User login
Hypogonadism is characterized by low testosterone levels that can result in symptoms such as reduced libido, erectile dysfunction (ED), fatigue, anemia, decreased bone density, decreased lean body mass, and increased body fat.1,2 The Endocrine Society defines male hypogonadism as serum total testosterone level (T) < 300 ng/dL, a threshold at which the likelihood of most symptoms associated with hypogonadism increases.1
Testosterone replacement therapy (TRT) is recommended for treatment of androgen deficiency in symptomatic men with unequivocally low serum T levels. Although current guidelines recommend against using TRT in men with a history of prostate cancer (PCa),1,3 and the FDA has a black box warning against prescribing TRT for these men, little evidence suggests that TRT stimulates tumor growth in patients treated for low-risk PCa.4-6 Retrospective studies and case series suggest that TRT can be safe in patients with low-risk PCa treated with radical prostatectomy, brachytherapy, or external beam radiation.7,8 Despite observed increases in prostate specific antigen (PSA), TRT does not seem to increase PCa recurrence rates when used cautiously, even in men with high-risk disease.9
Almost 3 million men living in the U.S. have been diagnosed with PCa. While 1 in 7 men will be diagnosed with PCa during their lifetime, most men diagnosed with PCa do not die of it.10 More than 90% of patients with PCa have localized or low-grade disease that does not result in PCa-related mortality.11 Active surveillance, brachytherapy, external beam radiation, and radical prostatectomy are considered appropriate monotherapy modalities for low-risk PCa.12 With successful treatment of early PCa, primary care providers may increasingly encounter PCa survivors with or without symptomatic hypogonadism. Surprisingly, the prevalence of hypogonadism in men with low-risk PCa has not been reported.
The primary objective of this study was to estimate the prevalence of hypogonadism in low-risk PCa survivors who received curative treatment. A second objective was to examine the presence of hypogonadism among subgroups of patients. The authors hypothesized that the prevalence in this population may be high enough to support prospective trials designed to determine the safety of TRT in selected hypogonadal men with a history of PCa.
Methods
This is a cross-sectional study conducted at the Edward Hines, Jr. VA Hospital (EHJVA) that included a convenience sample of 52 veterans aged 25 to 100 years who had been treated for low-risk PCa more than 12 months previously and were currently receiving medical care at EHJVA. Low-risk PCa was defined as tumors with a Gleason score ≤ 6 and PSA at diagnosis < 10 or a (clinical or pathologic) American Joint Committee on Cancer (AJCC) stage I or II. Patients were excluded if they had any of the following: Gleason score > 7, PSA at diagnosis > 10, prior TRT or androgen deprivation therapy, recurrent or active PCa, incomplete treatment of PCa, or history of breast cancer. The study was approved by the institutional review board at EHJVA.
Participant Identification and Recruitment
The EHJVA cancer registry provided a roster of about 600 patients who were diagnosed with AJCC stage I or II PCa after 2002 and had completed treatment by 2011. About 50% of the patients were excluded because they no longer were receiving care at EHJVA. More than 150 patients were excluded because they did not have upcoming appointments at EHJVA, they followed up at community-based outpatient clinics, or they did not meet the remaining parameters of the inclusion criteria.
Between April 2013 and August 2014, the authors approached 75 potentially eligible patients who had upcoming outpatient care appointments at EHJVA and invited them to participate in the study. After explaining the risks and benefits, 15 patients declined to participate. Although 60 eligible patients signed an informed consent, 6 later refused to consent to the medical record review. After the medical record review, 2 patients were excluded because their eligibility could not be confirmed (eg, missing tumor stage or PSA information). A total of 52 men were included in this study.
Data Collection
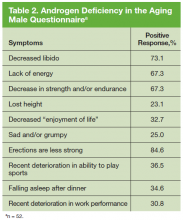
The study participants were asked to complete a brief validated Androgen Deficiency in the Aging Male (ADAM) questionnaire and provide a blood sample. The standard ADAM questionnaire consists of 10 yes/no questions concerning symptoms of androgen deficiency. A positive questionnaire was defined as a “yes” to any 3 questions.13 A blood sample was obtained between 7AM and 9AM for total and free testosterone level, follicle stimulating hormone, and luteinizing hormone (LH) to determine whether the hypogonadism was primary or secondary to a hypothalamicpituitary process. Additional data were collected, including age, height, weight, race, presence of diabetes mellitus (DM), tumor characteristics at diagnosis (ie, PSA, Gleason score, AJCC stage), and first course of cancer treatment.
Main Outcomes and Measures
The primary outcome was hypogonadism. Patients with serum T < 250 ng/dL were identified as having hypogonadism (the lower limit of the reference range in the EHJVA laboratory assay 250-1,100 ng/dL). The type of hypogonadism was further categorized as primary (LH > 10.6 IU/L) or secondary (LH < 10.6 IU/L). Patients with a body mass index (BMI) > 30 kg/m2 were considered obese.
The prevalence of hypogonadism was assessed overall and for subgroups of patients. The 95% confidence interval (CI) was calculated for each estimate. The correlation between BMI and serum T was also determined. Analyses were carried out using SAS 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
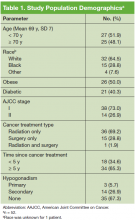
The mean age was 69.1 years and patients were primarily white (Table 1). Half the patients were obese, and 40% had DM. The majority of patients had been diagnosed with stage I PCa, had received radiation as their primary treatment, and had completed the PCa treatment more than 5 years ago.
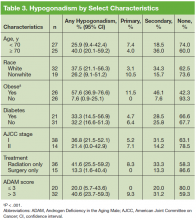
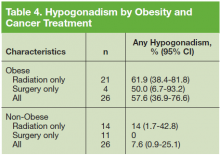
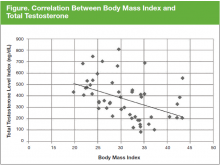
The prevalence of hypogonadism did not differ by race, DM, or tumor stage (Table 3). The oldest age group had the highest prevalence, but it was not statistically significant. However, obese patients were significantly more likely to have hypogonadism than were nonobese patients (57.6% vs 7.6%, P < .001). The negative association between BMI and serum T was more apparent when the correlation (r = -0.37, P < .01) between these 2 factors were examined (Figure). Patients with a higher ADAM score (> 3) were more likely to have hypogonadism than patients with a lower score (40.6% vs 20.0%, P = .14; Table 3). The prevalence of hypogonadism was higher in patients who were treated with radiation compared with patients who had surgery (41.6% vs 13.3%, P = .05). However, obese patients were more likely to have been treated with radiation (Table 4). After stratifying by obesity status, there was no difference in hypogonadism by treatment type.
Discussion
To the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism in treated low-risk PCa survivors. In this sample of 52 men in an outpatient setting, about 1 in 3 had low testosterone levels. Three patients had primary hypogonadism, and 14 had secondary
(hypogonadotrophic) hypogonadism. Obese patients were significantly more likely to have hypogonadism than were nonobese patients. There was no evidence of an associationbetween PCa treatment and hypogonadism.
Estimates of prevalence of hypogonadism in the general population vary in the literature based on different patient demographics, study designs, and geography. A recent review found that the prevalence of hypogonadism in adult men ≥ 18 years had a range of 2.1% to 31.2% since the authors included studies that were population-based, community-based, and primary care or screening-based.14 The prevalence of hypogonadism was 15.0% to 78.8% in obese people and 21.4% to 33% in men with newly diagnosed PCa.14
Mulligan and colleagues estimated that 38.7% of men aged ≥ 45 years who visited primary care clinics in the U.S. had hypogonadism.15 They also found that the prevalence of hypogonadism, defined as serum T < 300 ng/dL, was significantly higher in men with obesity, DM, hypertension, hyperlipidemia, asthma or chronic obstructive pulmonary disease, and prostate disease. The prevalence of hypogonadism in this study was slightly lower (32.6%), but the authors used a different serum T level (< 250 ng/dL).15 Using the Mulligan and colleagues’ definition of serum T level, there was a prevalence of 40.3% in this study’s population of men with treated low-risk PCa.
Other patient characteristics were examined to determine whether the prevalence of hypogonadism differed by age, tumor stage, or mode of treatment. Testosterone levels drop in all men, 1% per year on average after age 30 years.15 Hypogonadism has been consistently found to increase with age and certain comorbidities. Zarotsky and colleagues found a 17% increase in the risk for hypogonadism with every 10-year increase in age.14 In this study, the authors did not find a significant difference in the prevalence of hypogonadism in this group by age or DM.
In the Prostate Cancer Outcomes Study (PCOS), the prevalence of erectile dysfunction was almost 90% in the prostatectomy and radiotherapy group after 15 years. It was unclear whether this was due to PCa and its treatment, the normal aging process, or a combination of factors, but no significant differences were observed between the treatment groups. However, it is unclear whether the PCOS men had hypogonadism, because testosterone levels were not reported.16
In the current study, the association between obesity and hypogonadism is consistent with epidemiologic evidence that suggests complex multidirectional interactions between testosterone, sex hormone-binding globulin, obesity, metabolic syndrome, and type 2 DM (T2DM), mediated by cytokines and adipokines. Several studies show that obesity adversely affects testicular function and is associated with a reduction in sex hormone-binding globulin, serum T, and free testosterone levels.17,18 In addition, secondary hypogonadism has been shown to be higher in men who are overweight (25-29 kg/m2 BMI) and obese (≥ 30 kg/ m2 BMI) compared with normal weight men.19,20 Hypogonadism has also been shown to increase insulin resistance, thereby increasing the risk for developing metabolic syndrome, which is a precursor for cardiovascular disease (CVD) and T2DM.21 Furthermore, low testosterone concentration may be associated with increased insulin resistance, incidence of CVD events, anemia, and low bone density.19,22,23
The relationship between obesity, metabolic syndrome, and androgen deficiency remains unclear because of the complex mechanisms involved in this association. Impairment of hypothalamic-pituitary function by decreased LH pulse amplitude, inhibitory effects of estrogen at the
hypothalamus and pituitary, and the effects of leptin, ghrelin, and resistin, both centrally and on testicular Leydig cells, may explain lower testosterone level in obese males.24,25 Recent studies have suggested a possible increased risk of CV events among groups of men prescribed TRT.26,27 However, other studies demonstrate beneficial effects of TRT on CVD risk factors, and research over several decades suggests a strong beneficial relationship between normal T and CV health.26 The evidence to suggest that TRT increases CV morbidity and mortality risks is poor,28 but FDA is investigating the link between TRT and adverse CV outcomes.29
Testosterone replacement therapy is recommended for symptomatic men with androgen deficiency to induce or maintain secondary sex characteristics and improve their sexual function, sense of well-being, muscle mass, strength, and bone mineral density. In a recent study supported by the National Institutes of Health (NIH) in men aged 65 years, increasing serum testosterone levels to mid-normal range for 1 year was associated with a moderate benefit in sexual function and improved mood, but there was no significant benefit in vitality (as measured by a fatigue scale) or walking distance.28 In the NIH study, 4 men in the testosterone group and 1 in the placebo group received a diagnosis of PCa during or within the subsequent year of treatment, but the sample size was inadequate to reliably assess the effect of testosterone
on the risk of PCa.28
With increasing direct-to-consumer marketing of branded pharmaceutical products in the U.S., there is widening interest in testosterone levels and hypogonadism symptoms in middle-aged and older men. Indeed, 2.3 million patients received a prescription for testosterone in 2013, up from 1.3 million in 2010.29,30 This change has become important because whereas TRT is standard therapy in symptomatic hypogonadal men, it has long been considered taboo for men with a history of PCa, regardless of disease status. Transdermal testosterone is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate, according to the package insert.31 Androgens are contraindicated in men with known or suspected carcinoma of the prostate or breast, according to the testosterone cypionate injection package insert.32 Although TRT may increase serum PSA levels in some men, it often remains within clinically acceptable ranges and has not been shown to increase the risk for PCa. A recent observation from 3 registries of more than 1,000 hypogonadal men receiving TRT for up to 17 years concluded that TRT does not increase the risk for PCa.33
Prostate cancer encompasses a heterogeneous collection of androgen-dependent and independent cells. Androgens have been known to play an important role in PCa biology, but this relationship is more complex than the traditional view that androgens stimulate PCa growth. More than 7 decades ago, Huggins and colleagues showed that disseminated PCa was inhibited by eliminating androgens by castration and activated by androgen injections.34 Recently, the androgen hypothesis and the relationship of testosterone to PCa has been more clearly defined. Although it has been established that effective suppression of serum T levels with surgical or chemical castration remains an essential strategy in the management of advanced PCa, the assertion that testosterone causes growth of PCa has been challenged.35,36
Recent studies have shown that there may be a more complex relationship between serum T and PCa risk than was previously established.37 Although PCa cells have been shown to become androgen-independent as they progress into the castrate-resistant phase,9 several studies have indicated that low-serum T is associated with greater PCa risk and more worrisome features of PCa.38,39 Hence, a saturation model has been proposed: Changes in serum T concentrations below the point of maximal androgen-androgen receptor (AR) binding will elicit substantial changes in PCa growth, as seen with castration or with serum T administration to castrated men.40 However, once maximal androgen-AR binding is reached, the presence of additional androgen produces little further effect, suggesting that there is a limit to the ability of androgens to stimulate growth of PCa.40
A meta-analysis of 45 articles studying the relationship between serum T and PCa risk has reported conflicting results.4 Eugonadal testosterone levels, whether physiologically or pharmacologically replaced, do not seem to promote PCa growth. It is unclear whether the timing of sex hormone exposure affects PCa or whether the cancer may influence blood levels of sex hormones. Since 2004, there have been case series totaling almost 150 men treated with prostatectomy, brachytherapy, or external beam radiation who have been safely treated with TRT.6-9 These case studies suggest that after a thorough discussion of risks and benefits, TRT may be safer than previously thought for men who have been successfully treated for PCa, are deemed low risk for recurrence, and are monitored closely. However, no randomized controlled trials are available, and published guidelines recommend against starting TRT in patients with a history of breast or PCa.1
Limitations
This study has limitations. The sample size is small and, therefore, it may not have had enough power to show differences in prevalence by subgroups. In addition, the population may not be representative of all male veterans who seek care in the VA or of men in other health care settings, because this was a convenience sample of VA patients who received most of their care in the VA. However, the prevalence of hypogonadism, in this outpatient population is very similar to that found among men seen in primary care clinics.14 Finally, because pretreatment serum T levels were lacking, the authors were unable to assess whether hypogonadism was present before surgery or radiation therapy, and unable to determine whether treatment had any effect on serum T levels.
The study has several strengths. First, to the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism after patients with low-risk PCa have received treatment with curative intent. Second, the authors assessed hypogonadism in every patient by measuring serum T levels in the early morning when levels are known to be at their highest. Third, differentiating primary and secondary hypogonadism helped provide insight into the possible etiology of the low serum T levels. Fourth, this study was performed at a single institution that uses electronic medical records, with almost complete data on patient demographics, PCa treatment, and receipt of TRT or androgen deprivation therapy. Fifth, because this study had the participation of endocrinologists and urologists, the study design helped answer questions pertinent to both medical and surgical specialties.
Conclusion
As life expectancy increases, many survivors of treated PCa present with symptoms of hypogonadism associated with low serum T levels and request TRT. The prevalence of hypogonadism before or after treatment for PCa in this population is not known. This study suggests that many low-risk PCa survivors have hypogonadism. Because hypogonadism negatively impacts quality of life by increasing the risk for sexual dysfunction, mood disturbances, bone fracture, development of metabolic syndrome, frailty, and decline in the feeling of general well-being and may have significant deleterious effects on other body systems, consideration for treatment is warranted.
Patients with PCa may be untreated because the safety of TRT in this population is unknown. Clinical practice guidelines caution against using TRT in this population, and recent literature questions the benefits and risks associated with the long-term safety of TRT, particularly in older men.28,41 Although further studies are necessary before definitive conclusions can be drawn, increasing evidence, albeit small, suggests that TRT can be cautiously considered in selected hypogonadal men treated with curative intent for PCa and without evidence of active disease. However, because obese patients are at higher risk for aggressive PCa and mortality, it is unclear whether obese PCa survivors have an additional risk in regard to TRT.42,43 To help clinicians provide information and care for their patients, appropriately designed prospective randomized studies using a collaborative approach and long-term follow-up are urgently needed to determine the safety of TRT in hypogonadal men with a history of low-risk PCa.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Edward Hines Jr. VA Hospital. Dr. Silva’s work was carried out while she was a postdoctoral fellow supported by the VA Office of Academic Affiliations (TPP 42-013). The authors thank Ahmer V. Farooq, DO, Department of Surgery, Division of Urology, for his help in planning the design of the study. Dr. Agrawal affirms that all coauthors contributed significantly to the work and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
2. Shortridge EF, Polzer P, Donga P, et al. Experiences and treatment patterns of hypogonadal
men in a U.S. health system. Int J Clin Pract. 2014;68(10):1257-1263.
3. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated February 8, 2016. Accessed April 21, 2016.
4. Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol. 2015;193(2):403-414.
5. Landau D, Tsakok T, Aylwin S, Hughes S. Should testosterone replacement be offered to hypogonadal men treated previously for prostatic carcinoma? Clin Endocrinol (Oxf). 2012;76(2):179-181.
6. Dupree JM, Langille GM, Khera M, Lipshultz LI. The safety of testosterone supplementation therapy in prostate cancer. Nat Rev Urol. 2014;11(9):526-530.
7. Pastuszak AW, Pearlman AM, Godoy G, Miles BJ, Lipshultz LI, Khera M. Testosterone replacement therapy in the setting of prostate cancer treated with radiation. Int J Impot Res. 2013;25(1):24-28.
8. Pastuszak AW, Pearlman AM, Lai WS, et al. Testosterone replacement therapy in patients with prostate cancer after radical prostatectomy. J Urol. 2013;190(2):639-644.
9. Morgentaler A. Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol. 2013;189(1)(suppl):s26-s33.
10. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
11. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):104-117.
12. Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
13. Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49(9):1239-1242.
14. Zarotsky V, Huang M-Y, Carman W, et al. Systematic literature review of the epidemiology of nongenetic forms of hypogonadism in adult males. J Hormones. 2014;2014:190347.
15. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762-769.
16. Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436-445.
17. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamicpituitary- testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
18. MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293-311.
19. Dandona P, Dhindsa S. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96(9):2643-2651.
20. Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med. 2008;66(3):103-109.
21. Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91(3):843-850.
22. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68-75.
23. Orwoll ES, Klein RF. Osteoporosis in men. Endocrine Rev. 1995;16(1):87-116.
24. Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319-1340.
25. Mah PM, Wittert GA. Obesity and testicular function. Mol Cell Endocrinol. 2010;316(2):180-186.
26. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
27. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone
therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90(2):224-251.
28. Snyder PJ, Bhasin S, Cunningham GR, et al; Testosterone Trials Investigators. Effects
of testosterone treatment in older men. N Engl J Med. 2016;374(7):611-624.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products. U.S. Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm. Updated January 14, 2016. Accessed April 12, 2016.
30. Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
31. AndroGel [package insert]. North Chicago, IL: AbbVie; 2015.
32. Depo-Testosterone [package insert]. New York, NY: Pfizer; 2015.
33. Haider A, Zitzmann M, Doros G, Isbarn H, Hammerer P, Yassin A. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol. 2015;193(1):80-86.
34. Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232-240.
35. Gomella LG. Effective testosterone suppression for prostate cancer: is there a best castration therapy? Rev Urol. 2009;11(2):52-60.
36. Khera M, Crawford D, Morales A, Salonia A, Morgentaler A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65(1):115-123.
37. Morgentaler A. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol. 2006;50(5):935-939.
38. Morgentaler A, Rhoden EL. Prevalence of prostate cancer among hypogonadal men with prostate-specific antigen levels of 4.0 ng/mL or less. Urology. 2006;68(6):1263-1267.
39. Schatzl G, Madersbacher S, Thurridl T, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47(1):52-58.
40. Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55(2):310-320.
41. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
42. Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800-809.
43. Buschemeyer WC 3rd, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007;52(2):331-343.
Hypogonadism is characterized by low testosterone levels that can result in symptoms such as reduced libido, erectile dysfunction (ED), fatigue, anemia, decreased bone density, decreased lean body mass, and increased body fat.1,2 The Endocrine Society defines male hypogonadism as serum total testosterone level (T) < 300 ng/dL, a threshold at which the likelihood of most symptoms associated with hypogonadism increases.1
Testosterone replacement therapy (TRT) is recommended for treatment of androgen deficiency in symptomatic men with unequivocally low serum T levels. Although current guidelines recommend against using TRT in men with a history of prostate cancer (PCa),1,3 and the FDA has a black box warning against prescribing TRT for these men, little evidence suggests that TRT stimulates tumor growth in patients treated for low-risk PCa.4-6 Retrospective studies and case series suggest that TRT can be safe in patients with low-risk PCa treated with radical prostatectomy, brachytherapy, or external beam radiation.7,8 Despite observed increases in prostate specific antigen (PSA), TRT does not seem to increase PCa recurrence rates when used cautiously, even in men with high-risk disease.9
Almost 3 million men living in the U.S. have been diagnosed with PCa. While 1 in 7 men will be diagnosed with PCa during their lifetime, most men diagnosed with PCa do not die of it.10 More than 90% of patients with PCa have localized or low-grade disease that does not result in PCa-related mortality.11 Active surveillance, brachytherapy, external beam radiation, and radical prostatectomy are considered appropriate monotherapy modalities for low-risk PCa.12 With successful treatment of early PCa, primary care providers may increasingly encounter PCa survivors with or without symptomatic hypogonadism. Surprisingly, the prevalence of hypogonadism in men with low-risk PCa has not been reported.
The primary objective of this study was to estimate the prevalence of hypogonadism in low-risk PCa survivors who received curative treatment. A second objective was to examine the presence of hypogonadism among subgroups of patients. The authors hypothesized that the prevalence in this population may be high enough to support prospective trials designed to determine the safety of TRT in selected hypogonadal men with a history of PCa.
Methods
This is a cross-sectional study conducted at the Edward Hines, Jr. VA Hospital (EHJVA) that included a convenience sample of 52 veterans aged 25 to 100 years who had been treated for low-risk PCa more than 12 months previously and were currently receiving medical care at EHJVA. Low-risk PCa was defined as tumors with a Gleason score ≤ 6 and PSA at diagnosis < 10 or a (clinical or pathologic) American Joint Committee on Cancer (AJCC) stage I or II. Patients were excluded if they had any of the following: Gleason score > 7, PSA at diagnosis > 10, prior TRT or androgen deprivation therapy, recurrent or active PCa, incomplete treatment of PCa, or history of breast cancer. The study was approved by the institutional review board at EHJVA.
Participant Identification and Recruitment
The EHJVA cancer registry provided a roster of about 600 patients who were diagnosed with AJCC stage I or II PCa after 2002 and had completed treatment by 2011. About 50% of the patients were excluded because they no longer were receiving care at EHJVA. More than 150 patients were excluded because they did not have upcoming appointments at EHJVA, they followed up at community-based outpatient clinics, or they did not meet the remaining parameters of the inclusion criteria.
Between April 2013 and August 2014, the authors approached 75 potentially eligible patients who had upcoming outpatient care appointments at EHJVA and invited them to participate in the study. After explaining the risks and benefits, 15 patients declined to participate. Although 60 eligible patients signed an informed consent, 6 later refused to consent to the medical record review. After the medical record review, 2 patients were excluded because their eligibility could not be confirmed (eg, missing tumor stage or PSA information). A total of 52 men were included in this study.
Data Collection
The study participants were asked to complete a brief validated Androgen Deficiency in the Aging Male (ADAM) questionnaire and provide a blood sample. The standard ADAM questionnaire consists of 10 yes/no questions concerning symptoms of androgen deficiency. A positive questionnaire was defined as a “yes” to any 3 questions.13 A blood sample was obtained between 7AM and 9AM for total and free testosterone level, follicle stimulating hormone, and luteinizing hormone (LH) to determine whether the hypogonadism was primary or secondary to a hypothalamicpituitary process. Additional data were collected, including age, height, weight, race, presence of diabetes mellitus (DM), tumor characteristics at diagnosis (ie, PSA, Gleason score, AJCC stage), and first course of cancer treatment.
Main Outcomes and Measures
The primary outcome was hypogonadism. Patients with serum T < 250 ng/dL were identified as having hypogonadism (the lower limit of the reference range in the EHJVA laboratory assay 250-1,100 ng/dL). The type of hypogonadism was further categorized as primary (LH > 10.6 IU/L) or secondary (LH < 10.6 IU/L). Patients with a body mass index (BMI) > 30 kg/m2 were considered obese.
The prevalence of hypogonadism was assessed overall and for subgroups of patients. The 95% confidence interval (CI) was calculated for each estimate. The correlation between BMI and serum T was also determined. Analyses were carried out using SAS 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
The mean age was 69.1 years and patients were primarily white (Table 1). Half the patients were obese, and 40% had DM. The majority of patients had been diagnosed with stage I PCa, had received radiation as their primary treatment, and had completed the PCa treatment more than 5 years ago.
The prevalence of hypogonadism did not differ by race, DM, or tumor stage (Table 3). The oldest age group had the highest prevalence, but it was not statistically significant. However, obese patients were significantly more likely to have hypogonadism than were nonobese patients (57.6% vs 7.6%, P < .001). The negative association between BMI and serum T was more apparent when the correlation (r = -0.37, P < .01) between these 2 factors were examined (Figure). Patients with a higher ADAM score (> 3) were more likely to have hypogonadism than patients with a lower score (40.6% vs 20.0%, P = .14; Table 3). The prevalence of hypogonadism was higher in patients who were treated with radiation compared with patients who had surgery (41.6% vs 13.3%, P = .05). However, obese patients were more likely to have been treated with radiation (Table 4). After stratifying by obesity status, there was no difference in hypogonadism by treatment type.
Discussion
To the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism in treated low-risk PCa survivors. In this sample of 52 men in an outpatient setting, about 1 in 3 had low testosterone levels. Three patients had primary hypogonadism, and 14 had secondary
(hypogonadotrophic) hypogonadism. Obese patients were significantly more likely to have hypogonadism than were nonobese patients. There was no evidence of an associationbetween PCa treatment and hypogonadism.
Estimates of prevalence of hypogonadism in the general population vary in the literature based on different patient demographics, study designs, and geography. A recent review found that the prevalence of hypogonadism in adult men ≥ 18 years had a range of 2.1% to 31.2% since the authors included studies that were population-based, community-based, and primary care or screening-based.14 The prevalence of hypogonadism was 15.0% to 78.8% in obese people and 21.4% to 33% in men with newly diagnosed PCa.14
Mulligan and colleagues estimated that 38.7% of men aged ≥ 45 years who visited primary care clinics in the U.S. had hypogonadism.15 They also found that the prevalence of hypogonadism, defined as serum T < 300 ng/dL, was significantly higher in men with obesity, DM, hypertension, hyperlipidemia, asthma or chronic obstructive pulmonary disease, and prostate disease. The prevalence of hypogonadism in this study was slightly lower (32.6%), but the authors used a different serum T level (< 250 ng/dL).15 Using the Mulligan and colleagues’ definition of serum T level, there was a prevalence of 40.3% in this study’s population of men with treated low-risk PCa.
Other patient characteristics were examined to determine whether the prevalence of hypogonadism differed by age, tumor stage, or mode of treatment. Testosterone levels drop in all men, 1% per year on average after age 30 years.15 Hypogonadism has been consistently found to increase with age and certain comorbidities. Zarotsky and colleagues found a 17% increase in the risk for hypogonadism with every 10-year increase in age.14 In this study, the authors did not find a significant difference in the prevalence of hypogonadism in this group by age or DM.
In the Prostate Cancer Outcomes Study (PCOS), the prevalence of erectile dysfunction was almost 90% in the prostatectomy and radiotherapy group after 15 years. It was unclear whether this was due to PCa and its treatment, the normal aging process, or a combination of factors, but no significant differences were observed between the treatment groups. However, it is unclear whether the PCOS men had hypogonadism, because testosterone levels were not reported.16
In the current study, the association between obesity and hypogonadism is consistent with epidemiologic evidence that suggests complex multidirectional interactions between testosterone, sex hormone-binding globulin, obesity, metabolic syndrome, and type 2 DM (T2DM), mediated by cytokines and adipokines. Several studies show that obesity adversely affects testicular function and is associated with a reduction in sex hormone-binding globulin, serum T, and free testosterone levels.17,18 In addition, secondary hypogonadism has been shown to be higher in men who are overweight (25-29 kg/m2 BMI) and obese (≥ 30 kg/ m2 BMI) compared with normal weight men.19,20 Hypogonadism has also been shown to increase insulin resistance, thereby increasing the risk for developing metabolic syndrome, which is a precursor for cardiovascular disease (CVD) and T2DM.21 Furthermore, low testosterone concentration may be associated with increased insulin resistance, incidence of CVD events, anemia, and low bone density.19,22,23
The relationship between obesity, metabolic syndrome, and androgen deficiency remains unclear because of the complex mechanisms involved in this association. Impairment of hypothalamic-pituitary function by decreased LH pulse amplitude, inhibitory effects of estrogen at the
hypothalamus and pituitary, and the effects of leptin, ghrelin, and resistin, both centrally and on testicular Leydig cells, may explain lower testosterone level in obese males.24,25 Recent studies have suggested a possible increased risk of CV events among groups of men prescribed TRT.26,27 However, other studies demonstrate beneficial effects of TRT on CVD risk factors, and research over several decades suggests a strong beneficial relationship between normal T and CV health.26 The evidence to suggest that TRT increases CV morbidity and mortality risks is poor,28 but FDA is investigating the link between TRT and adverse CV outcomes.29
Testosterone replacement therapy is recommended for symptomatic men with androgen deficiency to induce or maintain secondary sex characteristics and improve their sexual function, sense of well-being, muscle mass, strength, and bone mineral density. In a recent study supported by the National Institutes of Health (NIH) in men aged 65 years, increasing serum testosterone levels to mid-normal range for 1 year was associated with a moderate benefit in sexual function and improved mood, but there was no significant benefit in vitality (as measured by a fatigue scale) or walking distance.28 In the NIH study, 4 men in the testosterone group and 1 in the placebo group received a diagnosis of PCa during or within the subsequent year of treatment, but the sample size was inadequate to reliably assess the effect of testosterone
on the risk of PCa.28
With increasing direct-to-consumer marketing of branded pharmaceutical products in the U.S., there is widening interest in testosterone levels and hypogonadism symptoms in middle-aged and older men. Indeed, 2.3 million patients received a prescription for testosterone in 2013, up from 1.3 million in 2010.29,30 This change has become important because whereas TRT is standard therapy in symptomatic hypogonadal men, it has long been considered taboo for men with a history of PCa, regardless of disease status. Transdermal testosterone is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate, according to the package insert.31 Androgens are contraindicated in men with known or suspected carcinoma of the prostate or breast, according to the testosterone cypionate injection package insert.32 Although TRT may increase serum PSA levels in some men, it often remains within clinically acceptable ranges and has not been shown to increase the risk for PCa. A recent observation from 3 registries of more than 1,000 hypogonadal men receiving TRT for up to 17 years concluded that TRT does not increase the risk for PCa.33
Prostate cancer encompasses a heterogeneous collection of androgen-dependent and independent cells. Androgens have been known to play an important role in PCa biology, but this relationship is more complex than the traditional view that androgens stimulate PCa growth. More than 7 decades ago, Huggins and colleagues showed that disseminated PCa was inhibited by eliminating androgens by castration and activated by androgen injections.34 Recently, the androgen hypothesis and the relationship of testosterone to PCa has been more clearly defined. Although it has been established that effective suppression of serum T levels with surgical or chemical castration remains an essential strategy in the management of advanced PCa, the assertion that testosterone causes growth of PCa has been challenged.35,36
Recent studies have shown that there may be a more complex relationship between serum T and PCa risk than was previously established.37 Although PCa cells have been shown to become androgen-independent as they progress into the castrate-resistant phase,9 several studies have indicated that low-serum T is associated with greater PCa risk and more worrisome features of PCa.38,39 Hence, a saturation model has been proposed: Changes in serum T concentrations below the point of maximal androgen-androgen receptor (AR) binding will elicit substantial changes in PCa growth, as seen with castration or with serum T administration to castrated men.40 However, once maximal androgen-AR binding is reached, the presence of additional androgen produces little further effect, suggesting that there is a limit to the ability of androgens to stimulate growth of PCa.40
A meta-analysis of 45 articles studying the relationship between serum T and PCa risk has reported conflicting results.4 Eugonadal testosterone levels, whether physiologically or pharmacologically replaced, do not seem to promote PCa growth. It is unclear whether the timing of sex hormone exposure affects PCa or whether the cancer may influence blood levels of sex hormones. Since 2004, there have been case series totaling almost 150 men treated with prostatectomy, brachytherapy, or external beam radiation who have been safely treated with TRT.6-9 These case studies suggest that after a thorough discussion of risks and benefits, TRT may be safer than previously thought for men who have been successfully treated for PCa, are deemed low risk for recurrence, and are monitored closely. However, no randomized controlled trials are available, and published guidelines recommend against starting TRT in patients with a history of breast or PCa.1
Limitations
This study has limitations. The sample size is small and, therefore, it may not have had enough power to show differences in prevalence by subgroups. In addition, the population may not be representative of all male veterans who seek care in the VA or of men in other health care settings, because this was a convenience sample of VA patients who received most of their care in the VA. However, the prevalence of hypogonadism, in this outpatient population is very similar to that found among men seen in primary care clinics.14 Finally, because pretreatment serum T levels were lacking, the authors were unable to assess whether hypogonadism was present before surgery or radiation therapy, and unable to determine whether treatment had any effect on serum T levels.
The study has several strengths. First, to the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism after patients with low-risk PCa have received treatment with curative intent. Second, the authors assessed hypogonadism in every patient by measuring serum T levels in the early morning when levels are known to be at their highest. Third, differentiating primary and secondary hypogonadism helped provide insight into the possible etiology of the low serum T levels. Fourth, this study was performed at a single institution that uses electronic medical records, with almost complete data on patient demographics, PCa treatment, and receipt of TRT or androgen deprivation therapy. Fifth, because this study had the participation of endocrinologists and urologists, the study design helped answer questions pertinent to both medical and surgical specialties.
Conclusion
As life expectancy increases, many survivors of treated PCa present with symptoms of hypogonadism associated with low serum T levels and request TRT. The prevalence of hypogonadism before or after treatment for PCa in this population is not known. This study suggests that many low-risk PCa survivors have hypogonadism. Because hypogonadism negatively impacts quality of life by increasing the risk for sexual dysfunction, mood disturbances, bone fracture, development of metabolic syndrome, frailty, and decline in the feeling of general well-being and may have significant deleterious effects on other body systems, consideration for treatment is warranted.
Patients with PCa may be untreated because the safety of TRT in this population is unknown. Clinical practice guidelines caution against using TRT in this population, and recent literature questions the benefits and risks associated with the long-term safety of TRT, particularly in older men.28,41 Although further studies are necessary before definitive conclusions can be drawn, increasing evidence, albeit small, suggests that TRT can be cautiously considered in selected hypogonadal men treated with curative intent for PCa and without evidence of active disease. However, because obese patients are at higher risk for aggressive PCa and mortality, it is unclear whether obese PCa survivors have an additional risk in regard to TRT.42,43 To help clinicians provide information and care for their patients, appropriately designed prospective randomized studies using a collaborative approach and long-term follow-up are urgently needed to determine the safety of TRT in hypogonadal men with a history of low-risk PCa.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Edward Hines Jr. VA Hospital. Dr. Silva’s work was carried out while she was a postdoctoral fellow supported by the VA Office of Academic Affiliations (TPP 42-013). The authors thank Ahmer V. Farooq, DO, Department of Surgery, Division of Urology, for his help in planning the design of the study. Dr. Agrawal affirms that all coauthors contributed significantly to the work and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Hypogonadism is characterized by low testosterone levels that can result in symptoms such as reduced libido, erectile dysfunction (ED), fatigue, anemia, decreased bone density, decreased lean body mass, and increased body fat.1,2 The Endocrine Society defines male hypogonadism as serum total testosterone level (T) < 300 ng/dL, a threshold at which the likelihood of most symptoms associated with hypogonadism increases.1
Testosterone replacement therapy (TRT) is recommended for treatment of androgen deficiency in symptomatic men with unequivocally low serum T levels. Although current guidelines recommend against using TRT in men with a history of prostate cancer (PCa),1,3 and the FDA has a black box warning against prescribing TRT for these men, little evidence suggests that TRT stimulates tumor growth in patients treated for low-risk PCa.4-6 Retrospective studies and case series suggest that TRT can be safe in patients with low-risk PCa treated with radical prostatectomy, brachytherapy, or external beam radiation.7,8 Despite observed increases in prostate specific antigen (PSA), TRT does not seem to increase PCa recurrence rates when used cautiously, even in men with high-risk disease.9
Almost 3 million men living in the U.S. have been diagnosed with PCa. While 1 in 7 men will be diagnosed with PCa during their lifetime, most men diagnosed with PCa do not die of it.10 More than 90% of patients with PCa have localized or low-grade disease that does not result in PCa-related mortality.11 Active surveillance, brachytherapy, external beam radiation, and radical prostatectomy are considered appropriate monotherapy modalities for low-risk PCa.12 With successful treatment of early PCa, primary care providers may increasingly encounter PCa survivors with or without symptomatic hypogonadism. Surprisingly, the prevalence of hypogonadism in men with low-risk PCa has not been reported.
The primary objective of this study was to estimate the prevalence of hypogonadism in low-risk PCa survivors who received curative treatment. A second objective was to examine the presence of hypogonadism among subgroups of patients. The authors hypothesized that the prevalence in this population may be high enough to support prospective trials designed to determine the safety of TRT in selected hypogonadal men with a history of PCa.
Methods
This is a cross-sectional study conducted at the Edward Hines, Jr. VA Hospital (EHJVA) that included a convenience sample of 52 veterans aged 25 to 100 years who had been treated for low-risk PCa more than 12 months previously and were currently receiving medical care at EHJVA. Low-risk PCa was defined as tumors with a Gleason score ≤ 6 and PSA at diagnosis < 10 or a (clinical or pathologic) American Joint Committee on Cancer (AJCC) stage I or II. Patients were excluded if they had any of the following: Gleason score > 7, PSA at diagnosis > 10, prior TRT or androgen deprivation therapy, recurrent or active PCa, incomplete treatment of PCa, or history of breast cancer. The study was approved by the institutional review board at EHJVA.
Participant Identification and Recruitment
The EHJVA cancer registry provided a roster of about 600 patients who were diagnosed with AJCC stage I or II PCa after 2002 and had completed treatment by 2011. About 50% of the patients were excluded because they no longer were receiving care at EHJVA. More than 150 patients were excluded because they did not have upcoming appointments at EHJVA, they followed up at community-based outpatient clinics, or they did not meet the remaining parameters of the inclusion criteria.
Between April 2013 and August 2014, the authors approached 75 potentially eligible patients who had upcoming outpatient care appointments at EHJVA and invited them to participate in the study. After explaining the risks and benefits, 15 patients declined to participate. Although 60 eligible patients signed an informed consent, 6 later refused to consent to the medical record review. After the medical record review, 2 patients were excluded because their eligibility could not be confirmed (eg, missing tumor stage or PSA information). A total of 52 men were included in this study.
Data Collection
The study participants were asked to complete a brief validated Androgen Deficiency in the Aging Male (ADAM) questionnaire and provide a blood sample. The standard ADAM questionnaire consists of 10 yes/no questions concerning symptoms of androgen deficiency. A positive questionnaire was defined as a “yes” to any 3 questions.13 A blood sample was obtained between 7AM and 9AM for total and free testosterone level, follicle stimulating hormone, and luteinizing hormone (LH) to determine whether the hypogonadism was primary or secondary to a hypothalamicpituitary process. Additional data were collected, including age, height, weight, race, presence of diabetes mellitus (DM), tumor characteristics at diagnosis (ie, PSA, Gleason score, AJCC stage), and first course of cancer treatment.
Main Outcomes and Measures
The primary outcome was hypogonadism. Patients with serum T < 250 ng/dL were identified as having hypogonadism (the lower limit of the reference range in the EHJVA laboratory assay 250-1,100 ng/dL). The type of hypogonadism was further categorized as primary (LH > 10.6 IU/L) or secondary (LH < 10.6 IU/L). Patients with a body mass index (BMI) > 30 kg/m2 were considered obese.
The prevalence of hypogonadism was assessed overall and for subgroups of patients. The 95% confidence interval (CI) was calculated for each estimate. The correlation between BMI and serum T was also determined. Analyses were carried out using SAS 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
The mean age was 69.1 years and patients were primarily white (Table 1). Half the patients were obese, and 40% had DM. The majority of patients had been diagnosed with stage I PCa, had received radiation as their primary treatment, and had completed the PCa treatment more than 5 years ago.
The prevalence of hypogonadism did not differ by race, DM, or tumor stage (Table 3). The oldest age group had the highest prevalence, but it was not statistically significant. However, obese patients were significantly more likely to have hypogonadism than were nonobese patients (57.6% vs 7.6%, P < .001). The negative association between BMI and serum T was more apparent when the correlation (r = -0.37, P < .01) between these 2 factors were examined (Figure). Patients with a higher ADAM score (> 3) were more likely to have hypogonadism than patients with a lower score (40.6% vs 20.0%, P = .14; Table 3). The prevalence of hypogonadism was higher in patients who were treated with radiation compared with patients who had surgery (41.6% vs 13.3%, P = .05). However, obese patients were more likely to have been treated with radiation (Table 4). After stratifying by obesity status, there was no difference in hypogonadism by treatment type.
Discussion
To the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism in treated low-risk PCa survivors. In this sample of 52 men in an outpatient setting, about 1 in 3 had low testosterone levels. Three patients had primary hypogonadism, and 14 had secondary
(hypogonadotrophic) hypogonadism. Obese patients were significantly more likely to have hypogonadism than were nonobese patients. There was no evidence of an associationbetween PCa treatment and hypogonadism.
Estimates of prevalence of hypogonadism in the general population vary in the literature based on different patient demographics, study designs, and geography. A recent review found that the prevalence of hypogonadism in adult men ≥ 18 years had a range of 2.1% to 31.2% since the authors included studies that were population-based, community-based, and primary care or screening-based.14 The prevalence of hypogonadism was 15.0% to 78.8% in obese people and 21.4% to 33% in men with newly diagnosed PCa.14
Mulligan and colleagues estimated that 38.7% of men aged ≥ 45 years who visited primary care clinics in the U.S. had hypogonadism.15 They also found that the prevalence of hypogonadism, defined as serum T < 300 ng/dL, was significantly higher in men with obesity, DM, hypertension, hyperlipidemia, asthma or chronic obstructive pulmonary disease, and prostate disease. The prevalence of hypogonadism in this study was slightly lower (32.6%), but the authors used a different serum T level (< 250 ng/dL).15 Using the Mulligan and colleagues’ definition of serum T level, there was a prevalence of 40.3% in this study’s population of men with treated low-risk PCa.
Other patient characteristics were examined to determine whether the prevalence of hypogonadism differed by age, tumor stage, or mode of treatment. Testosterone levels drop in all men, 1% per year on average after age 30 years.15 Hypogonadism has been consistently found to increase with age and certain comorbidities. Zarotsky and colleagues found a 17% increase in the risk for hypogonadism with every 10-year increase in age.14 In this study, the authors did not find a significant difference in the prevalence of hypogonadism in this group by age or DM.
In the Prostate Cancer Outcomes Study (PCOS), the prevalence of erectile dysfunction was almost 90% in the prostatectomy and radiotherapy group after 15 years. It was unclear whether this was due to PCa and its treatment, the normal aging process, or a combination of factors, but no significant differences were observed between the treatment groups. However, it is unclear whether the PCOS men had hypogonadism, because testosterone levels were not reported.16
In the current study, the association between obesity and hypogonadism is consistent with epidemiologic evidence that suggests complex multidirectional interactions between testosterone, sex hormone-binding globulin, obesity, metabolic syndrome, and type 2 DM (T2DM), mediated by cytokines and adipokines. Several studies show that obesity adversely affects testicular function and is associated with a reduction in sex hormone-binding globulin, serum T, and free testosterone levels.17,18 In addition, secondary hypogonadism has been shown to be higher in men who are overweight (25-29 kg/m2 BMI) and obese (≥ 30 kg/ m2 BMI) compared with normal weight men.19,20 Hypogonadism has also been shown to increase insulin resistance, thereby increasing the risk for developing metabolic syndrome, which is a precursor for cardiovascular disease (CVD) and T2DM.21 Furthermore, low testosterone concentration may be associated with increased insulin resistance, incidence of CVD events, anemia, and low bone density.19,22,23
The relationship between obesity, metabolic syndrome, and androgen deficiency remains unclear because of the complex mechanisms involved in this association. Impairment of hypothalamic-pituitary function by decreased LH pulse amplitude, inhibitory effects of estrogen at the
hypothalamus and pituitary, and the effects of leptin, ghrelin, and resistin, both centrally and on testicular Leydig cells, may explain lower testosterone level in obese males.24,25 Recent studies have suggested a possible increased risk of CV events among groups of men prescribed TRT.26,27 However, other studies demonstrate beneficial effects of TRT on CVD risk factors, and research over several decades suggests a strong beneficial relationship between normal T and CV health.26 The evidence to suggest that TRT increases CV morbidity and mortality risks is poor,28 but FDA is investigating the link between TRT and adverse CV outcomes.29
Testosterone replacement therapy is recommended for symptomatic men with androgen deficiency to induce or maintain secondary sex characteristics and improve their sexual function, sense of well-being, muscle mass, strength, and bone mineral density. In a recent study supported by the National Institutes of Health (NIH) in men aged 65 years, increasing serum testosterone levels to mid-normal range for 1 year was associated with a moderate benefit in sexual function and improved mood, but there was no significant benefit in vitality (as measured by a fatigue scale) or walking distance.28 In the NIH study, 4 men in the testosterone group and 1 in the placebo group received a diagnosis of PCa during or within the subsequent year of treatment, but the sample size was inadequate to reliably assess the effect of testosterone
on the risk of PCa.28
With increasing direct-to-consumer marketing of branded pharmaceutical products in the U.S., there is widening interest in testosterone levels and hypogonadism symptoms in middle-aged and older men. Indeed, 2.3 million patients received a prescription for testosterone in 2013, up from 1.3 million in 2010.29,30 This change has become important because whereas TRT is standard therapy in symptomatic hypogonadal men, it has long been considered taboo for men with a history of PCa, regardless of disease status. Transdermal testosterone is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate, according to the package insert.31 Androgens are contraindicated in men with known or suspected carcinoma of the prostate or breast, according to the testosterone cypionate injection package insert.32 Although TRT may increase serum PSA levels in some men, it often remains within clinically acceptable ranges and has not been shown to increase the risk for PCa. A recent observation from 3 registries of more than 1,000 hypogonadal men receiving TRT for up to 17 years concluded that TRT does not increase the risk for PCa.33
Prostate cancer encompasses a heterogeneous collection of androgen-dependent and independent cells. Androgens have been known to play an important role in PCa biology, but this relationship is more complex than the traditional view that androgens stimulate PCa growth. More than 7 decades ago, Huggins and colleagues showed that disseminated PCa was inhibited by eliminating androgens by castration and activated by androgen injections.34 Recently, the androgen hypothesis and the relationship of testosterone to PCa has been more clearly defined. Although it has been established that effective suppression of serum T levels with surgical or chemical castration remains an essential strategy in the management of advanced PCa, the assertion that testosterone causes growth of PCa has been challenged.35,36
Recent studies have shown that there may be a more complex relationship between serum T and PCa risk than was previously established.37 Although PCa cells have been shown to become androgen-independent as they progress into the castrate-resistant phase,9 several studies have indicated that low-serum T is associated with greater PCa risk and more worrisome features of PCa.38,39 Hence, a saturation model has been proposed: Changes in serum T concentrations below the point of maximal androgen-androgen receptor (AR) binding will elicit substantial changes in PCa growth, as seen with castration or with serum T administration to castrated men.40 However, once maximal androgen-AR binding is reached, the presence of additional androgen produces little further effect, suggesting that there is a limit to the ability of androgens to stimulate growth of PCa.40
A meta-analysis of 45 articles studying the relationship between serum T and PCa risk has reported conflicting results.4 Eugonadal testosterone levels, whether physiologically or pharmacologically replaced, do not seem to promote PCa growth. It is unclear whether the timing of sex hormone exposure affects PCa or whether the cancer may influence blood levels of sex hormones. Since 2004, there have been case series totaling almost 150 men treated with prostatectomy, brachytherapy, or external beam radiation who have been safely treated with TRT.6-9 These case studies suggest that after a thorough discussion of risks and benefits, TRT may be safer than previously thought for men who have been successfully treated for PCa, are deemed low risk for recurrence, and are monitored closely. However, no randomized controlled trials are available, and published guidelines recommend against starting TRT in patients with a history of breast or PCa.1
Limitations
This study has limitations. The sample size is small and, therefore, it may not have had enough power to show differences in prevalence by subgroups. In addition, the population may not be representative of all male veterans who seek care in the VA or of men in other health care settings, because this was a convenience sample of VA patients who received most of their care in the VA. However, the prevalence of hypogonadism, in this outpatient population is very similar to that found among men seen in primary care clinics.14 Finally, because pretreatment serum T levels were lacking, the authors were unable to assess whether hypogonadism was present before surgery or radiation therapy, and unable to determine whether treatment had any effect on serum T levels.
The study has several strengths. First, to the authors’ knowledge, this is the first study to assess the prevalence of hypogonadism after patients with low-risk PCa have received treatment with curative intent. Second, the authors assessed hypogonadism in every patient by measuring serum T levels in the early morning when levels are known to be at their highest. Third, differentiating primary and secondary hypogonadism helped provide insight into the possible etiology of the low serum T levels. Fourth, this study was performed at a single institution that uses electronic medical records, with almost complete data on patient demographics, PCa treatment, and receipt of TRT or androgen deprivation therapy. Fifth, because this study had the participation of endocrinologists and urologists, the study design helped answer questions pertinent to both medical and surgical specialties.
Conclusion
As life expectancy increases, many survivors of treated PCa present with symptoms of hypogonadism associated with low serum T levels and request TRT. The prevalence of hypogonadism before or after treatment for PCa in this population is not known. This study suggests that many low-risk PCa survivors have hypogonadism. Because hypogonadism negatively impacts quality of life by increasing the risk for sexual dysfunction, mood disturbances, bone fracture, development of metabolic syndrome, frailty, and decline in the feeling of general well-being and may have significant deleterious effects on other body systems, consideration for treatment is warranted.
Patients with PCa may be untreated because the safety of TRT in this population is unknown. Clinical practice guidelines caution against using TRT in this population, and recent literature questions the benefits and risks associated with the long-term safety of TRT, particularly in older men.28,41 Although further studies are necessary before definitive conclusions can be drawn, increasing evidence, albeit small, suggests that TRT can be cautiously considered in selected hypogonadal men treated with curative intent for PCa and without evidence of active disease. However, because obese patients are at higher risk for aggressive PCa and mortality, it is unclear whether obese PCa survivors have an additional risk in regard to TRT.42,43 To help clinicians provide information and care for their patients, appropriately designed prospective randomized studies using a collaborative approach and long-term follow-up are urgently needed to determine the safety of TRT in hypogonadal men with a history of low-risk PCa.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Edward Hines Jr. VA Hospital. Dr. Silva’s work was carried out while she was a postdoctoral fellow supported by the VA Office of Academic Affiliations (TPP 42-013). The authors thank Ahmer V. Farooq, DO, Department of Surgery, Division of Urology, for his help in planning the design of the study. Dr. Agrawal affirms that all coauthors contributed significantly to the work and had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
2. Shortridge EF, Polzer P, Donga P, et al. Experiences and treatment patterns of hypogonadal
men in a U.S. health system. Int J Clin Pract. 2014;68(10):1257-1263.
3. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated February 8, 2016. Accessed April 21, 2016.
4. Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol. 2015;193(2):403-414.
5. Landau D, Tsakok T, Aylwin S, Hughes S. Should testosterone replacement be offered to hypogonadal men treated previously for prostatic carcinoma? Clin Endocrinol (Oxf). 2012;76(2):179-181.
6. Dupree JM, Langille GM, Khera M, Lipshultz LI. The safety of testosterone supplementation therapy in prostate cancer. Nat Rev Urol. 2014;11(9):526-530.
7. Pastuszak AW, Pearlman AM, Godoy G, Miles BJ, Lipshultz LI, Khera M. Testosterone replacement therapy in the setting of prostate cancer treated with radiation. Int J Impot Res. 2013;25(1):24-28.
8. Pastuszak AW, Pearlman AM, Lai WS, et al. Testosterone replacement therapy in patients with prostate cancer after radical prostatectomy. J Urol. 2013;190(2):639-644.
9. Morgentaler A. Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol. 2013;189(1)(suppl):s26-s33.
10. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
11. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):104-117.
12. Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
13. Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49(9):1239-1242.
14. Zarotsky V, Huang M-Y, Carman W, et al. Systematic literature review of the epidemiology of nongenetic forms of hypogonadism in adult males. J Hormones. 2014;2014:190347.
15. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762-769.
16. Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436-445.
17. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamicpituitary- testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
18. MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293-311.
19. Dandona P, Dhindsa S. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96(9):2643-2651.
20. Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med. 2008;66(3):103-109.
21. Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91(3):843-850.
22. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68-75.
23. Orwoll ES, Klein RF. Osteoporosis in men. Endocrine Rev. 1995;16(1):87-116.
24. Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319-1340.
25. Mah PM, Wittert GA. Obesity and testicular function. Mol Cell Endocrinol. 2010;316(2):180-186.
26. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
27. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone
therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90(2):224-251.
28. Snyder PJ, Bhasin S, Cunningham GR, et al; Testosterone Trials Investigators. Effects
of testosterone treatment in older men. N Engl J Med. 2016;374(7):611-624.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products. U.S. Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm. Updated January 14, 2016. Accessed April 12, 2016.
30. Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
31. AndroGel [package insert]. North Chicago, IL: AbbVie; 2015.
32. Depo-Testosterone [package insert]. New York, NY: Pfizer; 2015.
33. Haider A, Zitzmann M, Doros G, Isbarn H, Hammerer P, Yassin A. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol. 2015;193(1):80-86.
34. Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232-240.
35. Gomella LG. Effective testosterone suppression for prostate cancer: is there a best castration therapy? Rev Urol. 2009;11(2):52-60.
36. Khera M, Crawford D, Morales A, Salonia A, Morgentaler A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65(1):115-123.
37. Morgentaler A. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol. 2006;50(5):935-939.
38. Morgentaler A, Rhoden EL. Prevalence of prostate cancer among hypogonadal men with prostate-specific antigen levels of 4.0 ng/mL or less. Urology. 2006;68(6):1263-1267.
39. Schatzl G, Madersbacher S, Thurridl T, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47(1):52-58.
40. Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55(2):310-320.
41. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
42. Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800-809.
43. Buschemeyer WC 3rd, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007;52(2):331-343.
1. Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536-2559.
2. Shortridge EF, Polzer P, Donga P, et al. Experiences and treatment patterns of hypogonadal
men in a U.S. health system. Int J Clin Pract. 2014;68(10):1257-1263.
3. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): prostate cancer. National Comprehensive Cancer Network website. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Updated February 8, 2016. Accessed April 21, 2016.
4. Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol. 2015;193(2):403-414.
5. Landau D, Tsakok T, Aylwin S, Hughes S. Should testosterone replacement be offered to hypogonadal men treated previously for prostatic carcinoma? Clin Endocrinol (Oxf). 2012;76(2):179-181.
6. Dupree JM, Langille GM, Khera M, Lipshultz LI. The safety of testosterone supplementation therapy in prostate cancer. Nat Rev Urol. 2014;11(9):526-530.
7. Pastuszak AW, Pearlman AM, Godoy G, Miles BJ, Lipshultz LI, Khera M. Testosterone replacement therapy in the setting of prostate cancer treated with radiation. Int J Impot Res. 2013;25(1):24-28.
8. Pastuszak AW, Pearlman AM, Lai WS, et al. Testosterone replacement therapy in patients with prostate cancer after radical prostatectomy. J Urol. 2013;190(2):639-644.
9. Morgentaler A. Testosterone therapy in men with prostate cancer: scientific and ethical considerations. J Urol. 2013;189(1)(suppl):s26-s33.
10. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5-29.
11. DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64(4):104-117.
12. Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106-2131.
13. Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism. 2000;49(9):1239-1242.
14. Zarotsky V, Huang M-Y, Carman W, et al. Systematic literature review of the epidemiology of nongenetic forms of hypogonadism in adult males. J Hormones. 2014;2014:190347.
15. Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60(7):762-769.
16. Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436-445.
17. Wu FC, Tajar A, Pye SR, et al; European Male Aging Study Group. Hypothalamicpituitary- testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93(7):2737-2745.
18. MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293-311.
19. Dandona P, Dhindsa S. Update: hypogonadotropic hypogonadism in type 2 diabetes and obesity. J Clin Endocrinol Metab. 2011;96(9):2643-2651.
20. Hofstra J, Loves S, van Wageningen B, Ruinemans-Koerts J, Jansen I, de Boer H. High prevalence of hypogonadotropic hypogonadism in men referred for obesity treatment. Neth J Med. 2008;66(3):103-109.
21. Kupelian V, Page ST, Araujo AB, Travison TG, Bremner WJ, McKinlay JB. Low sex hormone-binding globulin, total testosterone, and symptomatic androgen deficiency are associated with development of the metabolic syndrome in nonobese men. J Clin Endocrinol Metab. 2006;91(3):843-850.
22. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68-75.
23. Orwoll ES, Klein RF. Osteoporosis in men. Endocrine Rev. 1995;16(1):87-116.
24. Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319-1340.
25. Mah PM, Wittert GA. Obesity and testicular function. Mol Cell Endocrinol. 2010;316(2):180-186.
26. Vigen R, O’Donnell CI, Barón AE, et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829-1836.
27. Morgentaler A, Miner MM, Caliber M, Guay AT, Khera M, Traish AM. Testosterone
therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc. 2015;90(2):224-251.
28. Snyder PJ, Bhasin S, Cunningham GR, et al; Testosterone Trials Investigators. Effects
of testosterone treatment in older men. N Engl J Med. 2016;374(7):611-624.
29. U.S. Food and Drug Administration. FDA Drug Safety Communication: FDA evaluating risk of stroke, heart attack and death with FDA-approved testosterone products. U.S. Food and Drug Administration website. http://www.fda.gov/Drugs/DrugSafety/ucm436259.htm. Updated January 14, 2016. Accessed April 12, 2016.
30. Handelsman DJ. Global trends in testosterone prescribing, 2000-2011: expanding the spectrum of prescription drug misuse. Med J Aust. 2013;199(8):548-551.
31. AndroGel [package insert]. North Chicago, IL: AbbVie; 2015.
32. Depo-Testosterone [package insert]. New York, NY: Pfizer; 2015.
33. Haider A, Zitzmann M, Doros G, Isbarn H, Hammerer P, Yassin A. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol. 2015;193(1):80-86.
34. Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232-240.
35. Gomella LG. Effective testosterone suppression for prostate cancer: is there a best castration therapy? Rev Urol. 2009;11(2):52-60.
36. Khera M, Crawford D, Morales A, Salonia A, Morgentaler A. A new era of testosterone and prostate cancer: from physiology to clinical implications. Eur Urol. 2014;65(1):115-123.
37. Morgentaler A. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol. 2006;50(5):935-939.
38. Morgentaler A, Rhoden EL. Prevalence of prostate cancer among hypogonadal men with prostate-specific antigen levels of 4.0 ng/mL or less. Urology. 2006;68(6):1263-1267.
39. Schatzl G, Madersbacher S, Thurridl T, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47(1):52-58.
40. Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55(2):310-320.
41. Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109-122.
42. Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol. 2013;63(5):800-809.
43. Buschemeyer WC 3rd, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007;52(2):331-343.