User login
Primary Hepatic Lymphoma: A Rare Form of Diffuse Large B-Cell Lymphoma of the Liver
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
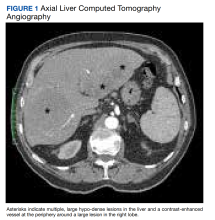
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
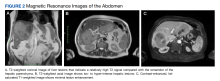
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
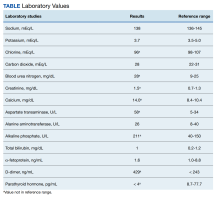
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
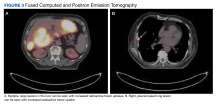
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
Primary hepatic lymphoma (PHL) is a rare, malignant lymphoma of the liver. It differs from the predominantly lymph nodal or splenic involvement associated with other types of lymphoma. It is usually detected incidentally on imaging examination, commonly computed tomography (CT), for nonspecific clinical presentation. However, it has important clinical implications for early diagnosis and treatment as indicated in our case.
Case Presentation
An 84-year-old man presented to the emergency department for evaluation of upper back pain. The patient had a history of hypertension, diabetes mellitus, and was a former smoker. He had normal vital signs, an unremarkable physical examination, and a body mass index of 25. His laboratory studies showed a normal blood cell count and serum chemistry, including serum calcium level and α-fetoprotein, but mildly elevated liver function tests.
The patient’s chest CT angiography showed no evidence of thoracic aortic dissection, penetrating atherosclerotic ulceration, or pulmonary artery embolism. Besides emphysematous changes in the lung, the chest CT was within normal limits.
Abdominal magnetic resonance imaging (MRI) showed hepatomegaly (the liver measured up to 19.3 cm in craniocaudal length) and multiple, large intrahepatic space-occupying lesions, the largest measuring 9.9 cm × 9.5 cm in the right lobe, as well as multiple lesions in the inferior right and left lobe with enhancing capsules surrounding the hepatic lesions (Figure 2).
An ultrasound-guided core needle biopsy of the liver was performed. Flow cytometry showed a monoclonal B-cell population that was mostly intermediate to large based on forward scattered light characteristics. Immunohistochemical staining was positive for CD20, BCL2, BCL6, and CD45 in the neoplastic cells. Anaplastic lymphoma kinase (ALK), CD15, CD30, and CD10 were negative, as were cytokeratin AE1/AE3 and pan-melanoma. CD3 highlighted background T cells. Ki-67 highlighted a proliferative index of approximately 75%, and the MYC stain demonstrated 50% positivity. This was consistent with diffuse large B-cell lymphoma (DLBCL). However, there was insufficient tissue on the MUM1-stained slide; therefore, it was inconclusive to distinguish a nongerminal center derived from germinal center–derived DLBCL.
Two weeks after the initial CT examination, the patient’s condition quickly deteriorated, and he was admitted for severe weakness with evidence of severe hypercalcemia, hyperuricemia, and renal insufficiency (Table).
To get additional tissue for further tumor characterization, a repeat liver biopsy was performed along with other diagnostic tests, including head MRI, bone marrow biopsy, and fluorodeoxyglucose (FDG) full-body positron emission tomography (PET). Repeat liver biopsy showed only necrotic debris with immunostaining positive for CD20 and negative for CD3. B-cell lymphomas tend to retain CD20 expression after necrosis, so the presence of CD20 staining was consistent with a necrotic tumor. Again, there was insufficient tissue on the MUM1-stained slide. Head MRI showed no evidence of tumor involvement. Full-body PET showed abnormally elevated standardized uptake value (SUV) of radioactive tracers in several areas: multifocal, large area uptake within both right (SUV, 19) and left (SUV, 24) hepatic lobe (Figure 3A), retroperitoneal lymph node (SUV, 3.9), and a right lateral pleural-based nodule (SUV, 17.9) (Figure 3B).
The diagnosis was primary DLBCL of the liver with retroperitoneal lymph nodes and right lung metastasis. The patient was started on systemic chemotherapy of R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone).
Discussion
Lymphoma is a tumor that originates from hematopoietic cells typically presented as a circumscribed solid tumor of lymphoid cells.1 Lymphomas are usually seen in the lymph nodes, spleen, blood, bone marrow, brain, gastrointestinal tract, skin, or other normal structures where lymphoreticular cells exist but very rarely in the liver.2 PHL is extremely rare due to the lack of abundant lymphoid tissue in the normal liver.3 It accounts for 0.4% of extra-nodal lymphomas and 0.016% of non-Hodgkin lymphoma.4-6 The etiology of PHL is unknown but usually it develops in patients with previous liver disease: viral infection (hepatitis B and C, Epstein-Barr, and HIV), autoimmune disease, immunosuppression, or liver cirrhosis.5-7
The diagnosis of PHL can be challenging due to its rarity, vague clinical features, and nonspecific radiologic findings. The common presenting symptoms are usually vague and include abdominal pain or discomfort, fatigue, jaundice, weight loss, and fever.5 Liver biopsy is essential to its diagnosis. The disease course is usually indolent among most patients with PHL. In our case, the patient presented with upper back pain but his condition deteriorated rapidly, likely due to the advanced stage of the disease. Diagnosis of liver lymphoma depends on a liver biopsy that should be compatible with the lymphoma. The criteria for diagnosis of PHL defined by Lei include (1) symptoms caused mainly by liver involvement at presentation; (2) absence of distant lymphadenopathy, palpable clinically at presentation or detected during staging radiologic studies; and (3) absence of leukemic blood involvement in the peripheral blood smear.7 Other authors define PHL as having major liver involvement without evidence of extrahepatic involvement for at least 6 months.8 In our case, the multiple large lesions of the liver are consistent with advanced stage PHL with retroperitoneal lymph nodes and right lung metastasis. DLBCL is the most common histopathological type of lymphoma (65.9%). Other types have been described less commonly, including diffuse mixed large- and small-cell, lymphoblastic, diffuse histiocytic, mantle cell, and small noncleaved or Burkitt lymphoma.5-7
Currently, there is no consensus on PHL treatment. The therapeutic options include surgery, chemotherapy, radiation therapy, or a combination of therapies.7 Most evidence regarding treatment and tumor response comes from case series, as PHLs are rare. Surgical resection in a series of 8 patients showed a cumulative 1- and 2-year survival rate of 66.7% and 55.6%, respectively.9 Chemotherapy is the recommended treatment option for extra-nodal DLBCL, making it a choice also for the treatment of PHL.10 Page and colleagues demonstrated that combination chemotherapy regimens helped achieve remission for 83.3% of patients.11 Since PHL is chemo-sensitive, most patients are treated with chemotherapy alone or in combination with surgery and radiotherapy. The most common chemotherapy regimen is R-CHOP for CD20-positive B-cell lymphoma. The use of the R-CHOP regimen has been reported to achieve complete remission in primary DLBCL of the liver.12
Conclusions
Primary DLBCL of the liver is a very rare disease without specific clinical manifestations, biochemical indicators, or radiologic features except for space-occupying liver lesions. However, patients’ conditions can deteriorate rapidly at an advanced stage, as demonstrated in our case. DLBCL requires a high level of suspicion for its early diagnosis and treatment and should be considered in the differential diagnosis for any hepatic space-occupying lesions.
Acknowledgments
We appreciate Lynne Dryer, ARNP, for her clinical assistance with this patient and in the preparation of the manuscript.
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
1. Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937-951. doi:10.1182/blood-2009-03-209262
2. Do TD, Neurohr C, Michl M, Reiser MF, Zech CJ. An unusual case of primary hepatic lymphoma mimicking sarcoidosis in MRI. Acta Radiol Short Rep. 2014;3(4):2047981613493625. Published 2014 May 10. doi:10.1177/2047981613493625
3. Laroia ST, Rastogi A, Panda D, Sarin SK. Primary hepatic non-Hodgkin’s lymphoma: an enigma beyond the liver, a case report. World J Oncol. 2015;6(2):338-344. doi:10.14740/wjon900W
4. Yousuf S, Szpejda M, Mody M, et al. A unique case of primary hepatic CD-30 positive, CD 15-negative classical Hodgkin’s lymphoma presenting as fever of unknown origin and acute hepatic failure. Haematol Int J. 2018;2(3):1-6. doi:10.23880/hij-16000127
5. Ugurluer G, Miller RC, Li Y, et al. Primary hepatic lymphoma: a retrospective, multicenter rare cancer network study. Rare Tumors. 2016;8(3):118-123. doi:10.4081/rt.2016.6502
6. Noronha V, Shafi NQ, Obando JÁ, Kummar S. Primary non-Hodgkin’s lymphoma of the liver. Crit Rev Oncol Hematol. 2005;53(3):199-207. doi:10.1016/j.critrevonc.2004.10.010
7. Lei KI. Primary non-Hodgkins lymphoma of the liver. Leuk Lymphoma. 1989;29(3-4):293-299. doi:10.3109/10428199809068566
8. Caccamo D, Pervez NK, Marchevsky A. Primary lymphoma of the liver in the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1986;110(6):553-555.
9. Yang XW, Tan WF, Yu WL, et al. Diagnosis and surgical treatment of primary hepatic lymphoma. World J Gastroenterol. 2010;16(47):6016-6019. doi:10.3748/wjg.v16.i47.6016
10. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23(22):5027-5033. doi:10.1200/JCO.2005.09.137
11. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination of chemotherapy. Cancer. 2001;92(8):2023-2029. doi:10.1002/1097-0142(20011015)92:8<2023::aid-cncr1540>3.0.co;2-b
12. Zafar MS, Aggarwal S, Bhalla S. Complete response to chemotherapy in primary hepatic lymphoma. J Cancer Res Ther. 2012;8(1):114-116. doi:10.4103/0973-1482.95187
Incidental Findings of Pulmonary and Hilar Malignancy by Low-Resolution Computed Tomography Used in Myocardial Perfusion Imaging (FULL)
Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is a well-established technique for the evaluation of coronary artery disease (CAD).1 To improve image quality, low-resolution computed tomography (CT) is used commonly for anatomical correct and artifact attenuation during SPECT MPI.2 The low resolution, unenhanced CT images are considered low quality and are, therefore, labeled by the manufacturer as nondiagnostic. The CT portion of the MPI in many centers is used only for image fusion and attenuation correction, and these images are not routinely reviewed or reported by cardiologists.
Incidental findings by these low-resolution CT were frequent. However, clinically significant findings, including lung cancer, although relatively infrequent, were serious enough for major clinical management.3-5 Currently, there are no consensus recommendations for reviewing low-resolution CT images or the interpretation of such incidental findings during cardiac MPI.6 Clinically, low-dose CT were used for early detection and screening of lung cancer and were associated with reduced lung-cancer and any cause mortality in National Lung Screening Trial (NLST).7,8 Therefore, low-dose CT is recommended for lung cancer screening of high-risk patients by the US Preventive Service Task Force (USPSTF).9 In the veteran population, current and past smoking history are more common when compared with the general population; therefore, veterans are potentially at increased risk of lung cancer.10 In this study, we did not intend to use low-resolution CT for lung cancer screening or detection but rather to identify and report incidental findings of pulmonary/hilar malignancy detected during cardiac MPI.
Methods
The Siemens’ (Munich, Germany) Symbia Intevo Excel SPECT/CT MPI cameras with dedicated cardiac collimators were used at both the Dwight D. Eisenhower VA Medical Center (VAMC) in Leavenworth, Kansas and Colmery-O'Neil VAMC in Topeka, Kansas. The integrated CT scanner (x-ray tube current 30 to 240 mA; voltage 110 Kv with a 40 kW power generator) has the capability to image up to a 2-slice/rotation, each of 5.0 mm per slice with a scan time of about 30 seconds. The SPECT/CT gamma camera has a low energy (140 KeV), high resolution, parallel hole collimator with IQ SPECT capabilities.
The radiation dose received by the patients were expressed in dose length product (DLP), which reflects the total energy absorbed by the patient and represents integrated dose in terms of the total scan length. Additionally, each patients received 2 injections of Technetium Tc 99m sestamibi (1-day Protocol: 10 mCi rest injection, 30 mCi stress injection: 2-day Protocol for patients weighing > 350 pounds: 30 mCi at rest injection and 30 mCi at stress injection) for myocardial perfusion imaging.
All CT images and cardiac MPI findings were reviewed and reported contemporaneously by 1 of 2 experienced, board-certified radiologists who were blinded to patients’ clinical information except the indication for the cardiac stress testing. When suspicious pulmonary/hilar nodules or masses were detected, these findings and recommendations for further evaluation were conveyed to primary care provider or ordering physician via the electronic health record system.
All CT images were reviewed with cardiac MPI from September 1, 2017 to August 31, 2018. When pulmonary/hilar malignancies were identified, the health records were reviewed. Patients with known history of prior pulmonary malignancy were excluded from the study.
Results
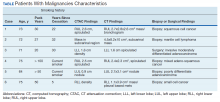
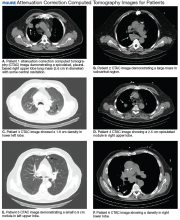
A total of 1,098 patients underwent cardiac MPI during the study period. When the CT imaging and cardiac MPI were reviewed, incidental findings led to the diagnosis of lung cancer in 5 patients and hilar mantle cell lymphoma in 1 patient. Their clinical characteristics, CT findings, and types of malignancies for these 6 patients are summarized in the Table and Figure. Only 0.55% (6 of 1,098) patients were found to have incidental pulmonary/hilar malignancy with the cardiac evaluation low-resolution CT. Four patients with prior, known history of lung cancer were excluded from the study.
For the 6 patients found to have cancer, the average CT radiation dose during the cardiac MPI was 100 mGy-cm (range, 77 -133 mCy-cm). The subsequent chest CT with or without contrast delivered a radiation dose of 726.4 mGy-cm (range, 279.4 - 1,075 mGy-cm).
A total of 79 (7.2%) patients were found to have significant pulmonary nodules that required further evaluation; after CT examination, 32 patients had findings of benign nature and required no further follow-up; the other 47 patients are being followed according to the Fleischner Society 2017 guidelines for pulmonary nodules.11 The follow-up findings on these patients are not within the scope of this report.
Discussion
Although incidental findings on low-resolution CT during cardiac MPI are frequent, clinically significant findings are less common. However, some incidental findings may be of important clinical significance.3-5 A multicenter analysis by Coward and colleagues reported that 2.4% findings on low-resolution CT were significant enough to warrant follow-up tests, but only 0.2% were deemed potentially detrimental to patient outcomes (ie, pathology confirmed malignancies).12 Thus, the authors suggested that routine reporting of incidental findings on low-dose CT images was not beneficial.12,13
Currently, the majority of cardiac MPIs are reviewed and interpreted by nuclear cardiologists, the use of hybrid SPECT/CT for attenuation correction give rise of issue of reviewing and interpreting these CT images during cardiac MPI. Since low-dose, low-resolution CT are considered nondiagnostic, these images are not routinely and readily reviewed by cardiologists who are not trained or skilled in CT interpretations.
Studies of high-resolution cardiac CT (including multidetector CT with contrast) suggest that incidental extracardiac findings should always be reported as there was a 0.7% incidence of previously unknown malignancies, while others have argued against “performing large field reconstructionsfor the explicit purpose of screening as it will lead to additional cost, liability and anxiety without proven benefits.”14-16 A review of incidental findings of cardiac CT by Earls suggested that all cardiac CT should be reconstructed in the maximal field of view available and images should be adequately reviewed to detect pathological findings.17 This led to an interesting discussion by Douglas and colleagues regarding the role of cardiologists and radiologists in this issue.18 Currently there is no uniform or consensus recommendations regarding incidental findings during cardiac CT imaging. Guidances range from no recommendations to optional reporting or mandatory reporting.19-23
Risk Factors for Veterans
Lung cancer is the second most common cancer and the leading cause of cancer-related death in the US.24 Smoking is the most important risk factor for lung cancer and CAD.25 Current or past smoking are more common among the veterans.10 According to a report for the US Centers for Disease Control and Prevention report, about 29.2% US veterans use tobacco products between 2010-2015, which is similar to the rate reported in 1997.26
When low-dose CT was used for lung cancer screening, it was associated with a 20.0% reduction in lung cancer mortality and a 6.7% reduction in any cause mortality.7 Currently, the US Preventive Services Task Force (USPSTF) recommends annual low-dose CT screening for lung cancer in high-risk adults that includes patients aged 55 to 80 years who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years.8
It is likely that the cardiac patients in this study might have pulmonary malignancy mortality similar to those reported in the NLST. While other studies have shown a low incidence (0.2%) of detection of malignancy by low-resolution CT during cardiac MPI,12,13 in this study we found pulmonary or hilar malignancy in 0.55% of patients.The higher incidence of malignancy in our study might be due in part to differences in the patient population studied (ie, our veterans patients have a higher proportion of current or past smoking history).10
The CT used in this study is part of the cardiac imaging process. Therefore, there was no additional radiation exposure besides that of the cardiac MPI for patients. Despite the limitations of low-resolution CT, which may miss small lesions, this study showed 0.55% incidence of incidental detection of pulmonary/hilar malignancy. This is comparable with 0.65%/year of diagnosing lung cancer using low-dose CT for lung cancer screening in NLST.8
Two of the 5 study patients who were found to have lung cancer, had quit smoking > 15 years previously and thus would not be considered as high-risk for lung cancer screening according to USPSTF guideline. These patients would not have been candidates for annual low-dose CT lung cancer screening. This study suggests that it is appropriate and necessary to review the low-resolution CT images for incidental findings during cardiac MPI.
Limitations
The study was retrospective in nature and limited by its small number of patients. The CT modality used in the study also has limitations, including low resolution, respiratory motion artifacts, and scans that did not include the entire chest area. Therefore, small and apical lesions may have been missed. However, both sets of CT at rest and after stress were reviewed to reduce or minimize the effects of respiratory motion artifacts. The true prevalence or incidence of pulmonary/hilar malignancies may have been higher than reported here. Our study population of veterans may not be representative of the general population with regards to gender (as most of our veteran patient population are of male gender, vs general population), smoking history, or lung cancer risk, thus the results should be interpreted with caution.
Conclusion
Low-resolution CTs used for attenuation correction during cardiac MPI should be routinely reviewed and interpreted by a physician or radiologist skilled in CT interpretation in order to identify incidental findings of pulmonary/hilar malignancy. This would require close collaboration between cardiologists and radiologists in the field to ensure unfragmented and high-quality patient care.
Acknowledgements
We want to thank all the staffs in cardiology and radiology department on both campuses for their dedication for our patients. Special thanks to Laura Knox, Radiation Safety Officer, Nuclear Medicine Supervisor for her technical assistance.
1. Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119(22):e561-e587.
2. Hendel RC, Corbett JR, Cullom SJ, DePuey EG, Garcia EV, Bateman TM. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: a joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Cardiol. 2002;9(1):135–143.
3. Coward J, Nightingale J, Hogg P. The clinical dilemma of incidental findings on the low-resolution CT images from SPECT/CT MPI studies. J Nucl Med Technol. 2016;44(3):167-172.
4. Osman MM, Cohade C, Fishman E, Wahl RL. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. J Nucl Med. 2005;46(8):1352-1355.
5. Goetze S, Pannu HK, Wahl RL. Clinically significant abnormal findings on the “nondiagnostic” CT portion of low-amperage-CT attenuation-corrected myocardial perfusion SPECT/CT studies. J Nucl Med. 2006;47(8):1312-1318.
6. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Mark DB, Berman DS, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2663-2699.
7. Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222(3):773-781.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
9. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338.
10. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-218.
11. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
12. Coward J, Lawson R, Kane T, et al. Multi-centre analysis of incidental findings on low-resolution CT attenuation correction images. Br J Radiol. 2014;87(1042):20130701.
13. Coward J, Lawson R, Kane T, et al. Multicentre analysis of incidental findings on low-resolution CT attenuation correction images: an extended study. Br J Radiol. 2015;88(1056):20150555.
14. Haller S, Kaiser C, Buser P, Bongartz G, Bremerich J. Coronary artery imaging with contrast-enhanced MDCT: extracardiac findings. AJR Am J Roentgenol. 2006;187(1):105-110.
15. Flor N, Di Leo G, Squarza SA, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. AJR Am J Roentgenol. 2013;201(3):555-564.
16. Budoff MJ, Gopal A. Incidental findings on cardiac computed tomography. Should we look? J Cardiovasc Comput Tomogr. 2007;1(2):97-105.
17. Earls JP. The pros and cons of searching for extracardiac findings at cardiac CT: studies should be reconstructed in the maximum field of view and adequately reviewed to detect pathologic findings. Radiology. 2011;261(2):342-346.
18. Douglas PS, Cerqueria M, Rubin GD, Chin AS. Extracardiac findings: what is a cardiologist to do? JACC Cardiovasc Imaging. 2008;1(5):682-687.
19. Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17(5):941-973.
20. Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25(5):1784-1846.
21. Tilkemeier PL, Bourque J, Doukky R, Sanghani R, Weinberg RL. ASNC imaging guidelines for nuclear cardiology procedures : Standardized reporting of nuclear cardiology procedures. J Nucl Cardiol. 2017;24(6):2064-2128.
22. Dorbala S, Di Carli MF, Delbeke D, et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med. 2013;54(8):1485-1507.
23. Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187-1226.
24. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9):djx030.
25. US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014.
26. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco Product Use Among Military Veterans - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12.
Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is a well-established technique for the evaluation of coronary artery disease (CAD).1 To improve image quality, low-resolution computed tomography (CT) is used commonly for anatomical correct and artifact attenuation during SPECT MPI.2 The low resolution, unenhanced CT images are considered low quality and are, therefore, labeled by the manufacturer as nondiagnostic. The CT portion of the MPI in many centers is used only for image fusion and attenuation correction, and these images are not routinely reviewed or reported by cardiologists.
Incidental findings by these low-resolution CT were frequent. However, clinically significant findings, including lung cancer, although relatively infrequent, were serious enough for major clinical management.3-5 Currently, there are no consensus recommendations for reviewing low-resolution CT images or the interpretation of such incidental findings during cardiac MPI.6 Clinically, low-dose CT were used for early detection and screening of lung cancer and were associated with reduced lung-cancer and any cause mortality in National Lung Screening Trial (NLST).7,8 Therefore, low-dose CT is recommended for lung cancer screening of high-risk patients by the US Preventive Service Task Force (USPSTF).9 In the veteran population, current and past smoking history are more common when compared with the general population; therefore, veterans are potentially at increased risk of lung cancer.10 In this study, we did not intend to use low-resolution CT for lung cancer screening or detection but rather to identify and report incidental findings of pulmonary/hilar malignancy detected during cardiac MPI.
Methods
The Siemens’ (Munich, Germany) Symbia Intevo Excel SPECT/CT MPI cameras with dedicated cardiac collimators were used at both the Dwight D. Eisenhower VA Medical Center (VAMC) in Leavenworth, Kansas and Colmery-O'Neil VAMC in Topeka, Kansas. The integrated CT scanner (x-ray tube current 30 to 240 mA; voltage 110 Kv with a 40 kW power generator) has the capability to image up to a 2-slice/rotation, each of 5.0 mm per slice with a scan time of about 30 seconds. The SPECT/CT gamma camera has a low energy (140 KeV), high resolution, parallel hole collimator with IQ SPECT capabilities.
The radiation dose received by the patients were expressed in dose length product (DLP), which reflects the total energy absorbed by the patient and represents integrated dose in terms of the total scan length. Additionally, each patients received 2 injections of Technetium Tc 99m sestamibi (1-day Protocol: 10 mCi rest injection, 30 mCi stress injection: 2-day Protocol for patients weighing > 350 pounds: 30 mCi at rest injection and 30 mCi at stress injection) for myocardial perfusion imaging.
All CT images and cardiac MPI findings were reviewed and reported contemporaneously by 1 of 2 experienced, board-certified radiologists who were blinded to patients’ clinical information except the indication for the cardiac stress testing. When suspicious pulmonary/hilar nodules or masses were detected, these findings and recommendations for further evaluation were conveyed to primary care provider or ordering physician via the electronic health record system.
All CT images were reviewed with cardiac MPI from September 1, 2017 to August 31, 2018. When pulmonary/hilar malignancies were identified, the health records were reviewed. Patients with known history of prior pulmonary malignancy were excluded from the study.
Results
A total of 1,098 patients underwent cardiac MPI during the study period. When the CT imaging and cardiac MPI were reviewed, incidental findings led to the diagnosis of lung cancer in 5 patients and hilar mantle cell lymphoma in 1 patient. Their clinical characteristics, CT findings, and types of malignancies for these 6 patients are summarized in the Table and Figure. Only 0.55% (6 of 1,098) patients were found to have incidental pulmonary/hilar malignancy with the cardiac evaluation low-resolution CT. Four patients with prior, known history of lung cancer were excluded from the study.
For the 6 patients found to have cancer, the average CT radiation dose during the cardiac MPI was 100 mGy-cm (range, 77 -133 mCy-cm). The subsequent chest CT with or without contrast delivered a radiation dose of 726.4 mGy-cm (range, 279.4 - 1,075 mGy-cm).
A total of 79 (7.2%) patients were found to have significant pulmonary nodules that required further evaluation; after CT examination, 32 patients had findings of benign nature and required no further follow-up; the other 47 patients are being followed according to the Fleischner Society 2017 guidelines for pulmonary nodules.11 The follow-up findings on these patients are not within the scope of this report.
Discussion
Although incidental findings on low-resolution CT during cardiac MPI are frequent, clinically significant findings are less common. However, some incidental findings may be of important clinical significance.3-5 A multicenter analysis by Coward and colleagues reported that 2.4% findings on low-resolution CT were significant enough to warrant follow-up tests, but only 0.2% were deemed potentially detrimental to patient outcomes (ie, pathology confirmed malignancies).12 Thus, the authors suggested that routine reporting of incidental findings on low-dose CT images was not beneficial.12,13
Currently, the majority of cardiac MPIs are reviewed and interpreted by nuclear cardiologists, the use of hybrid SPECT/CT for attenuation correction give rise of issue of reviewing and interpreting these CT images during cardiac MPI. Since low-dose, low-resolution CT are considered nondiagnostic, these images are not routinely and readily reviewed by cardiologists who are not trained or skilled in CT interpretations.
Studies of high-resolution cardiac CT (including multidetector CT with contrast) suggest that incidental extracardiac findings should always be reported as there was a 0.7% incidence of previously unknown malignancies, while others have argued against “performing large field reconstructionsfor the explicit purpose of screening as it will lead to additional cost, liability and anxiety without proven benefits.”14-16 A review of incidental findings of cardiac CT by Earls suggested that all cardiac CT should be reconstructed in the maximal field of view available and images should be adequately reviewed to detect pathological findings.17 This led to an interesting discussion by Douglas and colleagues regarding the role of cardiologists and radiologists in this issue.18 Currently there is no uniform or consensus recommendations regarding incidental findings during cardiac CT imaging. Guidances range from no recommendations to optional reporting or mandatory reporting.19-23
Risk Factors for Veterans
Lung cancer is the second most common cancer and the leading cause of cancer-related death in the US.24 Smoking is the most important risk factor for lung cancer and CAD.25 Current or past smoking are more common among the veterans.10 According to a report for the US Centers for Disease Control and Prevention report, about 29.2% US veterans use tobacco products between 2010-2015, which is similar to the rate reported in 1997.26
When low-dose CT was used for lung cancer screening, it was associated with a 20.0% reduction in lung cancer mortality and a 6.7% reduction in any cause mortality.7 Currently, the US Preventive Services Task Force (USPSTF) recommends annual low-dose CT screening for lung cancer in high-risk adults that includes patients aged 55 to 80 years who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years.8
It is likely that the cardiac patients in this study might have pulmonary malignancy mortality similar to those reported in the NLST. While other studies have shown a low incidence (0.2%) of detection of malignancy by low-resolution CT during cardiac MPI,12,13 in this study we found pulmonary or hilar malignancy in 0.55% of patients.The higher incidence of malignancy in our study might be due in part to differences in the patient population studied (ie, our veterans patients have a higher proportion of current or past smoking history).10
The CT used in this study is part of the cardiac imaging process. Therefore, there was no additional radiation exposure besides that of the cardiac MPI for patients. Despite the limitations of low-resolution CT, which may miss small lesions, this study showed 0.55% incidence of incidental detection of pulmonary/hilar malignancy. This is comparable with 0.65%/year of diagnosing lung cancer using low-dose CT for lung cancer screening in NLST.8
Two of the 5 study patients who were found to have lung cancer, had quit smoking > 15 years previously and thus would not be considered as high-risk for lung cancer screening according to USPSTF guideline. These patients would not have been candidates for annual low-dose CT lung cancer screening. This study suggests that it is appropriate and necessary to review the low-resolution CT images for incidental findings during cardiac MPI.
Limitations
The study was retrospective in nature and limited by its small number of patients. The CT modality used in the study also has limitations, including low resolution, respiratory motion artifacts, and scans that did not include the entire chest area. Therefore, small and apical lesions may have been missed. However, both sets of CT at rest and after stress were reviewed to reduce or minimize the effects of respiratory motion artifacts. The true prevalence or incidence of pulmonary/hilar malignancies may have been higher than reported here. Our study population of veterans may not be representative of the general population with regards to gender (as most of our veteran patient population are of male gender, vs general population), smoking history, or lung cancer risk, thus the results should be interpreted with caution.
Conclusion
Low-resolution CTs used for attenuation correction during cardiac MPI should be routinely reviewed and interpreted by a physician or radiologist skilled in CT interpretation in order to identify incidental findings of pulmonary/hilar malignancy. This would require close collaboration between cardiologists and radiologists in the field to ensure unfragmented and high-quality patient care.
Acknowledgements
We want to thank all the staffs in cardiology and radiology department on both campuses for their dedication for our patients. Special thanks to Laura Knox, Radiation Safety Officer, Nuclear Medicine Supervisor for her technical assistance.
Single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) is a well-established technique for the evaluation of coronary artery disease (CAD).1 To improve image quality, low-resolution computed tomography (CT) is used commonly for anatomical correct and artifact attenuation during SPECT MPI.2 The low resolution, unenhanced CT images are considered low quality and are, therefore, labeled by the manufacturer as nondiagnostic. The CT portion of the MPI in many centers is used only for image fusion and attenuation correction, and these images are not routinely reviewed or reported by cardiologists.
Incidental findings by these low-resolution CT were frequent. However, clinically significant findings, including lung cancer, although relatively infrequent, were serious enough for major clinical management.3-5 Currently, there are no consensus recommendations for reviewing low-resolution CT images or the interpretation of such incidental findings during cardiac MPI.6 Clinically, low-dose CT were used for early detection and screening of lung cancer and were associated with reduced lung-cancer and any cause mortality in National Lung Screening Trial (NLST).7,8 Therefore, low-dose CT is recommended for lung cancer screening of high-risk patients by the US Preventive Service Task Force (USPSTF).9 In the veteran population, current and past smoking history are more common when compared with the general population; therefore, veterans are potentially at increased risk of lung cancer.10 In this study, we did not intend to use low-resolution CT for lung cancer screening or detection but rather to identify and report incidental findings of pulmonary/hilar malignancy detected during cardiac MPI.
Methods
The Siemens’ (Munich, Germany) Symbia Intevo Excel SPECT/CT MPI cameras with dedicated cardiac collimators were used at both the Dwight D. Eisenhower VA Medical Center (VAMC) in Leavenworth, Kansas and Colmery-O'Neil VAMC in Topeka, Kansas. The integrated CT scanner (x-ray tube current 30 to 240 mA; voltage 110 Kv with a 40 kW power generator) has the capability to image up to a 2-slice/rotation, each of 5.0 mm per slice with a scan time of about 30 seconds. The SPECT/CT gamma camera has a low energy (140 KeV), high resolution, parallel hole collimator with IQ SPECT capabilities.
The radiation dose received by the patients were expressed in dose length product (DLP), which reflects the total energy absorbed by the patient and represents integrated dose in terms of the total scan length. Additionally, each patients received 2 injections of Technetium Tc 99m sestamibi (1-day Protocol: 10 mCi rest injection, 30 mCi stress injection: 2-day Protocol for patients weighing > 350 pounds: 30 mCi at rest injection and 30 mCi at stress injection) for myocardial perfusion imaging.
All CT images and cardiac MPI findings were reviewed and reported contemporaneously by 1 of 2 experienced, board-certified radiologists who were blinded to patients’ clinical information except the indication for the cardiac stress testing. When suspicious pulmonary/hilar nodules or masses were detected, these findings and recommendations for further evaluation were conveyed to primary care provider or ordering physician via the electronic health record system.
All CT images were reviewed with cardiac MPI from September 1, 2017 to August 31, 2018. When pulmonary/hilar malignancies were identified, the health records were reviewed. Patients with known history of prior pulmonary malignancy were excluded from the study.
Results
A total of 1,098 patients underwent cardiac MPI during the study period. When the CT imaging and cardiac MPI were reviewed, incidental findings led to the diagnosis of lung cancer in 5 patients and hilar mantle cell lymphoma in 1 patient. Their clinical characteristics, CT findings, and types of malignancies for these 6 patients are summarized in the Table and Figure. Only 0.55% (6 of 1,098) patients were found to have incidental pulmonary/hilar malignancy with the cardiac evaluation low-resolution CT. Four patients with prior, known history of lung cancer were excluded from the study.
For the 6 patients found to have cancer, the average CT radiation dose during the cardiac MPI was 100 mGy-cm (range, 77 -133 mCy-cm). The subsequent chest CT with or without contrast delivered a radiation dose of 726.4 mGy-cm (range, 279.4 - 1,075 mGy-cm).
A total of 79 (7.2%) patients were found to have significant pulmonary nodules that required further evaluation; after CT examination, 32 patients had findings of benign nature and required no further follow-up; the other 47 patients are being followed according to the Fleischner Society 2017 guidelines for pulmonary nodules.11 The follow-up findings on these patients are not within the scope of this report.
Discussion
Although incidental findings on low-resolution CT during cardiac MPI are frequent, clinically significant findings are less common. However, some incidental findings may be of important clinical significance.3-5 A multicenter analysis by Coward and colleagues reported that 2.4% findings on low-resolution CT were significant enough to warrant follow-up tests, but only 0.2% were deemed potentially detrimental to patient outcomes (ie, pathology confirmed malignancies).12 Thus, the authors suggested that routine reporting of incidental findings on low-dose CT images was not beneficial.12,13
Currently, the majority of cardiac MPIs are reviewed and interpreted by nuclear cardiologists, the use of hybrid SPECT/CT for attenuation correction give rise of issue of reviewing and interpreting these CT images during cardiac MPI. Since low-dose, low-resolution CT are considered nondiagnostic, these images are not routinely and readily reviewed by cardiologists who are not trained or skilled in CT interpretations.
Studies of high-resolution cardiac CT (including multidetector CT with contrast) suggest that incidental extracardiac findings should always be reported as there was a 0.7% incidence of previously unknown malignancies, while others have argued against “performing large field reconstructionsfor the explicit purpose of screening as it will lead to additional cost, liability and anxiety without proven benefits.”14-16 A review of incidental findings of cardiac CT by Earls suggested that all cardiac CT should be reconstructed in the maximal field of view available and images should be adequately reviewed to detect pathological findings.17 This led to an interesting discussion by Douglas and colleagues regarding the role of cardiologists and radiologists in this issue.18 Currently there is no uniform or consensus recommendations regarding incidental findings during cardiac CT imaging. Guidances range from no recommendations to optional reporting or mandatory reporting.19-23
Risk Factors for Veterans
Lung cancer is the second most common cancer and the leading cause of cancer-related death in the US.24 Smoking is the most important risk factor for lung cancer and CAD.25 Current or past smoking are more common among the veterans.10 According to a report for the US Centers for Disease Control and Prevention report, about 29.2% US veterans use tobacco products between 2010-2015, which is similar to the rate reported in 1997.26
When low-dose CT was used for lung cancer screening, it was associated with a 20.0% reduction in lung cancer mortality and a 6.7% reduction in any cause mortality.7 Currently, the US Preventive Services Task Force (USPSTF) recommends annual low-dose CT screening for lung cancer in high-risk adults that includes patients aged 55 to 80 years who have a 30-pack-year smoking history and currently smoke or have quit within the past 15 years.8
It is likely that the cardiac patients in this study might have pulmonary malignancy mortality similar to those reported in the NLST. While other studies have shown a low incidence (0.2%) of detection of malignancy by low-resolution CT during cardiac MPI,12,13 in this study we found pulmonary or hilar malignancy in 0.55% of patients.The higher incidence of malignancy in our study might be due in part to differences in the patient population studied (ie, our veterans patients have a higher proportion of current or past smoking history).10
The CT used in this study is part of the cardiac imaging process. Therefore, there was no additional radiation exposure besides that of the cardiac MPI for patients. Despite the limitations of low-resolution CT, which may miss small lesions, this study showed 0.55% incidence of incidental detection of pulmonary/hilar malignancy. This is comparable with 0.65%/year of diagnosing lung cancer using low-dose CT for lung cancer screening in NLST.8
Two of the 5 study patients who were found to have lung cancer, had quit smoking > 15 years previously and thus would not be considered as high-risk for lung cancer screening according to USPSTF guideline. These patients would not have been candidates for annual low-dose CT lung cancer screening. This study suggests that it is appropriate and necessary to review the low-resolution CT images for incidental findings during cardiac MPI.
Limitations
The study was retrospective in nature and limited by its small number of patients. The CT modality used in the study also has limitations, including low resolution, respiratory motion artifacts, and scans that did not include the entire chest area. Therefore, small and apical lesions may have been missed. However, both sets of CT at rest and after stress were reviewed to reduce or minimize the effects of respiratory motion artifacts. The true prevalence or incidence of pulmonary/hilar malignancies may have been higher than reported here. Our study population of veterans may not be representative of the general population with regards to gender (as most of our veteran patient population are of male gender, vs general population), smoking history, or lung cancer risk, thus the results should be interpreted with caution.
Conclusion
Low-resolution CTs used for attenuation correction during cardiac MPI should be routinely reviewed and interpreted by a physician or radiologist skilled in CT interpretation in order to identify incidental findings of pulmonary/hilar malignancy. This would require close collaboration between cardiologists and radiologists in the field to ensure unfragmented and high-quality patient care.
Acknowledgements
We want to thank all the staffs in cardiology and radiology department on both campuses for their dedication for our patients. Special thanks to Laura Knox, Radiation Safety Officer, Nuclear Medicine Supervisor for her technical assistance.
1. Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119(22):e561-e587.
2. Hendel RC, Corbett JR, Cullom SJ, DePuey EG, Garcia EV, Bateman TM. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: a joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Cardiol. 2002;9(1):135–143.
3. Coward J, Nightingale J, Hogg P. The clinical dilemma of incidental findings on the low-resolution CT images from SPECT/CT MPI studies. J Nucl Med Technol. 2016;44(3):167-172.
4. Osman MM, Cohade C, Fishman E, Wahl RL. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. J Nucl Med. 2005;46(8):1352-1355.
5. Goetze S, Pannu HK, Wahl RL. Clinically significant abnormal findings on the “nondiagnostic” CT portion of low-amperage-CT attenuation-corrected myocardial perfusion SPECT/CT studies. J Nucl Med. 2006;47(8):1312-1318.
6. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Mark DB, Berman DS, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2663-2699.
7. Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222(3):773-781.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
9. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338.
10. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-218.
11. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
12. Coward J, Lawson R, Kane T, et al. Multi-centre analysis of incidental findings on low-resolution CT attenuation correction images. Br J Radiol. 2014;87(1042):20130701.
13. Coward J, Lawson R, Kane T, et al. Multicentre analysis of incidental findings on low-resolution CT attenuation correction images: an extended study. Br J Radiol. 2015;88(1056):20150555.
14. Haller S, Kaiser C, Buser P, Bongartz G, Bremerich J. Coronary artery imaging with contrast-enhanced MDCT: extracardiac findings. AJR Am J Roentgenol. 2006;187(1):105-110.
15. Flor N, Di Leo G, Squarza SA, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. AJR Am J Roentgenol. 2013;201(3):555-564.
16. Budoff MJ, Gopal A. Incidental findings on cardiac computed tomography. Should we look? J Cardiovasc Comput Tomogr. 2007;1(2):97-105.
17. Earls JP. The pros and cons of searching for extracardiac findings at cardiac CT: studies should be reconstructed in the maximum field of view and adequately reviewed to detect pathologic findings. Radiology. 2011;261(2):342-346.
18. Douglas PS, Cerqueria M, Rubin GD, Chin AS. Extracardiac findings: what is a cardiologist to do? JACC Cardiovasc Imaging. 2008;1(5):682-687.
19. Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17(5):941-973.
20. Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25(5):1784-1846.
21. Tilkemeier PL, Bourque J, Doukky R, Sanghani R, Weinberg RL. ASNC imaging guidelines for nuclear cardiology procedures : Standardized reporting of nuclear cardiology procedures. J Nucl Cardiol. 2017;24(6):2064-2128.
22. Dorbala S, Di Carli MF, Delbeke D, et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med. 2013;54(8):1485-1507.
23. Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187-1226.
24. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9):djx030.
25. US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014.
26. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco Product Use Among Military Veterans - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12.
1. Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Circulation. 2009;119(22):e561-e587.
2. Hendel RC, Corbett JR, Cullom SJ, DePuey EG, Garcia EV, Bateman TM. The value and practice of attenuation correction for myocardial perfusion SPECT imaging: a joint position statement from the American Society of Nuclear Cardiology and the Society of Nuclear Medicine. J Nucl Cardiol. 2002;9(1):135–143.
3. Coward J, Nightingale J, Hogg P. The clinical dilemma of incidental findings on the low-resolution CT images from SPECT/CT MPI studies. J Nucl Med Technol. 2016;44(3):167-172.
4. Osman MM, Cohade C, Fishman E, Wahl RL. Clinically significant incidental findings on the unenhanced CT portion of PET/CT studies: frequency in 250 patients. J Nucl Med. 2005;46(8):1352-1355.
5. Goetze S, Pannu HK, Wahl RL. Clinically significant abnormal findings on the “nondiagnostic” CT portion of low-amperage-CT attenuation-corrected myocardial perfusion SPECT/CT studies. J Nucl Med. 2006;47(8):1312-1318.
6. American College of Cardiology Foundation Task Force on Expert Consensus Documents, Mark DB, Berman DS, et al. ACCF/ACR/AHA/NASCI/SAIP/SCAI/SCCT 2010 expert consensus document on coronary computed tomographic angiography: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol. 2010;55(23):2663-2699.
7. Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology. 2002;222(3):773-781.
8. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
9. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338.
10. McKinney WP, McIntire DD, Carmody TJ, Joseph A. Comparing the smoking behavior of veterans and nonveterans. Public Health Rep. 1997;112(3):212-218.
11. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
12. Coward J, Lawson R, Kane T, et al. Multi-centre analysis of incidental findings on low-resolution CT attenuation correction images. Br J Radiol. 2014;87(1042):20130701.
13. Coward J, Lawson R, Kane T, et al. Multicentre analysis of incidental findings on low-resolution CT attenuation correction images: an extended study. Br J Radiol. 2015;88(1056):20150555.
14. Haller S, Kaiser C, Buser P, Bongartz G, Bremerich J. Coronary artery imaging with contrast-enhanced MDCT: extracardiac findings. AJR Am J Roentgenol. 2006;187(1):105-110.
15. Flor N, Di Leo G, Squarza SA, et al. Malignant incidental extracardiac findings on cardiac CT: systematic review and meta-analysis. AJR Am J Roentgenol. 2013;201(3):555-564.
16. Budoff MJ, Gopal A. Incidental findings on cardiac computed tomography. Should we look? J Cardiovasc Comput Tomogr. 2007;1(2):97-105.
17. Earls JP. The pros and cons of searching for extracardiac findings at cardiac CT: studies should be reconstructed in the maximum field of view and adequately reviewed to detect pathologic findings. Radiology. 2011;261(2):342-346.
18. Douglas PS, Cerqueria M, Rubin GD, Chin AS. Extracardiac findings: what is a cardiologist to do? JACC Cardiovasc Imaging. 2008;1(5):682-687.
19. Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17(5):941-973.
20. Dorbala S, Ananthasubramaniam K, Armstrong IS, et al. Single photon emission computed tomography (SPECT) myocardial perfusion imaging guidelines: instrumentation, acquisition, processing, and interpretation. J Nucl Cardiol. 2018;25(5):1784-1846.
21. Tilkemeier PL, Bourque J, Doukky R, Sanghani R, Weinberg RL. ASNC imaging guidelines for nuclear cardiology procedures : Standardized reporting of nuclear cardiology procedures. J Nucl Cardiol. 2017;24(6):2064-2128.
22. Dorbala S, Di Carli MF, Delbeke D, et al. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med. 2013;54(8):1485-1507.
23. Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23(5):1187-1226.
24. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109(9):djx030.
25. US Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. Printed with corrections, January 2014.
26. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco Product Use Among Military Veterans - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12.