User login
To the Editor:
The organisms of the genus Nocardia are gram-positive, ubiquitous, aerobic actinomycetes found worldwide in soil, decaying organic material, and water.1 The genus Nocardia includes more than 50 species; some species, such as Nocardia asteroides, Nocardia farcinica, Nocardia nova, and Nocardia brasiliensis, are the cause of nocardiosis in humans and animals.2,3 Nocardiosis is a rare and opportunistic infection that predominantly affects immunocompromised individuals; however, up to 30% of infections can occur in immunocompetent hosts.4 Nocardiosis can manifest in 3 disease forms: cutaneous, pulmonary, or disseminated. Cutaneous nocardiosis commonly develops in immunocompetent individuals who have experienced a predisposing traumatic injury to the skin,5 and it can exhibit a diverse variety of clinical manifestations, making diagnosis difficult. We describe a case of serious progressive primary cutaneous nocardiosis with an unusual presentation in an immunocompetent patient.
A 26-year-old immunocompetent man presented with pain, swelling, nodules, abscesses, ulcers, and sinus drainage of the left arm. The left elbow lesion initially developed at the site of a trauma 6 years prior that was painless but was contaminated with mossy soil. The condition slowly progressed over the next 2 years, and the patient experienced increased swelling and eventually developed multiple draining sinus tracts. Over the next 4 years, the lesions multiplied, spreading to the forearm and upper arm; associated severe pain and swelling at the elbow and wrist joint developed. The patient sought medical care at a local hospital and subsequently was diagnosed with suspected cutaneous tuberculosis. The patient was empirically treated with a 6-month course of isoniazid, rifampicin, pyrazinamide, and ethambutol; however, the lesions continued to progress and worsen. The patient had to stop antibiotic treatment because of substantially elevated alanine aminotransferase and aspartate aminotransferase levels.
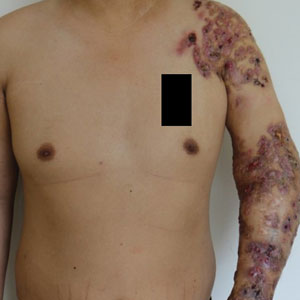
He subsequently was evaluated at our hospital. He had no notable medical history and was afebrile. Physical examination revealed multiple erythematous nodules, abscesses, and ulcers on the left arm. There were several nodules with open sinus tracts and seropurulent crusts along with numerous atrophic, ovoid, stellate scars. Other nodules and ulcers with purulent drainage were located along the lymphatic nodes extending up the patient’s left forearm (Figure 1A). The yellowish-white pus discharge from several active sinuses contained no apparent granules. The lesions were densely distributed along the elbow, wrist, and shoulder, which resulted in associated skin swelling and restricted joint movement. The left axillary lymph nodes were enlarged.

Laboratory analyses revealed a hemoglobin level of 9.6 g/dL (reference range, 13–17.5 g/dL), platelet count of 621×109/L (reference range, 125–350×109/L), and leukocyte count of 14.3×109/L (reference range, 3.5–9.5 ×109/L). C-reactive protein level was 88.4 mg/L (reference range, 0–10 mg/L). Blood, renal, and liver tests, as well as tumor marker, peripheral blood lymphocyte subset, immunoglobulin, and complement results were within reference ranges. Results for Treponema pallidum and HIV antibody tests were negative. Hepatitis B virus markers were positive for hepatitis B surface antigen, hepatitis B e antigen, and hepatitis B core antibody, and the serum concentration of hepatitis B virus DNA was 3.12×107 IU/mL (reference range, <5×102 IU/mL). Computed tomography of the chest and cranium were unremarkable. Ultrasonography of the left arm revealed multiple vertical sinus tracts and several horizontal communicating branches that were accompanied by worm-eaten bone destruction (Figure 2).

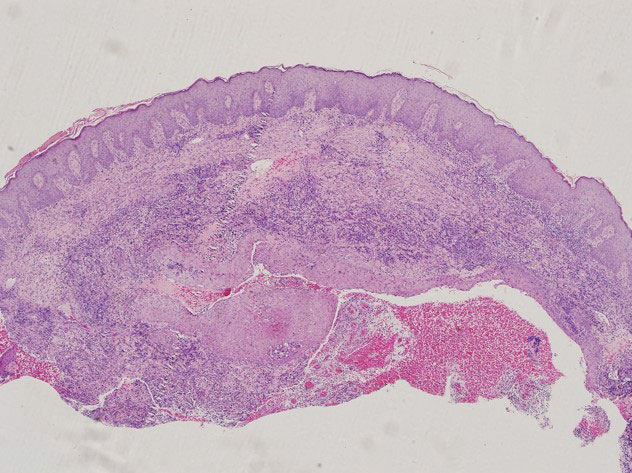
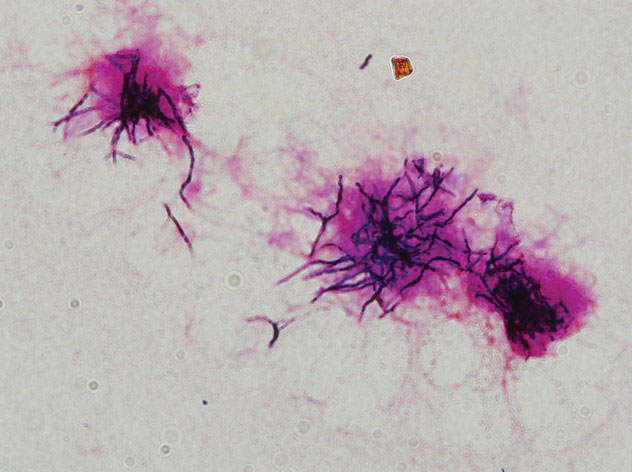
Additional testing included histopathologic staining of a skin tissue specimen—hematoxylin and eosin, periodic acid–Schiff, and acid-fast staining—showed nonspecific, diffuse, inflammatory cell infiltration suggestive of chronic suppurative granuloma (Figure 3) but failed to reveal any special strains or organisms. Gram stain examination of the purulent fluid collected from the subcutaneous tissue showed no apparent positive bacillus or filamentous granules. The specimen was then inoculated on Sabouraud dextrose agar and Lowenstein-Jensen medium for fungus and mycobacteria culture, respectively. After 5 days, chalky, yellow, adherent colonies were observed on the Löwenstein-Jensen medium, and after 26 days, yellow crinkled colonies were observed on Sabouraud dextrose agar. The colonies were then inoculated on Columbia blood agar and incubated for 1 week to aid in the identification of organisms. Growth of yellow colonies that were adherent to the agar, moist, and smooth with a velvety surface, as well as a characteristic moldy odor resulted. Gram staining revealed gram-positive, thin, and beaded branching filaments (Figure 4). Based on colony characteristics, physiological properties, and biochemical tests, the isolate was identified as Nocardia. Results of further investigations employing polymerase chain reaction analysis of the skin specimen and bacterial colonies using a Nocardia genus 596-bp fragment of 16S ribosomal RNA primer (forward primer NG1: 5’-ACCGACCACAAGGGG-3’, reverse primer NG2: 5’-GGTTGTAACCTCTTCGA-3’)6 were completely consistent with the reference for identification of N brasiliensis. Evaluation of these results led to a diagnosis of cutaneous nocardiosis after traumatic inoculation.
Because there was a high suspicion of actinophytosis or nocardiosis at admission, the patient received a combination antibiotic treatment with intravenous aqueous penicillin (4 million U every 4 hours) and oral trimethoprim-sulfamethoxazole (160/800 mg twice daily). Subsequently, treatment was changed to a combination of oral trimethoprim-sulfamethoxazole (160/800 mg twice daily) and moxifloxacin (400 mg once daily) based on pathogen identification and antibiotic sensitivity testing. After 1 month of treatment, the cutaneous lesions and left limb swelling dramatically improved and purulent drainage ceased, though some scarring occurred during the healing process. In addition, the mobility of the affected shoulder, elbow, and wrist joints slightly improved. Notable improvement in the mobility and swelling of the joints was observed at 6-month follow-up (Figure 1B). The patient continues to be monitored on an outpatient basis.
Cutaneous nocardiosis is a disfiguring granulomatous infection involving cutaneous and subcutaneous tissue that can progress to cause injury to viscera and bone.7 It has been called one of the great imitators because cutaneous nocardiosis can present in multiple forms,8,9 including mycetoma, sporotrichoid infection, superficial skin infection, and disseminated infection with cutaneous involvement. The differential diagnoses of cutaneous nocardiosis are broad and include tuberculosis; actinomycosis; deep fungal infections such as sporotrichosis, blastomycosis, phaeohyphomycosis, histoplasmosis, and coccidioidomycosis; other bacterial causes of cellulitis, abscess, or ecthyma; and malignancies.10 The principle method of diagnosis is the identification of Nocardia from the infection site.
Our patient ultimately was diagnosed with primary cutaneous nocardiosis resulting from a traumatic injury to the skin that was contaminated with soil. The clinical manifestation pattern was a compound type, including both mycetoma and sporotrichoid infections. Initially, Nocardia mycetoma occurred with subcutaneous infection by direct extension10,11 and appeared as dense, predominantly painless, swollen lesions. After 4 years, the skin lesions continued to spread linearly to the patient’s upper arm and forearm and manifested as the sporotrichoid infection type with painful swollen lesions at the site of inoculation and painful enlargement of the ipsilateral axillary lymph node.
Although nocardiosis is found worldwide, it is endemic to tropical and subtropical regions such as India, Africa, Southeast Asia, and Latin America.12 Nocardiosis most often is observed in individuals aged 20 to 40 years. It affects men more than women, and it commonly occurs in field laborers and cultivators whose occupations involve direct contact with the soil.13 Most lesions are found on the lower extremities, though localized nocardiosis infections can occur in other areas such as the neck, breasts, back, buttocks, and elbows.
Our patient initially was misdiagnosed, and treatment was delayed for several reasons. First, nocardiosis is not common in China, and most clinicians are unfamiliar with the disease. Second, the related lesions do not have specific features, and our patient had a complex clinical presentation that included mycetoma and sporotrichoid infection. Third, the characteristic grain of Nocardia species is small but that of N brasiliensis is even smaller (approximately 0.1–0.2 mm in diameter), which makes visualization difficult in both histopathologic and microbiologic examinations.14 The histopathologic examination results of our patient in the local hospital were nonspecific. Fourth, our patient did not initially go to the hospital but instead purchased some over-the-counter antibiotic ointments for external application because the lesions were painless. Moreover, microbiologic smear and culture examinations were not conducted in the local hospital before administering antituberculosis treatment to the patient. Instead, a polymerase chain reaction examination of skin lesion tissue for tubercle bacilli and atypical mycobacteria was negative. These findings imply that the traditional microbial smear and culture evaluations cannot be omitted. Furthermore, culture examinations should be conducted on multiple skin tissue and purulent fluid specimens to increase the likelihood of detection. These cultures should be monitored for at least 2 to 4 weeks because Nocardia is a slow-growing organism.10
The optimal antimicrobial treatment regimens for nocardiosis have not been firmly established.15 Trimethoprim-sulfamethoxazole is regarded as the first-line antimicrobial agent for treatment of nocardial infections. The optimal duration of antimicrobial therapy for nocardiosis also has not been determined, and the treatment regimen depends on the severity and extent of the infection as well as on the presence of infection-related complications. The main complication is bone involvement. Notable bony changes include periosteal thickening, osteoporosis, and osteolysis.
We considered the severity of skin lesions and bone marrow invasion in our patient and planned to treat him continually with oral trimethoprim-sulfamethoxazole according to the in vitro drug susceptibility test. The patient showed clinical improvement after 1 month of treatment, and he continued to improve after 6 months of treatment. To prevent recurrence, we found it necessary to treat the patient with a long-term antibiotic course over 6 to 12 months.16
Cutaneous nocardiosis remains a diagnostic challenge because of its nonspecific and diverse clinical and histopathological presentations. Diagnosis is further complicated by the inherent difficulty of cultivating and identifying the clinical isolate in the laboratory. A high degree of clinical suspicion followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Brown-Elliott BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
- Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. 2018;114:369-384.
- Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
- Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
- Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol. 1999;37:99-102.
- Nguyen NM, Sink JR, Carter AJ, et al. Nocardiosis incognito: primary cutaneous nocardiosis with extension to myositis and pleural infection. JAAD Case Rep. 2018;4:33-35.
- Sharna NL, Mahajan VK, Agarwal S, et al. Nocardial mycetoma: diverse clinical presentations. Indian J Dermatol Venereol Leprol. 2008;74:635-640.
- Huang L, Chen X, Xu H, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. 2019;94:165-172.
- Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis. 2014;8:E3102.
- Welsh O, Vero-Cabrera L, Salinas-Carmona MC. Mycetoma. Clin Dermatol. 2007;25:195-202.
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883.
- Emmanuel P, Dumre SP, John S, et al. Mycetoma: a clinical dilemma in resource limited settings. Ann Clin Microbiol Antimicrob. 2018;17:35.
- Reis CMS, Reis-Filho EGM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol. 2018;93:8-18.
- Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403-407.
- Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Current treatment for Nocardia infections. Expert Opin Pharmacother. 2013;14:2387-2398.
To the Editor:
The organisms of the genus Nocardia are gram-positive, ubiquitous, aerobic actinomycetes found worldwide in soil, decaying organic material, and water.1 The genus Nocardia includes more than 50 species; some species, such as Nocardia asteroides, Nocardia farcinica, Nocardia nova, and Nocardia brasiliensis, are the cause of nocardiosis in humans and animals.2,3 Nocardiosis is a rare and opportunistic infection that predominantly affects immunocompromised individuals; however, up to 30% of infections can occur in immunocompetent hosts.4 Nocardiosis can manifest in 3 disease forms: cutaneous, pulmonary, or disseminated. Cutaneous nocardiosis commonly develops in immunocompetent individuals who have experienced a predisposing traumatic injury to the skin,5 and it can exhibit a diverse variety of clinical manifestations, making diagnosis difficult. We describe a case of serious progressive primary cutaneous nocardiosis with an unusual presentation in an immunocompetent patient.
A 26-year-old immunocompetent man presented with pain, swelling, nodules, abscesses, ulcers, and sinus drainage of the left arm. The left elbow lesion initially developed at the site of a trauma 6 years prior that was painless but was contaminated with mossy soil. The condition slowly progressed over the next 2 years, and the patient experienced increased swelling and eventually developed multiple draining sinus tracts. Over the next 4 years, the lesions multiplied, spreading to the forearm and upper arm; associated severe pain and swelling at the elbow and wrist joint developed. The patient sought medical care at a local hospital and subsequently was diagnosed with suspected cutaneous tuberculosis. The patient was empirically treated with a 6-month course of isoniazid, rifampicin, pyrazinamide, and ethambutol; however, the lesions continued to progress and worsen. The patient had to stop antibiotic treatment because of substantially elevated alanine aminotransferase and aspartate aminotransferase levels.
He subsequently was evaluated at our hospital. He had no notable medical history and was afebrile. Physical examination revealed multiple erythematous nodules, abscesses, and ulcers on the left arm. There were several nodules with open sinus tracts and seropurulent crusts along with numerous atrophic, ovoid, stellate scars. Other nodules and ulcers with purulent drainage were located along the lymphatic nodes extending up the patient’s left forearm (Figure 1A). The yellowish-white pus discharge from several active sinuses contained no apparent granules. The lesions were densely distributed along the elbow, wrist, and shoulder, which resulted in associated skin swelling and restricted joint movement. The left axillary lymph nodes were enlarged.

Laboratory analyses revealed a hemoglobin level of 9.6 g/dL (reference range, 13–17.5 g/dL), platelet count of 621×109/L (reference range, 125–350×109/L), and leukocyte count of 14.3×109/L (reference range, 3.5–9.5 ×109/L). C-reactive protein level was 88.4 mg/L (reference range, 0–10 mg/L). Blood, renal, and liver tests, as well as tumor marker, peripheral blood lymphocyte subset, immunoglobulin, and complement results were within reference ranges. Results for Treponema pallidum and HIV antibody tests were negative. Hepatitis B virus markers were positive for hepatitis B surface antigen, hepatitis B e antigen, and hepatitis B core antibody, and the serum concentration of hepatitis B virus DNA was 3.12×107 IU/mL (reference range, <5×102 IU/mL). Computed tomography of the chest and cranium were unremarkable. Ultrasonography of the left arm revealed multiple vertical sinus tracts and several horizontal communicating branches that were accompanied by worm-eaten bone destruction (Figure 2).

Additional testing included histopathologic staining of a skin tissue specimen—hematoxylin and eosin, periodic acid–Schiff, and acid-fast staining—showed nonspecific, diffuse, inflammatory cell infiltration suggestive of chronic suppurative granuloma (Figure 3) but failed to reveal any special strains or organisms. Gram stain examination of the purulent fluid collected from the subcutaneous tissue showed no apparent positive bacillus or filamentous granules. The specimen was then inoculated on Sabouraud dextrose agar and Lowenstein-Jensen medium for fungus and mycobacteria culture, respectively. After 5 days, chalky, yellow, adherent colonies were observed on the Löwenstein-Jensen medium, and after 26 days, yellow crinkled colonies were observed on Sabouraud dextrose agar. The colonies were then inoculated on Columbia blood agar and incubated for 1 week to aid in the identification of organisms. Growth of yellow colonies that were adherent to the agar, moist, and smooth with a velvety surface, as well as a characteristic moldy odor resulted. Gram staining revealed gram-positive, thin, and beaded branching filaments (Figure 4). Based on colony characteristics, physiological properties, and biochemical tests, the isolate was identified as Nocardia. Results of further investigations employing polymerase chain reaction analysis of the skin specimen and bacterial colonies using a Nocardia genus 596-bp fragment of 16S ribosomal RNA primer (forward primer NG1: 5’-ACCGACCACAAGGGG-3’, reverse primer NG2: 5’-GGTTGTAACCTCTTCGA-3’)6 were completely consistent with the reference for identification of N brasiliensis. Evaluation of these results led to a diagnosis of cutaneous nocardiosis after traumatic inoculation.

Because there was a high suspicion of actinophytosis or nocardiosis at admission, the patient received a combination antibiotic treatment with intravenous aqueous penicillin (4 million U every 4 hours) and oral trimethoprim-sulfamethoxazole (160/800 mg twice daily). Subsequently, treatment was changed to a combination of oral trimethoprim-sulfamethoxazole (160/800 mg twice daily) and moxifloxacin (400 mg once daily) based on pathogen identification and antibiotic sensitivity testing. After 1 month of treatment, the cutaneous lesions and left limb swelling dramatically improved and purulent drainage ceased, though some scarring occurred during the healing process. In addition, the mobility of the affected shoulder, elbow, and wrist joints slightly improved. Notable improvement in the mobility and swelling of the joints was observed at 6-month follow-up (Figure 1B). The patient continues to be monitored on an outpatient basis.

Cutaneous nocardiosis is a disfiguring granulomatous infection involving cutaneous and subcutaneous tissue that can progress to cause injury to viscera and bone.7 It has been called one of the great imitators because cutaneous nocardiosis can present in multiple forms,8,9 including mycetoma, sporotrichoid infection, superficial skin infection, and disseminated infection with cutaneous involvement. The differential diagnoses of cutaneous nocardiosis are broad and include tuberculosis; actinomycosis; deep fungal infections such as sporotrichosis, blastomycosis, phaeohyphomycosis, histoplasmosis, and coccidioidomycosis; other bacterial causes of cellulitis, abscess, or ecthyma; and malignancies.10 The principle method of diagnosis is the identification of Nocardia from the infection site.
Our patient ultimately was diagnosed with primary cutaneous nocardiosis resulting from a traumatic injury to the skin that was contaminated with soil. The clinical manifestation pattern was a compound type, including both mycetoma and sporotrichoid infections. Initially, Nocardia mycetoma occurred with subcutaneous infection by direct extension10,11 and appeared as dense, predominantly painless, swollen lesions. After 4 years, the skin lesions continued to spread linearly to the patient’s upper arm and forearm and manifested as the sporotrichoid infection type with painful swollen lesions at the site of inoculation and painful enlargement of the ipsilateral axillary lymph node.
Although nocardiosis is found worldwide, it is endemic to tropical and subtropical regions such as India, Africa, Southeast Asia, and Latin America.12 Nocardiosis most often is observed in individuals aged 20 to 40 years. It affects men more than women, and it commonly occurs in field laborers and cultivators whose occupations involve direct contact with the soil.13 Most lesions are found on the lower extremities, though localized nocardiosis infections can occur in other areas such as the neck, breasts, back, buttocks, and elbows.
Our patient initially was misdiagnosed, and treatment was delayed for several reasons. First, nocardiosis is not common in China, and most clinicians are unfamiliar with the disease. Second, the related lesions do not have specific features, and our patient had a complex clinical presentation that included mycetoma and sporotrichoid infection. Third, the characteristic grain of Nocardia species is small but that of N brasiliensis is even smaller (approximately 0.1–0.2 mm in diameter), which makes visualization difficult in both histopathologic and microbiologic examinations.14 The histopathologic examination results of our patient in the local hospital were nonspecific. Fourth, our patient did not initially go to the hospital but instead purchased some over-the-counter antibiotic ointments for external application because the lesions were painless. Moreover, microbiologic smear and culture examinations were not conducted in the local hospital before administering antituberculosis treatment to the patient. Instead, a polymerase chain reaction examination of skin lesion tissue for tubercle bacilli and atypical mycobacteria was negative. These findings imply that the traditional microbial smear and culture evaluations cannot be omitted. Furthermore, culture examinations should be conducted on multiple skin tissue and purulent fluid specimens to increase the likelihood of detection. These cultures should be monitored for at least 2 to 4 weeks because Nocardia is a slow-growing organism.10
The optimal antimicrobial treatment regimens for nocardiosis have not been firmly established.15 Trimethoprim-sulfamethoxazole is regarded as the first-line antimicrobial agent for treatment of nocardial infections. The optimal duration of antimicrobial therapy for nocardiosis also has not been determined, and the treatment regimen depends on the severity and extent of the infection as well as on the presence of infection-related complications. The main complication is bone involvement. Notable bony changes include periosteal thickening, osteoporosis, and osteolysis.
We considered the severity of skin lesions and bone marrow invasion in our patient and planned to treat him continually with oral trimethoprim-sulfamethoxazole according to the in vitro drug susceptibility test. The patient showed clinical improvement after 1 month of treatment, and he continued to improve after 6 months of treatment. To prevent recurrence, we found it necessary to treat the patient with a long-term antibiotic course over 6 to 12 months.16
Cutaneous nocardiosis remains a diagnostic challenge because of its nonspecific and diverse clinical and histopathological presentations. Diagnosis is further complicated by the inherent difficulty of cultivating and identifying the clinical isolate in the laboratory. A high degree of clinical suspicion followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.
To the Editor:
The organisms of the genus Nocardia are gram-positive, ubiquitous, aerobic actinomycetes found worldwide in soil, decaying organic material, and water.1 The genus Nocardia includes more than 50 species; some species, such as Nocardia asteroides, Nocardia farcinica, Nocardia nova, and Nocardia brasiliensis, are the cause of nocardiosis in humans and animals.2,3 Nocardiosis is a rare and opportunistic infection that predominantly affects immunocompromised individuals; however, up to 30% of infections can occur in immunocompetent hosts.4 Nocardiosis can manifest in 3 disease forms: cutaneous, pulmonary, or disseminated. Cutaneous nocardiosis commonly develops in immunocompetent individuals who have experienced a predisposing traumatic injury to the skin,5 and it can exhibit a diverse variety of clinical manifestations, making diagnosis difficult. We describe a case of serious progressive primary cutaneous nocardiosis with an unusual presentation in an immunocompetent patient.
A 26-year-old immunocompetent man presented with pain, swelling, nodules, abscesses, ulcers, and sinus drainage of the left arm. The left elbow lesion initially developed at the site of a trauma 6 years prior that was painless but was contaminated with mossy soil. The condition slowly progressed over the next 2 years, and the patient experienced increased swelling and eventually developed multiple draining sinus tracts. Over the next 4 years, the lesions multiplied, spreading to the forearm and upper arm; associated severe pain and swelling at the elbow and wrist joint developed. The patient sought medical care at a local hospital and subsequently was diagnosed with suspected cutaneous tuberculosis. The patient was empirically treated with a 6-month course of isoniazid, rifampicin, pyrazinamide, and ethambutol; however, the lesions continued to progress and worsen. The patient had to stop antibiotic treatment because of substantially elevated alanine aminotransferase and aspartate aminotransferase levels.
He subsequently was evaluated at our hospital. He had no notable medical history and was afebrile. Physical examination revealed multiple erythematous nodules, abscesses, and ulcers on the left arm. There were several nodules with open sinus tracts and seropurulent crusts along with numerous atrophic, ovoid, stellate scars. Other nodules and ulcers with purulent drainage were located along the lymphatic nodes extending up the patient’s left forearm (Figure 1A). The yellowish-white pus discharge from several active sinuses contained no apparent granules. The lesions were densely distributed along the elbow, wrist, and shoulder, which resulted in associated skin swelling and restricted joint movement. The left axillary lymph nodes were enlarged.

Laboratory analyses revealed a hemoglobin level of 9.6 g/dL (reference range, 13–17.5 g/dL), platelet count of 621×109/L (reference range, 125–350×109/L), and leukocyte count of 14.3×109/L (reference range, 3.5–9.5 ×109/L). C-reactive protein level was 88.4 mg/L (reference range, 0–10 mg/L). Blood, renal, and liver tests, as well as tumor marker, peripheral blood lymphocyte subset, immunoglobulin, and complement results were within reference ranges. Results for Treponema pallidum and HIV antibody tests were negative. Hepatitis B virus markers were positive for hepatitis B surface antigen, hepatitis B e antigen, and hepatitis B core antibody, and the serum concentration of hepatitis B virus DNA was 3.12×107 IU/mL (reference range, <5×102 IU/mL). Computed tomography of the chest and cranium were unremarkable. Ultrasonography of the left arm revealed multiple vertical sinus tracts and several horizontal communicating branches that were accompanied by worm-eaten bone destruction (Figure 2).

Additional testing included histopathologic staining of a skin tissue specimen—hematoxylin and eosin, periodic acid–Schiff, and acid-fast staining—showed nonspecific, diffuse, inflammatory cell infiltration suggestive of chronic suppurative granuloma (Figure 3) but failed to reveal any special strains or organisms. Gram stain examination of the purulent fluid collected from the subcutaneous tissue showed no apparent positive bacillus or filamentous granules. The specimen was then inoculated on Sabouraud dextrose agar and Lowenstein-Jensen medium for fungus and mycobacteria culture, respectively. After 5 days, chalky, yellow, adherent colonies were observed on the Löwenstein-Jensen medium, and after 26 days, yellow crinkled colonies were observed on Sabouraud dextrose agar. The colonies were then inoculated on Columbia blood agar and incubated for 1 week to aid in the identification of organisms. Growth of yellow colonies that were adherent to the agar, moist, and smooth with a velvety surface, as well as a characteristic moldy odor resulted. Gram staining revealed gram-positive, thin, and beaded branching filaments (Figure 4). Based on colony characteristics, physiological properties, and biochemical tests, the isolate was identified as Nocardia. Results of further investigations employing polymerase chain reaction analysis of the skin specimen and bacterial colonies using a Nocardia genus 596-bp fragment of 16S ribosomal RNA primer (forward primer NG1: 5’-ACCGACCACAAGGGG-3’, reverse primer NG2: 5’-GGTTGTAACCTCTTCGA-3’)6 were completely consistent with the reference for identification of N brasiliensis. Evaluation of these results led to a diagnosis of cutaneous nocardiosis after traumatic inoculation.

Because there was a high suspicion of actinophytosis or nocardiosis at admission, the patient received a combination antibiotic treatment with intravenous aqueous penicillin (4 million U every 4 hours) and oral trimethoprim-sulfamethoxazole (160/800 mg twice daily). Subsequently, treatment was changed to a combination of oral trimethoprim-sulfamethoxazole (160/800 mg twice daily) and moxifloxacin (400 mg once daily) based on pathogen identification and antibiotic sensitivity testing. After 1 month of treatment, the cutaneous lesions and left limb swelling dramatically improved and purulent drainage ceased, though some scarring occurred during the healing process. In addition, the mobility of the affected shoulder, elbow, and wrist joints slightly improved. Notable improvement in the mobility and swelling of the joints was observed at 6-month follow-up (Figure 1B). The patient continues to be monitored on an outpatient basis.

Cutaneous nocardiosis is a disfiguring granulomatous infection involving cutaneous and subcutaneous tissue that can progress to cause injury to viscera and bone.7 It has been called one of the great imitators because cutaneous nocardiosis can present in multiple forms,8,9 including mycetoma, sporotrichoid infection, superficial skin infection, and disseminated infection with cutaneous involvement. The differential diagnoses of cutaneous nocardiosis are broad and include tuberculosis; actinomycosis; deep fungal infections such as sporotrichosis, blastomycosis, phaeohyphomycosis, histoplasmosis, and coccidioidomycosis; other bacterial causes of cellulitis, abscess, or ecthyma; and malignancies.10 The principle method of diagnosis is the identification of Nocardia from the infection site.
Our patient ultimately was diagnosed with primary cutaneous nocardiosis resulting from a traumatic injury to the skin that was contaminated with soil. The clinical manifestation pattern was a compound type, including both mycetoma and sporotrichoid infections. Initially, Nocardia mycetoma occurred with subcutaneous infection by direct extension10,11 and appeared as dense, predominantly painless, swollen lesions. After 4 years, the skin lesions continued to spread linearly to the patient’s upper arm and forearm and manifested as the sporotrichoid infection type with painful swollen lesions at the site of inoculation and painful enlargement of the ipsilateral axillary lymph node.
Although nocardiosis is found worldwide, it is endemic to tropical and subtropical regions such as India, Africa, Southeast Asia, and Latin America.12 Nocardiosis most often is observed in individuals aged 20 to 40 years. It affects men more than women, and it commonly occurs in field laborers and cultivators whose occupations involve direct contact with the soil.13 Most lesions are found on the lower extremities, though localized nocardiosis infections can occur in other areas such as the neck, breasts, back, buttocks, and elbows.
Our patient initially was misdiagnosed, and treatment was delayed for several reasons. First, nocardiosis is not common in China, and most clinicians are unfamiliar with the disease. Second, the related lesions do not have specific features, and our patient had a complex clinical presentation that included mycetoma and sporotrichoid infection. Third, the characteristic grain of Nocardia species is small but that of N brasiliensis is even smaller (approximately 0.1–0.2 mm in diameter), which makes visualization difficult in both histopathologic and microbiologic examinations.14 The histopathologic examination results of our patient in the local hospital were nonspecific. Fourth, our patient did not initially go to the hospital but instead purchased some over-the-counter antibiotic ointments for external application because the lesions were painless. Moreover, microbiologic smear and culture examinations were not conducted in the local hospital before administering antituberculosis treatment to the patient. Instead, a polymerase chain reaction examination of skin lesion tissue for tubercle bacilli and atypical mycobacteria was negative. These findings imply that the traditional microbial smear and culture evaluations cannot be omitted. Furthermore, culture examinations should be conducted on multiple skin tissue and purulent fluid specimens to increase the likelihood of detection. These cultures should be monitored for at least 2 to 4 weeks because Nocardia is a slow-growing organism.10
The optimal antimicrobial treatment regimens for nocardiosis have not been firmly established.15 Trimethoprim-sulfamethoxazole is regarded as the first-line antimicrobial agent for treatment of nocardial infections. The optimal duration of antimicrobial therapy for nocardiosis also has not been determined, and the treatment regimen depends on the severity and extent of the infection as well as on the presence of infection-related complications. The main complication is bone involvement. Notable bony changes include periosteal thickening, osteoporosis, and osteolysis.
We considered the severity of skin lesions and bone marrow invasion in our patient and planned to treat him continually with oral trimethoprim-sulfamethoxazole according to the in vitro drug susceptibility test. The patient showed clinical improvement after 1 month of treatment, and he continued to improve after 6 months of treatment. To prevent recurrence, we found it necessary to treat the patient with a long-term antibiotic course over 6 to 12 months.16
Cutaneous nocardiosis remains a diagnostic challenge because of its nonspecific and diverse clinical and histopathological presentations. Diagnosis is further complicated by the inherent difficulty of cultivating and identifying the clinical isolate in the laboratory. A high degree of clinical suspicion followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Brown-Elliott BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
- Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. 2018;114:369-384.
- Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
- Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
- Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol. 1999;37:99-102.
- Nguyen NM, Sink JR, Carter AJ, et al. Nocardiosis incognito: primary cutaneous nocardiosis with extension to myositis and pleural infection. JAAD Case Rep. 2018;4:33-35.
- Sharna NL, Mahajan VK, Agarwal S, et al. Nocardial mycetoma: diverse clinical presentations. Indian J Dermatol Venereol Leprol. 2008;74:635-640.
- Huang L, Chen X, Xu H, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. 2019;94:165-172.
- Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis. 2014;8:E3102.
- Welsh O, Vero-Cabrera L, Salinas-Carmona MC. Mycetoma. Clin Dermatol. 2007;25:195-202.
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883.
- Emmanuel P, Dumre SP, John S, et al. Mycetoma: a clinical dilemma in resource limited settings. Ann Clin Microbiol Antimicrob. 2018;17:35.
- Reis CMS, Reis-Filho EGM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol. 2018;93:8-18.
- Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403-407.
- Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Current treatment for Nocardia infections. Expert Opin Pharmacother. 2013;14:2387-2398.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Brown-Elliott BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
- Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. 2018;114:369-384.
- Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
- Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
- Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol. 1999;37:99-102.
- Nguyen NM, Sink JR, Carter AJ, et al. Nocardiosis incognito: primary cutaneous nocardiosis with extension to myositis and pleural infection. JAAD Case Rep. 2018;4:33-35.
- Sharna NL, Mahajan VK, Agarwal S, et al. Nocardial mycetoma: diverse clinical presentations. Indian J Dermatol Venereol Leprol. 2008;74:635-640.
- Huang L, Chen X, Xu H, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. 2019;94:165-172.
- Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis. 2014;8:E3102.
- Welsh O, Vero-Cabrera L, Salinas-Carmona MC. Mycetoma. Clin Dermatol. 2007;25:195-202.
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883.
- Emmanuel P, Dumre SP, John S, et al. Mycetoma: a clinical dilemma in resource limited settings. Ann Clin Microbiol Antimicrob. 2018;17:35.
- Reis CMS, Reis-Filho EGM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol. 2018;93:8-18.
- Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403-407.
- Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Current treatment for Nocardia infections. Expert Opin Pharmacother. 2013;14:2387-2398.
Practice Points
- Although unusual, cutaneous nocardiosis can present with both mycetoma and sporotrichoid infection, which should be treated based on pathogen identification and antibiotic sensitivity testing.
- A high degree of clinical suspicion by clinicians followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.