User login
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
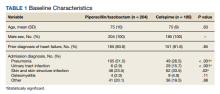
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
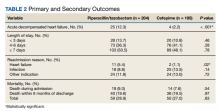
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
Piperacillin/tazobactam (PTZ) is a combination IV antibiotic comprised of the semisynthetic antipseudomonal β-lactam, piperacillin sodium, and the β-lactamase inhibitor, tazobactam sodium.1 PTZ is extensively prescribed in the hospital setting for a multitude of infections including but not limited to the US Food and Drug Administration–approved indications: intra-abdominal infection, skin and skin structure infection (SSTI), urinary tract infection (UTI), and pneumonia. Given its broad spectrum of activity and relative safety profile, PTZ is a mainstay of many empiric IV antibiotic regimens. The primary elimination pathway for PTZ is renal excretion, and dosage adjustments are recommended with reduced creatinine clearance. Additionally, PTZ use has been associated with acute renal injury and delayed renal recovery.1-3
There are various mechanisms through which medications can contribute to acute decomopensated heart failure (ADHF).4 These mechanisms include direct cardiotoxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; sodium loading; and drug-drug interactions that limit the benefits of heart failure (HF) medications. One potentially overlooked constituent of PTZ is the sodium content, with the standard formulation containing 65 mg of sodium per gram of piperacillin.1-3 Furthermore, PTZ must be diluted in 50 to 150 mL of diluent, commonly 0.9% sodium chloride, which can contribute an additional 177 to 531 mg of sodium per dose. PTZ prescribing information advises caution for use in patients with decreased renal, hepatic, and/or cardiac function and notes that geriatric patients, particularly with HF, may be at risk of impaired natriuresis in the setting of large sodium doses.
It is estimated that roughly 6.2 million adults in the United States have HF and prevalence continues to rise.5,6 Mortality rates after hospitalization due to HF are 20% to 25% at 1 year. Health care expenditures for the management of HF surpass $30 billion per year in the US, with most of this cost attributed to hospitalizations. Consequently, it is important to continue to identify and practice preventative strategies when managing patients with HF.
Methods
This single-center, retrospective, cohort study was conducted at James H. Quillen Veterans Affairs Medical Center (JHQVAMC) in Mountain Home, Tennessee, a 174-bed tertiary medical center. The purpose of this study was to compare the incidence of ADHF in patients who received PTZ vs cefepime (CFP). This project was reviewed by the JHQVAMC Institutional Review Board and deemed exempt as a clinical process improvement operations activity.
The antimicrobial stewardship team at JHQVAMC reviewed the use of PTZ in veterans between January 1, 2018, to December 31, 2019, and compared baseline demographics, history of HF, and outcomes in patients receiving analogous broad-spectrum empiric antibiotic therapy with CFP.
Statistical Analysis
Analysis was conducted with R Software. Pearson χ2 and t tests were used to compare baseline demographics, length of stay, readmission, and mortality. Significance used was α = .05.
Results
A retrospective chart review was performed on 389 veterans. Of the 389, 204 patients received at least 24 hours of PTZ, and 185 patients received CFP. The mean age in both groups was 75 years. Patients in the PTZ group were more likely to have been admitted with the diagnosis of pneumonia (105 vs 49, P < .001). However, 29 patients (15.7%) in the CFP group were admitted with a UTI diagnosis compared with 6 patients (2.9%) in the PTZ group (P < .001) and 62 patients (33.5%) in the CFP group were admitted with a SSTI diagnosis compared with 48 patients (23.5%) in the PTZ group (P = .03). Otherwise, there were no differences between other admitting diagnoses. Additionally, there was no difference in prior history of HF between groups (Table 1).
Twenty-five patients (12.3%) in the PTZ group and 4 patients (2.2%) in the CFP group were subsequently diagnosed with ADHF (P < .001). Hospital readmissions due to HF were higher in the PTZ group compared with the CFP group (11 vs 2, P = .02). Hospital readmission due to other causes was not significantly different between groups. Hospital readmission due to infection occurred in 18 patients who received PTZ and 25 who received CFP (8.8% vs 13.5%, P = .14). Hospital readmission due to any other indication occurred in 24 patients who received PTZ and 24 who received CFP (11.8% vs 13.0%, P = .72). There was no statistically significant difference in all-cause mortality during the associated admission or within 6 months of discharge between groups, with 59 total deaths in the PTZ group and 50 in the CFP group (28.9% vs 27.0%, P = .63).
There was no difference in length of stay outcomes between patients receiving PTZ compared with CFP. Twenty-eight patients in the PTZ group and 20 in the CFP group had a length of stay duration of < 3 days (13.7% vs 10.8%, P = .46). Seventy-three patients in the PTZ group and 76 in the CFP group had a length of stay duration of 4 to 6 days (36.3% vs 41.1%, P = .28). One hundred three patients in the PTZ group and 89 in the CFP group had a length of stay duration ≥ 7 days (50.5% vs 48.1%, P = .78). Table 2 includes a complete overview of primary and secondary endpoint results.
Discussion
The American Heart Association (AHA) lists PTZ as a medication that may cause or exacerbate HF, though no studies have identified a clear association between PTZ use and ADHF.4 Sodium restriction is consistently recommended as an important strategy for the prevention of ADHF. Accordingly, PTZ prescribing information and the AHA advise careful consideration with PTZ use in this patient population.1,4
The specific mechanism responsible for the association of PTZ with cardiac-related adverse outcomes is unclear. It is easy to presume that the sodium content of PTZ is solely responsible; however, other antibiotic regimens not included as agents of concern by the AHA, such as meropenem, can approach similar overall daily sodium amounts.4,7 Additionally, total sodium and volume can also be contributed by various IV medications and fluids. This study did not evaluate total sodium intake from all sources, but it is notable that this study identified a possible trend toward the risk of ADHF with PTZ use in a routine practice environment. It is reasonable to postulate additional intrinsic properties of PTZ may be contributing to the development of ADHF, such as its association with renal injury possibly resulting in increased fluid retainment and subsequent fluid volume overload.1,2,4 Other hypothesized mechanisms may include those previously described, such as direct myocardial toxicity; negative inotropic, lusitropic, or chronotropic effects; exacerbating hypertension; and drug-drug interactions that limit the benefits of HF medications, although these have not been overtly associated with PTZ in the literature to date.4,8
ADHF can present similarly to other acute pulmonary conditions, including pneumonia.9,10 It is important to acknowledge the challenge this creates for diagnosticians to differentiate between these conditions rapidly and precisely. As a result, this patient population is likely at increased risk of IV antibiotic exposure. Other studies have identified worse outcomes in patients who receive potentially unwarranted IV antibiotics in patients with ADHF.9,10 The results of this study further emphasize the importance of careful considerate antibiotic selection and overall avoidance of unnecessary antibiotic exposure to limit potential adverse outcomes.
Limitations
There are various limitations to this study. Firstly, no women were included due to the predominantly male population within the Veterans Health Administration system. Secondly, this study was retrospective in design and was therefore limited to the completeness and accuracy of the available data collected. Additionally, this study evaluated any ADHF episode during the associated hospitalization as the primary endpoint. The time to diagnosis of ADHF in relation to PTZ initiation was not evaluated, which may have helped better elucidate this possible association. Furthermore, while a significant statistical difference was identified, the smaller sample size may have limited the ability to accurately identify differences in lower event rate outcomes.
Conclusions
This study identifies an association between PTZ use and significant cardiac-related adverse outcomes, including increased incidence of ADHF and readmission due to HF exacerbation. While more research is needed to define the exact mechanisms by which PTZ may precipitate acute decompensation in patients with HF, it is judicious to consider close monitoring or the avoidance of PTZ when appropriate antibiotic alternatives are available in patients with a known history of HF.
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220
1. Zosyn. Package insert. Wyeth Pharmaceuticals; 2020.
2. Jensen JU, Hein L, Lundgren B, et al. Kidney failure related to broad-spectrum antibiotics in critically ill patients: secondary end point results from a 1200 patient randomised trial. BMJ Open. 2012;2(2):e000635. Published 2012 Mar 11. doi:10.1136/bmjopen-2011-000635
3. Kadomura S, Takekuma Y, Sato Y, et al. Higher incidence of acute kidney injury in patients treated with piperacillin/tazobactam than in patients treated with cefepime: a single-center retrospective cohort study. J Pharm Health Care Sci. 2019;5:13. Published 2019 Jun 12. doi:10.1186/s40780-019-0142-6
4. Page RL 2nd, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(6):e32-e69. doi:10.1161/CIR.0000000000000426
5. Bozkurt B, Hershberger RE, Butler J, et al. 2021 ACC/AHA key data elements and definitions for heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical data standards. J Am Coll Cardiol. 2021;77(16):2053-2150.
6. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254-e743. doi:10.1161/CIR.0000000000000950
7. Merrem. Package insert. Pfizer Labs; 2021.
8. Keller GA, Alvarez PA, Ponte ML, et al. Drug-induced QTc interval prolongation: a multicenter study to detect drugs and clinical factors involved in every day practice. Curr Drug Saf. 2016;11(1):86-98. doi:10.2174/1574886311207040262
9. Wu S, Alikhil M, Forsyth R, Allen B. Impact of potentially unwarranted intravenous antibiotics targeting pulmonary infections in acute decompensated heart failure. J Pharm Technol. 2021;37(6):298-303. doi:10.1177/87551225211038020
10. Frisbee J, Heidel RH, Rasnake MS. Adverse outcomes associated with potentially inappropriate antibiotic use in heart failure admissions. Open Forum Infect Dis. 2019;6(6):ofz220. doi:10.1093/ofid/ofz220