User login
The Perfect Storm: Delivery system reform and precision medicine for all
Editor’s Note: This is the fifth and final installment of a five-part monthly series that will discuss the biologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series was adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society.
As discussed in the last installment of this series, multifaceted interventions that address all stakeholders are needed to close the racial disparity gap in breast cancer. The Patient Protection and Affordable Care Act (PPACA) emphasizes delivery system reform with a focus on the triple aim of better health, better health care, and lower costs.2 One component of this reform will be accountable care organizations (ACOs). ACOs potentially could assist in closing the racial mortality gap, because provider groups will take responsibility for improving the health of a defined population and will be held accountable for the quality of care delivered.
In the ACO model, an integrated network of providers, led by primary care practitioners, will evaluate the necessity, quality, value, and accountable delivery of specialty diagnostic and therapeutic procedures, including cancer care.3 ACOs will also collect extensive patient data through the meaningful use of medical records.3 These detailed data can then be used to shape locoregional protocols for clinical decision making in oncology and evaluate physician performance. Intermountain Healthcare is an example of an organization that has had success with instituting these clinical protocols to highlight best practices and improve the quality of care.4 In breast cancer, oncologists will need to be prepared to develop and follow protocols tailored for their communities, which will lead to standardized, improved care for minority populations.
The oncology medical home is one example of an ACO delivery system reform that has the potential to reduce the racial mortality gap. The oncology medical home replaces episodic care with long-term coordinated care and replaces the fee-for-service model with a performance and outcomes-based system. A key trait of the oncology medical home is care that is continuously improved by measurement against quality standards.5 The model oncology home accomplishes this by incorporating software to extract clinical data as well as provider compliance with locoregional guidelines to give oncologists feedback regarding the quality of care that they are providing.6 Through this system reform, oncologists will be held accountable for the care they deliver, and it is hoped that this will eliminate the delays, misuse, and underuse of treatment. This could be especially important for optimizing use of hormone therapy for estrogen receptor-positive breast cancer. Trial oncology medical homes in North Carolina and Michigan have yielded promising results regarding improved care (fewer emergency department visits and inpatient admissions) and high adherence to national and practice-selected guidelines.7,8
PPACA also increases funding for community health centers and provides grants to support community health workers; this highlights again the importance of place in racial health care disparities.9 Encouraging collaboration between community health centers and academic institutions, this funding could build bridges between minority communities and high-quality health care institutions while also improving patient communication and education.9 As this series has discussed, a failure to provide culturally appropriate clinical information can lead to issues with follow-up and adjuvant treatment compliance and further widen the breast cancer racial mortality gap.
Delivery system reform has the potential to help close the disparity gap by improving the quality of care delivered to minority breast cancer patients. As Chin et al10 describe in their analysis of effective strategies for reducing health disparities, successful interventions are “culturally tailored to meet patients’ needs, employ multidisciplinary teams of care providers, and target multiple leverage points along a patient’s pathway of care.” ACOs have the financial incentive to meet these features of a successful intervention and improve quality across the continuum of breast cancer care. In addition, the PPACA “incentivized experimentation” with health care delivery, such as the oncology medical home and novel telemedicine interventions, to provide higher quality care outside of hospital settings, which could impact the disparity gap.11
In the face of this new era of organizational structures focused on coordinated, population-based care, oncology providers put themselves at financial risk if they do not position themselves for policy and reimbursement changes that reduce disparities.10 However, ongoing research will be needed to ensure that as these changes are implemented, the racial mortality gap in breast cancer decreases, and that no vulnerable patient populations are left out.
Precision medicine for all
In addition, as discussed earlier in this series, there are differences in the tumor biology and genomics of breast cancer in African American patients. Beyond quality interventions, initiatives to reduce the mortality gap should focus on precision medicine for all. These initiatives should allow researchers to better understand biologic and genomic differences among breast cancer patients and tailor treatments accordingly. The PPACA has taken steps in this direction and is the first federal law to require group health plans and state-licensed health insurance companies to cover standard-of-care costs associated with participation in clinical trials as well as genetic testing for prevention.12
The clinical trial regulations also expressly require plans to show that administrative burdens are not used to create barriers to cancer care for anyone who might benefit from participation in a clinical trial.9 The overarching goal of this push to eliminate financial and administrative barriers is to increase the enrollment of minority patients, especially those who do not live close to academic medical centers. In his April 2016 address at the annual meeting of the American Association for Cancer Research, Vice President Joseph Biden identified increased clinical trial participation as a key component of the administration’s cancer “moonshot” as well. Community medical oncologists will be called upon to facilitate and encourage clinical trial participation by their minority patients and should be supported in this endeavor by academic medical centers. With greater minority patient involvement, however, there also should be further research on how trial designs can better lead to clinically significant findings for minority patients. As Polite et al13 argue, at a bare minimum, basic sociodemographic and detailed comorbidity information should be prospectively collected and integrated with tumor and host biology data to better examine racial differences in cancer outcomes.
Initiatives also are needed to address the gap in referrals to cancer risk clinics so that more data are available on African American genetic variants, allowing the creation of more robust risk assessment models. Risk assessment relies on predictive statistical models to estimate an individual’s risk of developing cancer, and without accurate estimates of mutation prevalence in minority subgroups, these models’ reliability is compromised.14 As shown in a recent study at the University of Chicago’s Comprehensive Cancer Risk and Prevention Clinic using targeted genomic capture and next-generation sequencing, nearly one in four African American breast cancer patients referred to the clinic had inherited at least one damaging mutation that increased their risk for the most aggressive type of breast cancer.15
To identify damaging mutations only after a diagnosis of incurable breast cancer is a failure of prevention. As has been documented in Ashkenazi Jewish populations, there is evidence of high rates of inherited mutations in genes that increase the risk for aggressive breast cancers in populations of African ancestry. This is a fertile area for further research to better understand how these mutations affect the clinical course of breast cancer, what targeted interventions will increase the proportion of breast cancer diagnosed at stage 1, and what molecularly targeted treatments will produce a response in these tumors. Churpek et al15 also demonstrated the need for continued technological innovation to reduce the disparity gap, because next-generation sequencing is a faster and more cost-efficient way to evaluate multiple variants in many genes. This approach is particularly valuable for African Americans, who tend to have greater genetic diversity.16 The current administration is also heralding this approach to cancer care. In his 2015 State of the Union address, President Obama announced a precision medicine initiative, including a request for $70 million for the National Cancer Institute to investigate genes that may contribute to the risk of developing cancer.17 African American women should no longer be left behind in the push for personalized medicine that caters to a patient’s tumor biology and genetic profile. As Subbiah and Kurzrock state, universal genomic testing is not necessarily cost prohibitive, as the cost to obtain a “complete diagnosis and to select appropriate therapy may be miniscule compared with the money wasted on ill-chosen therapies.”18
In conclusion, there is an opportunity in the current climate of health care reform ushered in by the Affordable Care Act to address many of the discussed elements leading to the persistent racial mortality gap in breast cancer. We have argued that two substantial factors lead to this eroding gap. One is differences in tumor biology and genomics, and the second is a quality difference in patterns of care. In describing the perfect storm, Sebastian Junger19 wrote of the collision of two forces – a hurricane’s warm-air, low-pressure system and an anticyclone’s cool-air, high-pressure system – that combined to create a more powerful and devastating meteorological force. Similarly, we argue that it is the collision of these two factors – tumor biology and genomics with patterns of care – that leads to the breast cancer mortality gap. The delays, misuse, and underuse of treatment that we have underscored are of increased significance when patients present with more aggressive forms of breast cancer. Interventions to close this gap will take leaders at the patient, provider, payer, and community levels to drive system change.
1. Daly B, Olopade OI: A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 Apr;65:221-38.
2. Fox J: Lessons from an oncology medical home collaborative. Am J Manag Care. 2013; 19:SP5-9.
3. Mehta AJ, Macklis RM: Overview of accountable care organizations for oncology specialists. J Oncol Pract. 2013 Jul; 9(4):216-21.
4. Daly B, Mort EA: A decade after to Err is Human: what should health care leaders be doing? Physician Exec. 2014 May-Jun; 40(3):50-2, 54.
5. Dangi-Garimella S: Oncology medical home: improved quality and cost of care. Am J Manag Care. 2014 Sep.
6. McAneny BL: The future of oncology? COME HOME, the oncology medical home. Am J Manag Care. 2013 Feb.
7. Goyal RK, Wheeler SB, Kohler RE, et al: Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014 Jul-Aug;75(4):231-8.
8. Kuntz G, Tozer JM, Snegosky J, et al: Michigan Oncology Medical Home Demonstration Project: first-year results. J Oncol Pract. 2014 Sep. 10:294-7.
9. Moy B, Polite BN, Halpern MT, et al: American Society of Clinical Oncology policy statement: opportunities in the patient protection and affordable care act to reduce cancer care disparities. J Clin Oncol. 2011 Oct;29(28):3816-24.
10. Chin MH, Clarke AR, Nocon RS, et al: A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med. 2012 Aug; 27(8):992-1000.
11. Emanuel EJ: How Well Is the Affordable Care Act Doing?: Reasons for Optimism. JAMA 315:1331-2, 2016.
12. Zhang SQ, Polite BN: Achieving a deeper understanding of the implemented provisions of the Affordable Care Act. Am Soc Clin Oncol Educ Book. 2014:e472-7.
13. Polite BN, Sylvester BE, Olopade OI: Race and subset analyses in clinical trials: time to get serious about data integration. J Natl Cancer Inst. 2011 Oct. 103(20):1486-8.
14. Hall MJ, Olopade OI: Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol. 2006 May; 24(14):2197-203.
15. Churpek JE, Walsh T, Zheng Y, et al: Inherited predisposition to breast cancer among African American women. Breast Cancer Res Treat. 2015 Jan;149(1):31-9.
16. Easton J: Genetic mutations more common among African American women with breast cancer: early testing could protect patients and their relatives. news.uchicago.edu/article/2013/06/03/genetic-mutations-more-common-among-african-american-women-breast-cancer. Published June 3, 2013. Accessed April 20, 2016.
17. Pear R: U.S. to collect genetic data to hone care. New York Times. January 30, 2015.
18. Subbiah V, Kurzrock R: Universal Genomic Testing Needed to Win the War Against Cancer: Genomics IS the Diagnosis. JAMA Oncol, 2016.
19. Junger S: The Perfect Storm. New York, NY: WW Norton and Co; 2009.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
Editor’s Note: This is the fifth and final installment of a five-part monthly series that will discuss the biologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series was adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society.
As discussed in the last installment of this series, multifaceted interventions that address all stakeholders are needed to close the racial disparity gap in breast cancer. The Patient Protection and Affordable Care Act (PPACA) emphasizes delivery system reform with a focus on the triple aim of better health, better health care, and lower costs.2 One component of this reform will be accountable care organizations (ACOs). ACOs potentially could assist in closing the racial mortality gap, because provider groups will take responsibility for improving the health of a defined population and will be held accountable for the quality of care delivered.
In the ACO model, an integrated network of providers, led by primary care practitioners, will evaluate the necessity, quality, value, and accountable delivery of specialty diagnostic and therapeutic procedures, including cancer care.3 ACOs will also collect extensive patient data through the meaningful use of medical records.3 These detailed data can then be used to shape locoregional protocols for clinical decision making in oncology and evaluate physician performance. Intermountain Healthcare is an example of an organization that has had success with instituting these clinical protocols to highlight best practices and improve the quality of care.4 In breast cancer, oncologists will need to be prepared to develop and follow protocols tailored for their communities, which will lead to standardized, improved care for minority populations.
The oncology medical home is one example of an ACO delivery system reform that has the potential to reduce the racial mortality gap. The oncology medical home replaces episodic care with long-term coordinated care and replaces the fee-for-service model with a performance and outcomes-based system. A key trait of the oncology medical home is care that is continuously improved by measurement against quality standards.5 The model oncology home accomplishes this by incorporating software to extract clinical data as well as provider compliance with locoregional guidelines to give oncologists feedback regarding the quality of care that they are providing.6 Through this system reform, oncologists will be held accountable for the care they deliver, and it is hoped that this will eliminate the delays, misuse, and underuse of treatment. This could be especially important for optimizing use of hormone therapy for estrogen receptor-positive breast cancer. Trial oncology medical homes in North Carolina and Michigan have yielded promising results regarding improved care (fewer emergency department visits and inpatient admissions) and high adherence to national and practice-selected guidelines.7,8
PPACA also increases funding for community health centers and provides grants to support community health workers; this highlights again the importance of place in racial health care disparities.9 Encouraging collaboration between community health centers and academic institutions, this funding could build bridges between minority communities and high-quality health care institutions while also improving patient communication and education.9 As this series has discussed, a failure to provide culturally appropriate clinical information can lead to issues with follow-up and adjuvant treatment compliance and further widen the breast cancer racial mortality gap.
Delivery system reform has the potential to help close the disparity gap by improving the quality of care delivered to minority breast cancer patients. As Chin et al10 describe in their analysis of effective strategies for reducing health disparities, successful interventions are “culturally tailored to meet patients’ needs, employ multidisciplinary teams of care providers, and target multiple leverage points along a patient’s pathway of care.” ACOs have the financial incentive to meet these features of a successful intervention and improve quality across the continuum of breast cancer care. In addition, the PPACA “incentivized experimentation” with health care delivery, such as the oncology medical home and novel telemedicine interventions, to provide higher quality care outside of hospital settings, which could impact the disparity gap.11
In the face of this new era of organizational structures focused on coordinated, population-based care, oncology providers put themselves at financial risk if they do not position themselves for policy and reimbursement changes that reduce disparities.10 However, ongoing research will be needed to ensure that as these changes are implemented, the racial mortality gap in breast cancer decreases, and that no vulnerable patient populations are left out.
Precision medicine for all
In addition, as discussed earlier in this series, there are differences in the tumor biology and genomics of breast cancer in African American patients. Beyond quality interventions, initiatives to reduce the mortality gap should focus on precision medicine for all. These initiatives should allow researchers to better understand biologic and genomic differences among breast cancer patients and tailor treatments accordingly. The PPACA has taken steps in this direction and is the first federal law to require group health plans and state-licensed health insurance companies to cover standard-of-care costs associated with participation in clinical trials as well as genetic testing for prevention.12
The clinical trial regulations also expressly require plans to show that administrative burdens are not used to create barriers to cancer care for anyone who might benefit from participation in a clinical trial.9 The overarching goal of this push to eliminate financial and administrative barriers is to increase the enrollment of minority patients, especially those who do not live close to academic medical centers. In his April 2016 address at the annual meeting of the American Association for Cancer Research, Vice President Joseph Biden identified increased clinical trial participation as a key component of the administration’s cancer “moonshot” as well. Community medical oncologists will be called upon to facilitate and encourage clinical trial participation by their minority patients and should be supported in this endeavor by academic medical centers. With greater minority patient involvement, however, there also should be further research on how trial designs can better lead to clinically significant findings for minority patients. As Polite et al13 argue, at a bare minimum, basic sociodemographic and detailed comorbidity information should be prospectively collected and integrated with tumor and host biology data to better examine racial differences in cancer outcomes.
Initiatives also are needed to address the gap in referrals to cancer risk clinics so that more data are available on African American genetic variants, allowing the creation of more robust risk assessment models. Risk assessment relies on predictive statistical models to estimate an individual’s risk of developing cancer, and without accurate estimates of mutation prevalence in minority subgroups, these models’ reliability is compromised.14 As shown in a recent study at the University of Chicago’s Comprehensive Cancer Risk and Prevention Clinic using targeted genomic capture and next-generation sequencing, nearly one in four African American breast cancer patients referred to the clinic had inherited at least one damaging mutation that increased their risk for the most aggressive type of breast cancer.15
To identify damaging mutations only after a diagnosis of incurable breast cancer is a failure of prevention. As has been documented in Ashkenazi Jewish populations, there is evidence of high rates of inherited mutations in genes that increase the risk for aggressive breast cancers in populations of African ancestry. This is a fertile area for further research to better understand how these mutations affect the clinical course of breast cancer, what targeted interventions will increase the proportion of breast cancer diagnosed at stage 1, and what molecularly targeted treatments will produce a response in these tumors. Churpek et al15 also demonstrated the need for continued technological innovation to reduce the disparity gap, because next-generation sequencing is a faster and more cost-efficient way to evaluate multiple variants in many genes. This approach is particularly valuable for African Americans, who tend to have greater genetic diversity.16 The current administration is also heralding this approach to cancer care. In his 2015 State of the Union address, President Obama announced a precision medicine initiative, including a request for $70 million for the National Cancer Institute to investigate genes that may contribute to the risk of developing cancer.17 African American women should no longer be left behind in the push for personalized medicine that caters to a patient’s tumor biology and genetic profile. As Subbiah and Kurzrock state, universal genomic testing is not necessarily cost prohibitive, as the cost to obtain a “complete diagnosis and to select appropriate therapy may be miniscule compared with the money wasted on ill-chosen therapies.”18
In conclusion, there is an opportunity in the current climate of health care reform ushered in by the Affordable Care Act to address many of the discussed elements leading to the persistent racial mortality gap in breast cancer. We have argued that two substantial factors lead to this eroding gap. One is differences in tumor biology and genomics, and the second is a quality difference in patterns of care. In describing the perfect storm, Sebastian Junger19 wrote of the collision of two forces – a hurricane’s warm-air, low-pressure system and an anticyclone’s cool-air, high-pressure system – that combined to create a more powerful and devastating meteorological force. Similarly, we argue that it is the collision of these two factors – tumor biology and genomics with patterns of care – that leads to the breast cancer mortality gap. The delays, misuse, and underuse of treatment that we have underscored are of increased significance when patients present with more aggressive forms of breast cancer. Interventions to close this gap will take leaders at the patient, provider, payer, and community levels to drive system change.
1. Daly B, Olopade OI: A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 Apr;65:221-38.
2. Fox J: Lessons from an oncology medical home collaborative. Am J Manag Care. 2013; 19:SP5-9.
3. Mehta AJ, Macklis RM: Overview of accountable care organizations for oncology specialists. J Oncol Pract. 2013 Jul; 9(4):216-21.
4. Daly B, Mort EA: A decade after to Err is Human: what should health care leaders be doing? Physician Exec. 2014 May-Jun; 40(3):50-2, 54.
5. Dangi-Garimella S: Oncology medical home: improved quality and cost of care. Am J Manag Care. 2014 Sep.
6. McAneny BL: The future of oncology? COME HOME, the oncology medical home. Am J Manag Care. 2013 Feb.
7. Goyal RK, Wheeler SB, Kohler RE, et al: Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014 Jul-Aug;75(4):231-8.
8. Kuntz G, Tozer JM, Snegosky J, et al: Michigan Oncology Medical Home Demonstration Project: first-year results. J Oncol Pract. 2014 Sep. 10:294-7.
9. Moy B, Polite BN, Halpern MT, et al: American Society of Clinical Oncology policy statement: opportunities in the patient protection and affordable care act to reduce cancer care disparities. J Clin Oncol. 2011 Oct;29(28):3816-24.
10. Chin MH, Clarke AR, Nocon RS, et al: A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med. 2012 Aug; 27(8):992-1000.
11. Emanuel EJ: How Well Is the Affordable Care Act Doing?: Reasons for Optimism. JAMA 315:1331-2, 2016.
12. Zhang SQ, Polite BN: Achieving a deeper understanding of the implemented provisions of the Affordable Care Act. Am Soc Clin Oncol Educ Book. 2014:e472-7.
13. Polite BN, Sylvester BE, Olopade OI: Race and subset analyses in clinical trials: time to get serious about data integration. J Natl Cancer Inst. 2011 Oct. 103(20):1486-8.
14. Hall MJ, Olopade OI: Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol. 2006 May; 24(14):2197-203.
15. Churpek JE, Walsh T, Zheng Y, et al: Inherited predisposition to breast cancer among African American women. Breast Cancer Res Treat. 2015 Jan;149(1):31-9.
16. Easton J: Genetic mutations more common among African American women with breast cancer: early testing could protect patients and their relatives. news.uchicago.edu/article/2013/06/03/genetic-mutations-more-common-among-african-american-women-breast-cancer. Published June 3, 2013. Accessed April 20, 2016.
17. Pear R: U.S. to collect genetic data to hone care. New York Times. January 30, 2015.
18. Subbiah V, Kurzrock R: Universal Genomic Testing Needed to Win the War Against Cancer: Genomics IS the Diagnosis. JAMA Oncol, 2016.
19. Junger S: The Perfect Storm. New York, NY: WW Norton and Co; 2009.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
Editor’s Note: This is the fifth and final installment of a five-part monthly series that will discuss the biologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series was adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society.
As discussed in the last installment of this series, multifaceted interventions that address all stakeholders are needed to close the racial disparity gap in breast cancer. The Patient Protection and Affordable Care Act (PPACA) emphasizes delivery system reform with a focus on the triple aim of better health, better health care, and lower costs.2 One component of this reform will be accountable care organizations (ACOs). ACOs potentially could assist in closing the racial mortality gap, because provider groups will take responsibility for improving the health of a defined population and will be held accountable for the quality of care delivered.
In the ACO model, an integrated network of providers, led by primary care practitioners, will evaluate the necessity, quality, value, and accountable delivery of specialty diagnostic and therapeutic procedures, including cancer care.3 ACOs will also collect extensive patient data through the meaningful use of medical records.3 These detailed data can then be used to shape locoregional protocols for clinical decision making in oncology and evaluate physician performance. Intermountain Healthcare is an example of an organization that has had success with instituting these clinical protocols to highlight best practices and improve the quality of care.4 In breast cancer, oncologists will need to be prepared to develop and follow protocols tailored for their communities, which will lead to standardized, improved care for minority populations.
The oncology medical home is one example of an ACO delivery system reform that has the potential to reduce the racial mortality gap. The oncology medical home replaces episodic care with long-term coordinated care and replaces the fee-for-service model with a performance and outcomes-based system. A key trait of the oncology medical home is care that is continuously improved by measurement against quality standards.5 The model oncology home accomplishes this by incorporating software to extract clinical data as well as provider compliance with locoregional guidelines to give oncologists feedback regarding the quality of care that they are providing.6 Through this system reform, oncologists will be held accountable for the care they deliver, and it is hoped that this will eliminate the delays, misuse, and underuse of treatment. This could be especially important for optimizing use of hormone therapy for estrogen receptor-positive breast cancer. Trial oncology medical homes in North Carolina and Michigan have yielded promising results regarding improved care (fewer emergency department visits and inpatient admissions) and high adherence to national and practice-selected guidelines.7,8
PPACA also increases funding for community health centers and provides grants to support community health workers; this highlights again the importance of place in racial health care disparities.9 Encouraging collaboration between community health centers and academic institutions, this funding could build bridges between minority communities and high-quality health care institutions while also improving patient communication and education.9 As this series has discussed, a failure to provide culturally appropriate clinical information can lead to issues with follow-up and adjuvant treatment compliance and further widen the breast cancer racial mortality gap.
Delivery system reform has the potential to help close the disparity gap by improving the quality of care delivered to minority breast cancer patients. As Chin et al10 describe in their analysis of effective strategies for reducing health disparities, successful interventions are “culturally tailored to meet patients’ needs, employ multidisciplinary teams of care providers, and target multiple leverage points along a patient’s pathway of care.” ACOs have the financial incentive to meet these features of a successful intervention and improve quality across the continuum of breast cancer care. In addition, the PPACA “incentivized experimentation” with health care delivery, such as the oncology medical home and novel telemedicine interventions, to provide higher quality care outside of hospital settings, which could impact the disparity gap.11
In the face of this new era of organizational structures focused on coordinated, population-based care, oncology providers put themselves at financial risk if they do not position themselves for policy and reimbursement changes that reduce disparities.10 However, ongoing research will be needed to ensure that as these changes are implemented, the racial mortality gap in breast cancer decreases, and that no vulnerable patient populations are left out.
Precision medicine for all
In addition, as discussed earlier in this series, there are differences in the tumor biology and genomics of breast cancer in African American patients. Beyond quality interventions, initiatives to reduce the mortality gap should focus on precision medicine for all. These initiatives should allow researchers to better understand biologic and genomic differences among breast cancer patients and tailor treatments accordingly. The PPACA has taken steps in this direction and is the first federal law to require group health plans and state-licensed health insurance companies to cover standard-of-care costs associated with participation in clinical trials as well as genetic testing for prevention.12
The clinical trial regulations also expressly require plans to show that administrative burdens are not used to create barriers to cancer care for anyone who might benefit from participation in a clinical trial.9 The overarching goal of this push to eliminate financial and administrative barriers is to increase the enrollment of minority patients, especially those who do not live close to academic medical centers. In his April 2016 address at the annual meeting of the American Association for Cancer Research, Vice President Joseph Biden identified increased clinical trial participation as a key component of the administration’s cancer “moonshot” as well. Community medical oncologists will be called upon to facilitate and encourage clinical trial participation by their minority patients and should be supported in this endeavor by academic medical centers. With greater minority patient involvement, however, there also should be further research on how trial designs can better lead to clinically significant findings for minority patients. As Polite et al13 argue, at a bare minimum, basic sociodemographic and detailed comorbidity information should be prospectively collected and integrated with tumor and host biology data to better examine racial differences in cancer outcomes.
Initiatives also are needed to address the gap in referrals to cancer risk clinics so that more data are available on African American genetic variants, allowing the creation of more robust risk assessment models. Risk assessment relies on predictive statistical models to estimate an individual’s risk of developing cancer, and without accurate estimates of mutation prevalence in minority subgroups, these models’ reliability is compromised.14 As shown in a recent study at the University of Chicago’s Comprehensive Cancer Risk and Prevention Clinic using targeted genomic capture and next-generation sequencing, nearly one in four African American breast cancer patients referred to the clinic had inherited at least one damaging mutation that increased their risk for the most aggressive type of breast cancer.15
To identify damaging mutations only after a diagnosis of incurable breast cancer is a failure of prevention. As has been documented in Ashkenazi Jewish populations, there is evidence of high rates of inherited mutations in genes that increase the risk for aggressive breast cancers in populations of African ancestry. This is a fertile area for further research to better understand how these mutations affect the clinical course of breast cancer, what targeted interventions will increase the proportion of breast cancer diagnosed at stage 1, and what molecularly targeted treatments will produce a response in these tumors. Churpek et al15 also demonstrated the need for continued technological innovation to reduce the disparity gap, because next-generation sequencing is a faster and more cost-efficient way to evaluate multiple variants in many genes. This approach is particularly valuable for African Americans, who tend to have greater genetic diversity.16 The current administration is also heralding this approach to cancer care. In his 2015 State of the Union address, President Obama announced a precision medicine initiative, including a request for $70 million for the National Cancer Institute to investigate genes that may contribute to the risk of developing cancer.17 African American women should no longer be left behind in the push for personalized medicine that caters to a patient’s tumor biology and genetic profile. As Subbiah and Kurzrock state, universal genomic testing is not necessarily cost prohibitive, as the cost to obtain a “complete diagnosis and to select appropriate therapy may be miniscule compared with the money wasted on ill-chosen therapies.”18
In conclusion, there is an opportunity in the current climate of health care reform ushered in by the Affordable Care Act to address many of the discussed elements leading to the persistent racial mortality gap in breast cancer. We have argued that two substantial factors lead to this eroding gap. One is differences in tumor biology and genomics, and the second is a quality difference in patterns of care. In describing the perfect storm, Sebastian Junger19 wrote of the collision of two forces – a hurricane’s warm-air, low-pressure system and an anticyclone’s cool-air, high-pressure system – that combined to create a more powerful and devastating meteorological force. Similarly, we argue that it is the collision of these two factors – tumor biology and genomics with patterns of care – that leads to the breast cancer mortality gap. The delays, misuse, and underuse of treatment that we have underscored are of increased significance when patients present with more aggressive forms of breast cancer. Interventions to close this gap will take leaders at the patient, provider, payer, and community levels to drive system change.
1. Daly B, Olopade OI: A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 Apr;65:221-38.
2. Fox J: Lessons from an oncology medical home collaborative. Am J Manag Care. 2013; 19:SP5-9.
3. Mehta AJ, Macklis RM: Overview of accountable care organizations for oncology specialists. J Oncol Pract. 2013 Jul; 9(4):216-21.
4. Daly B, Mort EA: A decade after to Err is Human: what should health care leaders be doing? Physician Exec. 2014 May-Jun; 40(3):50-2, 54.
5. Dangi-Garimella S: Oncology medical home: improved quality and cost of care. Am J Manag Care. 2014 Sep.
6. McAneny BL: The future of oncology? COME HOME, the oncology medical home. Am J Manag Care. 2013 Feb.
7. Goyal RK, Wheeler SB, Kohler RE, et al: Health care utilization from chemotherapy-related adverse events among low-income breast cancer patients: effect of enrollment in a medical home program. N C Med J. 2014 Jul-Aug;75(4):231-8.
8. Kuntz G, Tozer JM, Snegosky J, et al: Michigan Oncology Medical Home Demonstration Project: first-year results. J Oncol Pract. 2014 Sep. 10:294-7.
9. Moy B, Polite BN, Halpern MT, et al: American Society of Clinical Oncology policy statement: opportunities in the patient protection and affordable care act to reduce cancer care disparities. J Clin Oncol. 2011 Oct;29(28):3816-24.
10. Chin MH, Clarke AR, Nocon RS, et al: A roadmap and best practices for organizations to reduce racial and ethnic disparities in health care. J Gen Intern Med. 2012 Aug; 27(8):992-1000.
11. Emanuel EJ: How Well Is the Affordable Care Act Doing?: Reasons for Optimism. JAMA 315:1331-2, 2016.
12. Zhang SQ, Polite BN: Achieving a deeper understanding of the implemented provisions of the Affordable Care Act. Am Soc Clin Oncol Educ Book. 2014:e472-7.
13. Polite BN, Sylvester BE, Olopade OI: Race and subset analyses in clinical trials: time to get serious about data integration. J Natl Cancer Inst. 2011 Oct. 103(20):1486-8.
14. Hall MJ, Olopade OI: Disparities in genetic testing: thinking outside the BRCA box. J Clin Oncol. 2006 May; 24(14):2197-203.
15. Churpek JE, Walsh T, Zheng Y, et al: Inherited predisposition to breast cancer among African American women. Breast Cancer Res Treat. 2015 Jan;149(1):31-9.
16. Easton J: Genetic mutations more common among African American women with breast cancer: early testing could protect patients and their relatives. news.uchicago.edu/article/2013/06/03/genetic-mutations-more-common-among-african-american-women-breast-cancer. Published June 3, 2013. Accessed April 20, 2016.
17. Pear R: U.S. to collect genetic data to hone care. New York Times. January 30, 2015.
18. Subbiah V, Kurzrock R: Universal Genomic Testing Needed to Win the War Against Cancer: Genomics IS the Diagnosis. JAMA Oncol, 2016.
19. Junger S: The Perfect Storm. New York, NY: WW Norton and Co; 2009.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
A Perfect Storm: Interventions – Closing the survival gap
Editor’s Note: This is the fourth installment of a five-part monthly series that will discuss the biologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series was adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society. Eliminating racial disparities in cancer mortality through effective interventions has become an increasingly important imperative of federal, state, and community health care programs. This month’s column reviews interventions to close this survival gap.
Insurance
It has been posited that interventions aimed at providing insurance coverage to minority patients will be able to reduce racial health care disparities.2 Studies have indicated that women without insurance present with more-advanced disease,3,4 and are more likely to receive nonstandard treatment.5 However, outside of cancer care, a large study of Medicaid expansion in Oregon demonstrated that Medicaid coverage alone generated no significant improvement in measured physical health outcomes in the first 2 years.6 Thus, coverage alone does not ensure that patients will be able to navigate the health care system and that quality care will be provided.
In breast cancer, Hoffman et al.7 evaluated the effect of race and health insurance on diagnostic time, which was defined as the number of days from suspicious finding to diagnostic resolution (either no evidence of malignancy on diagnostic mammogram or definitive diagnosis by biopsy) in a large, urban setting. The authors’ hypothesis was that every insured patient would receive the same timely diagnosis as any other patient with equivalent insurance, regardless of race or ethnicity. The study found that non-Hispanic whites with government insurance had significantly shorter diagnostic times than did non-Hispanic African Americans with government insurance: The average diagnostic times were 12 and 39 days, respectively. In addition, privately insured non-Hispanic whites also had significantly shorter diagnostic times than did privately insured non-Hispanic African Americans (16 vs. 27 days). In addition, Short et al.8 demonstrated that when the health plan status was held constant in a retrospective study of 476 white patients and 99 African American patients with newly diagnosed breast cancer, African American patients had a higher mortality rate (8.1% vs. 3.6%) and were diagnosed at a later stage. Accordingly, interventions must go beyond just providing health insurance to minorities in order to have a significant impact on the mortality gap.
Patient education and physician communication
An underlying cause frequently cited for the delayed diagnosis and treatment of African American patients with breast cancer is a lack of patient education and physician communication. These elements are essential components of quality care. In a qualitative study of low-income, ethnically diverse women older than 40 years, Allen et al.9 identified salient themes differentiating women who received timely follow-up from those who did not. For the women who delayed follow-up, prominent themes were dissatisfaction with the communication of results, disrespect on the part of providers and clinical staff, logistical barriers to accessing services, anxiety and fear about a possible cancer diagnosis, and a lack of information about breast cancer screening and symptoms.
In another study, Masi and Gehlert10 employed focus group interviews of African American adults to characterize their perceptions of breast cancer treatment. The analysis revealed a core set of themes, including mistrust of the medical establishment and concern about the effect of racism on treatment quality; the researchers concluded that “in the eyes of many study participants, the issues of trust, race, and quality of care were closely intertwined.”10 Thus, this knot that is created by underlying issues of trust can be untied only by interventions that address improved physician communication and patient education.
Janz et al.11 examined racial differences in the adequacy of information and support for women with breast cancer. The researchers used survey data from a population of 1,766 women diagnosed with nonmetastatic breast cancer and reported to the Los Angeles County Surveillance, Epidemiology, and End Results (SEER) registry. The study found that across treatment- and survivorship-related issues, African American women desired more information than white women did. One of the explanations for the unmet information needs posited by the authors is a failure to provide culturally appropriate information related to health issues. This breakdown in patient education and communication was demonstrated by Hawley et al. to hold across providers and locations.12
Hawley et al.12 evaluated the association between minority patients’ knowledge of breast cancer treatment risks and benefits and provider characteristics and treatment locations. The provider characteristics included surgeon-level independent variables, such as breast cancer procedure volume and demographics (years in practice and sex). The treatment location variable was categorized into one of three groups: National Cancer Institute–designated cancer center, American College of Surgeons cancer program, or no specific cancer program. Provider characteristics and treatment locations are factors previously identified as being associated with high-quality care.
The study employed a multivariable regression to identify associations between patient, surgeon, and treatment-setting factors and accurate knowledge of the survival benefit and recurrence risk related to mastectomy and breast-conserving surgery with radiation. The authors found that minority women were significantly less likely to have adequate knowledge and more likely to be uncertain about recurrence risk than were white patients. In the multivariate logistic regression model, neither provider characteristics nor treatment setting attenuated observed racial disparities in knowledge. Quality health care depends on the ability to make an informed treatment decision. As the authors concluded, this study underscores the need for providers to communicate information effectively to all patients, and effective communication relies on the cultural competency of providers.13 Without effective, culturally competent communication, there are treatment delays and omissions that result in poor-quality cancer care. Currently, the research has established that these communication deficits are found across providers and treatment center types.
Patient Navigation
Patient navigation has been championed as a method of improving care in breast cancer by enhancing patient communication and education, and removing barriers to timely care. Patient navigation empowers patients to become knowledgeable about their own health and supports them through the course of care.14 Patient navigation programs have been developed to address the patient communication breakdowns and underuse and misuse of treatment among vulnerable populations, which were detailed earlier in this series and are thought to contribute to the racial mortality gap.15
A benefit of patient navigation has been suggested in studies evaluating the time to diagnosis and follow-up from an abnormal screening. Markossian et al.16 evaluated the efficacy of a Chicago-based cancer patient navigation program developed to reduce the time from abnormal screening to definitive diagnostic testing. The majority of patients in this study were Hispanic (66%) and African American (32%). Compared with controls without navigation, the breast navigation group had a shorter time to diagnostic resolution. Hoffman et al.17 evaluated patient navigation in the District of Columbia to determine its ability to reduce the breast cancer diagnostic time (number of days from abnormal screening to a definitive diagnosis). African American women comprised 48% of the study population. The investigators found that women in the navigation group reached their diagnostic resolution significantly faster than did other women. Among women with breast cancer, there was a nearly fourfold reduction in time to diagnostic resolution for women in the navigation group versus women without a navigator.
In a national multicenter study, Ko et al.14 were the first to evaluate whether patient navigation can improve the quality of breast cancer care. The authors hypothesized that breast cancer patients assigned a navigator would be more likely to receive recommended standard treatment than were those without a navigator. Three separate quality measures of breast cancer care, including initiation of antiestrogen therapy, radiation therapy, and chemotherapy, were evaluated. Study participants were racially and ethnically diverse, with a plurality being African American (37.5%). The study produced mixed results: Patients in the navigation group had a statistically higher likelihood of receiving antiestrogen therapy than were non–navigated controls, but navigation patients eligible for radiation therapy were no more likely to receive it than were controls. The initiation of chemotherapy could not be accurately assessed because of a limited sample size. The study concluded that navigation alone does not remove all of the barriers to quality care for breast cancer patients, and barriers are diverse and potentially specific to the modality of treatment.
A study by Tejeda et al.18 used a systematic framework to characterize barriers faced by minority patients with breast and cervical cancer. The investigators categorized barriers as intrapersonal (defined by characteristics of the individual, such as knowledge, belief, attitudes, and transportation and financial barriers), interpersonal (defined by processes that involve other people, such as social support systems, child care, and employment issues), or institutional (defined by characteristics and policies of organizations). The authors found that although navigators were able to easily resolve intrapersonal barriers, ongoing navigation was needed to address institutional barriers. Thus, patient navigation in a vacuum does not work, and it is only in examining the entire health care system that changes can be implemented to eliminate barriers to quality care and close the racial mortality chasm.
System Change
To this effect, Clarke et al.19 performed a review of the disparities intervention literature to understand which interventions are being evaluated to improve minority health. The authors found that the majority of such interventions are focused on changing the patient rather than the system that serves her, with the most common strategy being education and training (37% of strategies studied). Interventions aimed at health care system improvements were surprisingly few, with the responsibility for change resting with the patient rather than the care delivery system. Interventions incorporating community involvement were also severely lacking and reflected only 6.5% of the reviewed intervention tactics. The majority of interventions failed to involve major stakeholders, including providers, health care institutions, community organizers, and policy makers, and accordingly, were unlikely to succeed in creating meaningful change.
In breast cancer, there have been examples of successful system-based approaches to reducing the racial mortality disparity. At New York area hospitals, Bickell et al.20 implemented a tracking and feedback registry to close the referral loop between surgeons and oncologists to decrease the underuse of valuable adjuvant treatments.
The intervention targeted important quality issues in both communication (the breakdown in dialogue among providers of different specialties and between providers and patients) and the underuse of adjuvant treatment in minorities. The approach was designed to address failures in the health care system through the involvement of leadership from pathology, surgery, and oncology. The intervention also incorporated technology, with tracking software prompting contact with patients who had failed to follow up. Among African American and Hispanic women, there were statistically significant decreases in the underuse of radiotherapy (23% before the intervention vs. 10% after the intervention), chemotherapy (26% vs. 6%), and hormonal therapy (27% vs. 11%). After the intervention, minority race was no longer a risk factor for low rates of oncology consultation or underuse of adjuvant therapy. Interestingly, four of the six hospitals involved in this study had a patient navigation system in place; however, as discussed, the navigation system alone was not enough to address the system failures that led to disparities in care.
Ansell et al.21 also described a system-based approach to reducing the breast cancer mortality disparity in Chicago. The Metropolitan Chicago Breast Cancer Task Force comprised 102 individuals and 74 Chicago area organizations to address the growing disparity in breast cancer mortality between African American and white patients. The task force identified a number of themes underlying the disparity gap, including a need for breast cancer education and outreach programs for African American women, a broken mammography process leading to quality differences between African American and white patients, and a number of barriers to diagnosis and treatment, including fear, a lack of primary care, the burden of insurance copays/deductibles, and the noncompletion of treatment for social and economic reasons. After identifying these underlying causes, the task force proposed that addressing one aspect of the health care system would not correct the problem, but rather quality improvement initiatives would have to occur across the continuum of care for breast cancer.
In Delaware, such a broad system-based intervention was implemented to eliminate health disparities in colorectal cancer.22 Delaware created a comprehensive statewide colorectal screening and treatment program, combining many of the interventions discussed previously, including insurance coverage, patient education and communication, and patient navigation, to address the entire health care system and its treatment of African Americans with colorectal cancer. The state also partnered with underserved community organizations to tailor programs locally and create targeted marketing campaigns.
The results of this system-based approach were impressive, with screening rates among African American increasing from 48% to 74% and equaling the rate among whites. In addition, among African American patients, the percentage diagnosed at advanced and regional stages declined from 79% to 40%, and the percentage diagnosed at a local stage increased from 16% to 50%. Most importantly, the mortality rate declined by 42% for African Americans, resulting in a rate almost equal to that among whites. Significantly, this program was also found to be economically viable, because the cost savings from reduced cancer incidence and the stage shift to cancers requiring less-aggressive treatment offset the program cost. As the authors concluded, this model of a comprehensive, system-wide approach to the racial mortality difference would lend itself to other cancers, and more research is needed to assess and build such an approach to breast cancer.
As discussed in the aforementioned studies, multifaceted interventions that address all stakeholders are needed to close the racial survival disparity in breast cancer. In the final installment of this series, we will address how the changing care models ushered in by the Patient Protection and Affordable Care Act have the potential to advance this agenda of creating an intervention that works across the breast cancer care continuum to reduce disparities.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 May-Jun;65(3):221-38.
2. Lillie-Blanton M, Hoffman C. The role of health insurance coverage in reducing racial/ethnic disparities in health care. Health Aff (Millwood). 2005 Mar-Apr;24(2):398-408.
3. Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993 Jul 29;329(5):326-31.
4. Coburn N, Fulton J, Pearlman DN, Law C, DiPaolo B, Cady B. Treatment variation by insurance status for breast cancer patients. Breast J. 2008 Mar-Apr;14(2):128-34.
5. Voti L, Richardson LC, Reis I, Fleming LE, Mackinnon J, Coebergh JW. The effect of race/ethnicity and insurance in the administration of standard therapy for local breast cancer in Florida. Breast Cancer Res Treat. 2006 Jan;95(1):89-95.
6. Baicker K, Taubman SL, Allen HL, et al. The Oregon experiment – effects of Medicaid on clinical outcomes. N Engl J Med. 2013 May 2;368(18):1713-22.
7. Hoffman HJ, LaVerda NL, Levine PH, et al. Having health insurance does not eliminate race/ethnicity-associated delays in breast cancer diagnosis in the District of Columbia. Cancer. 2011 Aug 15;117(16):3824-32.
8. Short LJ, Fisher MD, Wahl PM, et al. Disparities in medical care among commercially insured patients with newly diagnosed breast cancer: opportunities for intervention. Cancer. 2010 Jan 1;116(1):193-202.
9. Allen JD, Shelton RC, Harden E, Goldman RE. Follow-up of abnormal screening mammograms among low-income ethnically diverse women: findings from a qualitative study. Patient Educ Couns. 2008 Aug;72(2):283-92.
10. Masi CM, Gehlert S. Perceptions of breast cancer treatment among African-American women and men: implications for interventions. J Gen Intern Med. 2009 Mar;24(3):408-14.
11. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008 Sep 1;113(5):1058-67.
12. Hawley ST, Fagerlin A, Janz NK, Katz SJ. Racial/ethnic disparities in knowledge about risks and benefits of breast cancer treatment: does it matter where you go? Health Serv Res. 2008 Aug;43(4):1366-87.
13. Lannin DR, Mathews HF, Mitchell J, Swanson MS. Impacting cultural attitudes in African-American women to decrease breast cancer mortality. Am J Surg. 2002 Nov;184(5):418-23.
14. Ko NY, Darnell JS, Calhoun E, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Navigation Research Program. J Clin Oncol. 2014 Sep 1;32(25):2758-64.
15. Vargas RB, Ryan GW, Jackson CA, Rodriguez R, Freeman HP. Characteristics of the original patient navigation programs to reduce disparities in the diagnosis and treatment of breast cancer. Cancer. 2008 Jul 15;113(2):426-33.
16. Markossian TW, Darnell JS, Calhoun EA. Follow-up and timeliness after an abnormal cancer screening among underserved, urban women in a patient navigation program. Cancer Epidemiol Biomarkers Prev. 2012 Oct;21(10):1691-700.
17. Hoffman HJ, LaVerda NL, Young HA, et al. Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomarkers Prev. 2012 Oct;21(10):1655-63.
18. Tejeda S, Darnell JS, Cho YI, Stolley MR, Markossian TW, Calhoun EA. Patient barriers to follow-up care for breast and cervical cancer abnormalities. J Womens Health (Larchmt). 2013 Jun;22(6):507-17.
19. Clarke AR, Goddu AP, Nocon RS, et al. Thirty years of disparities intervention research: what are we doing to close racial and ethnic gaps in health care? Med Care. 2013 Nov;51(11):1020-26.
20. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008 Dec 3;100(23):1717-23.
21. Ansell D, Grabler P, Whitman S, et al. A community effort to reduce the black/white breast cancer mortality disparity in Chicago. Cancer Causes Control. 2009 Nov;20(9):1681-88.
22. Grubbs SS, Polite BN, Carney J, Jr., et al. Eliminating racial disparities in colorectal cancer in the real world: it took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
Editor’s Note: This is the fourth installment of a five-part monthly series that will discuss the biologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series was adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society. Eliminating racial disparities in cancer mortality through effective interventions has become an increasingly important imperative of federal, state, and community health care programs. This month’s column reviews interventions to close this survival gap.
Insurance
It has been posited that interventions aimed at providing insurance coverage to minority patients will be able to reduce racial health care disparities.2 Studies have indicated that women without insurance present with more-advanced disease,3,4 and are more likely to receive nonstandard treatment.5 However, outside of cancer care, a large study of Medicaid expansion in Oregon demonstrated that Medicaid coverage alone generated no significant improvement in measured physical health outcomes in the first 2 years.6 Thus, coverage alone does not ensure that patients will be able to navigate the health care system and that quality care will be provided.
In breast cancer, Hoffman et al.7 evaluated the effect of race and health insurance on diagnostic time, which was defined as the number of days from suspicious finding to diagnostic resolution (either no evidence of malignancy on diagnostic mammogram or definitive diagnosis by biopsy) in a large, urban setting. The authors’ hypothesis was that every insured patient would receive the same timely diagnosis as any other patient with equivalent insurance, regardless of race or ethnicity. The study found that non-Hispanic whites with government insurance had significantly shorter diagnostic times than did non-Hispanic African Americans with government insurance: The average diagnostic times were 12 and 39 days, respectively. In addition, privately insured non-Hispanic whites also had significantly shorter diagnostic times than did privately insured non-Hispanic African Americans (16 vs. 27 days). In addition, Short et al.8 demonstrated that when the health plan status was held constant in a retrospective study of 476 white patients and 99 African American patients with newly diagnosed breast cancer, African American patients had a higher mortality rate (8.1% vs. 3.6%) and were diagnosed at a later stage. Accordingly, interventions must go beyond just providing health insurance to minorities in order to have a significant impact on the mortality gap.
Patient education and physician communication
An underlying cause frequently cited for the delayed diagnosis and treatment of African American patients with breast cancer is a lack of patient education and physician communication. These elements are essential components of quality care. In a qualitative study of low-income, ethnically diverse women older than 40 years, Allen et al.9 identified salient themes differentiating women who received timely follow-up from those who did not. For the women who delayed follow-up, prominent themes were dissatisfaction with the communication of results, disrespect on the part of providers and clinical staff, logistical barriers to accessing services, anxiety and fear about a possible cancer diagnosis, and a lack of information about breast cancer screening and symptoms.
In another study, Masi and Gehlert10 employed focus group interviews of African American adults to characterize their perceptions of breast cancer treatment. The analysis revealed a core set of themes, including mistrust of the medical establishment and concern about the effect of racism on treatment quality; the researchers concluded that “in the eyes of many study participants, the issues of trust, race, and quality of care were closely intertwined.”10 Thus, this knot that is created by underlying issues of trust can be untied only by interventions that address improved physician communication and patient education.
Janz et al.11 examined racial differences in the adequacy of information and support for women with breast cancer. The researchers used survey data from a population of 1,766 women diagnosed with nonmetastatic breast cancer and reported to the Los Angeles County Surveillance, Epidemiology, and End Results (SEER) registry. The study found that across treatment- and survivorship-related issues, African American women desired more information than white women did. One of the explanations for the unmet information needs posited by the authors is a failure to provide culturally appropriate information related to health issues. This breakdown in patient education and communication was demonstrated by Hawley et al. to hold across providers and locations.12
Hawley et al.12 evaluated the association between minority patients’ knowledge of breast cancer treatment risks and benefits and provider characteristics and treatment locations. The provider characteristics included surgeon-level independent variables, such as breast cancer procedure volume and demographics (years in practice and sex). The treatment location variable was categorized into one of three groups: National Cancer Institute–designated cancer center, American College of Surgeons cancer program, or no specific cancer program. Provider characteristics and treatment locations are factors previously identified as being associated with high-quality care.
The study employed a multivariable regression to identify associations between patient, surgeon, and treatment-setting factors and accurate knowledge of the survival benefit and recurrence risk related to mastectomy and breast-conserving surgery with radiation. The authors found that minority women were significantly less likely to have adequate knowledge and more likely to be uncertain about recurrence risk than were white patients. In the multivariate logistic regression model, neither provider characteristics nor treatment setting attenuated observed racial disparities in knowledge. Quality health care depends on the ability to make an informed treatment decision. As the authors concluded, this study underscores the need for providers to communicate information effectively to all patients, and effective communication relies on the cultural competency of providers.13 Without effective, culturally competent communication, there are treatment delays and omissions that result in poor-quality cancer care. Currently, the research has established that these communication deficits are found across providers and treatment center types.
Patient Navigation
Patient navigation has been championed as a method of improving care in breast cancer by enhancing patient communication and education, and removing barriers to timely care. Patient navigation empowers patients to become knowledgeable about their own health and supports them through the course of care.14 Patient navigation programs have been developed to address the patient communication breakdowns and underuse and misuse of treatment among vulnerable populations, which were detailed earlier in this series and are thought to contribute to the racial mortality gap.15
A benefit of patient navigation has been suggested in studies evaluating the time to diagnosis and follow-up from an abnormal screening. Markossian et al.16 evaluated the efficacy of a Chicago-based cancer patient navigation program developed to reduce the time from abnormal screening to definitive diagnostic testing. The majority of patients in this study were Hispanic (66%) and African American (32%). Compared with controls without navigation, the breast navigation group had a shorter time to diagnostic resolution. Hoffman et al.17 evaluated patient navigation in the District of Columbia to determine its ability to reduce the breast cancer diagnostic time (number of days from abnormal screening to a definitive diagnosis). African American women comprised 48% of the study population. The investigators found that women in the navigation group reached their diagnostic resolution significantly faster than did other women. Among women with breast cancer, there was a nearly fourfold reduction in time to diagnostic resolution for women in the navigation group versus women without a navigator.
In a national multicenter study, Ko et al.14 were the first to evaluate whether patient navigation can improve the quality of breast cancer care. The authors hypothesized that breast cancer patients assigned a navigator would be more likely to receive recommended standard treatment than were those without a navigator. Three separate quality measures of breast cancer care, including initiation of antiestrogen therapy, radiation therapy, and chemotherapy, were evaluated. Study participants were racially and ethnically diverse, with a plurality being African American (37.5%). The study produced mixed results: Patients in the navigation group had a statistically higher likelihood of receiving antiestrogen therapy than were non–navigated controls, but navigation patients eligible for radiation therapy were no more likely to receive it than were controls. The initiation of chemotherapy could not be accurately assessed because of a limited sample size. The study concluded that navigation alone does not remove all of the barriers to quality care for breast cancer patients, and barriers are diverse and potentially specific to the modality of treatment.
A study by Tejeda et al.18 used a systematic framework to characterize barriers faced by minority patients with breast and cervical cancer. The investigators categorized barriers as intrapersonal (defined by characteristics of the individual, such as knowledge, belief, attitudes, and transportation and financial barriers), interpersonal (defined by processes that involve other people, such as social support systems, child care, and employment issues), or institutional (defined by characteristics and policies of organizations). The authors found that although navigators were able to easily resolve intrapersonal barriers, ongoing navigation was needed to address institutional barriers. Thus, patient navigation in a vacuum does not work, and it is only in examining the entire health care system that changes can be implemented to eliminate barriers to quality care and close the racial mortality chasm.
System Change
To this effect, Clarke et al.19 performed a review of the disparities intervention literature to understand which interventions are being evaluated to improve minority health. The authors found that the majority of such interventions are focused on changing the patient rather than the system that serves her, with the most common strategy being education and training (37% of strategies studied). Interventions aimed at health care system improvements were surprisingly few, with the responsibility for change resting with the patient rather than the care delivery system. Interventions incorporating community involvement were also severely lacking and reflected only 6.5% of the reviewed intervention tactics. The majority of interventions failed to involve major stakeholders, including providers, health care institutions, community organizers, and policy makers, and accordingly, were unlikely to succeed in creating meaningful change.
In breast cancer, there have been examples of successful system-based approaches to reducing the racial mortality disparity. At New York area hospitals, Bickell et al.20 implemented a tracking and feedback registry to close the referral loop between surgeons and oncologists to decrease the underuse of valuable adjuvant treatments.
The intervention targeted important quality issues in both communication (the breakdown in dialogue among providers of different specialties and between providers and patients) and the underuse of adjuvant treatment in minorities. The approach was designed to address failures in the health care system through the involvement of leadership from pathology, surgery, and oncology. The intervention also incorporated technology, with tracking software prompting contact with patients who had failed to follow up. Among African American and Hispanic women, there were statistically significant decreases in the underuse of radiotherapy (23% before the intervention vs. 10% after the intervention), chemotherapy (26% vs. 6%), and hormonal therapy (27% vs. 11%). After the intervention, minority race was no longer a risk factor for low rates of oncology consultation or underuse of adjuvant therapy. Interestingly, four of the six hospitals involved in this study had a patient navigation system in place; however, as discussed, the navigation system alone was not enough to address the system failures that led to disparities in care.
Ansell et al.21 also described a system-based approach to reducing the breast cancer mortality disparity in Chicago. The Metropolitan Chicago Breast Cancer Task Force comprised 102 individuals and 74 Chicago area organizations to address the growing disparity in breast cancer mortality between African American and white patients. The task force identified a number of themes underlying the disparity gap, including a need for breast cancer education and outreach programs for African American women, a broken mammography process leading to quality differences between African American and white patients, and a number of barriers to diagnosis and treatment, including fear, a lack of primary care, the burden of insurance copays/deductibles, and the noncompletion of treatment for social and economic reasons. After identifying these underlying causes, the task force proposed that addressing one aspect of the health care system would not correct the problem, but rather quality improvement initiatives would have to occur across the continuum of care for breast cancer.
In Delaware, such a broad system-based intervention was implemented to eliminate health disparities in colorectal cancer.22 Delaware created a comprehensive statewide colorectal screening and treatment program, combining many of the interventions discussed previously, including insurance coverage, patient education and communication, and patient navigation, to address the entire health care system and its treatment of African Americans with colorectal cancer. The state also partnered with underserved community organizations to tailor programs locally and create targeted marketing campaigns.
The results of this system-based approach were impressive, with screening rates among African American increasing from 48% to 74% and equaling the rate among whites. In addition, among African American patients, the percentage diagnosed at advanced and regional stages declined from 79% to 40%, and the percentage diagnosed at a local stage increased from 16% to 50%. Most importantly, the mortality rate declined by 42% for African Americans, resulting in a rate almost equal to that among whites. Significantly, this program was also found to be economically viable, because the cost savings from reduced cancer incidence and the stage shift to cancers requiring less-aggressive treatment offset the program cost. As the authors concluded, this model of a comprehensive, system-wide approach to the racial mortality difference would lend itself to other cancers, and more research is needed to assess and build such an approach to breast cancer.
As discussed in the aforementioned studies, multifaceted interventions that address all stakeholders are needed to close the racial survival disparity in breast cancer. In the final installment of this series, we will address how the changing care models ushered in by the Patient Protection and Affordable Care Act have the potential to advance this agenda of creating an intervention that works across the breast cancer care continuum to reduce disparities.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 May-Jun;65(3):221-38.
2. Lillie-Blanton M, Hoffman C. The role of health insurance coverage in reducing racial/ethnic disparities in health care. Health Aff (Millwood). 2005 Mar-Apr;24(2):398-408.
3. Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993 Jul 29;329(5):326-31.
4. Coburn N, Fulton J, Pearlman DN, Law C, DiPaolo B, Cady B. Treatment variation by insurance status for breast cancer patients. Breast J. 2008 Mar-Apr;14(2):128-34.
5. Voti L, Richardson LC, Reis I, Fleming LE, Mackinnon J, Coebergh JW. The effect of race/ethnicity and insurance in the administration of standard therapy for local breast cancer in Florida. Breast Cancer Res Treat. 2006 Jan;95(1):89-95.
6. Baicker K, Taubman SL, Allen HL, et al. The Oregon experiment – effects of Medicaid on clinical outcomes. N Engl J Med. 2013 May 2;368(18):1713-22.
7. Hoffman HJ, LaVerda NL, Levine PH, et al. Having health insurance does not eliminate race/ethnicity-associated delays in breast cancer diagnosis in the District of Columbia. Cancer. 2011 Aug 15;117(16):3824-32.
8. Short LJ, Fisher MD, Wahl PM, et al. Disparities in medical care among commercially insured patients with newly diagnosed breast cancer: opportunities for intervention. Cancer. 2010 Jan 1;116(1):193-202.
9. Allen JD, Shelton RC, Harden E, Goldman RE. Follow-up of abnormal screening mammograms among low-income ethnically diverse women: findings from a qualitative study. Patient Educ Couns. 2008 Aug;72(2):283-92.
10. Masi CM, Gehlert S. Perceptions of breast cancer treatment among African-American women and men: implications for interventions. J Gen Intern Med. 2009 Mar;24(3):408-14.
11. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008 Sep 1;113(5):1058-67.
12. Hawley ST, Fagerlin A, Janz NK, Katz SJ. Racial/ethnic disparities in knowledge about risks and benefits of breast cancer treatment: does it matter where you go? Health Serv Res. 2008 Aug;43(4):1366-87.
13. Lannin DR, Mathews HF, Mitchell J, Swanson MS. Impacting cultural attitudes in African-American women to decrease breast cancer mortality. Am J Surg. 2002 Nov;184(5):418-23.
14. Ko NY, Darnell JS, Calhoun E, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Navigation Research Program. J Clin Oncol. 2014 Sep 1;32(25):2758-64.
15. Vargas RB, Ryan GW, Jackson CA, Rodriguez R, Freeman HP. Characteristics of the original patient navigation programs to reduce disparities in the diagnosis and treatment of breast cancer. Cancer. 2008 Jul 15;113(2):426-33.
16. Markossian TW, Darnell JS, Calhoun EA. Follow-up and timeliness after an abnormal cancer screening among underserved, urban women in a patient navigation program. Cancer Epidemiol Biomarkers Prev. 2012 Oct;21(10):1691-700.
17. Hoffman HJ, LaVerda NL, Young HA, et al. Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomarkers Prev. 2012 Oct;21(10):1655-63.
18. Tejeda S, Darnell JS, Cho YI, Stolley MR, Markossian TW, Calhoun EA. Patient barriers to follow-up care for breast and cervical cancer abnormalities. J Womens Health (Larchmt). 2013 Jun;22(6):507-17.
19. Clarke AR, Goddu AP, Nocon RS, et al. Thirty years of disparities intervention research: what are we doing to close racial and ethnic gaps in health care? Med Care. 2013 Nov;51(11):1020-26.
20. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008 Dec 3;100(23):1717-23.
21. Ansell D, Grabler P, Whitman S, et al. A community effort to reduce the black/white breast cancer mortality disparity in Chicago. Cancer Causes Control. 2009 Nov;20(9):1681-88.
22. Grubbs SS, Polite BN, Carney J, Jr., et al. Eliminating racial disparities in colorectal cancer in the real world: it took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
Editor’s Note: This is the fourth installment of a five-part monthly series that will discuss the biologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series was adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians,1 a journal of the American Cancer Society. Eliminating racial disparities in cancer mortality through effective interventions has become an increasingly important imperative of federal, state, and community health care programs. This month’s column reviews interventions to close this survival gap.
Insurance
It has been posited that interventions aimed at providing insurance coverage to minority patients will be able to reduce racial health care disparities.2 Studies have indicated that women without insurance present with more-advanced disease,3,4 and are more likely to receive nonstandard treatment.5 However, outside of cancer care, a large study of Medicaid expansion in Oregon demonstrated that Medicaid coverage alone generated no significant improvement in measured physical health outcomes in the first 2 years.6 Thus, coverage alone does not ensure that patients will be able to navigate the health care system and that quality care will be provided.
In breast cancer, Hoffman et al.7 evaluated the effect of race and health insurance on diagnostic time, which was defined as the number of days from suspicious finding to diagnostic resolution (either no evidence of malignancy on diagnostic mammogram or definitive diagnosis by biopsy) in a large, urban setting. The authors’ hypothesis was that every insured patient would receive the same timely diagnosis as any other patient with equivalent insurance, regardless of race or ethnicity. The study found that non-Hispanic whites with government insurance had significantly shorter diagnostic times than did non-Hispanic African Americans with government insurance: The average diagnostic times were 12 and 39 days, respectively. In addition, privately insured non-Hispanic whites also had significantly shorter diagnostic times than did privately insured non-Hispanic African Americans (16 vs. 27 days). In addition, Short et al.8 demonstrated that when the health plan status was held constant in a retrospective study of 476 white patients and 99 African American patients with newly diagnosed breast cancer, African American patients had a higher mortality rate (8.1% vs. 3.6%) and were diagnosed at a later stage. Accordingly, interventions must go beyond just providing health insurance to minorities in order to have a significant impact on the mortality gap.
Patient education and physician communication
An underlying cause frequently cited for the delayed diagnosis and treatment of African American patients with breast cancer is a lack of patient education and physician communication. These elements are essential components of quality care. In a qualitative study of low-income, ethnically diverse women older than 40 years, Allen et al.9 identified salient themes differentiating women who received timely follow-up from those who did not. For the women who delayed follow-up, prominent themes were dissatisfaction with the communication of results, disrespect on the part of providers and clinical staff, logistical barriers to accessing services, anxiety and fear about a possible cancer diagnosis, and a lack of information about breast cancer screening and symptoms.
In another study, Masi and Gehlert10 employed focus group interviews of African American adults to characterize their perceptions of breast cancer treatment. The analysis revealed a core set of themes, including mistrust of the medical establishment and concern about the effect of racism on treatment quality; the researchers concluded that “in the eyes of many study participants, the issues of trust, race, and quality of care were closely intertwined.”10 Thus, this knot that is created by underlying issues of trust can be untied only by interventions that address improved physician communication and patient education.
Janz et al.11 examined racial differences in the adequacy of information and support for women with breast cancer. The researchers used survey data from a population of 1,766 women diagnosed with nonmetastatic breast cancer and reported to the Los Angeles County Surveillance, Epidemiology, and End Results (SEER) registry. The study found that across treatment- and survivorship-related issues, African American women desired more information than white women did. One of the explanations for the unmet information needs posited by the authors is a failure to provide culturally appropriate information related to health issues. This breakdown in patient education and communication was demonstrated by Hawley et al. to hold across providers and locations.12
Hawley et al.12 evaluated the association between minority patients’ knowledge of breast cancer treatment risks and benefits and provider characteristics and treatment locations. The provider characteristics included surgeon-level independent variables, such as breast cancer procedure volume and demographics (years in practice and sex). The treatment location variable was categorized into one of three groups: National Cancer Institute–designated cancer center, American College of Surgeons cancer program, or no specific cancer program. Provider characteristics and treatment locations are factors previously identified as being associated with high-quality care.
The study employed a multivariable regression to identify associations between patient, surgeon, and treatment-setting factors and accurate knowledge of the survival benefit and recurrence risk related to mastectomy and breast-conserving surgery with radiation. The authors found that minority women were significantly less likely to have adequate knowledge and more likely to be uncertain about recurrence risk than were white patients. In the multivariate logistic regression model, neither provider characteristics nor treatment setting attenuated observed racial disparities in knowledge. Quality health care depends on the ability to make an informed treatment decision. As the authors concluded, this study underscores the need for providers to communicate information effectively to all patients, and effective communication relies on the cultural competency of providers.13 Without effective, culturally competent communication, there are treatment delays and omissions that result in poor-quality cancer care. Currently, the research has established that these communication deficits are found across providers and treatment center types.
Patient Navigation
Patient navigation has been championed as a method of improving care in breast cancer by enhancing patient communication and education, and removing barriers to timely care. Patient navigation empowers patients to become knowledgeable about their own health and supports them through the course of care.14 Patient navigation programs have been developed to address the patient communication breakdowns and underuse and misuse of treatment among vulnerable populations, which were detailed earlier in this series and are thought to contribute to the racial mortality gap.15
A benefit of patient navigation has been suggested in studies evaluating the time to diagnosis and follow-up from an abnormal screening. Markossian et al.16 evaluated the efficacy of a Chicago-based cancer patient navigation program developed to reduce the time from abnormal screening to definitive diagnostic testing. The majority of patients in this study were Hispanic (66%) and African American (32%). Compared with controls without navigation, the breast navigation group had a shorter time to diagnostic resolution. Hoffman et al.17 evaluated patient navigation in the District of Columbia to determine its ability to reduce the breast cancer diagnostic time (number of days from abnormal screening to a definitive diagnosis). African American women comprised 48% of the study population. The investigators found that women in the navigation group reached their diagnostic resolution significantly faster than did other women. Among women with breast cancer, there was a nearly fourfold reduction in time to diagnostic resolution for women in the navigation group versus women without a navigator.
In a national multicenter study, Ko et al.14 were the first to evaluate whether patient navigation can improve the quality of breast cancer care. The authors hypothesized that breast cancer patients assigned a navigator would be more likely to receive recommended standard treatment than were those without a navigator. Three separate quality measures of breast cancer care, including initiation of antiestrogen therapy, radiation therapy, and chemotherapy, were evaluated. Study participants were racially and ethnically diverse, with a plurality being African American (37.5%). The study produced mixed results: Patients in the navigation group had a statistically higher likelihood of receiving antiestrogen therapy than were non–navigated controls, but navigation patients eligible for radiation therapy were no more likely to receive it than were controls. The initiation of chemotherapy could not be accurately assessed because of a limited sample size. The study concluded that navigation alone does not remove all of the barriers to quality care for breast cancer patients, and barriers are diverse and potentially specific to the modality of treatment.
A study by Tejeda et al.18 used a systematic framework to characterize barriers faced by minority patients with breast and cervical cancer. The investigators categorized barriers as intrapersonal (defined by characteristics of the individual, such as knowledge, belief, attitudes, and transportation and financial barriers), interpersonal (defined by processes that involve other people, such as social support systems, child care, and employment issues), or institutional (defined by characteristics and policies of organizations). The authors found that although navigators were able to easily resolve intrapersonal barriers, ongoing navigation was needed to address institutional barriers. Thus, patient navigation in a vacuum does not work, and it is only in examining the entire health care system that changes can be implemented to eliminate barriers to quality care and close the racial mortality chasm.
System Change
To this effect, Clarke et al.19 performed a review of the disparities intervention literature to understand which interventions are being evaluated to improve minority health. The authors found that the majority of such interventions are focused on changing the patient rather than the system that serves her, with the most common strategy being education and training (37% of strategies studied). Interventions aimed at health care system improvements were surprisingly few, with the responsibility for change resting with the patient rather than the care delivery system. Interventions incorporating community involvement were also severely lacking and reflected only 6.5% of the reviewed intervention tactics. The majority of interventions failed to involve major stakeholders, including providers, health care institutions, community organizers, and policy makers, and accordingly, were unlikely to succeed in creating meaningful change.
In breast cancer, there have been examples of successful system-based approaches to reducing the racial mortality disparity. At New York area hospitals, Bickell et al.20 implemented a tracking and feedback registry to close the referral loop between surgeons and oncologists to decrease the underuse of valuable adjuvant treatments.
The intervention targeted important quality issues in both communication (the breakdown in dialogue among providers of different specialties and between providers and patients) and the underuse of adjuvant treatment in minorities. The approach was designed to address failures in the health care system through the involvement of leadership from pathology, surgery, and oncology. The intervention also incorporated technology, with tracking software prompting contact with patients who had failed to follow up. Among African American and Hispanic women, there were statistically significant decreases in the underuse of radiotherapy (23% before the intervention vs. 10% after the intervention), chemotherapy (26% vs. 6%), and hormonal therapy (27% vs. 11%). After the intervention, minority race was no longer a risk factor for low rates of oncology consultation or underuse of adjuvant therapy. Interestingly, four of the six hospitals involved in this study had a patient navigation system in place; however, as discussed, the navigation system alone was not enough to address the system failures that led to disparities in care.
Ansell et al.21 also described a system-based approach to reducing the breast cancer mortality disparity in Chicago. The Metropolitan Chicago Breast Cancer Task Force comprised 102 individuals and 74 Chicago area organizations to address the growing disparity in breast cancer mortality between African American and white patients. The task force identified a number of themes underlying the disparity gap, including a need for breast cancer education and outreach programs for African American women, a broken mammography process leading to quality differences between African American and white patients, and a number of barriers to diagnosis and treatment, including fear, a lack of primary care, the burden of insurance copays/deductibles, and the noncompletion of treatment for social and economic reasons. After identifying these underlying causes, the task force proposed that addressing one aspect of the health care system would not correct the problem, but rather quality improvement initiatives would have to occur across the continuum of care for breast cancer.
In Delaware, such a broad system-based intervention was implemented to eliminate health disparities in colorectal cancer.22 Delaware created a comprehensive statewide colorectal screening and treatment program, combining many of the interventions discussed previously, including insurance coverage, patient education and communication, and patient navigation, to address the entire health care system and its treatment of African Americans with colorectal cancer. The state also partnered with underserved community organizations to tailor programs locally and create targeted marketing campaigns.
The results of this system-based approach were impressive, with screening rates among African American increasing from 48% to 74% and equaling the rate among whites. In addition, among African American patients, the percentage diagnosed at advanced and regional stages declined from 79% to 40%, and the percentage diagnosed at a local stage increased from 16% to 50%. Most importantly, the mortality rate declined by 42% for African Americans, resulting in a rate almost equal to that among whites. Significantly, this program was also found to be economically viable, because the cost savings from reduced cancer incidence and the stage shift to cancers requiring less-aggressive treatment offset the program cost. As the authors concluded, this model of a comprehensive, system-wide approach to the racial mortality difference would lend itself to other cancers, and more research is needed to assess and build such an approach to breast cancer.
As discussed in the aforementioned studies, multifaceted interventions that address all stakeholders are needed to close the racial survival disparity in breast cancer. In the final installment of this series, we will address how the changing care models ushered in by the Patient Protection and Affordable Care Act have the potential to advance this agenda of creating an intervention that works across the breast cancer care continuum to reduce disparities.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 May-Jun;65(3):221-38.
2. Lillie-Blanton M, Hoffman C. The role of health insurance coverage in reducing racial/ethnic disparities in health care. Health Aff (Millwood). 2005 Mar-Apr;24(2):398-408.
3. Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993 Jul 29;329(5):326-31.
4. Coburn N, Fulton J, Pearlman DN, Law C, DiPaolo B, Cady B. Treatment variation by insurance status for breast cancer patients. Breast J. 2008 Mar-Apr;14(2):128-34.
5. Voti L, Richardson LC, Reis I, Fleming LE, Mackinnon J, Coebergh JW. The effect of race/ethnicity and insurance in the administration of standard therapy for local breast cancer in Florida. Breast Cancer Res Treat. 2006 Jan;95(1):89-95.
6. Baicker K, Taubman SL, Allen HL, et al. The Oregon experiment – effects of Medicaid on clinical outcomes. N Engl J Med. 2013 May 2;368(18):1713-22.
7. Hoffman HJ, LaVerda NL, Levine PH, et al. Having health insurance does not eliminate race/ethnicity-associated delays in breast cancer diagnosis in the District of Columbia. Cancer. 2011 Aug 15;117(16):3824-32.
8. Short LJ, Fisher MD, Wahl PM, et al. Disparities in medical care among commercially insured patients with newly diagnosed breast cancer: opportunities for intervention. Cancer. 2010 Jan 1;116(1):193-202.
9. Allen JD, Shelton RC, Harden E, Goldman RE. Follow-up of abnormal screening mammograms among low-income ethnically diverse women: findings from a qualitative study. Patient Educ Couns. 2008 Aug;72(2):283-92.
10. Masi CM, Gehlert S. Perceptions of breast cancer treatment among African-American women and men: implications for interventions. J Gen Intern Med. 2009 Mar;24(3):408-14.
11. Janz NK, Mujahid MS, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. Racial/ethnic differences in adequacy of information and support for women with breast cancer. Cancer. 2008 Sep 1;113(5):1058-67.
12. Hawley ST, Fagerlin A, Janz NK, Katz SJ. Racial/ethnic disparities in knowledge about risks and benefits of breast cancer treatment: does it matter where you go? Health Serv Res. 2008 Aug;43(4):1366-87.
13. Lannin DR, Mathews HF, Mitchell J, Swanson MS. Impacting cultural attitudes in African-American women to decrease breast cancer mortality. Am J Surg. 2002 Nov;184(5):418-23.
14. Ko NY, Darnell JS, Calhoun E, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Navigation Research Program. J Clin Oncol. 2014 Sep 1;32(25):2758-64.
15. Vargas RB, Ryan GW, Jackson CA, Rodriguez R, Freeman HP. Characteristics of the original patient navigation programs to reduce disparities in the diagnosis and treatment of breast cancer. Cancer. 2008 Jul 15;113(2):426-33.
16. Markossian TW, Darnell JS, Calhoun EA. Follow-up and timeliness after an abnormal cancer screening among underserved, urban women in a patient navigation program. Cancer Epidemiol Biomarkers Prev. 2012 Oct;21(10):1691-700.
17. Hoffman HJ, LaVerda NL, Young HA, et al. Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomarkers Prev. 2012 Oct;21(10):1655-63.
18. Tejeda S, Darnell JS, Cho YI, Stolley MR, Markossian TW, Calhoun EA. Patient barriers to follow-up care for breast and cervical cancer abnormalities. J Womens Health (Larchmt). 2013 Jun;22(6):507-17.
19. Clarke AR, Goddu AP, Nocon RS, et al. Thirty years of disparities intervention research: what are we doing to close racial and ethnic gaps in health care? Med Care. 2013 Nov;51(11):1020-26.
20. Bickell NA, Shastri K, Fei K, et al. A tracking and feedback registry to reduce racial disparities in breast cancer care. J Natl Cancer Inst. 2008 Dec 3;100(23):1717-23.
21. Ansell D, Grabler P, Whitman S, et al. A community effort to reduce the black/white breast cancer mortality disparity in Chicago. Cancer Causes Control. 2009 Nov;20(9):1681-88.
22. Grubbs SS, Polite BN, Carney J, Jr., et al. Eliminating racial disparities in colorectal cancer in the real world: it took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
A Perfect Storm: Patterns of care
Editor’s Note: This is the third installment of a five-part monthly series that will discuss the pathologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians1, a journal of the American Cancer Society, will also review exciting and innovative interventions to close the survival gap. This month’s column reviews patterns of care – the second element in the perfect storm.
Mammography
Despite advances in breast cancer imaging technology, the mainstay of breast cancer screening has remained mammography. Chu et al.2 found that African American women have less early-stage disease in every age group for each hormone receptor status, and this raises the concern that mammography screening might be inadequate in this population. Although historically, African American women used mammography less than did white women, this difference has fortunately disappeared with time.3 According to results from the 2010 National Health Interview Survey, among women who were 40 years or older, 50.6% of non-Hispanic African Americans and 51.5% of non-Hispanic whites reported having had a mammogram within the past year.4
Although mammography uptake may be similar between these groups, there are still differences both in quality and in follow-up of abnormal imaging results. A study of mammography capacity and quality in a large urban setting found that the facilities that served predominantly minority women were more likely to be public institutions (31% vs. 0%) and less likely to be academic (27% vs. 71%), less likely to have digital mammography (18% vs. 71%), and less likely to have dedicated breast imaging specialists reading the films (23% vs. 87%). The authors concluded that the mammography process was broken, with quality differences in the manner in which the centers provided care and reported results.5

The accompanying graphic illustrates the disparities seen in breast cancer mammography and care for women in underserved communities on Chicago’s South Side. As the figure demonstrates, there are fewer mammography centers on the city’s South Side, with the concentration of breast cancer imaging and treatment resources localized in the more affluent communities of central and northern Chicago. A total of 300,000 women who were eligible for screening went unscreened because of improper management of resources.
Highlighting the importance of location in breast cancer care, Gehlert et al.6 asserted that ensuring that inner-city health facilities have up-to-date, well-maintained equipment and that mammographers have access to continuing training and opportunities for consultation should help reduce breast cancer mortality in African Americans.
With respect to follow-up of abnormal imaging results, a large retrospective cohort study of 6,722 women with abnormal mammogram results seen at a New York academic medical center from January 2002 through December 2002 found longer times to diagnostic follow-up for African American versus white women. The median number of days to diagnostic follow-up was 20 for African American patients versus 14 for white patients. In addition, racial disparities remained significant after the researchers controlled for age, Breast Imaging Reporting and Data System (BI-RADS) category, insurance status, provider practice location, and median household income. More important, in women with a BI-RADS classification of 4 or 5 – signifying a lesion seen on mammography that is either suspicious for or highly suggestive of malignancy, respectively – the median number of days to follow-up among those without same-day additional imaging was 26 for African Americans and 14 for whites (P < .05).7
Delays in treatment
A cascade of delays also has been documented in breast cancer care for African American women. Silber et al.8 investigated factors associated with differences in breast cancer outcomes in a large population-based study using Surveillance, Epidemiology, and End Results (SEER)-Medicare data. The mean time from diagnosis to treatment was 29.2 days for African Americans versus 22.5 days for whites (P < .001). The authors also found that African Americans were more likely to have very-long treatment delays. At least 6% of African Americans did not initiate treatment within the first 3 months of diagnosis, whereas only 3% of whites failed to start treatment (P < .001). Gwyn et al.9 also found potentially clinically significant treatment delays more often for African American women than for white women. The time from medical consultation to the initiation of treatment was longer than 3 months for 22.4% of African American women versus 14.3% of white women. Three months was chosen as a clinically significant time period, because Richards et al.10 demonstrated that a delay ≥ to 3 months affects survival. Thus, delays in the diagnosis and treatment of African American women are factors that worsen the survival gap.
Misuse of treatment
Once treatment is initiated, African Americans often receive inappropriate therapy, studies have demonstrated. In a prospective analysis of 957 patients in 101 oncology practices, Griggs et al.11 found more frequent use of non–guideline concordant adjuvant chemotherapy regimens in African American women. In a univariate analysis, African American patients were more likely than were whites to receive a nonstandard regimen (19% vs. 11%; P = .047). Although we will discuss further in this column whether guidelines based on clinical trials are appropriate for African American patients, the study demonstrates that these women are not uniformly receiving standard-of-care treatment.
Underuse of treatment
In addition to misuse of treatment, studies also have examined undertreatment of African American patients with breast cancer. One study investigated chemotherapy administration among African American patients with stage I-III breast cancer at 10 different treatment sites. Compared with white patients, African Americans received a lower dose proportion (actual vs. expected dose) and lower relative dose intensity.
The authors found that between-group differences in biological and medical characteristics, such as tolerance of therapy, comorbidities, and leukocyte counts, did not explain these variations in treatment. In fact, despite the association between lower leukocyte counts and African American ethnicity, there was no evidence that white blood cell levels accounted for the difference in dose proportion or relative dose intensity. Significantly, the authors discovered that more African Americans had chemotherapy dose reductions in the first cycle of treatment, perhaps indicating physician assumptions regarding African American patients’ ability to tolerate chemotherapy.12
Silber et al.8 also examined differences in the administration of chemotherapy between white and African American breast cancer patients. The authors found that 3.7% of African Americans received both an anthracycline and a taxane; that figure rose to 5.0% among whites who were matched to African Americans at presentation.
Bickell et al.13 explored further racial disparity in the underuse of adjuvant breast cancer treatment. The researchers examined the medical records of 677 women treated surgically for stage I or II breast cancer. The study defined underuse as omissions of radiotherapy after breast-conserving surgery, adjuvant chemotherapy after resection of hormone receptor–negative tumors ≥ 1 cm, or hormonal therapy for receptor-positive tumors ≥ 1 cm. Underuse of appropriate adjuvant treatment was found in 34% of African American patients versus 16% of white patients (P less than .001). There were racial disparities present in all three adjuvant therapies assessed.
Hormonal therapy has been shown effective in clinical trials for preventing breast cancer recurrence and death in women with early-stage breast cancer.14 The study by Bickell et al.13 documented underuse of this treatment in African American patients. Partridge et al.15 conducted the largest study of oral antineoplastic use outside of a clinical trial setting. Their study consisted of 2,378 primary breast cancer patients enrolled in New Jersey’s Medicaid or pharmaceutical assistance program; the main outcome was the number of days covered by filled tamoxifen prescriptions in the first year of therapy. The study found that nonwhite patients had significantly lower adherence rates than did whites. Although further investigation is needed to determine the drivers of this nonadherence in African American patients, medication cost has been proposed as a significant factor leading to underuse of these agents. Streeter et al.16 analyzed a nationally representative pharmacy claims database for oral antineoplastics and calculated abandonment rates for the initial claim. Not surprisingly, high cost sharing and low incomes were associated with a higher abandonment rate (P < .05). Despite being an important component of health equity research, treatment adherence has been identified by the Association of American Medical Colleges as a critically underrepresented area of disparities-focused health services research.17 More attention to this area is needed to understand the underuse of hormonal therapies in African American breast cancer patients.
The treatment strategies that have been shown to be delayed, underused, or misused in African American patients in the aforementioned studies have improved disease-free and overall survival in large randomized trials. Furthermore, diminished total dose and dose intensity of adjuvant chemotherapy both have been associated with lower breast cancer survival rates.18,19 These quality-of-care failures in breast cancer treatment for minority patients are thought to partially explain the survival disparity between African Americans and whites. It has been proposed that patients in both groups derive a similar benefit from systemic therapy when it is administered in accordance with their clinical and pathologic presentation,20 but that assumption becomes more nuanced when the clinical trial experience is reviewed.
Clinical trial experience
Dignam20 examined survival by race in several National Surgical Adjuvant Breast and Bowel Project trials. He found that the benefit from systemic adjuvant therapy for reductions in disease recurrence and mortality was comparable between African American and white patients. His survey of trials consistently indicated equivalent disease-free survival, but a mortality deficit for African Americans also was found consistently. Among African Americans, the excess risk of mortality was 21% for those who were lymph node–negative and 17% for those who were lymph node–positive. The excess mortality risk was thought to be attributable to greater mortality from noncancer causes among African American patients rather than a failure of African Americans to respond to breast cancer treatment.
In contrast to Dignam’s findings20, Hershman et al.21 assessed the association between race and treatment discontinuation/delay, white blood cell counts, and survival in women enrolled in the Southwest Oncology Group adjuvant breast cancer trials. The study found that African American women were significantly more likely to experience treatment discontinuation/delay than were white women (87% vs. 81%, respectively; P = .04). These delays were not accounted for by toxicities, which were experienced in similar proportions by race. African American women also were more likely to miss appointments (19% vs. 9%; P = .0002); perhaps, as Hassett and Griggs22 speculated, this finding speaks to economic barriers, including the inability to arrange alternate child care, miss work, or afford transportation to the clinic. Despite these barriers to care for African American patients, they still received the same mean relative dose intensity (87% vs. 86%).
In their survival analysis, Hershman et al.21 controlled for treatment-related factors such as dose reductions and delays, body surface area, baseline white blood cell counts, and other predictors of survival and still found that African Americans had worse disease-free and overall survival than did white women. The authors concluded that the study was “unable to demonstrate that any factor related to treatment quality or delivery contributed to racial differences in survival between the groups.”21 The study thus established two important findings related to the disparity gap. First, even in the controlled setting of a clinical trial, African American patients faced barriers to optimal treatment,22 and second, despite attempts to control for treatment quality and delivery, African American women still had worse outcomes. These findings suggest that tumor biology and genomics remain important.
In next month’s installment, we will discuss interventions aimed at closing the racial survival disparity in breast cancer. Eliminating racial disparities in cancer mortality through effective interventions has become an increasingly important imperative in federal, state, and community health care programs.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 May-Jun;65(3):221-38.
2. Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates: Separating factors that affect diagnosis from factors that affect treatment. Cancer. 2003 Jun;97(11):2853-60.
3. DeLancey JO, Thun MJ, Jemal A, Ward EM. Recent trends in black-white disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 2008 Nov;17(11):2908-12.
4. DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013 Nov;63(3):151-66.
5. Ansell D, Grabler P, Whitman S, et al. A community effort to reduce the black/white breast cancer mortality disparity in Chicago. Cancer Causes Control. 2009 Nov;20(9):1681-8.
6. Gehlert S, Sohmer D, Sacks T, Mininger C, McClintock M, Olopade O. Targeting health disparities: a model linking upstream determinants to downstream interventions. Health Aff (Millwood). 2008 Mar-Apr;27(2):339-49.
7. Press R, Carrasquillo O, Sciacca RR, Giardina EG. Racial/ethnic disparities in time to follow-up after an abnormal mammogram. J Womens Health (Larchmt). 2008 Jul;17(6):923-30.
8. Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013 Jul;310(4):389-397.
9. Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004 Apr;100(8):1595-604.
10. Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999 Apr 3;353(9159):1119-26.
11. Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007 Jun 20;25(18):2522-7.
12. Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003 Sep;81(1):21-31.
13. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006 Mar 20;24(9):1357-62. 14. Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989 Feb 23;320(8):479-84.
15. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003 Feb 15;21(4):602-6.
16. Streeter SB, Schwartzberg L, Husain N, Johnsrud M. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. J Oncol Pract. 2011 Jul;7(3 Suppl):46s-51s.
17. Alberti PM KN, Sutton K, Johnson BH, Holve E. The state of health equity research: closing knowledge gaps to address inequities. ©2014 Association of American Medical Colleges. May not be reproduced or distributed without prior permission.
18. Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994 May 5;330(18):1253-9.
19. Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998 Aug 19;90(16):1205-11.
20. Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001(30):36-43.
21. Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009 May;27(13):2157-62.
22. Hassett MJ, Griggs JJ. Disparities in breast cancer adjuvant chemotherapy: moving beyond yes or no. J Clin Oncol. 2009 May 1;27(13):2120-1.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
Editor’s Note: This is the third installment of a five-part monthly series that will discuss the pathologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians1, a journal of the American Cancer Society, will also review exciting and innovative interventions to close the survival gap. This month’s column reviews patterns of care – the second element in the perfect storm.
Mammography
Despite advances in breast cancer imaging technology, the mainstay of breast cancer screening has remained mammography. Chu et al.2 found that African American women have less early-stage disease in every age group for each hormone receptor status, and this raises the concern that mammography screening might be inadequate in this population. Although historically, African American women used mammography less than did white women, this difference has fortunately disappeared with time.3 According to results from the 2010 National Health Interview Survey, among women who were 40 years or older, 50.6% of non-Hispanic African Americans and 51.5% of non-Hispanic whites reported having had a mammogram within the past year.4
Although mammography uptake may be similar between these groups, there are still differences both in quality and in follow-up of abnormal imaging results. A study of mammography capacity and quality in a large urban setting found that the facilities that served predominantly minority women were more likely to be public institutions (31% vs. 0%) and less likely to be academic (27% vs. 71%), less likely to have digital mammography (18% vs. 71%), and less likely to have dedicated breast imaging specialists reading the films (23% vs. 87%). The authors concluded that the mammography process was broken, with quality differences in the manner in which the centers provided care and reported results.5

The accompanying graphic illustrates the disparities seen in breast cancer mammography and care for women in underserved communities on Chicago’s South Side. As the figure demonstrates, there are fewer mammography centers on the city’s South Side, with the concentration of breast cancer imaging and treatment resources localized in the more affluent communities of central and northern Chicago. A total of 300,000 women who were eligible for screening went unscreened because of improper management of resources.
Highlighting the importance of location in breast cancer care, Gehlert et al.6 asserted that ensuring that inner-city health facilities have up-to-date, well-maintained equipment and that mammographers have access to continuing training and opportunities for consultation should help reduce breast cancer mortality in African Americans.
With respect to follow-up of abnormal imaging results, a large retrospective cohort study of 6,722 women with abnormal mammogram results seen at a New York academic medical center from January 2002 through December 2002 found longer times to diagnostic follow-up for African American versus white women. The median number of days to diagnostic follow-up was 20 for African American patients versus 14 for white patients. In addition, racial disparities remained significant after the researchers controlled for age, Breast Imaging Reporting and Data System (BI-RADS) category, insurance status, provider practice location, and median household income. More important, in women with a BI-RADS classification of 4 or 5 – signifying a lesion seen on mammography that is either suspicious for or highly suggestive of malignancy, respectively – the median number of days to follow-up among those without same-day additional imaging was 26 for African Americans and 14 for whites (P < .05).7
Delays in treatment
A cascade of delays also has been documented in breast cancer care for African American women. Silber et al.8 investigated factors associated with differences in breast cancer outcomes in a large population-based study using Surveillance, Epidemiology, and End Results (SEER)-Medicare data. The mean time from diagnosis to treatment was 29.2 days for African Americans versus 22.5 days for whites (P < .001). The authors also found that African Americans were more likely to have very-long treatment delays. At least 6% of African Americans did not initiate treatment within the first 3 months of diagnosis, whereas only 3% of whites failed to start treatment (P < .001). Gwyn et al.9 also found potentially clinically significant treatment delays more often for African American women than for white women. The time from medical consultation to the initiation of treatment was longer than 3 months for 22.4% of African American women versus 14.3% of white women. Three months was chosen as a clinically significant time period, because Richards et al.10 demonstrated that a delay ≥ to 3 months affects survival. Thus, delays in the diagnosis and treatment of African American women are factors that worsen the survival gap.
Misuse of treatment
Once treatment is initiated, African Americans often receive inappropriate therapy, studies have demonstrated. In a prospective analysis of 957 patients in 101 oncology practices, Griggs et al.11 found more frequent use of non–guideline concordant adjuvant chemotherapy regimens in African American women. In a univariate analysis, African American patients were more likely than were whites to receive a nonstandard regimen (19% vs. 11%; P = .047). Although we will discuss further in this column whether guidelines based on clinical trials are appropriate for African American patients, the study demonstrates that these women are not uniformly receiving standard-of-care treatment.
Underuse of treatment
In addition to misuse of treatment, studies also have examined undertreatment of African American patients with breast cancer. One study investigated chemotherapy administration among African American patients with stage I-III breast cancer at 10 different treatment sites. Compared with white patients, African Americans received a lower dose proportion (actual vs. expected dose) and lower relative dose intensity.
The authors found that between-group differences in biological and medical characteristics, such as tolerance of therapy, comorbidities, and leukocyte counts, did not explain these variations in treatment. In fact, despite the association between lower leukocyte counts and African American ethnicity, there was no evidence that white blood cell levels accounted for the difference in dose proportion or relative dose intensity. Significantly, the authors discovered that more African Americans had chemotherapy dose reductions in the first cycle of treatment, perhaps indicating physician assumptions regarding African American patients’ ability to tolerate chemotherapy.12
Silber et al.8 also examined differences in the administration of chemotherapy between white and African American breast cancer patients. The authors found that 3.7% of African Americans received both an anthracycline and a taxane; that figure rose to 5.0% among whites who were matched to African Americans at presentation.
Bickell et al.13 explored further racial disparity in the underuse of adjuvant breast cancer treatment. The researchers examined the medical records of 677 women treated surgically for stage I or II breast cancer. The study defined underuse as omissions of radiotherapy after breast-conserving surgery, adjuvant chemotherapy after resection of hormone receptor–negative tumors ≥ 1 cm, or hormonal therapy for receptor-positive tumors ≥ 1 cm. Underuse of appropriate adjuvant treatment was found in 34% of African American patients versus 16% of white patients (P less than .001). There were racial disparities present in all three adjuvant therapies assessed.
Hormonal therapy has been shown effective in clinical trials for preventing breast cancer recurrence and death in women with early-stage breast cancer.14 The study by Bickell et al.13 documented underuse of this treatment in African American patients. Partridge et al.15 conducted the largest study of oral antineoplastic use outside of a clinical trial setting. Their study consisted of 2,378 primary breast cancer patients enrolled in New Jersey’s Medicaid or pharmaceutical assistance program; the main outcome was the number of days covered by filled tamoxifen prescriptions in the first year of therapy. The study found that nonwhite patients had significantly lower adherence rates than did whites. Although further investigation is needed to determine the drivers of this nonadherence in African American patients, medication cost has been proposed as a significant factor leading to underuse of these agents. Streeter et al.16 analyzed a nationally representative pharmacy claims database for oral antineoplastics and calculated abandonment rates for the initial claim. Not surprisingly, high cost sharing and low incomes were associated with a higher abandonment rate (P < .05). Despite being an important component of health equity research, treatment adherence has been identified by the Association of American Medical Colleges as a critically underrepresented area of disparities-focused health services research.17 More attention to this area is needed to understand the underuse of hormonal therapies in African American breast cancer patients.
The treatment strategies that have been shown to be delayed, underused, or misused in African American patients in the aforementioned studies have improved disease-free and overall survival in large randomized trials. Furthermore, diminished total dose and dose intensity of adjuvant chemotherapy both have been associated with lower breast cancer survival rates.18,19 These quality-of-care failures in breast cancer treatment for minority patients are thought to partially explain the survival disparity between African Americans and whites. It has been proposed that patients in both groups derive a similar benefit from systemic therapy when it is administered in accordance with their clinical and pathologic presentation,20 but that assumption becomes more nuanced when the clinical trial experience is reviewed.
Clinical trial experience
Dignam20 examined survival by race in several National Surgical Adjuvant Breast and Bowel Project trials. He found that the benefit from systemic adjuvant therapy for reductions in disease recurrence and mortality was comparable between African American and white patients. His survey of trials consistently indicated equivalent disease-free survival, but a mortality deficit for African Americans also was found consistently. Among African Americans, the excess risk of mortality was 21% for those who were lymph node–negative and 17% for those who were lymph node–positive. The excess mortality risk was thought to be attributable to greater mortality from noncancer causes among African American patients rather than a failure of African Americans to respond to breast cancer treatment.
In contrast to Dignam’s findings20, Hershman et al.21 assessed the association between race and treatment discontinuation/delay, white blood cell counts, and survival in women enrolled in the Southwest Oncology Group adjuvant breast cancer trials. The study found that African American women were significantly more likely to experience treatment discontinuation/delay than were white women (87% vs. 81%, respectively; P = .04). These delays were not accounted for by toxicities, which were experienced in similar proportions by race. African American women also were more likely to miss appointments (19% vs. 9%; P = .0002); perhaps, as Hassett and Griggs22 speculated, this finding speaks to economic barriers, including the inability to arrange alternate child care, miss work, or afford transportation to the clinic. Despite these barriers to care for African American patients, they still received the same mean relative dose intensity (87% vs. 86%).
In their survival analysis, Hershman et al.21 controlled for treatment-related factors such as dose reductions and delays, body surface area, baseline white blood cell counts, and other predictors of survival and still found that African Americans had worse disease-free and overall survival than did white women. The authors concluded that the study was “unable to demonstrate that any factor related to treatment quality or delivery contributed to racial differences in survival between the groups.”21 The study thus established two important findings related to the disparity gap. First, even in the controlled setting of a clinical trial, African American patients faced barriers to optimal treatment,22 and second, despite attempts to control for treatment quality and delivery, African American women still had worse outcomes. These findings suggest that tumor biology and genomics remain important.
In next month’s installment, we will discuss interventions aimed at closing the racial survival disparity in breast cancer. Eliminating racial disparities in cancer mortality through effective interventions has become an increasingly important imperative in federal, state, and community health care programs.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 May-Jun;65(3):221-38.
2. Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates: Separating factors that affect diagnosis from factors that affect treatment. Cancer. 2003 Jun;97(11):2853-60.
3. DeLancey JO, Thun MJ, Jemal A, Ward EM. Recent trends in black-white disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 2008 Nov;17(11):2908-12.
4. DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013 Nov;63(3):151-66.
5. Ansell D, Grabler P, Whitman S, et al. A community effort to reduce the black/white breast cancer mortality disparity in Chicago. Cancer Causes Control. 2009 Nov;20(9):1681-8.
6. Gehlert S, Sohmer D, Sacks T, Mininger C, McClintock M, Olopade O. Targeting health disparities: a model linking upstream determinants to downstream interventions. Health Aff (Millwood). 2008 Mar-Apr;27(2):339-49.
7. Press R, Carrasquillo O, Sciacca RR, Giardina EG. Racial/ethnic disparities in time to follow-up after an abnormal mammogram. J Womens Health (Larchmt). 2008 Jul;17(6):923-30.
8. Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013 Jul;310(4):389-397.
9. Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004 Apr;100(8):1595-604.
10. Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999 Apr 3;353(9159):1119-26.
11. Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007 Jun 20;25(18):2522-7.
12. Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003 Sep;81(1):21-31.
13. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006 Mar 20;24(9):1357-62. 14. Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989 Feb 23;320(8):479-84.
15. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003 Feb 15;21(4):602-6.
16. Streeter SB, Schwartzberg L, Husain N, Johnsrud M. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. J Oncol Pract. 2011 Jul;7(3 Suppl):46s-51s.
17. Alberti PM KN, Sutton K, Johnson BH, Holve E. The state of health equity research: closing knowledge gaps to address inequities. ©2014 Association of American Medical Colleges. May not be reproduced or distributed without prior permission.
18. Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994 May 5;330(18):1253-9.
19. Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998 Aug 19;90(16):1205-11.
20. Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001(30):36-43.
21. Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009 May;27(13):2157-62.
22. Hassett MJ, Griggs JJ. Disparities in breast cancer adjuvant chemotherapy: moving beyond yes or no. J Clin Oncol. 2009 May 1;27(13):2120-1.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
Editor’s Note: This is the third installment of a five-part monthly series that will discuss the pathologic, genomic, and health system factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians1, a journal of the American Cancer Society, will also review exciting and innovative interventions to close the survival gap. This month’s column reviews patterns of care – the second element in the perfect storm.
Mammography
Despite advances in breast cancer imaging technology, the mainstay of breast cancer screening has remained mammography. Chu et al.2 found that African American women have less early-stage disease in every age group for each hormone receptor status, and this raises the concern that mammography screening might be inadequate in this population. Although historically, African American women used mammography less than did white women, this difference has fortunately disappeared with time.3 According to results from the 2010 National Health Interview Survey, among women who were 40 years or older, 50.6% of non-Hispanic African Americans and 51.5% of non-Hispanic whites reported having had a mammogram within the past year.4
Although mammography uptake may be similar between these groups, there are still differences both in quality and in follow-up of abnormal imaging results. A study of mammography capacity and quality in a large urban setting found that the facilities that served predominantly minority women were more likely to be public institutions (31% vs. 0%) and less likely to be academic (27% vs. 71%), less likely to have digital mammography (18% vs. 71%), and less likely to have dedicated breast imaging specialists reading the films (23% vs. 87%). The authors concluded that the mammography process was broken, with quality differences in the manner in which the centers provided care and reported results.5

The accompanying graphic illustrates the disparities seen in breast cancer mammography and care for women in underserved communities on Chicago’s South Side. As the figure demonstrates, there are fewer mammography centers on the city’s South Side, with the concentration of breast cancer imaging and treatment resources localized in the more affluent communities of central and northern Chicago. A total of 300,000 women who were eligible for screening went unscreened because of improper management of resources.
Highlighting the importance of location in breast cancer care, Gehlert et al.6 asserted that ensuring that inner-city health facilities have up-to-date, well-maintained equipment and that mammographers have access to continuing training and opportunities for consultation should help reduce breast cancer mortality in African Americans.
With respect to follow-up of abnormal imaging results, a large retrospective cohort study of 6,722 women with abnormal mammogram results seen at a New York academic medical center from January 2002 through December 2002 found longer times to diagnostic follow-up for African American versus white women. The median number of days to diagnostic follow-up was 20 for African American patients versus 14 for white patients. In addition, racial disparities remained significant after the researchers controlled for age, Breast Imaging Reporting and Data System (BI-RADS) category, insurance status, provider practice location, and median household income. More important, in women with a BI-RADS classification of 4 or 5 – signifying a lesion seen on mammography that is either suspicious for or highly suggestive of malignancy, respectively – the median number of days to follow-up among those without same-day additional imaging was 26 for African Americans and 14 for whites (P < .05).7
Delays in treatment
A cascade of delays also has been documented in breast cancer care for African American women. Silber et al.8 investigated factors associated with differences in breast cancer outcomes in a large population-based study using Surveillance, Epidemiology, and End Results (SEER)-Medicare data. The mean time from diagnosis to treatment was 29.2 days for African Americans versus 22.5 days for whites (P < .001). The authors also found that African Americans were more likely to have very-long treatment delays. At least 6% of African Americans did not initiate treatment within the first 3 months of diagnosis, whereas only 3% of whites failed to start treatment (P < .001). Gwyn et al.9 also found potentially clinically significant treatment delays more often for African American women than for white women. The time from medical consultation to the initiation of treatment was longer than 3 months for 22.4% of African American women versus 14.3% of white women. Three months was chosen as a clinically significant time period, because Richards et al.10 demonstrated that a delay ≥ to 3 months affects survival. Thus, delays in the diagnosis and treatment of African American women are factors that worsen the survival gap.
Misuse of treatment
Once treatment is initiated, African Americans often receive inappropriate therapy, studies have demonstrated. In a prospective analysis of 957 patients in 101 oncology practices, Griggs et al.11 found more frequent use of non–guideline concordant adjuvant chemotherapy regimens in African American women. In a univariate analysis, African American patients were more likely than were whites to receive a nonstandard regimen (19% vs. 11%; P = .047). Although we will discuss further in this column whether guidelines based on clinical trials are appropriate for African American patients, the study demonstrates that these women are not uniformly receiving standard-of-care treatment.
Underuse of treatment
In addition to misuse of treatment, studies also have examined undertreatment of African American patients with breast cancer. One study investigated chemotherapy administration among African American patients with stage I-III breast cancer at 10 different treatment sites. Compared with white patients, African Americans received a lower dose proportion (actual vs. expected dose) and lower relative dose intensity.
The authors found that between-group differences in biological and medical characteristics, such as tolerance of therapy, comorbidities, and leukocyte counts, did not explain these variations in treatment. In fact, despite the association between lower leukocyte counts and African American ethnicity, there was no evidence that white blood cell levels accounted for the difference in dose proportion or relative dose intensity. Significantly, the authors discovered that more African Americans had chemotherapy dose reductions in the first cycle of treatment, perhaps indicating physician assumptions regarding African American patients’ ability to tolerate chemotherapy.12
Silber et al.8 also examined differences in the administration of chemotherapy between white and African American breast cancer patients. The authors found that 3.7% of African Americans received both an anthracycline and a taxane; that figure rose to 5.0% among whites who were matched to African Americans at presentation.
Bickell et al.13 explored further racial disparity in the underuse of adjuvant breast cancer treatment. The researchers examined the medical records of 677 women treated surgically for stage I or II breast cancer. The study defined underuse as omissions of radiotherapy after breast-conserving surgery, adjuvant chemotherapy after resection of hormone receptor–negative tumors ≥ 1 cm, or hormonal therapy for receptor-positive tumors ≥ 1 cm. Underuse of appropriate adjuvant treatment was found in 34% of African American patients versus 16% of white patients (P less than .001). There were racial disparities present in all three adjuvant therapies assessed.
Hormonal therapy has been shown effective in clinical trials for preventing breast cancer recurrence and death in women with early-stage breast cancer.14 The study by Bickell et al.13 documented underuse of this treatment in African American patients. Partridge et al.15 conducted the largest study of oral antineoplastic use outside of a clinical trial setting. Their study consisted of 2,378 primary breast cancer patients enrolled in New Jersey’s Medicaid or pharmaceutical assistance program; the main outcome was the number of days covered by filled tamoxifen prescriptions in the first year of therapy. The study found that nonwhite patients had significantly lower adherence rates than did whites. Although further investigation is needed to determine the drivers of this nonadherence in African American patients, medication cost has been proposed as a significant factor leading to underuse of these agents. Streeter et al.16 analyzed a nationally representative pharmacy claims database for oral antineoplastics and calculated abandonment rates for the initial claim. Not surprisingly, high cost sharing and low incomes were associated with a higher abandonment rate (P < .05). Despite being an important component of health equity research, treatment adherence has been identified by the Association of American Medical Colleges as a critically underrepresented area of disparities-focused health services research.17 More attention to this area is needed to understand the underuse of hormonal therapies in African American breast cancer patients.
The treatment strategies that have been shown to be delayed, underused, or misused in African American patients in the aforementioned studies have improved disease-free and overall survival in large randomized trials. Furthermore, diminished total dose and dose intensity of adjuvant chemotherapy both have been associated with lower breast cancer survival rates.18,19 These quality-of-care failures in breast cancer treatment for minority patients are thought to partially explain the survival disparity between African Americans and whites. It has been proposed that patients in both groups derive a similar benefit from systemic therapy when it is administered in accordance with their clinical and pathologic presentation,20 but that assumption becomes more nuanced when the clinical trial experience is reviewed.
Clinical trial experience
Dignam20 examined survival by race in several National Surgical Adjuvant Breast and Bowel Project trials. He found that the benefit from systemic adjuvant therapy for reductions in disease recurrence and mortality was comparable between African American and white patients. His survey of trials consistently indicated equivalent disease-free survival, but a mortality deficit for African Americans also was found consistently. Among African Americans, the excess risk of mortality was 21% for those who were lymph node–negative and 17% for those who were lymph node–positive. The excess mortality risk was thought to be attributable to greater mortality from noncancer causes among African American patients rather than a failure of African Americans to respond to breast cancer treatment.
In contrast to Dignam’s findings20, Hershman et al.21 assessed the association between race and treatment discontinuation/delay, white blood cell counts, and survival in women enrolled in the Southwest Oncology Group adjuvant breast cancer trials. The study found that African American women were significantly more likely to experience treatment discontinuation/delay than were white women (87% vs. 81%, respectively; P = .04). These delays were not accounted for by toxicities, which were experienced in similar proportions by race. African American women also were more likely to miss appointments (19% vs. 9%; P = .0002); perhaps, as Hassett and Griggs22 speculated, this finding speaks to economic barriers, including the inability to arrange alternate child care, miss work, or afford transportation to the clinic. Despite these barriers to care for African American patients, they still received the same mean relative dose intensity (87% vs. 86%).
In their survival analysis, Hershman et al.21 controlled for treatment-related factors such as dose reductions and delays, body surface area, baseline white blood cell counts, and other predictors of survival and still found that African Americans had worse disease-free and overall survival than did white women. The authors concluded that the study was “unable to demonstrate that any factor related to treatment quality or delivery contributed to racial differences in survival between the groups.”21 The study thus established two important findings related to the disparity gap. First, even in the controlled setting of a clinical trial, African American patients faced barriers to optimal treatment,22 and second, despite attempts to control for treatment quality and delivery, African American women still had worse outcomes. These findings suggest that tumor biology and genomics remain important.
In next month’s installment, we will discuss interventions aimed at closing the racial survival disparity in breast cancer. Eliminating racial disparities in cancer mortality through effective interventions has become an increasingly important imperative in federal, state, and community health care programs.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015 May-Jun;65(3):221-38.
2. Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates: Separating factors that affect diagnosis from factors that affect treatment. Cancer. 2003 Jun;97(11):2853-60.
3. DeLancey JO, Thun MJ, Jemal A, Ward EM. Recent trends in black-white disparities in cancer mortality. Cancer Epidemiol Biomarkers Prev. 2008 Nov;17(11):2908-12.
4. DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013 Nov;63(3):151-66.
5. Ansell D, Grabler P, Whitman S, et al. A community effort to reduce the black/white breast cancer mortality disparity in Chicago. Cancer Causes Control. 2009 Nov;20(9):1681-8.
6. Gehlert S, Sohmer D, Sacks T, Mininger C, McClintock M, Olopade O. Targeting health disparities: a model linking upstream determinants to downstream interventions. Health Aff (Millwood). 2008 Mar-Apr;27(2):339-49.
7. Press R, Carrasquillo O, Sciacca RR, Giardina EG. Racial/ethnic disparities in time to follow-up after an abnormal mammogram. J Womens Health (Larchmt). 2008 Jul;17(6):923-30.
8. Silber JH, Rosenbaum PR, Clark AS, et al. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013 Jul;310(4):389-397.
9. Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004 Apr;100(8):1595-604.
10. Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999 Apr 3;353(9159):1119-26.
11. Griggs JJ, Culakova E, Sorbero ME, et al. Social and racial differences in selection of breast cancer adjuvant chemotherapy regimens. J Clin Oncol. 2007 Jun 20;25(18):2522-7.
12. Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003 Sep;81(1):21-31.
13. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006 Mar 20;24(9):1357-62. 14. Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989 Feb 23;320(8):479-84.
15. Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003 Feb 15;21(4):602-6.
16. Streeter SB, Schwartzberg L, Husain N, Johnsrud M. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. J Oncol Pract. 2011 Jul;7(3 Suppl):46s-51s.
17. Alberti PM KN, Sutton K, Johnson BH, Holve E. The state of health equity research: closing knowledge gaps to address inequities. ©2014 Association of American Medical Colleges. May not be reproduced or distributed without prior permission.
18. Wood WC, Budman DR, Korzun AH, et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. N Engl J Med. 1994 May 5;330(18):1253-9.
19. Budman DR, Berry DA, Cirrincione CT, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst. 1998 Aug 19;90(16):1205-11.
20. Dignam JJ. Efficacy of systemic adjuvant therapy for breast cancer in African-American and Caucasian women. J Natl Cancer Inst Monogr. 2001(30):36-43.
21. Hershman DL, Unger JM, Barlow WE, et al. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. J Clin Oncol. 2009 May;27(13):2157-62.
22. Hassett MJ, Griggs JJ. Disparities in breast cancer adjuvant chemotherapy: moving beyond yes or no. J Clin Oncol. 2009 May 1;27(13):2120-1.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
A Perfect Storm: Tumor biology and genomics
This is the second installment of a five-part monthly series that will discuss the pathologic, genomic, and clinical factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians1, a journal of the American Cancer Society, will also review exciting and innovative interventions to close this survival gap. This month’s column reviews tumor biology and genomics—the first element in the perfect storm.
Hormone receptor status and human epidermal growth factor receptor 2 (HER2)/neu
Breast cancer is not a single disease, and breast cancer subtype classifications are used in the clinical setting to determine prognosis and guide management. These different molecular subtypes are based on tumor markers, which include the presence or absence of three proteins: estrogen receptor (ER), progesterone receptor (PR), and HER2/neu. Hormone receptor status is a main factor in planning breast cancer treatment. Hormone receptor–positive breast tumors benefit from hormone therapies, such as selective ER modulators (for example, tamoxifen) and aromatase inhibitors (for example, anastrozole). Thus, these tumors have a more favorable disease-specific survival than do hormone receptor–negative tumors.2
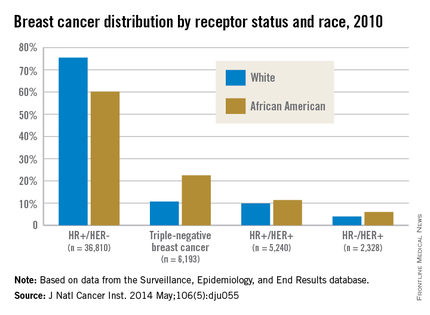
African American women are more likely to present with hormone receptor-negative tumors. In an analysis of the California Cancer Registry, which has collected patient ER and PR status since 1990, whites had a higher proportion of tumors that were ER positive or PR positive (or both) and HER2 negative (72% vs. 53%).3 DeSantis et al.4 reported similar results for this tumor type, with 76% of non-Hispanic whites having hormone receptor–positive, HER2-negative tumors vs. 62% of non-Hispanic blacks. Even with stratification by tumor stage, African Americans continue to have a significantly higher proportion of hormone receptor–negative tumors than do whites for localized and advanced disease.5
Although hormone receptor status varies significantly by race, HER2 status does not show the same divergence. HER2 overexpression is present in approximately 20% of invasive breast cancers. HER2-positive, hormone receptor–negative tumors demonstrate more-aggressive features and worse breast cancer–specific survival than do hormone receptor–positive and HER2-negative tumors,2 although survival has vastly improved with new HER2-targeted therapies such as trastuzumab and pertuzumab. Unlike hormone receptor status, there was no association between race and HER2-positive/ER-negative tumors in the Carolina Breast Cancer Study.2
Triple-negative breast cancer (TNBC)
TNBC is the subtype of breast cancer with the worst prognosis. TNBC gets its name because its tumor cells lack the markers for ER, PR, and HER2 overexpression. Thus, TNBC tumors are estrogen receptor negative (ER), progesterone receptor negative (PR), and HER2/neu negative (HER2). While other subtypes of breast cancer have benefited from drug development regarding hormonal therapies and HER2-targeted treatments, TNBC has not experienced the same pharmacologic breakthroughs.
As such, even after analyses control for the stage at diagnosis, women with this subtype have poorer survival than those with other breast cancers.6 African American women have a higher incidence of TNBC than white women.7 DeSantis et al.4 reported that 22% of breast cancers were triple negative in non-Hispanic black patients vs. only 11% in non-Hispanic white patients. The Carolina Breast Cancer Study found that 26% of African American women had TNBC, whereas 16% of non-African American women did.2 This subtype was most common among younger, premenopausal African American women (39% of diagnosed cancer subtypes). When TNBC patients were excluded from analysis in the Carolina Breast Cancer Study, breast cancer–specific survival remained significantly worse among premenopausal African American women, suggesting that although tumor biology in part explains the poor outcomes, the survival disparity story is more complex.
Germline mutations: BRCA1 and BRCA2 Mutations
In addition to tumor biology, cancer genomics has become increasingly important in determining cancer prognosis and guiding treatment. Approximately 5%-10% of breast cancer cases present in individuals with inherited mutations in autosomal dominant, highly penetrant breast cancer susceptibility genes.8 Accounting for 80%-90% of families containing multiple cases of breast and ovarian cancer, BRCA1 and BRCA2 germline mutations are the most common of the breast cancer susceptibility genes.9 These patients often are younger and have a higher-grade tumor that is hormone receptor negative, which also often matches the profile of the African American breast cancer patient.10
Despite similarities between BRCA1-associated breast cancers and breast cancer in African Americans, genetic abnormalities in African American breast cancer patients remain underresearched. Nanda et al.11 found that BRCA1 and BRCA2 mutations occur with appreciable frequency in high-risk families of African ancestry, with 28% testing positive for a deleterious mutation in one of these genes. This frequency was at a lower rate than that found in non-Hispanic, non-Jewish whites, who had a rate of 46%, because African Americans had a higher rate of polymorphisms or variants of unknown significance (44% vs. 12%). This large percentage of variants of unknown significance indicates that more analysis is needed to understand the clinical implications of these genetic variations. In another study from the Northern California site of the Breast Cancer Family Registry, the BRCA1 mutation prevalence was 16.7% in African American cases diagnosed under the age of 35 years vs. 7.2% in non-Hispanic, non-Ashkenazi Jewish whites in the same age category.12 High frequencies of mutations in BRCA1 and BRCA2 have also been reported in breast cancer patients of African ancestry from Nigeria and the Bahamas.13, 14
These results in African American patients highlight the need for further study of breast cancer genomics in minority populations. However, Armstrong et al.15 illuminated the existence of racial/ethnic disparities in patterns of referral to cancer risk clinics. In their study, African American women with a family history of breast or ovarian cancer were significantly less likely to undergo genetic counseling for BRCA1/2 testing than were white women with this family history. The results of this study were noteworthy for the magnitude of the disparity, with white women having almost five times greater odds of undergoing this clinically important evaluation. More than two decades after BRCA1 and BRCA2 genes were identified, larger studies are still needed in diverse populations to derive true estimates of the burden of mutations in both genes in underserved and understudied populations.
Although these differences in tumor biology and genomics tell part of the mortality disparity story, there is more to be told. In a study of African American and white patients in South Carolina, Adams et al.16 determined survival rates by ethnicity that were adjusted for disease stage and other prognostic characteristics. After they controlled for age, stage, ER, and HER2 expression as well as insurance status, African American women still had a twofold excess risk of death from breast cancer. Thus, in addition to differences in the innate characteristics of the breast tumors, racial differences in patterns of care for women with breast cancer must be considered in unraveling the observed disparity in mortality. The third installment of this series will discuss the second element of the perfect storm – patterns of care.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221-238.
2. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492-502.
3. Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12(6):R99.
4. DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2015 Oct 29. doi: 10.3322/caac.21320. [Epub ahead of print]
5. Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169(10):1251-9.
6. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721-8.
7. Ray M, Polite BN. Triple-negative breast cancers: a view from 10,000 feet. Cancer J. 2010;16(1):17-22.
8. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77(11):2318-24.
9. Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993;52(4):678-701.
10. Polite BN, Olopade OI. Breast cancer and race: a rising tide does not lift all boats equally. Perspect Biol Med. 2005;48(1 Suppl):S166-75.
11. Nanda R, Schumm LP, Cummings S, et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA. 2005;294(15):1925-33.
12. John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298(24):2869-76.
13. Fackenthal JD, Zhang J, Zhang B, et al. High prevalence of BRCA1 and BRCA2 mutations in unselected Nigerian breast cancer patients. Int J Cancer. 2012;131(5):1114-23.
14. Donenberg T, Lunn J, Curling D, et al. A high prevalence of BRCA1 mutations among breast cancer patients from the Bahamas. Breast Cancer Res Treat. 2011;125(2):591-6.
15. Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729-36.
16. Adams SA, Butler WM, Fulton J, et al. Racial disparities in breast cancer mortality in a multi-ethnic cohort in the Southeast. Cancer. 2012;118(10):2693-9.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
This is the second installment of a five-part monthly series that will discuss the pathologic, genomic, and clinical factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians1, a journal of the American Cancer Society, will also review exciting and innovative interventions to close this survival gap. This month’s column reviews tumor biology and genomics—the first element in the perfect storm.
Hormone receptor status and human epidermal growth factor receptor 2 (HER2)/neu
Breast cancer is not a single disease, and breast cancer subtype classifications are used in the clinical setting to determine prognosis and guide management. These different molecular subtypes are based on tumor markers, which include the presence or absence of three proteins: estrogen receptor (ER), progesterone receptor (PR), and HER2/neu. Hormone receptor status is a main factor in planning breast cancer treatment. Hormone receptor–positive breast tumors benefit from hormone therapies, such as selective ER modulators (for example, tamoxifen) and aromatase inhibitors (for example, anastrozole). Thus, these tumors have a more favorable disease-specific survival than do hormone receptor–negative tumors.2

African American women are more likely to present with hormone receptor-negative tumors. In an analysis of the California Cancer Registry, which has collected patient ER and PR status since 1990, whites had a higher proportion of tumors that were ER positive or PR positive (or both) and HER2 negative (72% vs. 53%).3 DeSantis et al.4 reported similar results for this tumor type, with 76% of non-Hispanic whites having hormone receptor–positive, HER2-negative tumors vs. 62% of non-Hispanic blacks. Even with stratification by tumor stage, African Americans continue to have a significantly higher proportion of hormone receptor–negative tumors than do whites for localized and advanced disease.5
Although hormone receptor status varies significantly by race, HER2 status does not show the same divergence. HER2 overexpression is present in approximately 20% of invasive breast cancers. HER2-positive, hormone receptor–negative tumors demonstrate more-aggressive features and worse breast cancer–specific survival than do hormone receptor–positive and HER2-negative tumors,2 although survival has vastly improved with new HER2-targeted therapies such as trastuzumab and pertuzumab. Unlike hormone receptor status, there was no association between race and HER2-positive/ER-negative tumors in the Carolina Breast Cancer Study.2
Triple-negative breast cancer (TNBC)
TNBC is the subtype of breast cancer with the worst prognosis. TNBC gets its name because its tumor cells lack the markers for ER, PR, and HER2 overexpression. Thus, TNBC tumors are estrogen receptor negative (ER), progesterone receptor negative (PR), and HER2/neu negative (HER2). While other subtypes of breast cancer have benefited from drug development regarding hormonal therapies and HER2-targeted treatments, TNBC has not experienced the same pharmacologic breakthroughs.
As such, even after analyses control for the stage at diagnosis, women with this subtype have poorer survival than those with other breast cancers.6 African American women have a higher incidence of TNBC than white women.7 DeSantis et al.4 reported that 22% of breast cancers were triple negative in non-Hispanic black patients vs. only 11% in non-Hispanic white patients. The Carolina Breast Cancer Study found that 26% of African American women had TNBC, whereas 16% of non-African American women did.2 This subtype was most common among younger, premenopausal African American women (39% of diagnosed cancer subtypes). When TNBC patients were excluded from analysis in the Carolina Breast Cancer Study, breast cancer–specific survival remained significantly worse among premenopausal African American women, suggesting that although tumor biology in part explains the poor outcomes, the survival disparity story is more complex.
Germline mutations: BRCA1 and BRCA2 Mutations
In addition to tumor biology, cancer genomics has become increasingly important in determining cancer prognosis and guiding treatment. Approximately 5%-10% of breast cancer cases present in individuals with inherited mutations in autosomal dominant, highly penetrant breast cancer susceptibility genes.8 Accounting for 80%-90% of families containing multiple cases of breast and ovarian cancer, BRCA1 and BRCA2 germline mutations are the most common of the breast cancer susceptibility genes.9 These patients often are younger and have a higher-grade tumor that is hormone receptor negative, which also often matches the profile of the African American breast cancer patient.10
Despite similarities between BRCA1-associated breast cancers and breast cancer in African Americans, genetic abnormalities in African American breast cancer patients remain underresearched. Nanda et al.11 found that BRCA1 and BRCA2 mutations occur with appreciable frequency in high-risk families of African ancestry, with 28% testing positive for a deleterious mutation in one of these genes. This frequency was at a lower rate than that found in non-Hispanic, non-Jewish whites, who had a rate of 46%, because African Americans had a higher rate of polymorphisms or variants of unknown significance (44% vs. 12%). This large percentage of variants of unknown significance indicates that more analysis is needed to understand the clinical implications of these genetic variations. In another study from the Northern California site of the Breast Cancer Family Registry, the BRCA1 mutation prevalence was 16.7% in African American cases diagnosed under the age of 35 years vs. 7.2% in non-Hispanic, non-Ashkenazi Jewish whites in the same age category.12 High frequencies of mutations in BRCA1 and BRCA2 have also been reported in breast cancer patients of African ancestry from Nigeria and the Bahamas.13, 14
These results in African American patients highlight the need for further study of breast cancer genomics in minority populations. However, Armstrong et al.15 illuminated the existence of racial/ethnic disparities in patterns of referral to cancer risk clinics. In their study, African American women with a family history of breast or ovarian cancer were significantly less likely to undergo genetic counseling for BRCA1/2 testing than were white women with this family history. The results of this study were noteworthy for the magnitude of the disparity, with white women having almost five times greater odds of undergoing this clinically important evaluation. More than two decades after BRCA1 and BRCA2 genes were identified, larger studies are still needed in diverse populations to derive true estimates of the burden of mutations in both genes in underserved and understudied populations.
Although these differences in tumor biology and genomics tell part of the mortality disparity story, there is more to be told. In a study of African American and white patients in South Carolina, Adams et al.16 determined survival rates by ethnicity that were adjusted for disease stage and other prognostic characteristics. After they controlled for age, stage, ER, and HER2 expression as well as insurance status, African American women still had a twofold excess risk of death from breast cancer. Thus, in addition to differences in the innate characteristics of the breast tumors, racial differences in patterns of care for women with breast cancer must be considered in unraveling the observed disparity in mortality. The third installment of this series will discuss the second element of the perfect storm – patterns of care.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221-238.
2. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492-502.
3. Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12(6):R99.
4. DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2015 Oct 29. doi: 10.3322/caac.21320. [Epub ahead of print]
5. Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169(10):1251-9.
6. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721-8.
7. Ray M, Polite BN. Triple-negative breast cancers: a view from 10,000 feet. Cancer J. 2010;16(1):17-22.
8. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77(11):2318-24.
9. Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993;52(4):678-701.
10. Polite BN, Olopade OI. Breast cancer and race: a rising tide does not lift all boats equally. Perspect Biol Med. 2005;48(1 Suppl):S166-75.
11. Nanda R, Schumm LP, Cummings S, et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA. 2005;294(15):1925-33.
12. John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298(24):2869-76.
13. Fackenthal JD, Zhang J, Zhang B, et al. High prevalence of BRCA1 and BRCA2 mutations in unselected Nigerian breast cancer patients. Int J Cancer. 2012;131(5):1114-23.
14. Donenberg T, Lunn J, Curling D, et al. A high prevalence of BRCA1 mutations among breast cancer patients from the Bahamas. Breast Cancer Res Treat. 2011;125(2):591-6.
15. Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729-36.
16. Adams SA, Butler WM, Fulton J, et al. Racial disparities in breast cancer mortality in a multi-ethnic cohort in the Southeast. Cancer. 2012;118(10):2693-9.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.
This is the second installment of a five-part monthly series that will discuss the pathologic, genomic, and clinical factors that contribute to the racial survival disparity in breast cancer. The series, which is adapted from an article that originally appeared in CA: A Cancer Journal for Clinicians1, a journal of the American Cancer Society, will also review exciting and innovative interventions to close this survival gap. This month’s column reviews tumor biology and genomics—the first element in the perfect storm.
Hormone receptor status and human epidermal growth factor receptor 2 (HER2)/neu
Breast cancer is not a single disease, and breast cancer subtype classifications are used in the clinical setting to determine prognosis and guide management. These different molecular subtypes are based on tumor markers, which include the presence or absence of three proteins: estrogen receptor (ER), progesterone receptor (PR), and HER2/neu. Hormone receptor status is a main factor in planning breast cancer treatment. Hormone receptor–positive breast tumors benefit from hormone therapies, such as selective ER modulators (for example, tamoxifen) and aromatase inhibitors (for example, anastrozole). Thus, these tumors have a more favorable disease-specific survival than do hormone receptor–negative tumors.2

African American women are more likely to present with hormone receptor-negative tumors. In an analysis of the California Cancer Registry, which has collected patient ER and PR status since 1990, whites had a higher proportion of tumors that were ER positive or PR positive (or both) and HER2 negative (72% vs. 53%).3 DeSantis et al.4 reported similar results for this tumor type, with 76% of non-Hispanic whites having hormone receptor–positive, HER2-negative tumors vs. 62% of non-Hispanic blacks. Even with stratification by tumor stage, African Americans continue to have a significantly higher proportion of hormone receptor–negative tumors than do whites for localized and advanced disease.5
Although hormone receptor status varies significantly by race, HER2 status does not show the same divergence. HER2 overexpression is present in approximately 20% of invasive breast cancers. HER2-positive, hormone receptor–negative tumors demonstrate more-aggressive features and worse breast cancer–specific survival than do hormone receptor–positive and HER2-negative tumors,2 although survival has vastly improved with new HER2-targeted therapies such as trastuzumab and pertuzumab. Unlike hormone receptor status, there was no association between race and HER2-positive/ER-negative tumors in the Carolina Breast Cancer Study.2
Triple-negative breast cancer (TNBC)
TNBC is the subtype of breast cancer with the worst prognosis. TNBC gets its name because its tumor cells lack the markers for ER, PR, and HER2 overexpression. Thus, TNBC tumors are estrogen receptor negative (ER), progesterone receptor negative (PR), and HER2/neu negative (HER2). While other subtypes of breast cancer have benefited from drug development regarding hormonal therapies and HER2-targeted treatments, TNBC has not experienced the same pharmacologic breakthroughs.
As such, even after analyses control for the stage at diagnosis, women with this subtype have poorer survival than those with other breast cancers.6 African American women have a higher incidence of TNBC than white women.7 DeSantis et al.4 reported that 22% of breast cancers were triple negative in non-Hispanic black patients vs. only 11% in non-Hispanic white patients. The Carolina Breast Cancer Study found that 26% of African American women had TNBC, whereas 16% of non-African American women did.2 This subtype was most common among younger, premenopausal African American women (39% of diagnosed cancer subtypes). When TNBC patients were excluded from analysis in the Carolina Breast Cancer Study, breast cancer–specific survival remained significantly worse among premenopausal African American women, suggesting that although tumor biology in part explains the poor outcomes, the survival disparity story is more complex.
Germline mutations: BRCA1 and BRCA2 Mutations
In addition to tumor biology, cancer genomics has become increasingly important in determining cancer prognosis and guiding treatment. Approximately 5%-10% of breast cancer cases present in individuals with inherited mutations in autosomal dominant, highly penetrant breast cancer susceptibility genes.8 Accounting for 80%-90% of families containing multiple cases of breast and ovarian cancer, BRCA1 and BRCA2 germline mutations are the most common of the breast cancer susceptibility genes.9 These patients often are younger and have a higher-grade tumor that is hormone receptor negative, which also often matches the profile of the African American breast cancer patient.10
Despite similarities between BRCA1-associated breast cancers and breast cancer in African Americans, genetic abnormalities in African American breast cancer patients remain underresearched. Nanda et al.11 found that BRCA1 and BRCA2 mutations occur with appreciable frequency in high-risk families of African ancestry, with 28% testing positive for a deleterious mutation in one of these genes. This frequency was at a lower rate than that found in non-Hispanic, non-Jewish whites, who had a rate of 46%, because African Americans had a higher rate of polymorphisms or variants of unknown significance (44% vs. 12%). This large percentage of variants of unknown significance indicates that more analysis is needed to understand the clinical implications of these genetic variations. In another study from the Northern California site of the Breast Cancer Family Registry, the BRCA1 mutation prevalence was 16.7% in African American cases diagnosed under the age of 35 years vs. 7.2% in non-Hispanic, non-Ashkenazi Jewish whites in the same age category.12 High frequencies of mutations in BRCA1 and BRCA2 have also been reported in breast cancer patients of African ancestry from Nigeria and the Bahamas.13, 14
These results in African American patients highlight the need for further study of breast cancer genomics in minority populations. However, Armstrong et al.15 illuminated the existence of racial/ethnic disparities in patterns of referral to cancer risk clinics. In their study, African American women with a family history of breast or ovarian cancer were significantly less likely to undergo genetic counseling for BRCA1/2 testing than were white women with this family history. The results of this study were noteworthy for the magnitude of the disparity, with white women having almost five times greater odds of undergoing this clinically important evaluation. More than two decades after BRCA1 and BRCA2 genes were identified, larger studies are still needed in diverse populations to derive true estimates of the burden of mutations in both genes in underserved and understudied populations.
Although these differences in tumor biology and genomics tell part of the mortality disparity story, there is more to be told. In a study of African American and white patients in South Carolina, Adams et al.16 determined survival rates by ethnicity that were adjusted for disease stage and other prognostic characteristics. After they controlled for age, stage, ER, and HER2 expression as well as insurance status, African American women still had a twofold excess risk of death from breast cancer. Thus, in addition to differences in the innate characteristics of the breast tumors, racial differences in patterns of care for women with breast cancer must be considered in unraveling the observed disparity in mortality. The third installment of this series will discuss the second element of the perfect storm – patterns of care.
Other installments of this column can be found in the Related Content box.
1. Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65(3):221-238.
2. Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492-502.
3. Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12(6):R99.
4. DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2015 Oct 29. doi: 10.3322/caac.21320. [Epub ahead of print]
5. Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE. Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol. 2009;169(10):1251-9.
6. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721-8.
7. Ray M, Polite BN. Triple-negative breast cancers: a view from 10,000 feet. Cancer J. 2010;16(1):17-22.
8. Claus EB, Schildkraut JM, Thompson WD, Risch NJ. The genetic attributable risk of breast and ovarian cancer. Cancer. 1996;77(11):2318-24.
9. Easton DF, Bishop DT, Ford D, Crockford GP. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993;52(4):678-701.
10. Polite BN, Olopade OI. Breast cancer and race: a rising tide does not lift all boats equally. Perspect Biol Med. 2005;48(1 Suppl):S166-75.
11. Nanda R, Schumm LP, Cummings S, et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA. 2005;294(15):1925-33.
12. John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298(24):2869-76.
13. Fackenthal JD, Zhang J, Zhang B, et al. High prevalence of BRCA1 and BRCA2 mutations in unselected Nigerian breast cancer patients. Int J Cancer. 2012;131(5):1114-23.
14. Donenberg T, Lunn J, Curling D, et al. A high prevalence of BRCA1 mutations among breast cancer patients from the Bahamas. Breast Cancer Res Treat. 2011;125(2):591-6.
15. Armstrong K, Micco E, Carney A, Stopfer J, Putt M. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293(14):1729-36.
16. Adams SA, Butler WM, Fulton J, et al. Racial disparities in breast cancer mortality in a multi-ethnic cohort in the Southeast. Cancer. 2012;118(10):2693-9.
Bobby Daly, MD, MBA, is the chief fellow in the section of hematology/oncology at the University of Chicago Medicine. His clinical focus is breast and thoracic oncology, and his research focus is health services. Specifically, Dr. Daly researches disparities in oncology care delivery, oncology health care utilization, aggressive end-of-life oncology care, and oncology payment models. He received his MD and MBA from Harvard Medical School and Harvard Business School, both in Boston, and a BA in Economics and History from Stanford (Calif.) University. He was the recipient of the Dean’s Award at Harvard Medical and Business Schools.
Olufunmilayo Olopade, MD, FACP, OON, is the Walter L. Palmer Distinguished Service Professor of Medicine and Human Genetics, and director, Center for Global Health at the University of Chicago. She is adopting emerging high throughput genomic and informatics strategies to identify genetic and nongenetic risk factors for breast cancer in order to implement precision health care in diverse populations. This innovative approach has the potential to improve the quality of care and reduce costs while saving more lives.
Disclosures: Dr. Olopade serves on the Medical Advisory Board for CancerIQ. Dr. Daly serves as a director of Quadrant Holdings Corporation and receives compensation from this entity. Frontline Medical Communications is a subsidiary of Quadrant Holdings Corporation.
Published in conjunction with Susan G. Komen®.















