specific survival than do hormone receptor–positive and HER2-negative tumors,2 although survival has vastly improved with new HER2-targeted therapies such as trastuzumab and pertuzumab. Unlike hormone receptor status, there was no association between race and HER2-positive/ER-negative tumors in the Carolina Breast Cancer Study.2
Triple-negative breast cancer (TNBC)
TNBC is the subtype of breast cancer with the worst prognosis. TNBC gets its name because its tumor cells lack the markers for ER, PR, and HER2 overexpression. Thus, TNBC tumors are estrogen receptor negative (ER), progesterone receptor negative (PR), and HER2/neu negative (HER2). While other subtypes of breast cancer have benefited from drug development regarding hormonal therapies and HER2-targeted treatments, TNBC has not experienced the same pharmacologic breakthroughs.
As such, even after analyses control for the stage at diagnosis, women with this subtype have poorer survival than those with other breast cancers.6 African American women have a higher incidence of TNBC than white women.7 DeSantis et al.4 reported that 22% of breast cancers were triple negative in non-Hispanic black patients vs. only 11% in non-Hispanic white patients. The Carolina Breast Cancer Study found that 26% of African American women had TNBC, whereas 16% of non-African American women did.2 This subtype was most common among younger, premenopausal African American women (39% of diagnosed cancer subtypes). When TNBC patients were excluded from analysis in the Carolina Breast Cancer Study, breast cancer–specific survival remained significantly worse among premenopausal African American women, suggesting that although tumor biology in part explains the poor outcomes, the survival disparity story is more complex.
Germline mutations: BRCA1 and BRCA2 Mutations
In addition to tumor biology, cancer genomics has become increasingly important in determining cancer prognosis and guiding treatment. Approximately 5%-10% of breast cancer cases present in individuals with inherited mutations in autosomal dominant, highly penetrant breast cancer susceptibility genes.8 Accounting for 80%-90% of families containing multiple cases of breast and ovarian cancer, BRCA1 and BRCA2 germline mutations are the most common of the breast cancer susceptibility genes.9 These patients often are younger and have a higher-grade tumor that is hormone receptor negative, which also often matches the profile of the African American breast cancer patient.10
Despite similarities between BRCA1-associated breast cancers and breast cancer in African Americans, genetic abnormalities in African American breast cancer patients remain underresearched. Nanda et al.11 found that BRCA1 and BRCA2 mutations occur with appreciable frequency in high-risk families of African ancestry, with 28% testing positive for a deleterious mutation in one of these genes. This frequency was at a lower rate than that found in non-Hispanic, non-Jewish whites, who had a rate of 46%, because African Americans had a higher rate of polymorphisms or variants of unknown significance (44% vs. 12%). This large percentage of variants of unknown significance indicates that more analysis is needed to understand the clinical implications of these genetic variations. In another study from the Northern California site of the Breast Cancer Family Registry, the BRCA1 mutation prevalence was 16.7% in African American cases diagnosed under the age of 35 years vs. 7.2% in non-Hispanic, non-Ashkenazi Jewish whites in the same age category.12 High frequencies of mutations in BRCA1 and BRCA2 have also been reported in breast cancer patients of African ancestry from Nigeria and the Bahamas.13, 14
These results in African American patients highlight the need for further study of breast cancer genomics in minority populations. However, Armstrong et al.15 illuminated the existence of racial/ethnic disparities in patterns of referral to cancer risk clinics. In their study, African American women with a family history of breast or ovarian cancer were significantly less likely to undergo genetic counseling for BRCA1/2 testing than were white women with this family history. The results of this study were noteworthy for the magnitude of the disparity, with white women having almost five times greater odds of undergoing this clinically important evaluation. More than two decades after BRCA1 and BRCA2 genes were identified, larger studies are still needed in diverse populations to derive true estimates of the burden of mutations in both genes in underserved and understudied populations.
Although these differences in tumor biology and genomics tell part of the mortality disparity story, there is more to be told. In a study of African American and white patients in South Carolina, Adams et al.16 determined survival rates by ethnicity that were adjusted for disease stage and other prognostic characteristics. After they controlled for age, stage, ER, and HER2 expression as well as insurance status, African American women still had a twofold excess risk of death from breast cancer. Thus, in addition to differences in the innate characteristics of the breast tumors, racial differences in patterns of care for women with breast cancer must be considered in unraveling the observed disparity in mortality. The third installment of this series will discuss the second element of the perfect storm – patterns of care.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221-238.
2. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492-502.
3. Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12(6):R99.
4. DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2015 Oct 29. doi: 10.3322/caac.21320. [Epub ahead of print]
5. Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169(10):1251-9.
6. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721-8.
7. Ray M, Polite BN. Triple-negative breast cancers: a view from 10,000 feet. Cancer J. 2010;16(1):17-22.
8. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77(11):2318-24.
9. Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993;52(4):678-701.
10. Polite BN, Olopade OI. Breast cancer and race: a rising tide does not lift all boats equally. Perspect Biol Med. 2005;48(1 Suppl):S166-75.
11. Nanda R, Schumm LP, Cummings S, et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA. 2005;294(15):1925-33.
12. John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298(24):2869-76.
13. Fackenthal JD, Zhang J, Zhang B, et al. High prevalence of BRCA1 and BRCA2 mutations in unselected Nigerian breast cancer patients. Int J Cancer. 2012;131(5):1114-23.
14. Donenberg T, Lunn J, Curling D, et al. A high prevalence of BRCA1 mutations among breast cancer patients from the Bahamas. Breast Cancer Res Treat. 2011;125(2):591-6.
15. Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729-36.
16. Adams SA, Butler WM, Fulton J, et al. Racial disparities in breast cancer mortality in a multi-ethnic cohort in the Southeast. Cancer. 2012;118(10):2693-9.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
This is the second installment of a five-part monthly series that will discuss the pathologic, genomic, and clinical factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians1, a journal of the American Cancer Society, will also review exciting and innovative interventions to close this survival gap. This month’s column reviews tumor biology and genomics—the first element in the perfect storm.
Hormone receptor status and human epidermal growth factor receptor 2 (HER2)/neu
Breast cancer is not a single disease, and breast cancer subtype classifications are used in the clinical setting to determine prognosis and guide management. These different molecular subtypes are based on tumor markers, which include the presence or absence of three proteins: estrogen receptor (ER), progesterone receptor (PR), and HER2/neu. Hormone receptor status is a main factor in planning breast cancer treatment. Hormone receptor–positive breast tumors benefit from hormone therapies, such as selective ER modulators (for example, tamoxifen) and aromatase inhibitors (for example, anastrozole). Thus, these tumors have a more favorable disease-specific survival than do hormone receptor–negative tumors.2

African American women are more likely to present with hormone receptor-negative tumors. In an analysis of the California Cancer Registry, which has collected patient ER and PR status since 1990, whites had a higher proportion of tumors that were ER positive or PR positive (or both) and HER2 negative (72% vs. 53%).3 DeSantis et al.4 reported similar results for this tumor type, with 76% of non-Hispanic whites having hormone receptor–positive, HER2-negative tumors vs. 62% of non-Hispanic blacks. Even with stratification by tumor stage, African Americans continue to have a significantly higher proportion of hormone receptor–negative tumors than do whites for localized and advanced disease.5
Although hormone receptor status varies significantly by race, HER2 status does not show the same divergence. HER2 overexpression is present in approximately 20% of invasive breast cancers. HER2-positive, hormone receptor–negative tumors demonstrate more-aggressive features and worse breast cancer–specific survival than do hormone receptor–positive and HER2-negative tumors,2 although survival has vastly improved with new HER2-targeted therapies such as trastuzumab and pertuzumab. Unlike hormone receptor status, there was no association between race and HER2-positive/ER-negative tumors in the Carolina Breast Cancer Study.2
Triple-negative breast cancer (TNBC)
TNBC is the subtype of breast cancer with the worst prognosis. TNBC gets its name because its tumor cells lack the markers for ER, PR, and HER2 overexpression. Thus, TNBC tumors are estrogen receptor negative (ER), progesterone receptor negative (PR), and HER2/neu negative (HER2). While other subtypes of breast cancer have benefited from drug development regarding hormonal therapies and HER2-targeted treatments, TNBC has not experienced the same pharmacologic breakthroughs.
As such, even after analyses control for the stage at diagnosis, women with this subtype have poorer survival than those with other breast cancers.6 African American women have a higher incidence of TNBC than white women.7 DeSantis et al.4 reported that 22% of breast cancers were triple negative in non-Hispanic black patients vs. only 11% in non-Hispanic white patients. The Carolina Breast Cancer Study found that 26% of African American women had TNBC, whereas 16% of non-African American women did.2 This subtype was most common among younger, premenopausal African American women (39% of diagnosed cancer subtypes). When TNBC patients were excluded from analysis in the Carolina Breast Cancer Study, breast cancer–specific survival remained significantly worse among premenopausal African American women, suggesting that although tumor biology in part explains the poor outcomes, the survival disparity story is more complex.
Germline mutations: BRCA1 and BRCA2 Mutations
In addition to tumor biology, cancer genomics has become increasingly important in determining cancer prognosis and guiding treatment. Approximately 5%-10% of breast cancer cases present in individuals with inherited mutations in autosomal dominant, highly penetrant breast cancer susceptibility genes.8 Accounting for 80%-90% of families containing multiple cases of breast and ovarian cancer, BRCA1 and BRCA2 germline mutations are the most common of the breast cancer susceptibility genes.9 These patients often are younger and have a higher-grade tumor that is hormone receptor negative, which also often matches the profile of the African American breast cancer patient.10
Despite similarities between BRCA1-associated breast cancers and breast cancer in African Americans, genetic abnormalities in African American breast cancer patients remain underresearched. Nanda et al.11 found that BRCA1 and BRCA2 mutations occur with appreciable frequency in high-risk families of African ancestry, with 28% testing positive for a deleterious mutation in one of these genes. This frequency was at a lower rate than that found in non-Hispanic, non-Jewish whites, who had a rate of 46%, because African Americans had a higher rate of polymorphisms or variants of unknown significance (44% vs. 12%). This large percentage of variants of unknown significance indicates that more analysis is needed to understand the clinical implications of these genetic variations. In another study from the Northern California site of the Breast Cancer Family Registry, the BRCA1 mutation prevalence was 16.7% in African American cases diagnosed under the age of 35 years vs. 7.2% in non-Hispanic, non-Ashkenazi Jewish whites in the same age category.12 High frequencies of mutations in BRCA1 and BRCA2 have also been reported in breast cancer patients of African ancestry from Nigeria and the Bahamas.13, 14
These results in African American patients highlight the need for further study of breast cancer genomics in minority populations. However, Armstrong et al.15 illuminated the existence of racial/ethnic disparities in patterns of referral to cancer risk clinics. In their study, African American women with a family history of breast or ovarian cancer were significantly less likely to undergo genetic counseling for BRCA1/2 testing than were white women with this family history. The results of this study were noteworthy for the magnitude of the disparity, with white women having almost five times greater odds of undergoing this clinically important evaluation. More than two decades after BRCA1 and BRCA2 genes were identified, larger studies are still needed in diverse populations to derive true estimates of the burden of mutations in both genes in underserved and understudied populations.
Although these differences in tumor biology and genomics tell part of the mortality disparity story, there is more to be told. In a study of African American and white patients in South Carolina, Adams et al.16 determined survival rates by ethnicity that were adjusted for disease stage and other prognostic characteristics. After they controlled for age, stage, ER, and HER2 expression as well as insurance status, African American women still had a twofold excess risk of death from breast cancer. Thus, in addition to differences in the innate characteristics of the breast tumors, racial differences in patterns of care for women with breast cancer must be considered in unraveling the observed disparity in mortality. The third installment of this series will discuss the second element of the perfect storm – patterns of care.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221-238.
2. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492-502.
3. Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12(6):R99.
4. DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2015 Oct 29. doi: 10.3322/caac.21320. [Epub ahead of print]
5. Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169(10):1251-9.
6. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721-8.
7. Ray M, Polite BN. Triple-negative breast cancers: a view from 10,000 feet. Cancer J. 2010;16(1):17-22.
8. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77(11):2318-24.
9. Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993;52(4):678-701.
10. Polite BN, Olopade OI. Breast cancer and race: a rising tide does not lift all boats equally. Perspect Biol Med. 2005;48(1 Suppl):S166-75.
11. Nanda R, Schumm LP, Cummings S, et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA. 2005;294(15):1925-33.
12. John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298(24):2869-76.
13. Fackenthal JD, Zhang J, Zhang B, et al. High prevalence of BRCA1 and BRCA2 mutations in unselected Nigerian breast cancer patients. Int J Cancer. 2012;131(5):1114-23.
14. Donenberg T, Lunn J, Curling D, et al. A high prevalence of BRCA1 mutations among breast cancer patients from the Bahamas. Breast Cancer Res Treat. 2011;125(2):591-6.
15. Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729-36.
16. Adams SA, Butler WM, Fulton J, et al. Racial disparities in breast cancer mortality in a multi-ethnic cohort in the Southeast. Cancer. 2012;118(10):2693-9.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.



