User login
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
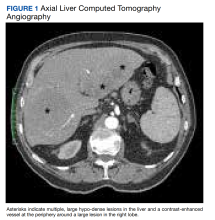
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
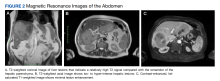
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
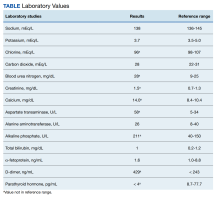
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
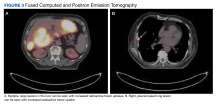
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187